Changes in Proteolytic System Activity Due to Varroa destructor Infestation in Apis mellifera Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting Honeybees for Experiments
2.2. Fat Body and Hemolymph Collection
2.3. Determination of Protein Concentration
2.4. Determination of Proteolytic Activity and Protease Inhibitor Activity
2.5. Statistical Analysis
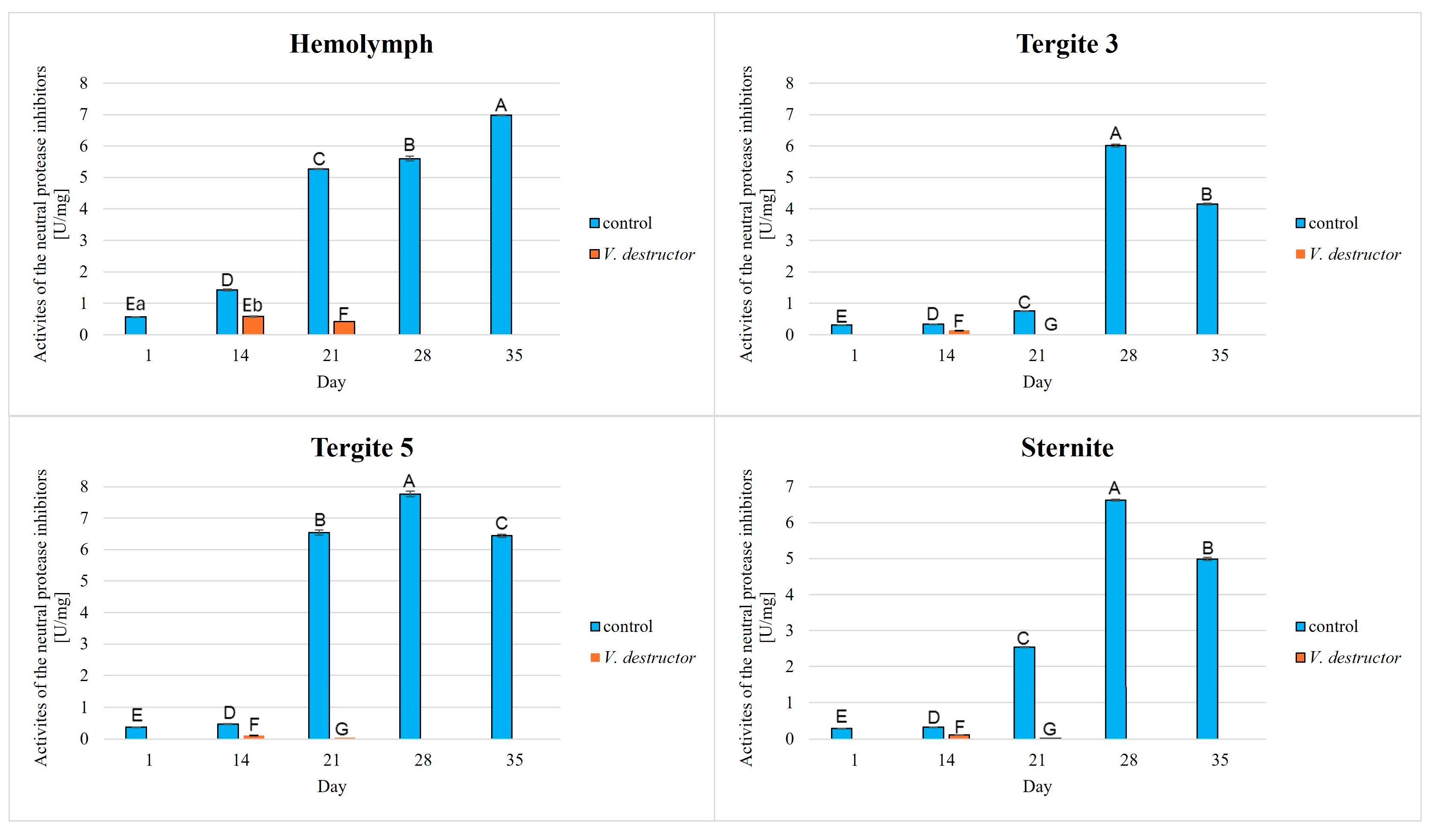
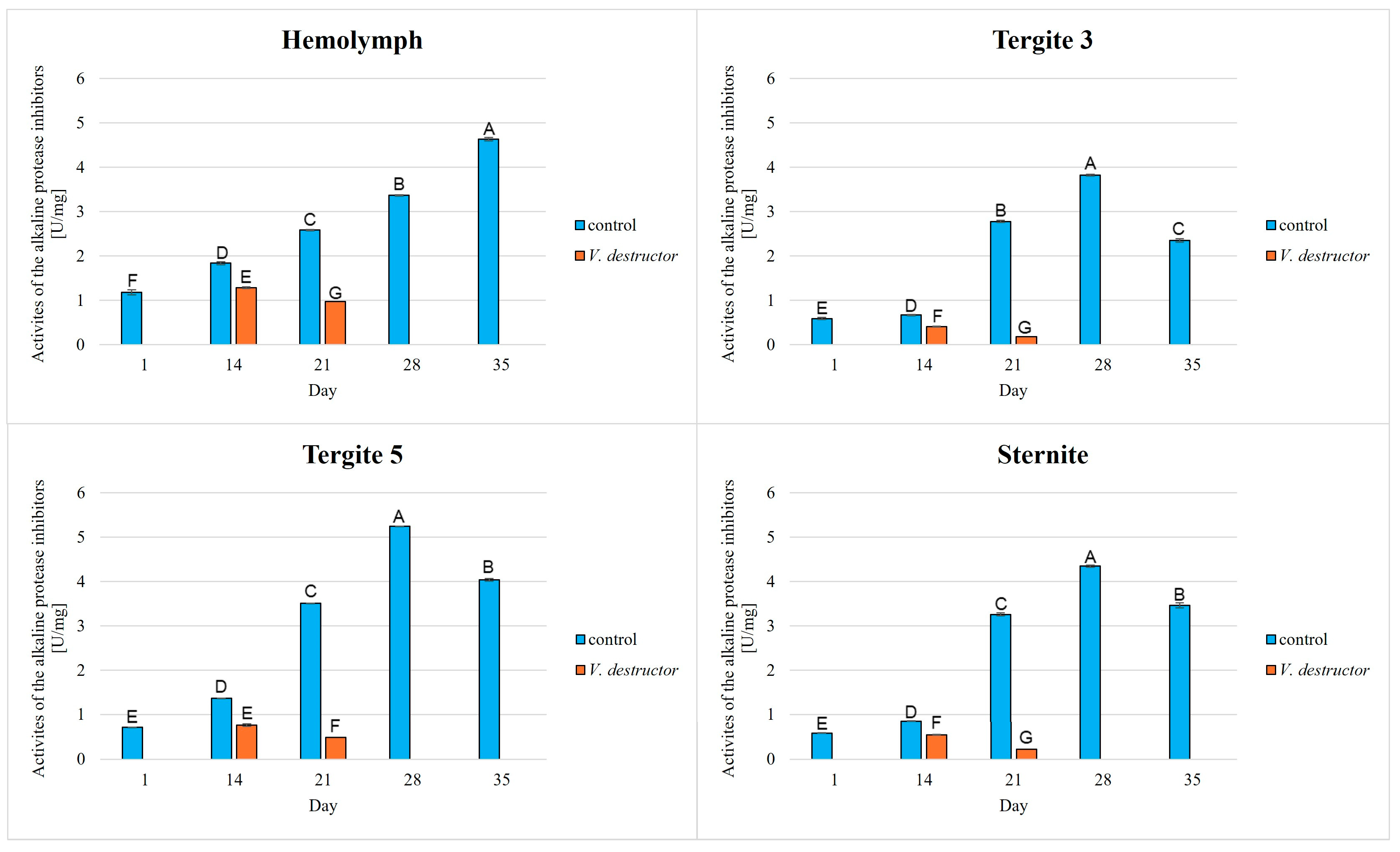
3. Results
4. Discussion
4.1. Host (Honeybee)—Pathogen/Parasite Interaction
4.2. The Relationship Between the Proteolytic System, Tissue Location and Aging Processes in the Workers
5. Conclusions
- Our results showed that the activities of acidic, neutral, and alkaline proteases, and their inhibitors were highest in the fat body of tergite 5. Therefore, the fat body of tergite 5 not only plays a significant role in the antioxidant defence but also in other mechanisms, such as the activation of the proteolytic system. Understanding these various (other) mechanisms is crucial, particularly in the context of immunity and aging in bees. It is worthwhile to focus on analyzing the fat body from this segment in the future, as it may provide valuable information on bee colony health.
- V. destructor reduces the activities of proteases and their inhibitors, which in turn causes an accelerated aging of the bees. It is important to monitor bee colonies for infestations with this parasite and to develop effective methods of combating this parasite, as it leads to the decline of bee colonies.
- Serine proteases were the most active among these enzymes. Perhaps they could become a new indicator of immunity, which could help monitor the condition of bees and relatively quickly identify emerging stressors, such as pathogens or pesticides that threaten bee colonies.
- Changes in the proteolytic system are one of the effects of aging. Therefore, it is important to develop strategies for the early detection of disturbances in the proteolytic system. It is also crucial to create new supplements and management methods for honey bee colonies that enhance immunity while simultaneously slowing the aging process.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morfin, N.; Anguiano-Baez, R.; Guzman-Novoa, E. Honey bee (Apis mellifera) immunity. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 521–533. [Google Scholar] [CrossRef]
- Strachecka, A.; Kuszewska, K.; Olszewski, K.; Skowronek, P.; Grzybek, M.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Activities of antioxidant and proteolytic systems and biomarkers in the fat body and hemolymph of young Apis mellifera females. Animals 2022, 12, 1121. [Google Scholar] [CrossRef]
- Larsen, A.; Reynaldi, F.J.; Guzmán-Novoa, E. Fundaments of the honey bee (Apis mellifera) immune system. Review. Rev. Mex. Cienc. Pecu. 2019, 10, 705–728. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. CBD supplementation has a positive effect on the activity of the proteolytic system and biochemical markers of honey bees (Apis mellifera) in the apiary. Animals 2022, 12, 2313. [Google Scholar] [CrossRef]
- Rai, M.; Curley, M.; Coleman, Z.; Demontis, F. Contribution of proteases to the hallmarks of aging and to age-related neurodegeneration. Aging Cell 2022, 21, e13603. [Google Scholar] [CrossRef]
- Zou, Z.; Lopez, D.L.; Kanost, M.R.; Evans, J.D.; Jiang, H. Comparative analysis of serine protease-related genes in the honey bee genome: Possible involvement in embryonic development and innate immunity. Insect Mol. Biol. 2006, 15, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Baggerman, G.; Vierstraete, E.; Schoofs, L.; Verleyen, P. The hemolymph proteome of the honeybee: Gel-based or gel-free? Proteomics 2009, 9, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Honey bee proteolytic system and behavior parameters under the influence of an electric field at 50 Hz and variable intensities for a long exposure time. Animals 2021, 11, 863. [Google Scholar] [CrossRef]
- Bryś, M.S.; Olszewski, K.; Strachecka, A. The relationship between pollen monodiets and the activities of proteolytic systems in the fat body and hemolymph of honeybee workers. PLoS ONE 2025, 20, e0326175. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Zhang, G.; Zareie, R.; Schmidt, O. A Serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Wan, H.; Zou, F.M.; Choi, Y.S.; Yoon, H.J.; Kwon, H.W.; Je, Y.H.; Jin, B.R. Anti-elastolytic activity of a honeybee (Apis cerana) chymotrypsin inhibitor. Biochem. Biophys. Res. Commun. 2013, 430, 144–149. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Strachecka, A.; Borsuk, G.; Olszewski, K. Workers of honey bee (Apis mellifera L.) reared in small-cell combs in apiary conditions show higher activity of the proteolytic system and lower protein concentrations on the cuticle surface than workers reared in standard-cell combs. Pol. J. Vet. Sci. 2025, 28, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Bajda, M.; Łoś, A.; Schulz, M.; Kasperek, K. Metaloproteazy ssaków i owadów. Med. Weter 2016, 72, 408–412. (In Polish) [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Wang, L.; Guo, P.; Tang, Z.; Sun, X.; Tang, X.; Wang, W.; Wang, L.; Cao, Y.; et al. Serpin-1a and Serpin-6 regulate the toll pathway immune homeostasis by synergistically inhibiting the Spätzle-processing enzyme CLIP2 in silkworm, Bombyx mori. PLoS Pathog. 2023, 19, e1011740. [Google Scholar] [CrossRef]
- Dmitryjuk, M.; Żółtowska, K.; Frączek, R.; Lipiński, Z. Esterases of Varroa destructor (Acari: Varroidae), parasitic mite of the honeybee. Exp. Appl. Acarol. 2014, 62, 499–510. [Google Scholar] [CrossRef] [PubMed]
- van Dooremalen, C.; Stam, E.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.; van Langevelde, F.; Blacquière, T. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 2013, 59, 487–493. [Google Scholar] [CrossRef]
- Cournoyer, A.; Plamondon, L.; Bau-Gaudreault, L.; Deschamps, A.; Dubreuil, P.; Benoit-Biancamano, M.O. Effects of Varroa destructor on hemolymph sugars and secondary infections in honeybees (Apis mellifera). Appl. Sci. 2022, 12, 11630. [Google Scholar] [CrossRef]
- Strachecka, A.; Borsuk, G.; Olszewski, K.; Paleolog, J.; Lipiński, Z. Proteolysis on the body surface of pyrethroid-sensitive and resistant Varroa destructor. Acta Parasitol. 2013, 58, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Piou, V.; Arafah, K.; Bocquet, M.; Bulet, P.; Vétillard, A. The proteomic content of Varroa destructor gut varies according to the developmental stage of its host. PLoS Pathog. 2024, 20, e1012802. [Google Scholar] [CrossRef]
- Colin, M.; Tchamitchian, M.; Bonmatin, J.M.; Di Pasquale, S. Presence of chitinase in adult Varroa destructor, an ectoparasitic mite of Apis mellifera. Exp. Appl. Acarol. 2001, 25, 947–955. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie 2004, 35, 419–430. [Google Scholar] [CrossRef]
- Pinto, F.; Souza, G.; Sanches, M.; Serrão, J. Parasitic effects of Varroa destructor (Acari: Varroidae) on hypopharyngeal glands of africanized Apis mellifera (Hymenoptera: Apidae). Sociobiology 2011, 58, 769–778. [Google Scholar]
- Zakaria, M.E.; Abd El-Wahab, T.E. Scanning electron microscopic (SEM) of hypopharyngeal and mandibular glands of honey bee workers infested by Varroa mites (Varroa destructor). Egypt. J. Agric. Sci. 2004, 55, 375–384. [Google Scholar] [CrossRef]
- Abd El-Wahab, T.E.; Zakaria, M.E.; Nour, M.E. Influence of the infestation by Varroa mite Varroa destructor on some antennal sense organs of the worker and drone honey bees Apis mellifera L. J. Appl. Sci. Res. 2006, 2, 80–85. [Google Scholar]
- Power, K.; Martano, M.; Altamura, G.; Piscopo, N.; Maiolino, P. Histopathological features of symptomatic and asymptomatic honeybees naturally infected by deformed wing virus. Pathogens 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Kunat-Budzyńska, M.; Staniszewska, P.; Olszewski, K.; Strachecka, A. Antioxidant activities in the hemolymph and fat body of physiologically and prematurely aging bees (Apis mellifera). Antioxidants 2025, 14, 373. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Staniszewska, P.; Olszewski, K.; Cytryńska, M.; Strachecka, A. The efficiency of the krebs cycle and the respiratory chain in physiologically and prematurely aging bees (Apis mellifera). Int. J. Mol. Sci. 2025, 26, 7294. [Google Scholar] [CrossRef]
- Olszewski, K. Biotechniczne Zwalczanie Warrozy-Współczesne Możliwości „Archaicznych” Metod, 1st ed.; Bee & Honey Sp z o.o.: Klecza Dolna, Poland, 2025; pp. 1–114. [Google Scholar]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “blood” and body surface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, Ł.; Chęć, M.; Skowronek, P.; Grabowski, M.; Persona, K.; Strachecka, A. Do the different life history strategies of ants and honeybees determine fat body morphology? Arthropod Struct. Dev. 2022, 69, 101186. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the subcuticular fat body in Apis mellifera females with different reproductive potentials. Sci. Rep. 2021, 11, 13887. [Google Scholar] [CrossRef]
- Schacterle, G.R.; Pollack, R.L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Lin, Y.H. Trypsin inhibitor and trypsin-like protease activity in air- or submergence-grown rice (Oryza sativa L.) coleoptiles. Plant Sci. 1995, 106, 43–54. [Google Scholar] [CrossRef]
- Grzywnowicz, K.; Ciołek, A.; Tabor, A.; Jaszek, M. Profiles of the body-surface proteolytic system of honey bee queens, workers and drones: Ontogenetic and seasonal changes in proteases and their natural inhibitors. Apidologie 2009, 40, 4–19. [Google Scholar] [CrossRef]
- Haloi, K.; Kalita, M.K.; Devi, D. Regulation and characterization of amylase enzyme secretion in the digestive tract of Antheraea assamensis Helfer. Indian J. Entomol. 2023, 85, 556–562. [Google Scholar] [CrossRef]
- Sharifi, M.; Chitgar, G.; Ghadamyari, M.; Ajamhasani, M. Identification and characterization of midgut digestive proteases from the rosaceous branch borer, Osphranteria coerulescens redtenbacher (Coleoptera: Cerambycidae). Rom. J. Biochem. 2012, 49, 33–37. [Google Scholar]
- Murawska, A.; Migdał, P.; Roman, A. Effects of plant protection products on biochemical markers in honey bees. Agriculture 2021, 11, 648. [Google Scholar] [CrossRef]
- Wessler, I.; Gärtner, H.A.; Michel-Schmidt, R.; Brochhausen, C.; Schmitz, L.; Anspach, L.; Grünewald, B.; Kirkpatrick, C.J. Honeybees produce millimolar concentrations of non-neuronal acetylcholine for breeding: Possible adverse effects of neonicotinoids. PLoS ONE 2016, 11, e0156886. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Changes in the honeybee antioxidant system after 12 h of exposure to electromagnetic field frequency of 50 Hz and variable intensity. Insects 2020, 11, 713. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Strachecka, A.; Roman, A. Effect of the electric field at 50 Hz and variable intensities on biochemical markers in the honey bee’s hemolymph. PLoS ONE 2021, 16, e0252858. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid markedly affects hemolymph proteolysis, biomarkers, DNA global methylation, and the cuticle proteolytic layer in western honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Strachecka, A.; Borsuk, G.; Olszewski, K. Effect of rearing in small-cell combs on activities of catalase and superoxide dismutase and total antioxidant capacity in the hemolymph of Apis mellifera workers. Antioxidants 2023, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, J.; Wei, Q.; Liu, F.; Cui, L.; Rueppell, O.; Xu, S. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef]
- Tewarson, N.C.; Jany, K.D. Determination of proteolytic activity in Varroa jacobsoni an ectoparasitic hemophagous mite of honey bees (Apis sp.). Apidologie 1982, 13, 383–389. [Google Scholar] [CrossRef]
- Zhou, H.; Duan, X.; Sun, C.; Huang, H.; Yang, M.; Huang, S.; Li, J. Salivary cystatin-L2-like of Varroa destructor causes lower metabolism activity and abnormal development in Apis Mellifera pupae. Animals 2023, 13, 3660. [Google Scholar] [CrossRef] [PubMed]
- Frączek, R.J.; Żółtowska, K.; Lipiński, Z.; Dmitryjuk, M. The mutual influence of proteins from Varroa destructor extracts and from honeybee haemolymph on their proteolytic activity-in vitro study. Acta Parasitol. 2013, 58, 317–323. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Malone, L.A.; Gatehouse, H.S. Effects of Nosema apis infection on honey bee (Apis Mellifera) digestive proteolytic enzyme activity. J. Invertebr. Pathol. 1998, 71, 169–174. [Google Scholar] [CrossRef]
- Doghuzlu, M.A.; Nabian, S.; Paghaleh, G.A.N.; Taheri, M.; Asadollahi, Z.; Akhzari, S. Zymography of proteases in honey bees (Apis mellifera) infected with Nosema ceranae. Vet. Glas. 2024, 78, 155–167. [Google Scholar] [CrossRef]
- Antúnez, K.; Anido, M.; Schlapp, G.; Evans, J.D.; Zunino, P. Characterization of secreted proteases of Paenibacillus larvae, potential virulence factors involved in honeybee larval infection. J. Invertebr. Pathol. 2009, 102, 129–132. [Google Scholar] [CrossRef]
- Pellegrini, M.C.; Zalazar, L.; Fuselli, S.R.; Ponce, A.G. Inhibitory action of essential oils against proteases activity of Paenibacillus larvae, the Etiological Agent of American Foulbrood Disease. Span. J. Agric. Res. 2017, 15, e0504. [Google Scholar] [CrossRef]
- Elfar, S.A.; Bahgat, I.M.; Shebl, M.A.; Lihoreau, M.; Tawfik, M.M. Intraspecific variability in proteomic profiles and biological activities of the honey bee hemolymph. Insects 2023, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Cabbri, R.; Ferlizza, E.; Nanetti, A.; Monari, E.; Andreani, G.; Galuppi, R.; Isani, G. Biomarkers of nutritional status in honeybee haemolymph: Effects of different biotechnical approaches for Varroa destructor treatment and wintering phase. Apidologie 2018, 49, 606–618. [Google Scholar] [CrossRef]
- Hashim, K.N.; Chin, K.Y.; Ahmad, F. The mechanism of honey in reversing metabolic syndrome. Molecules 2021, 26, 808. [Google Scholar] [CrossRef]
- Schilder, R.J.; Marden, J.H. Metabolic syndrome and obesity in an insect. Proc. Natl. Acad. Sci. USA 2006, 103, 18805–18809. [Google Scholar] [CrossRef]
- Bryś, M.S.; Staniec, B.; Strachecka, A. The effect of pollen monodiets on fat body morphology parameters and energy substrate levels in the fat body and hemolymph of Apis mellifera L. workers. Sci. Rep. 2024, 14, 15177. [Google Scholar] [CrossRef]
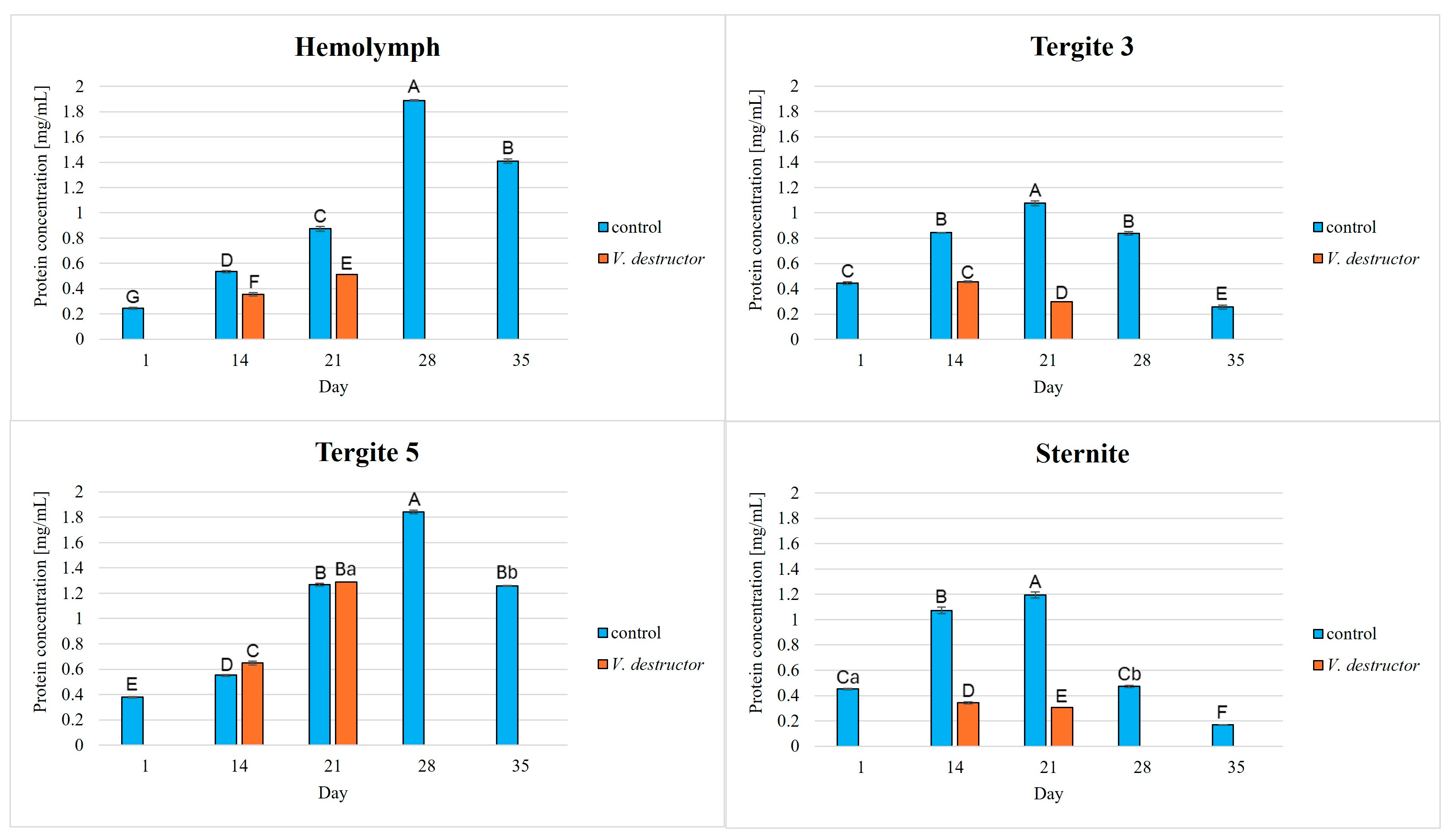
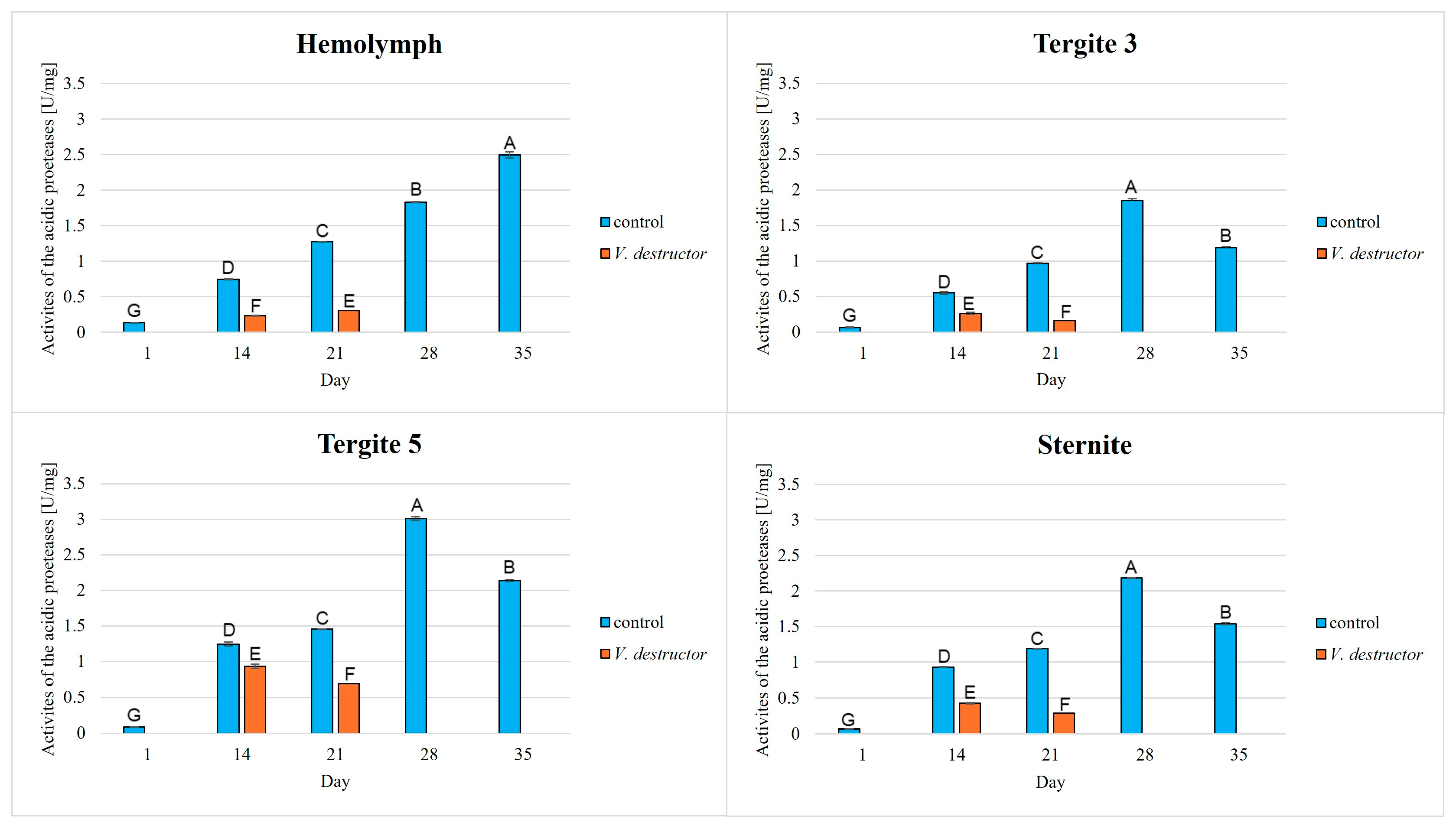
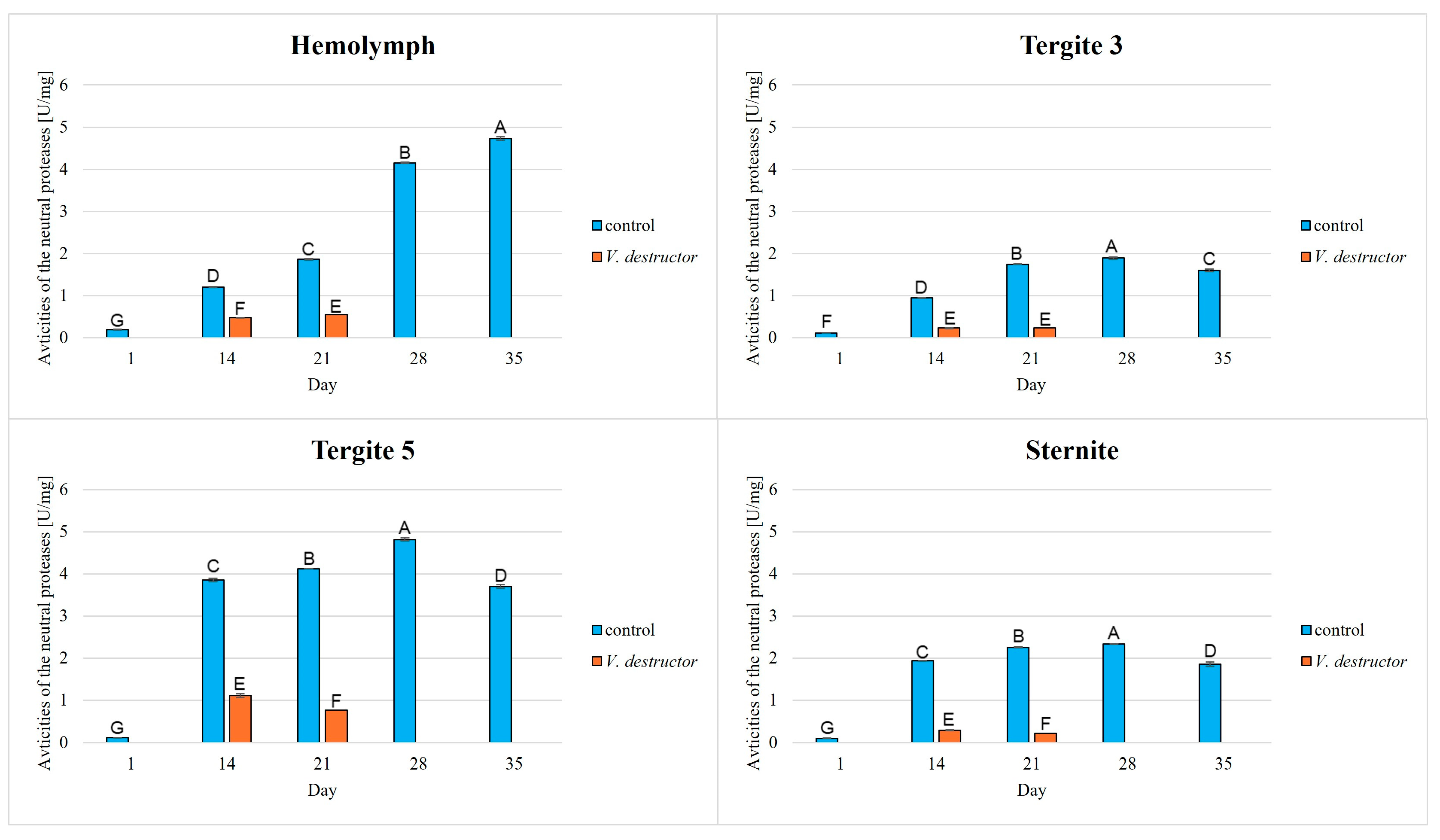
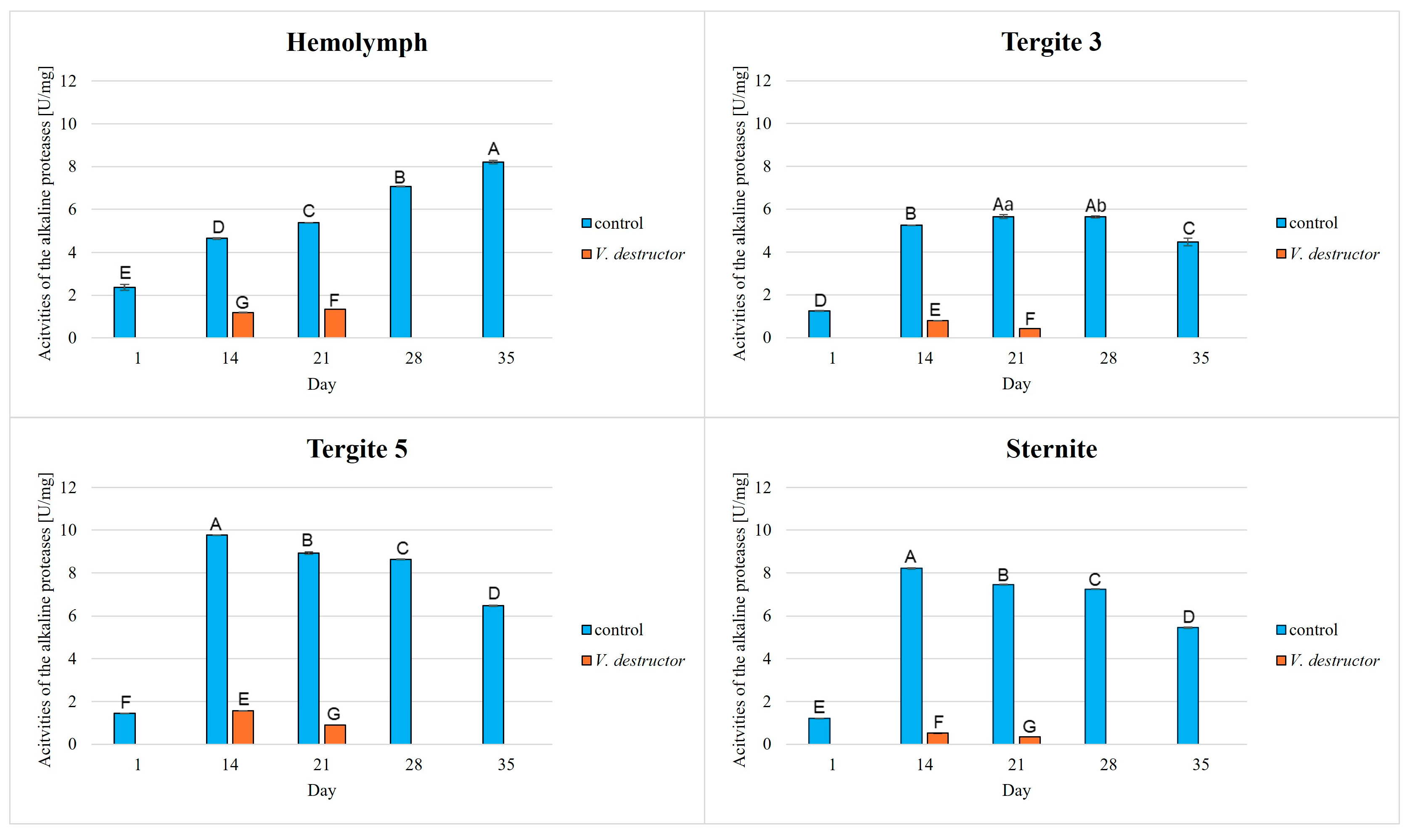


| Acidic Proteases | Neutral Proteases | Alkaline Proteases | Protein Concentration | |
|---|---|---|---|---|
| Hemolymph | H = 175.49 p < 0.001 | H = 175.19 p < 0.001 | H = 138.93 p < 0.001 | H = 181.96 p < 0.001 |
| Tergite 3 | H = 168.18 p < 0.001 | H = 146.89 p < 0.001 | H = 62.45 p < 0.001 | H = 78.56 p < 0.001 |
| Tergite 5 | H = 168.18 p < 0.001 | H = 131.76 p < 0.001 | H = 53.56 p < 0.001 | H = 187.20 p < 0.001 |
| Sternite | H = 168.17 p < 0.001 | H = 129.95 p < 0.001 | H = 22.83 p < 0.001 | H = 76.66 p < 0.001 |
| Acidic Protease Inhibitors | Neutral Protease Inhibitors | Alkaline Protease Inhibitors | ||
| Hemolymph | H = 141.16 p < 0.001 | H = 135.04 p < 0.001 | H = 138.01 p < 0.001 | |
| Tergite 3 | H = 124.71 p < 0.001 | H = 132.38 p < 0.001 | H = 98.88 p < 0.001 | |
| Tergite 5 | H = 125.14 p < 0.001 | H = 101.75 p < 0.001 | H = 141.32 p < 0.001 | |
| Sternite | H = 131.60 p < 0.001 | H = 131.62 p < 0.001 | H = 131.43 p < 0.001 |
| Group | Age (Days) | Acidic Proteases | Neutral Proteases | Alkaline Proteases | Protein Concentration |
|---|---|---|---|---|---|
| control | 1 | H = 100.45 p < 0.001 | H = 92.51 p < 0.001 | H = 100.85 p < 0.001 | H = 100.62 p < 0.001 |
| control | 14 | H = 111.57 p < 0.001 | H = 111.55 p < 0.001 | H = 111.58 p < 0.001 | H = 100.98 p < 0.001 |
| V. destructor | 14 | H = 111.42 p < 0.001 | H = 104.53 p < 0.001 | H = 111.57 p < 0.001 | H = 100.72 p < 0.001 |
| control | 21 | H = 111.57 p < 0.001 | H = 111.53 p < 0.001 | H = 109.45 p < 0.001 | H = 108.02 p < 0.001 |
| V. destructor | 21 | H = 102.09 p < 0.001 | H = 100.78 p < 0.001 | H = 111.58 p < 0.001 | H = 100.04 p < 0.001 |
| control | 28 | H = 102.79 p < 0.001 | H = 111.57 p < 0.001 | H = 109.81 p < 0.001 | H = 104.07 p < 0.001 |
| control | 35 | H = 111.38 p < 0.001 | H = 111.59 p < 0.001 | H = 111.58 p < 0.001 | H = 107.13 p < 0.001 |
| Group | Age (Days) | Acidic protease inhibitors | Neutral protease inhibitors | Alkaline protease inhibitors | |
| control | 1 | H = 103.75 p < 0.001 | H = 102.10 p < 0.001 | H = 100.59 p < 0.001 | |
| control | 14 | H = 111.53 p < 0.001 | H = 100.45 p < 0.001 | H = 111.57 p < 0.001 | |
| V. destructor | 14 | H = 111.57 p < 0.001 | H = 85.47 p < 0.001 | H = 110.60 p < 0.001 | |
| control | 21 | H = 110.55 p < 0.001 | H = 111.57 p < 0.001 | H = 110.316 p < 0.001 | |
| V. destructor | 21 | H = 111.57 p < 0.001 | H = 99.37 p < 0.001 | H = 108.79 p < 0.001 | |
| control | 28 | H = 111.57 p < 0.001 | H = 111.56 p < 0.001 | H = 111.57 p < 0.001 | |
| control | 35 | H = 111.57 p < 0.001 | H = 111.56 p < 0.001 | H = 111.58 p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunat-Budzyńska, M.; Staniszewska, P.; Olszewski, K.; Strachecka, A. Changes in Proteolytic System Activity Due to Varroa destructor Infestation in Apis mellifera Workers. Agriculture 2025, 15, 1942. https://doi.org/10.3390/agriculture15181942
Kunat-Budzyńska M, Staniszewska P, Olszewski K, Strachecka A. Changes in Proteolytic System Activity Due to Varroa destructor Infestation in Apis mellifera Workers. Agriculture. 2025; 15(18):1942. https://doi.org/10.3390/agriculture15181942
Chicago/Turabian StyleKunat-Budzyńska, Magdalena, Patrycja Staniszewska, Krzysztof Olszewski, and Aneta Strachecka. 2025. "Changes in Proteolytic System Activity Due to Varroa destructor Infestation in Apis mellifera Workers" Agriculture 15, no. 18: 1942. https://doi.org/10.3390/agriculture15181942
APA StyleKunat-Budzyńska, M., Staniszewska, P., Olszewski, K., & Strachecka, A. (2025). Changes in Proteolytic System Activity Due to Varroa destructor Infestation in Apis mellifera Workers. Agriculture, 15(18), 1942. https://doi.org/10.3390/agriculture15181942








