1. Introduction
On the cut flower market, forced lilacs still hold an important position on the list of autumn–winter–spring flowering species. They are available for six months of the year if a grower/producer can release timely shrubs or cut branches from dormancy. In common lilac formation of generative buds begins in July and continues into autumn, after which the buds enter winter dormancy brought about by inner factors (endodormancy). Vegetation continues in spring when plants pass into the state of ecodormancy which depends only on the environmental conditions. Special treatments are necessary to overcome endodormancy in lilac so the procedure to obtain flowers in autumn/winter is called forcing [
1,
2]. For the earliest blooming date (November), shrubs have to be artificially cooled as the natural period of low temperatures outdoor is too short to release buds from dormancy or they are treated in a greenhouse with high temperatures of 35–37 °C. Such drastic conditions may damage flower buds on different levels (cytological, anatomical, morphological, biochemical, physiological) which was reported on the white cultivar ‘Mme Florent Stepman’ [
3,
4,
5,
6]. To make lilacs bloom in March/April, i.e., at a date much less distant from the natural flowering period is simpler, cheaper and safer. Plants do not have to be “forced” to flower as they have already been released from winter dormancy and no special treatments to break it are needed [
7]. It is therefore a procedure aiming to accelerate blooming by creating the right environmental conditions which may be performed either in a greenhouse or outside for the soil-grown shrubs, using foil if needed. We introduce here the term “to accelerate” blooming to distinguish two procedures leading to the production of cut lilacs in the autumn/winter/spring period though we are aware that practically the term “to force” is commonly used in the production of lilac in all the above abnormal blooming dates.
The quality of the lilac inflorescences is determined by the date of forcing and the choice of cultivar. The earliest-flowering, white-flowered, single-flowered cultivar ‘Mme Florent Stepman’ is recommended for the November–December date. A good cultivar for early forcing is also white ‘Marie Legraye’. For January–March forcing, ‘Mme Lemoine’, with white, double flowers, and ‘Andenken an Ludwig Spaeth’, with single, lilac-blue flowers and long, narrow panicles, are recommended [
8]. All the above-listed cultivars show the longest vase life when forced on their appropriate dates. Practically, only the cultivars ‘Mme Florent Stepman’ and ‘Andenken an Ludwig Spaeth’, are commercially forced in Poland, for the winter and spring period, respectively.
The quality of forced lilacs depends not only on growing conditions, but also on conditions after harvesting the flowers and it is water and carbohydrate availability which are decisive here. The concentration of endogenous total soluble sugars decreases in wilting lilac florets on stems held in water [
7,
9], while floral preservatives can significantly increase the longevity of cut lilac stems and promote sugar accumulation in petals [
4,
10]. Sucrose absorbed from a vase solution is broken down by the action of invertase and sucrose synthase, releasing glucose and fructose, causing their concentration to be increased in the rose flowers [
11], which was confirmed also in studies on eustoma [
12] and sweet pea [
13]. Floral preservatives containing sugars, biocides and acidifying agents provide essential nutrients and inhibit microbial growth. Adding sugars to a vase solution provides an immediate source of energy for cut stems, which can promote better metabolic activity. Sucrose-containing solutions, in the range of 2% to 10%, can increase the vase life of lilac stems by providing the necessary carbohydrates to maintain cellular function and promote better hydration thus enabling flower opening [
8]. Increasing the pool of energy compounds is an important aspect of ‘feeding’ cut flowers, because once they have been cut from the mother plant they no longer assimilate, and their vitality depends on reserves stored in the petals or supplied from the leaves and shoots [
14]. As cut forced lilacs are devoid of foliage this extra supply of carbohydrates is especially important.
The longevity of cut flowers after harvesting is closely connected to their water balance, as the opening of the flower buds is not related to cell divisions in the petals, but depends on the free flow of water to them and the increase in the volume of already existing cells [
15]. The water balance of cut flowers is mainly determined by water uptake and conduction in the shoot, transpiration, the ability to retain water in the cells of the perianth and the flower’s competition for water with other organs, especially under stress conditions [
16,
17].
Water uptake of cut flowers decreases primarily due to the appearance of obstructions in their vascular system [
18,
19,
20]. A direct cause of blockage of conduction vessels is the presence of microorganisms [
21,
22], which multiply in the water and enter the vessels, limiting conduction and additionally secrete toxic metabolites, especially ethylene, which is dangerous for flowers, or enzymes that degrade cell walls [
18,
20]. Sometimes physiological xylem blockage occurs in cut flowers: it can appear even under sterile conditions and occurs in the part of the shoot above the water level in the vessel. It causes gums and gels to leak out of the shoot and enter the conductive vessels, limiting water uptake. In plants with woody shoots, polyphenolic compounds are primarily responsible for the physiological blockade, as the lignification process is accompanied by an increase in suberin, cutin and other metabolic substances [
23]. Cutting of the lilac inflorescences stimulates as well the formation of tyloses (outgrowths of parenchyma cells) that effectively block water transport. The number of tyloses formed in the vessels of lilac shoots is related to the flowering date of the shrubs and the cut panicles longevity [
20].
Water stress resulting from vessel obstructing hastens petal wilting leading to premature death. The onset of symptoms of petal cell death is preceded by rapid ion leakage due to disorganization of the cytoplasmic membranes and loss of their semi-permeable properties. Such parameters as electrical conductivity and pH are good indicators of perianth ageing and, by studying changes in their values, it is possible to predict the longevity of flowers after cutting what is used in practice in rose breeding [
24].
The work aimed was to investigate changes occurring in spring (April) blooming lilacs and to compared them to the phenomena occurring in the winter forced lilacs. The effects of flower preservatives on their vase life were also checked. For practical (commercial) reason changes occurring during vase life were analyzed in the best cultivar recommended for the given blooming periods, i.e. ‘Andeken an Ludwig Spaeth’.
4. Discussion
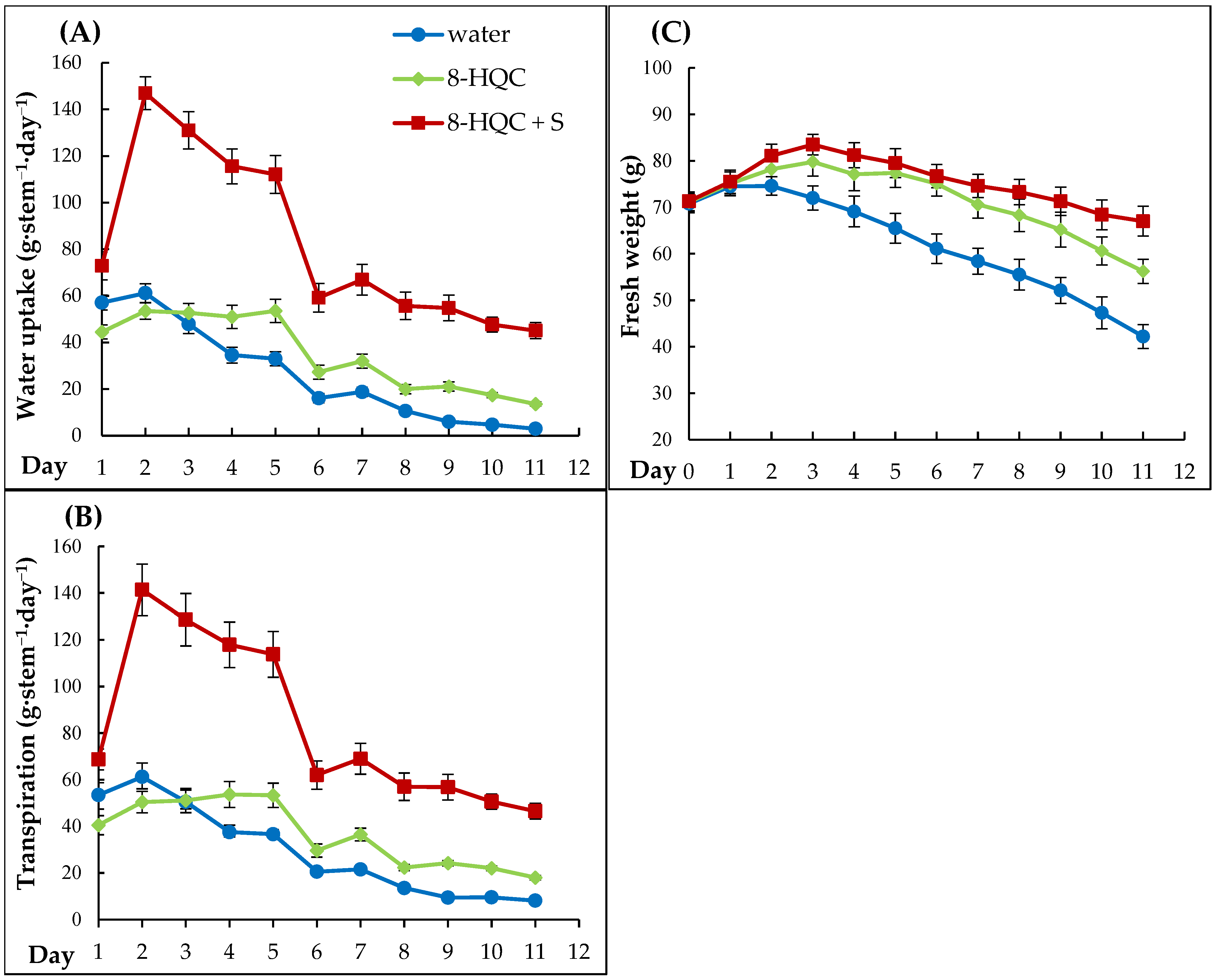
Lilac vase life varies from 4 to 12 d, depending on a cultivar, initial conditioning, and use of preservative and 5 d is a typical length of vase life for cut lilac stems [
8,
32]. The loss of vase life of cut lilac inflorescences is due to a number of factors. The tested purple cultivar ‘Andenken an Ludwig Spaeth’ cut in March and early April stood in water for only about 4 d (4.0–4.7), while already in late April it stood for 6.4 d and in early May for 9.9 d. Early forcing dates and high temperatures of forcing procedures significantly affect the postharvest life of lilac, especially of cultivars forced in November, as confirmed by Jędrzejuk’s and co-workers research on the white ‘Mme Florent Stepman’ cultivar [
3,
6]. Their vase life during this period was only 2.4 d in water and 6.8 in preservative solution (8-HQC + 2% S). But already with the alternative method of forcing at 15 °C (flowering in January), the lilacs stood in water for 9.2 d and in preservative for 12 d. This confirms how clearly the forcing temperature affects the longevity of flowers after harvesting.
To prolong this short vase life the preservative solutions can be used. The applied biocide, especially with the addition of sucrose, significantly prolonged the vase life of the cut lilacs ‘Andenken an Ludwig Spaeth’ on each of the forcing dates. The standard preservatives were particularly effective on the last dates of blooming A positive effect of exogenous sugar on postharvest vase life has been found for many cut flowers: rose [
33], hydrangea [
34], limonium [
35] and peony [
36]. This group of carbohydrates not only provides a substrate for respiration but also controls water balance in cut flowers. The sugar content of plant organs changes during their development and some organs, especially flowers, act as a sink.
When the stem is cut off, there is a reduction in the import of sucrose into the sink tissues of cut flowers [
37]. The content of soluble sugars decreases in the petals as the flowers age, as the flowers usually no longer assimilate after cutting, but use their own reserves or exogenous sugar supplied with the preservative solution to the vase [
38,
39]. In the lilacs the contents of total sugars, including reducing sugars, decreased. Therefore, the use of sucrose in a vase solution had a significant effect on the physiological state of the flower tissues and endogenous sucrose levels. When placed in the standard preservative, they accumulated soluble carbohydrates in amounts sometimes up to twice as high as the control flowers standing in water. Younger lilac florets in the upper part of the inflorescence showed greater sink strength and accumulated more reducing sugars than older lower flowers. The phenomenon of sugar accumulation was not observed in lilac flowers placed in a biocide (8-HQC) only, due to the lack of sugars in this solution. This was also presented on the forced white lilac ‘Mme Florent Stepman’ [
4]. In both cultivars the increase in the vase life of flowers placed into preservative solutions with sucrose was connected with increases in the diameter of the individual florets and their weight. Studies on lily also have shown that exogenous sucrose affects the longevity of cut flowers, due to increasing the endogenous carbohydrate pool [
38]. The treatments with sugars promoted flower opening and extended vase life also in cut roses. The use of sucrose increases the concentration of glucose and fructose in the vacuole, which may reduce the osmotic potential of the symplast and increase water uptake, leading to cell enlargement during flower opening [
33].
Water stress accelerates the senescence process of cut flowers, as water does not reach the inflorescence/florets in sufficient quantity due to the difficulties in transport through the stem. Flowers start to wilt when the fresh mass of the florets decreases due to the predominance of transpiration over water uptake. The results show that lilacs put into the standard preservative uptake significantly more solution from the vase than shoots put into water, which was directly translated into the vase life and floret opening. This confirms the important role of the undisturbed water uptake and transport towards the inflorescences in maintaining a good postharvest quality of cut lilacs.
However, on the based on the measurements and observations the conclusion is that the wilting of the lilacs does not appear to be related neither to the absolute amount of water uptake on the day of wilting, nor to the amount of water transpired at that point, or even to the fact that a positive water balance was maintained. In a previous study by Skutnik et al. [
9], the holding solution (Chrysal Professional) which extended the longevity of the lilacs ‘Andenken an Ludwig Spaeth’ forced between March and April, in the vase from 5.2 d (water control) to 10.7 d, improved water balance parameters (fresh weight, water uptake and transpiration), delaying the appearance of negative water balance values. Also, in the white lilac cultivar ‘Mme Florent Stepman’ forced between December and March the uptake of preservative solution (Chrysal Professional 2 and 8-HQC 200 mg·dm
−3 + sucrose 2%) was higher, what promoted flower opening and prolonged lilac vase life [
40].
Flower senescence after cutting is strictly related to protein degradation [
41]. In general, this relationship has been observed in many ornamental plant species during postharvest ageing, for example, in carnation [
42], gladiolus [
43] or dendrobium [
44]. Sometimes the opposite happens, as observed by Zhao et al. [
45] in cut peony flowers, where soluble protein content increased during the early vase life of the flowers and then decreased. In this experiment, an increase in soluble protein content in control lilac flowers and those placed in 8-HQC solution in both the lower and upper panicles was noticed. Only in flowers placed in the standard preservative a decrease in the protein content during inflorescence senescence was recorded, despite the higher longevity recorded in this treatment. In many species of plants, protein degradation and remobilization are mediated through protein ubiquitination and the action of specific proteases [
46,
47] transferring various amino acids to phloem. It is possible that some of the proteins decomposed into free amino acids were converted into sugars, essential for flower development and maintaining good postharvest vase life. Similarly, the white lilac flowers blooming at the natural May date had a significantly lower soluble protein content in open flowers in comparison to initial value in bud phases. While in the same white lilac cultivar forced in November under 37 °C at different stages of inflorescence development the protein content was at a similar, unchanged level [
48]. It shows, how important the temperature during growth of lilac flowers is.
Accumulation of free proline is a characteristic change that takes place in plants under water stress conditions [
49]. The content of this amino acid increases in stressed plants by up to several tens of times, which enables plants to function under unfavourable water stress conditions. Flowers are exposed to such stress after cutting. During their ageing, an increase in the level of free proline has been found [
50], and vase life extension treatments have had the effect of reducing this phenomenon, as found, for example, in carnation by Yakimova at al. [
51], and in cut lisianthus by Kazemi et al. [
52]. There was an increase in free proline content in lilac inflorescences, but it was not as significant as that described in the literature. In lilacs placed in the standard preservative solution and in biocide solution in lower part of panicle, proline levels were generally lower than in control flowers. This suggests that the increase in proline content is a symptom of stress rather than an expression of adaptation to harsh conditions, since stress-reducing and flower-prolonging treatments reduce free proline levels in florets.
Durkin et al. [
53] report that objective indicators useful for determining physicochemical changes in petal cut flowers are cell sap parameters, its pH and electroconductivity. Their research showed that from the time the cut roses (‘Sweet Promise’ and ‘Royalty’) were placed in the vase until they wilted, the values of these parameters kept increasing [
54]. Similar changes in the cell sap and electrical conductivity values occurred during the lilac postharvest life. Here, almost 2-fold increase in electroconductivity was observed, resulting from changes in cytoplasmic membrane permeability. The holding solution did not limit this rise but affected the pH of the cell sap, in the upper part of inflorescences. These parameters are known and used as indicators of cut flower senescence, and often as predictors of their potential longevity [
24] but in the case of lilacs flowers they have proven to be of little use.
Another cause of cut flower senescence is oxidative stress, which causes disruption of homeostasis. It results from an imbalance between the generation of reactive oxygen species and the antioxidant capacity in the plant [
55]. Oxidative processes are accelerated by the increased water deficit in the plant, and result in the accumulation of large amounts of hydrogen peroxide, leading to cell damage [
56]. In this study changes in the hydrogen peroxide contents were relatively small and senescing control flowers from the upper panicles contained less H
2O
2 than flowers placed into the standard preservative and biocide. Slightly higher amounts of this reactive oxygen species were in florets from the lower part of the panicles, but still much lower than on the day of harvesting. Such a relationship was confirmed by an earlier study by Jędrzejuk et al. [
5] on the white cultivar ‘Mme Florent Stepman’. In the naturally flowering plants, the highest H
2O
2 content was reported in the open flower phase and the lowest in an unopen flower bud, while in the standard forced plants in November hydrogen peroxide level was more than 6 times higher than in florets from the naturally flowering shrubs in May in the same phase.
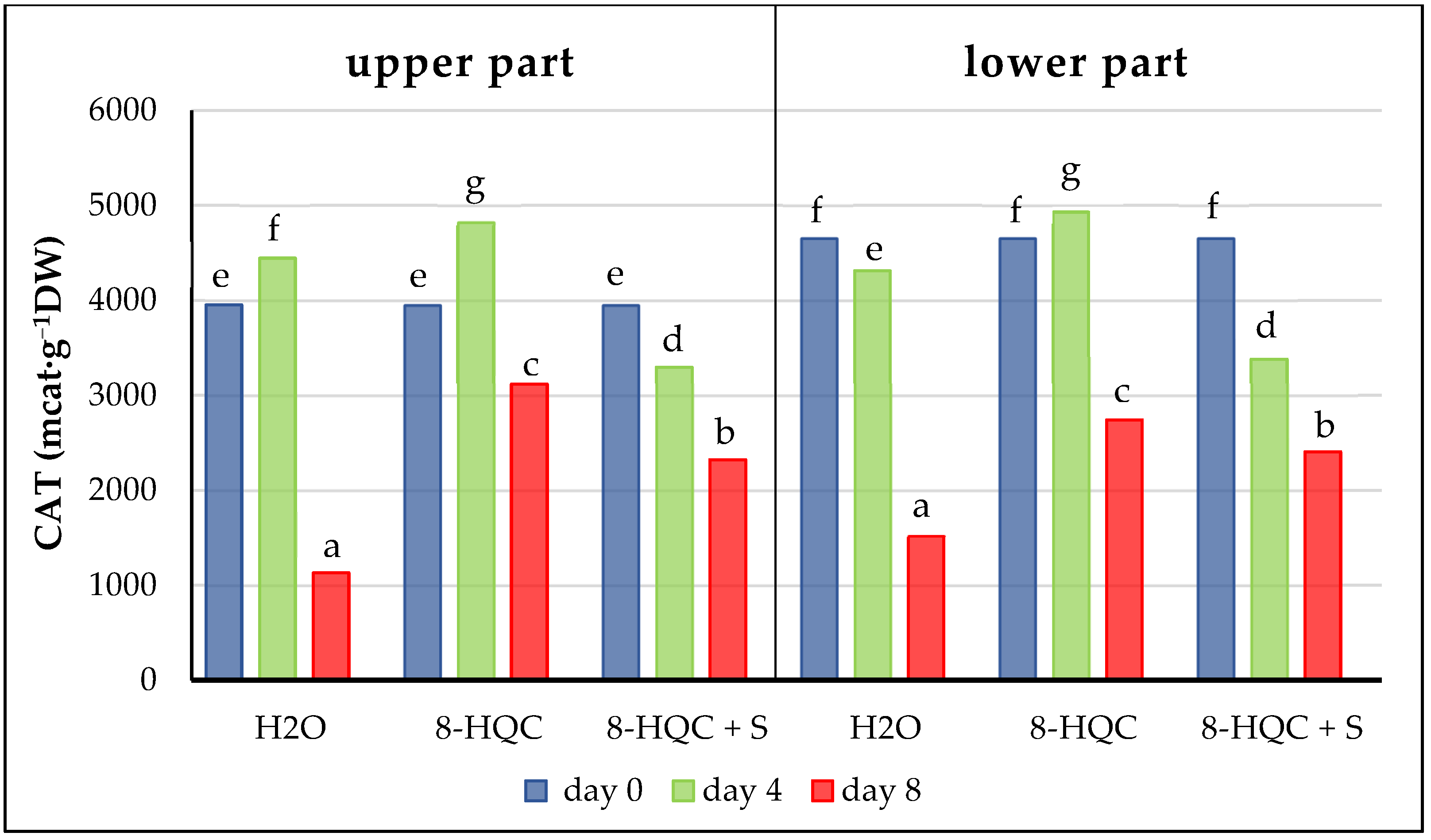
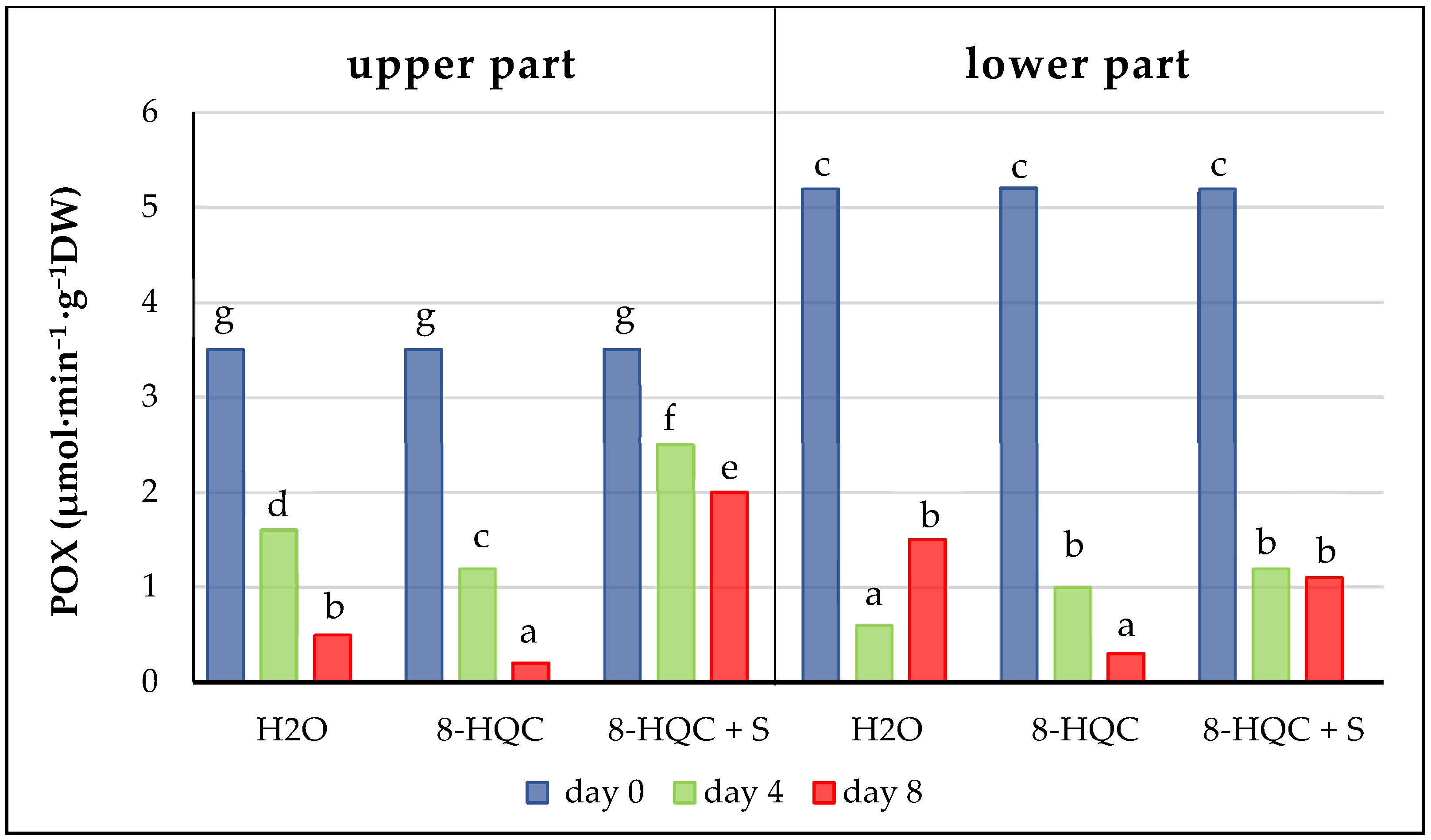
Plants have developed various protective mechanisms against the effects of oxidative stress in cut flowers. During the senescence of cut flowers antioxidant enzymes such as catalase, peroxidases and superoxide dismutase show increased activity [
57]. Here, CAT and POX activities decreased with time in the lower and upper florets of all treatments, reaching values at the end of the vase life period lower than the initial values. This is most likely correlated with the level of hydrogen peroxide; the higher it is, the higher the activity of CAT and POX. The high activity of these enzymes, activated after harvesting due to the accumulation of hydrogen peroxide, reduced the level of this compound while simultaneously decreasing its own level. Similar observations were made by Jędrzejuk et al. [
6] on a white cultivar ‘Mme Florent Stepman’ treated with the standard preservative and a solution with nanosilver (1 mg·L
−1) and 2% S. These values for the activity of both enzymes decreased at subsequent stages of inflorescence development for the standard and alternative forcing method as well as at the natural May flowering date in all treatments.
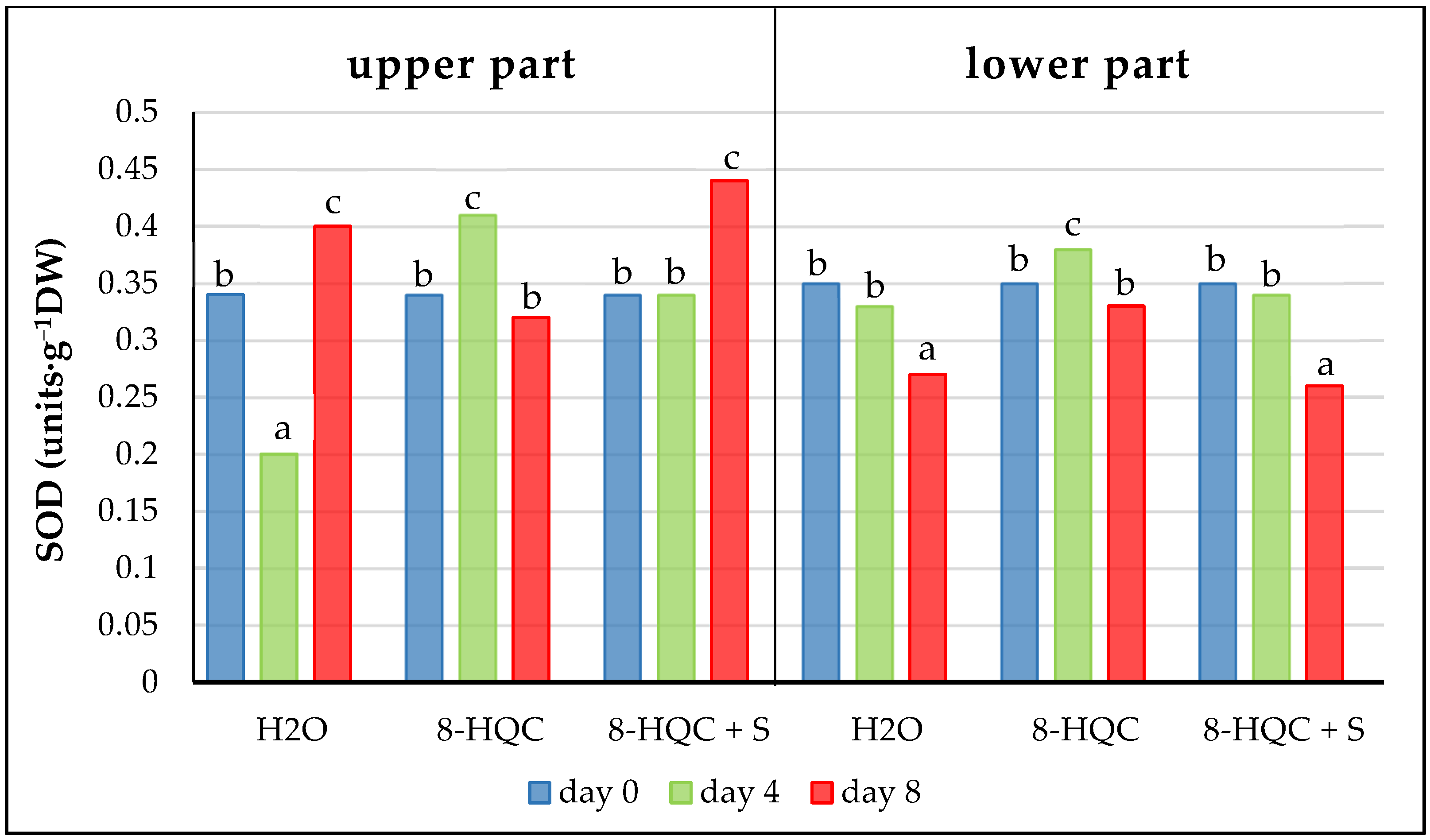
In this study SOD activity decreased on subsequent dates in the lower, older parts of the lilac panicles. Research by Jedrzejuk et al. [
5] shows the same pattern in standard November forcing when the drastic decrease started already from the flower opening stage. However, in the branches forced under 15 °C it increased above the initial value, similarly as in this experiment where at the end of vase life these values in the upper, younger parts of the inflorescences were higher than the initial ones. This shows that forcing lilac shrubs under low temperature better protects the antioxidant defence system thus delaying the senescence process.