Optimizing Controlled-Release Urea and Conventional Urea Ratios Enhances Nitrogen Use Efficiency and Yield in Peanut
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Materials
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Yield-Related Traits and Quality of Peanut
2.3.2. Sampling
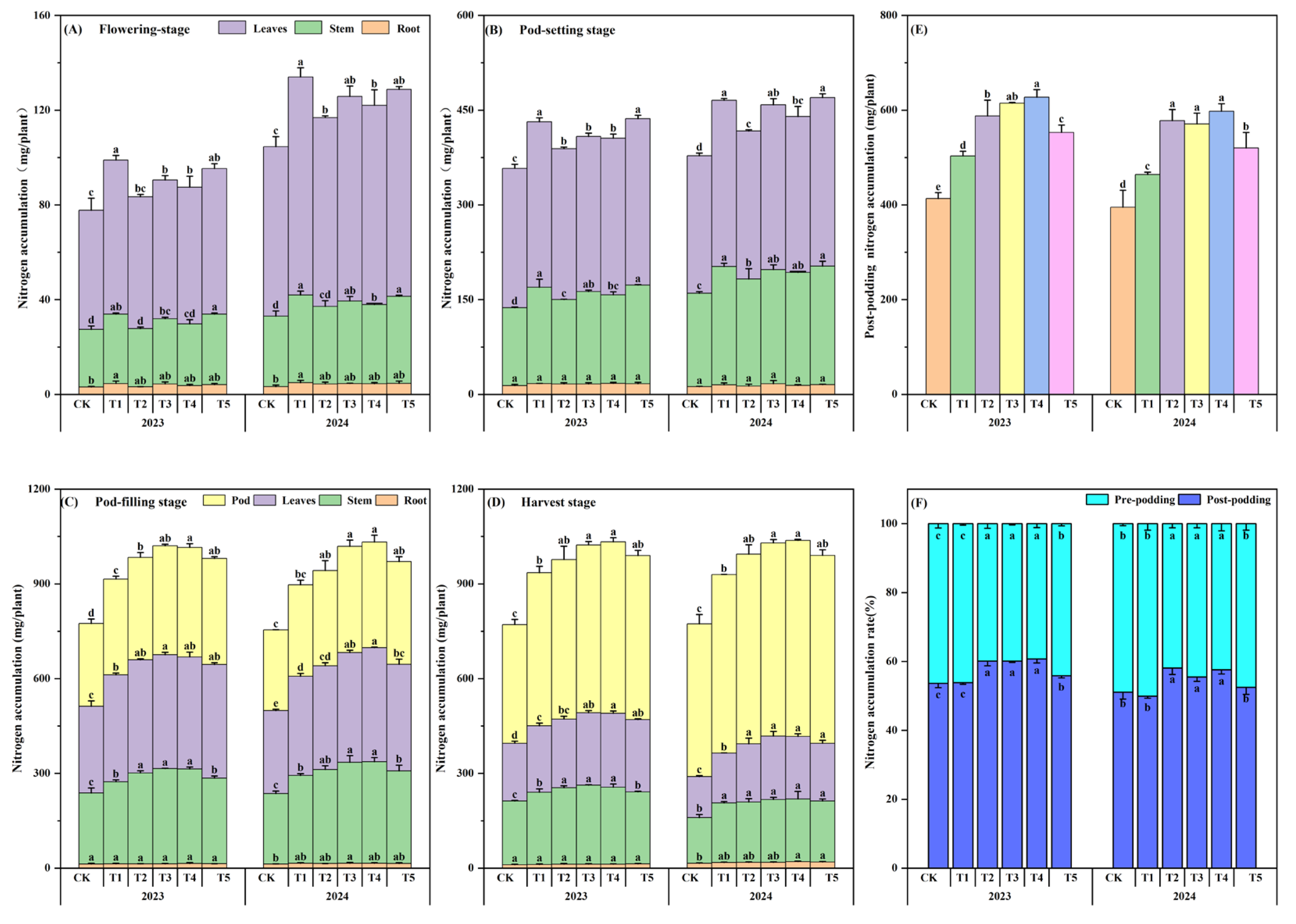
2.3.3. Peanut Agronomic Traits, Dry Matter Accumulation, Nitrogen Accumulation, and Nitrogen Fertilizer Efficiency
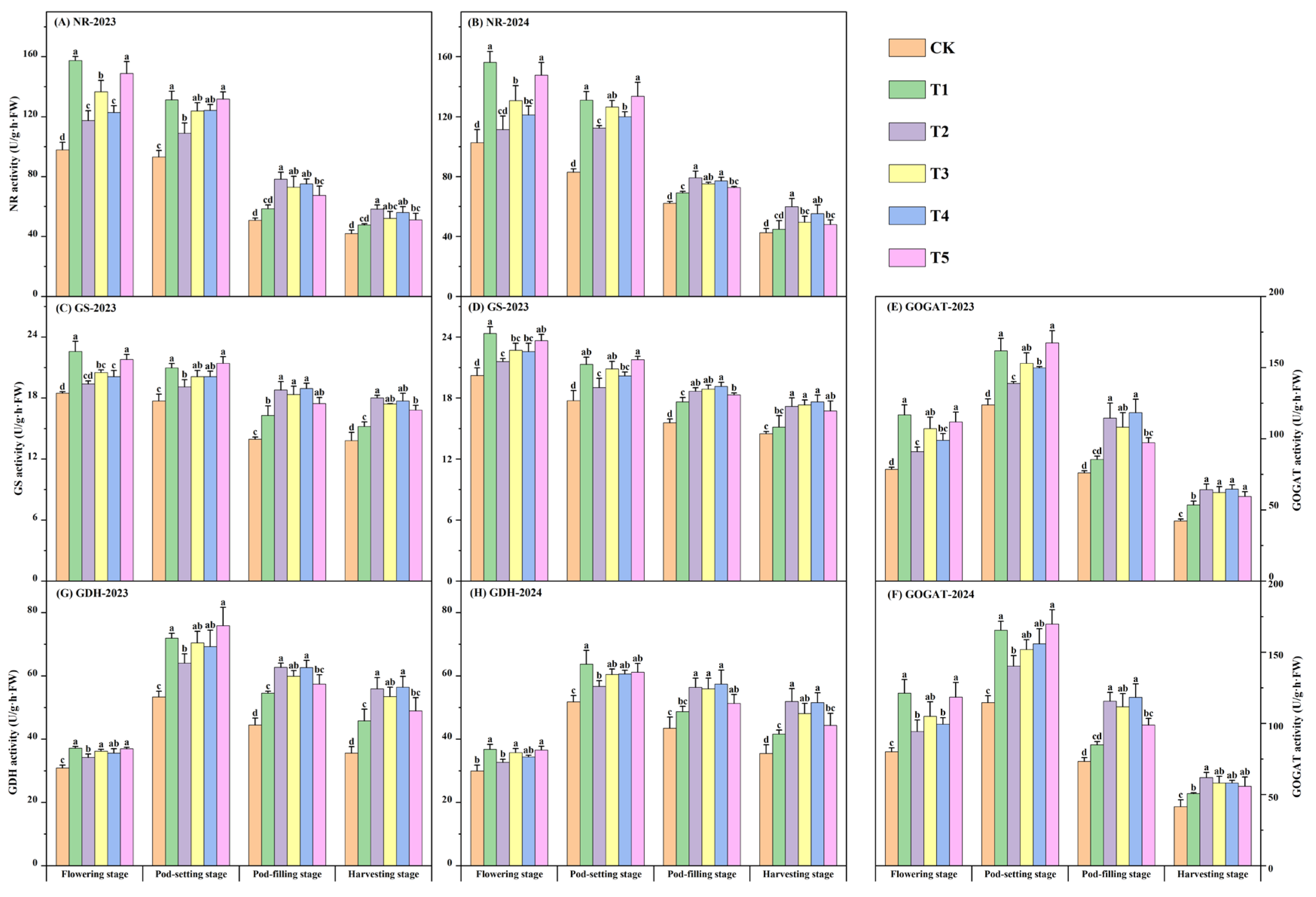
2.3.4. Determination of Leaf Nitrogen Metabolism Enzyme Activity
2.3.5. Determination of Leaf Antioxidant Enzyme Activity
2.3.6. Determination of Soil Nitrogen Content
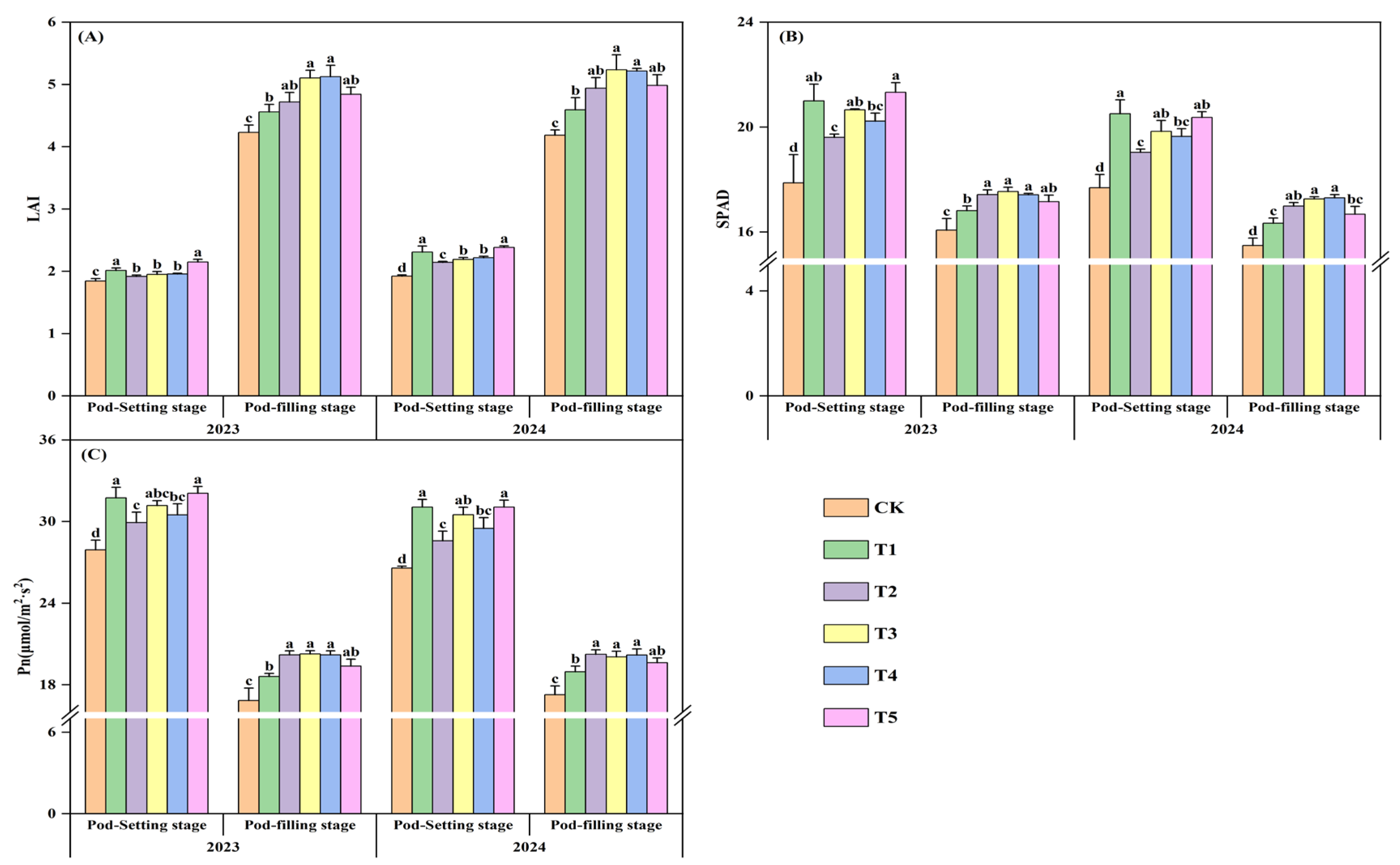
2.3.7. Leaf Area Index (LAI), SPAD Value, Photosynthetic Intensity (Pn)
2.4. Statistical Analysis
3. Results
3.1. Peanut Yield and Constituent Factors
3.2. Peanut Quality
3.3. Soil Nitrogen Content
3.4. Agronomic Traits of Peanut
3.5. Leaf Antioxidant Enzyme Activity
3.6. Leaf Nitrogen Metabolism Enzyme Activity
3.7. Photosynthetic Performance
3.8. Dry Matter Accumulatio
3.9. Nitrogen Accumulation
3.10. Nitrogen Fertilizer Efficiency
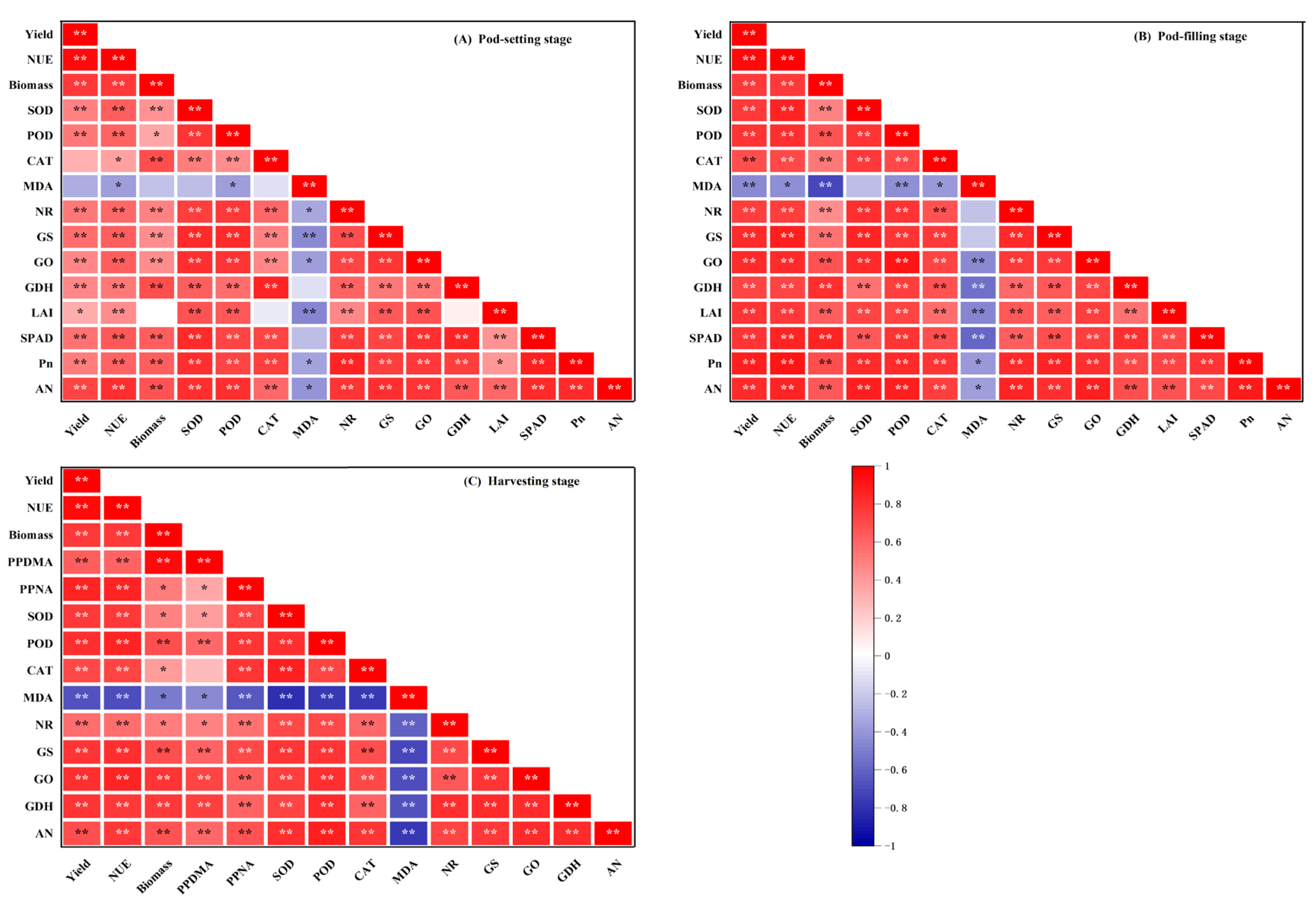
3.11. Correlation Analysis
4. Discussion
4.1. Effects of Combined Application of CRU and U on Nitrogen Supply Dynamics
4.2. Effects of Combined Application of CRU and U on Peanut Growth and Nitrogen Utilization
4.3. Effects of Combined Application of CRU and U on Peanut Senescence
4.4. Effects of Combined Application of CRU and U on Biomass, Yield, and Quality of Peanuts
4.5. Technology Promotion and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; Wang, B.; Hu, Z.; Yang, H. Mechanism and Experiment of Full-Feeding Tangential-Flow Picking for Peanut Harvesting. Agriculture 2022, 12, 179. [Google Scholar] [CrossRef]
- Li, L.; Cheng, X.; Zhang, Y.; Kohtz, D.; Wang, X.; Zhang, X.; Kong, X.; Xue, H.; Jia, P.; Bai, N.; et al. Exogenous melatonin improves peanut field productivity and quality at reduced nitrogen application. Field Crops Res. 2024, 319, 109650. [Google Scholar] [CrossRef]
- Toomer, O.T. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Wang, F.; Miao, H.; Zhang, S.; Hu, X.; Li, C.; Yang, W.; Chen, J. Identification of a New Major Oil Content QTL Overlapped with FAD2B in Cultivated Peanut (Arachis hypogaea L.). Plants 2025, 14, 615. [Google Scholar] [CrossRef]
- Guo, P.; Ren, J.; Shi, X.; Xu, A.; Zhang, P.; Guo, F.; Feng, Y.; Zhao, X.; Yu, H.; Jiang, C. Optimized nitrogen application ameliorates the photosynthetic performance and yield potential in peanuts as revealed by OJIP chlorophyll fluorescence kinetics. BMC Plant Biol. 2024, 24, 774. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, C.; Zhang, L.; Guo, F.; Cross, P.; Zhang, Z.; Wan, S.; Zhang, F. Morphological responses in peanut pod development to intercropping and nitrogen application rates. Field Crops Res. 2023, 302, 109101. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Wan, Y.; Liu, F.; Zhang, K. Rational Nitrogen Strategies Can Improve Peanut Source Supply Capacity and Pod Yield. Agron. J. 2017, 109, 2927–2935. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Lai, H.; Zhao, M.; Zhu, Q.; Zhao, C.; Yang, D.; Li, X. The impacts of soil tillage combined with plastic film management practices on soil quality, carbon footprint, and peanut yield. Eur. J. Agron. 2023, 148, 126881. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Q.; Yuan, Y.; Zhang, X.; Sun, R.; Hao, R.; Yang, X.; Li, C.; Qin, X.; Song, F.; et al. Effects of plastic film mulching on yield, water use efficiency, and nitrogen use efficiency of different crops in China: A meta-analysis. Field Crops Res. 2024, 312, 109407. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, R.; Cao, Y.; Li, J.; Xiao, D.; Hou, J.; Wang, X. Integrated assessment of yield, nitrogen use efficiency and ecosystem economic benefits of use of controlled-release and common urea in ratoon rice production. J. Integr. Agric. 2024, 23, 3186–3199. [Google Scholar] [CrossRef]
- Pierozan, J.C.; Favarin, J.L.; Baptistella, J.L.C.; Almeida, R.E.M.; Maciel, O.S.; Lago, B.C.; Tezotto, T. Controlled release urea increases soybean yield without compromising symbiotic nitrogen fixation. Exp. Agric. 2023, 59, e1. [Google Scholar] [CrossRef]
- Shibru, D.; Mitiku, H. Effects of Rhizobial inoculant and nitrogen fertilizer on yield and nodulation of common bean. J. Plant Nutr. 2000, 41, 55–63. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Blaylock, A.D.; Chen, X. Mixture of controlled release and normal urea to optimize nitrogen management for high-yielding (>15 mg·ha −1 ) maize. Field Crops Res. 2017, 204, 23–30. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Zhang, Y.; Chen, L.; Wang, T.; Li, H.; Liao, Y.; Li, Y.; Zhang, G.; Han, J. Controlled release urea combined with normal urea maintains the N balance and improves the environmental and economic benefits in wheat–maize multiple cropping. Eur. J. Agron. 2025, 163, 127446. [Google Scholar] [CrossRef]
- Zheng, C.; Li, C.; Tian, L.; Shen, Z.; Feng, G.; Hou, W.; Liu, F.; Gao, Q.; Wang, Y. Mixture of controlled-release and normal urea to improve maize root development, post-silking plant growth, and grain filling. Eur. J. Agron. 2023, 151, 126994. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Wang, G.; Liu, R.; Huang, D.; Song, M.; Zhang, Y.; Wang, H.; Wang, Y.; Shao, R.; et al. Maize yield increased by matching canopy light and nitrogen distribution via controlled-release urea /urea adjustment. Field Crops Res. 2024, 308, 109284. [Google Scholar] [CrossRef]
- Ji, P.; Du, X.; Zhou, J.; Peng, Y.; Li, X.; Tao, P.; Zhang, Y. Network Analysis Reveals the Combination of Controlled-Release and Regular Urea Enhances Microbial Interactions and Improves Maize Yields. Front. Microbiol. 2022, 13, 825787. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, F.; Hassan, J.H.; Peng, X.; Chen, H.; Tang, W.; Lai, Y.; Wu, Y. Controlled-Release Nitrogen Mixed with Common Nitrogen Fertilizer Can Maintain High Yield of Rapeseed and Improve Nitrogen Utilization Efficiency. Plants 2023, 12, 4105. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Han, M.; Yang, X.; Shen, T.; Gao, Y.; Wang, Y.; Zhang, S.; Chen, D.; He, D.; Li, C. Gene Expression, Enzyme Activity, Nitrogen Use Efficiency, and Yield of Rice Affected by Controlled-Release Nitrogen. ACS Omega 2023, 8, 23772–23781. [Google Scholar] [CrossRef]
- Yang, L.; Lakshmanan, P.; Tu, D.; Sun, X.; Zhou, Y.; Xu, Y.; Li, Z.; Wu, W.; Liu, X.; Luo, T.; et al. Co-Benefits of Yield and Nitrogen Use Efficiency Gains Through Combined Use of Controlled-Release Urea and Conventional Urea in Rice. Food Energy Secur. 2025, 14, e70043. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Zhai, S.; Ding, X.; Zhang, H.; Sun, S.; Tian, X. Optimal Blends of Controlled-Release Urea and Conventional Urea Improved Nitrogen Use Efficiency in Wheat and Maize with Reduced Nitrogen Application. J. Soil Sci. Plant Nutr. 2021, 21, 1103–1111. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, F.; Li, X.; Zhang, J. Source-sink coordinated peanut cultivar increases yield and kernel protein content through enhancing photosynthetic characteristics and regulating carbon and nitrogen metabolisms. Plant Physiol. Biochem. 2024, 206, 108311. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Zhang, Q.; Liu, Z.; Zhao, J.; Yang, D.; Li, X. Regulating effect of spraying stage of ethephon on the formation of source-sink in peanut. Ying Yong Sheng Tai Xue Bao 2021, 32, 951–958. [Google Scholar] [CrossRef]
- Li, G.; Guo, X.; Sun, W.; Hou, L.; Wang, G.; Tian, R.; Wang, X.; Qu, C.; Zhao, C. Nitrogen application in pod zone improves yield and quality of two peanut cultivars by modulating nitrogen accumulation and metabolism. BMC Plant Biol. 2024, 24, 48. [Google Scholar] [CrossRef]
- Wu, L.; Wang, S.; Tian, L.; Wu, L.; Li, M.; Zhang, J.; Li, P.; Zhang, W.; Chen, Y. Comparative proteomic analysis of the maize responses to early leaf senescence induced by preventing pollination. J. Proteom. 2018, 177, 75–87. [Google Scholar] [CrossRef]
- Ahmad, I.; Minkina, T.; Mandzhieva, S.; Rajput, V.D.; Sushkova, S.; Tarigholizadeh, S.; Ikram, K. Effect of Planting Patterns and Fertilization Optimization on Hormonal Profile, Leaf Senescence and Yield in Maize. J. Soil Sci. Plant Nutr. 2024, 25, 195–212. [Google Scholar] [CrossRef]
- Du, K.; Zhao, W.; Lv, Z.; Xu, B.; Hu, W.; Zhou, Z.; Wang, Y. Optimal rate of nitrogen fertilizer improves maize grain yield by delaying the senescence of ear leaves and thereby altering their nitrogen remobilization. Field Crops Res. 2024, 310, 109359. [Google Scholar] [CrossRef]
- Lan, T.; Du, L.; Wang, X.; Zhan, X.; Liu, Q.; Wei, G.; Lyu, C.; Liu, F.; Gao, J.; Feng, D.; et al. Synergistic effects of planting density and nitrogen fertilization on chlorophyll degradation and leaf senescence after silking in maize. Crop J. 2024, 12, 605–613. [Google Scholar] [CrossRef]
- Zhang, W. Regulatory Effects of Different Humic acid Fertilizers on Peanut Growth and Yield. Master’s thesis, Shandong Agricultural University, Taian, China, 2020. [Google Scholar] [CrossRef]
- Li, H.; Qiong, Y.; Fang, C.; Tang, J.; Huang, K. Integrated use of cultivation practices increases the Tartary buckwheat yield by improving the photosynthetic capacity and nitrogen utilization rate. Front. Plant Sci. 2025, 16, 1651635. [Google Scholar] [CrossRef]
- Bories, P.N.; Bories, C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin. Chem. 1995, 41, 904–907. [Google Scholar] [CrossRef]
- Singh, R.P.; Srivastava, H.S.M.D. Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, S.; Peng, X.; Chavez, A.Q.; Bennett, J. Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza sativa L.) roots. Plant Sci. 1997, 125, 163–170. [Google Scholar] [CrossRef]
- Wu, P.; Liu, F.; Chen, G.; Wang, J.; Huang, F.; Cai, T.; Zhang, P.; Jia, Z. Can deep fertilizer application enhance maize productivity by delaying leaf senescence and decreasing nitrate residue levels? Field Crops Res. 2022, 277, 108417. [Google Scholar] [CrossRef]
- Shang, S.; Yu, X.; Qi, Y. Comparison of determination methods of hydrolyzable nitrogen in soil and precautions. Agric. Technol. 2022, 42, 97–99. [Google Scholar] [CrossRef]
- Junior, P.C.; Kawakami, J. Efficiency of the leaf disc method for estimating the leaf area index of soybean plants. Acta Sci. Agron. 2013, 35, 487–493. [Google Scholar] [CrossRef]
- Noorzai, A.; Suresh, K.; Subbarao, A.S.; Kumar, K.A.; Reddy, K.P.C.; Satish, P. Nutrient Uptake of the Individual Crops in Maize and Groundnut Cropping System Grown under Different Irrigation Systems with Varied Irrigation and Nitrogen Levels. J. Sci. Res. Rep. 2024, 30, 369–382. [Google Scholar] [CrossRef]
- Noorzai, A.; Suresh, K.; Subbarao, A.S.; Kumar, K.A.; Reddy, K.P.C.; Satish, P. Profitability of Maize and Groundnut Crop Production under Different Irrigation Systems with Varied Irrigation and Nitrogen Level Application in Southern India. Arch. Curr. Res. Int. 2024, 24, 109–122. [Google Scholar] [CrossRef]
- Meng, C.; Wu, M.; Wang, X.; Yang, L.; Liang, H.; Wu, Q.; Shen, P. Slow-release fertilisers increased microflora and nitrogen use efficiency and thus promoted peanut growth and yield. Plant Soil Environ. 2024, 70, 61–71. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Wang, C.; Wen, Y.; Wang, W.; Zhu, K.; Zhang, W.; Gu, J.; Liu, L.; Zhang, J.; et al. Optimized controlled-release nitrogen strategy achieves high yield and nitrogen use efficiency of wheat following rice in the lower reaches of Yangtze River of China. Field Crops Res. 2024, 317, 109567. [Google Scholar] [CrossRef]
- Nagaraju, K.; Prasad, T.N.V.K.; Chari, M.S.; Ramu, Y.R.; Murthy, B.R.; Naidu, M.V.S.; Leelavathy, G.P.; Mohan, P.R.; Damu, A.G.; Gopal, D. Effects of Soil Application of Nanobiochar-Based Nitrogen and Potassium Fertilizers on the Growth and Yield of Groundnut (Arachis Hypogaea L.). J. Soil Sci. Plant Nutr. 2024, 24, 5759–5771. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, M.K.; Roychoudhry, S.; Nayyar, H.; Kepinski, S.; Varshney, R.K. Peg Biology: Deciphering the Molecular Regulations Involved During Peanut Peg Development. Front. Plant Sci. 2019, 10, 1289. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.O.; Liu, Z.; Sun, X. The interplay of carbon and nitrogen distribution: Prospects for improved crop yields. Mod. Agric. 2023, 1, 57–75. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, J.; Zhang, Y.; Zhang, Y.; Peng, S.; Chen, H.; Zhu, D. Effects of post-anthesis nitrogen uptake and translocation on photosynthetic production and rice yield. Sci. Rep. 2018, 8, 12891. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.; Upadhayaya, B.T.; Pokharel, B.; Pandey, B.P.; Gyawaly, P.; Moi, T. Effect of Different Nitrogen Levels on Growth, Yield, and Nitrogen Use Efficiency (NUE) of Hardinath Hybrid Dhan-1 in Madhesh Province, Nepal. Int. J. Plant Soil Sci. 2025, 37, 56–66. [Google Scholar] [CrossRef]
- Liu, C.; Pang, S.; Li, X.; Liu, P.; Zhou, Y.; Lin, X.; Gu, S.; Wang, D. Layered nitrogen fertilization regulates root morphology to promote synergistic nitrogen and phosphorus absorption in maize (Zea mays L.). Field Crops Res. 2025, 322, 109737. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, C.; van der Werf, W.; Ning, P.; Zhang, Z.; Wan, S.; Zhang, F. Intercropping modulates the accumulation and translocation of dry matter and nitrogen in maize and peanut. Field Crops Res. 2022, 284, 108561. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, F.; Yang, J.; Yang, H. Nitric oxide alleviates lipid peroxidation induced by osmotic stress during senescence of detached leaves of Malus hupehensis Rehd. J. Hortic. Sci. Biotechnol. 2010, 85, 367–373. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Sun, X.; Deng, Y.; Guo, H.; Wang, C.; Zhao, L. Study on the Mechanism of Slow-Release Fertilizer and Nitrogen Fertilizer on the Senescence Characteristics of Quinoa Leaves. Agronomy 2024, 14, 884. [Google Scholar] [CrossRef]
- Shehab, A.E.S.A.; Guo, Y. Effects of nitrogen fertilization and drought on hydrocyanic acid accumulation and morpho-physiological parameters of sorghums. J. Sci. Food Agric. 2021, 101, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Vasanthan, B.; Arora, A. Calcium regulates Gladiolus flower senescence by influencing antioxidative enzymes activity. Acta Physiol. Plant. 2011, 33, 1897–1904. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Fudjoe, S.K.; Zhang, R.; Luo, Z.; Anwar, S. Nitrogen Supply Affects Grain Yield by Regulating Antioxidant Enzyme Activity and Photosynthetic Capacity of Maize Plant in the Loess Plateau. Agronomy 2021, 11, 1094. [Google Scholar] [CrossRef]
- Tian, G.; Qi, D.; Zhu, J.; Xu, Y. Effects of nitrogen fertilizer rates and waterlogging on leaf physiological characteristics and grain yield of maize. Arch. Für Acker-Pflanzenbau Bodenkd. 2021, 67, 863–875. [Google Scholar] [CrossRef]
- Qiang, B.; Chen, S.; Fan, Z.; Cao, L.; Li, X.; Fu, C.; Zhang, Y.; Jin, X. Effects of nitrogen application levels on soybean photosynthetic performance and yield: Insights from canopy nitrogen allocation studies. Field Crops Res. 2025, 326, 109871. [Google Scholar] [CrossRef]
- Zhao, S.; LÜ, J.; Xu, X.; Lin, X.; Luiz, M.R.; Qiu, S.; Ciampitti, I.; He, P. Peanut yield, nutrient uptake and nutrient requirements in different regions of China. J. Integr. Agric. 2021, 20, 2502–2511. [Google Scholar] [CrossRef]
- Liao, Z.; Lai, Z.; Kou, H.; Zhang, H.; Li, Z.; Zhang, F.; Fan, J. Soil Mulching Practices Increased Grain-Filling Capacity of Rainfed Maize (Zea mays L.) by Improving Soil Hydrothermal Condition and Leaf Photosynthetic Potential. J. Agron. Crop Sci. 2024, 210, e12781. [Google Scholar] [CrossRef]
- Jegadeeswari, D.; Chinnappan, S.; Mathiyazhagan, V.; Mahalingam, M.; Damodharan, Y.; Ganesan, D. Unlocking bottlenecks of groundnut productivity and quality: Opportunities for foliar micronutrient mixture. J. Plant Nutr. 2025, 48, 1134–1143. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, F.; Lin, Y.; Wang, Y.; Yang, C.T.; Zhang, F.; Li, Y.; Li, X. Differences in seed kernel qualityand related enzyme activities of different quality type peanut cultivars. Chin. J. Appl. Ecol. 2013, 24, 481–487. [Google Scholar] [CrossRef]
- Lothier, J.; Gaufichon, L.; Sormani, R.; Lemaître, T.; Azzopardi, M.; Morin, H.; Chardon, F.; Reisdorf-Cren, M.; Avice, J.C.; Masclaux-Daubresse, C. The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot. 2011, 62, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, Z.; Wang, J.; Zhao, J.; Liu, Y.; Zou, J.; Li, L.; Zhang, J.; Wan, S. Optimizing Initial Nitrogen Application Rates to Improve Peanut (Arachis hypogaea L.) Biological Nitrogen Fixation. Agronomy 2023, 13, 3020. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, Q.; Zhou, P.; Li, H.; Liu, Y.; Lu, Y.; Li, B. Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment. Agronomy 2024, 14, 2114. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T. Runoff and accumulation of microplastics derived from polymer-coated fertilizer in Japanese paddy fields. Environ. Toxicol. Chem. 2025, 44, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, L.; Gopakumar, A.N.; Beheshtimaal, A.; Jazaei, F.; Ccanccapa-Cartagena, A.; Salehi, M. Mechanisms of microplastic generation from polymer-coated controlled-release fertilizers (PC-CRFs). J. Hazard. Mater. 2025, 486, 137082. [Google Scholar] [CrossRef] [PubMed]
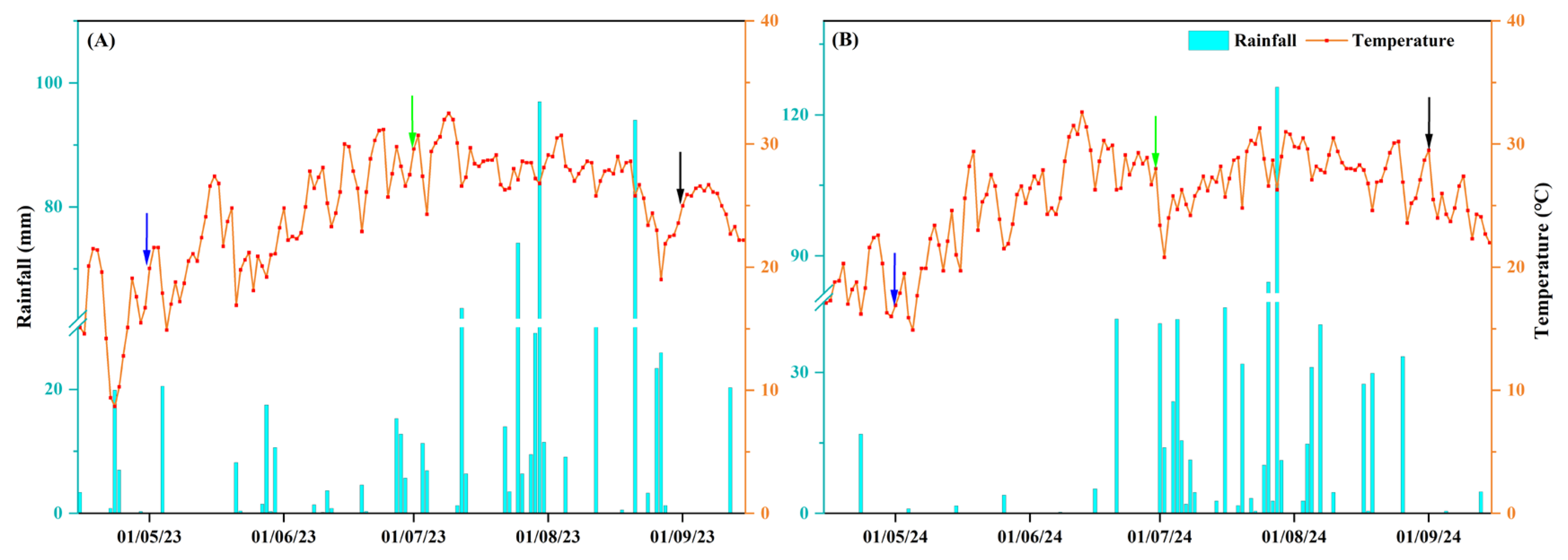
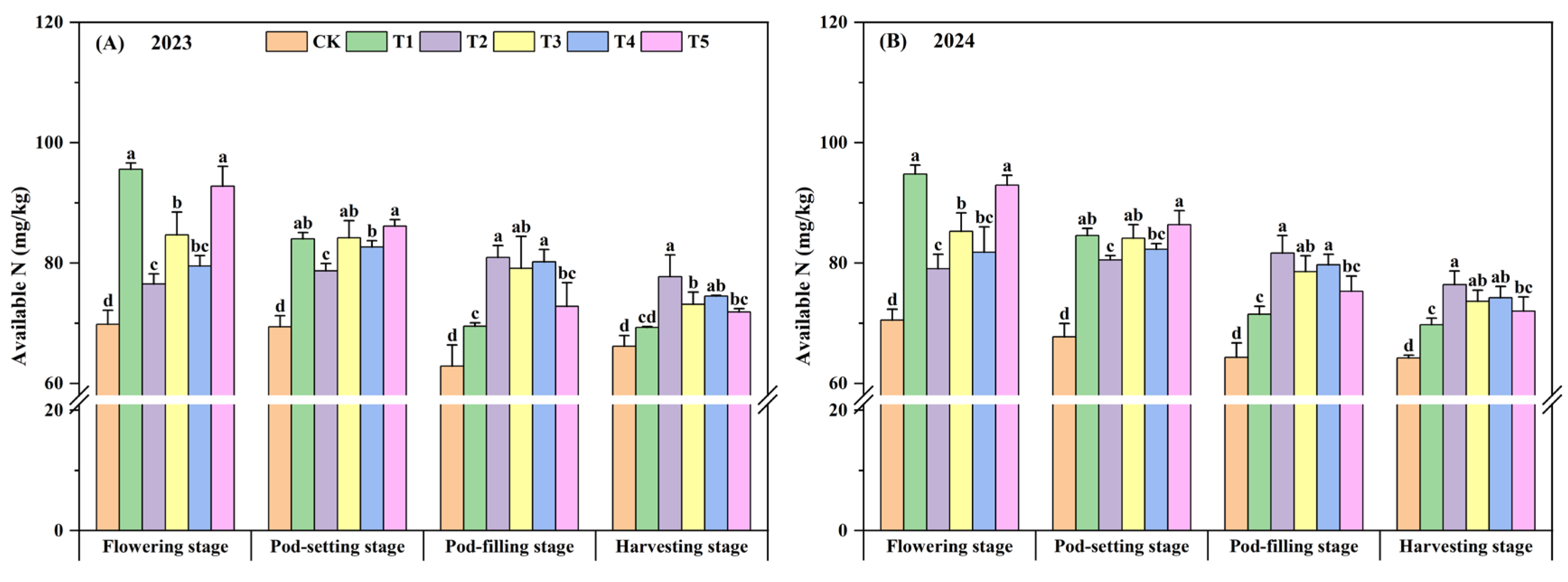
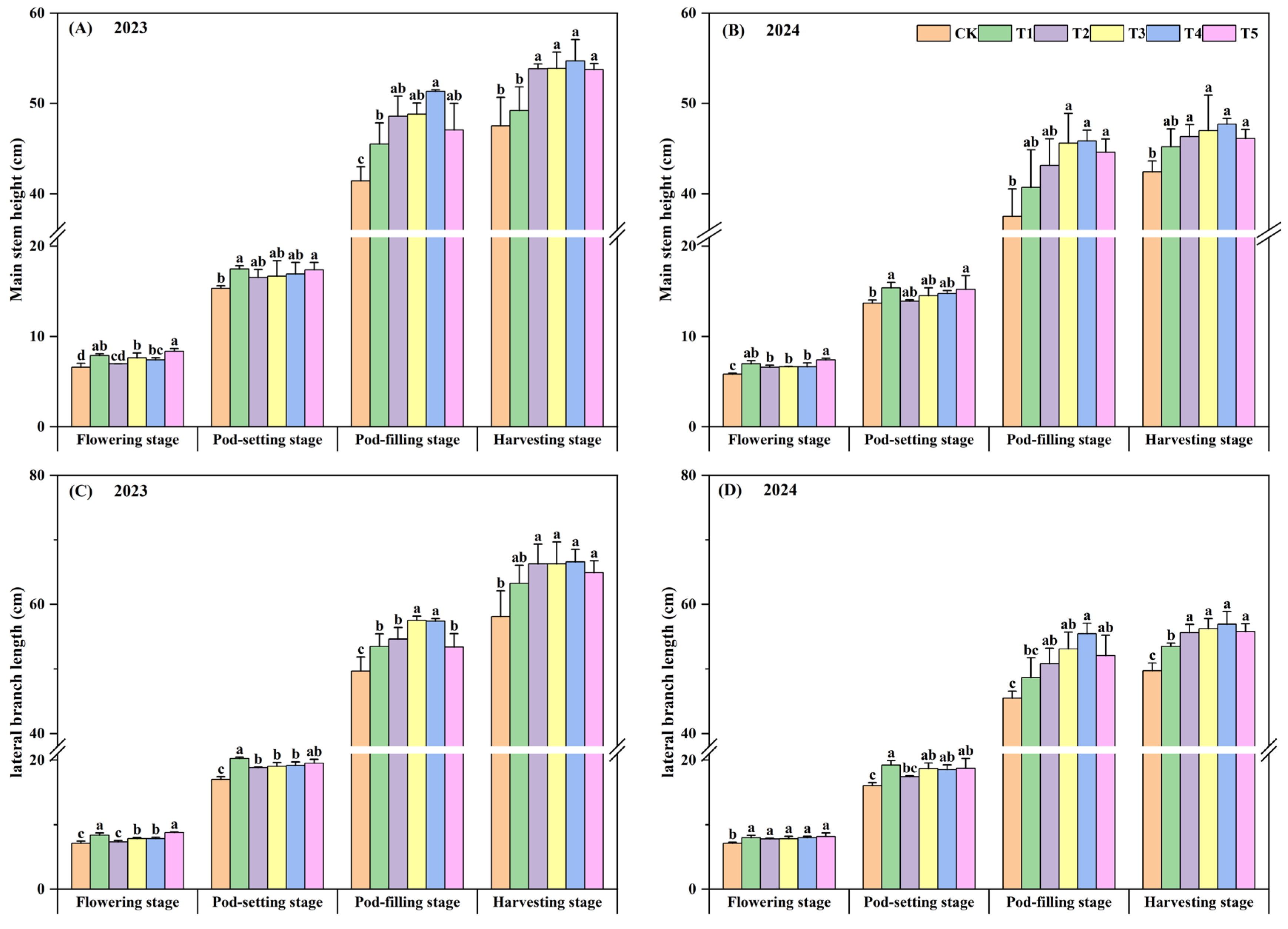
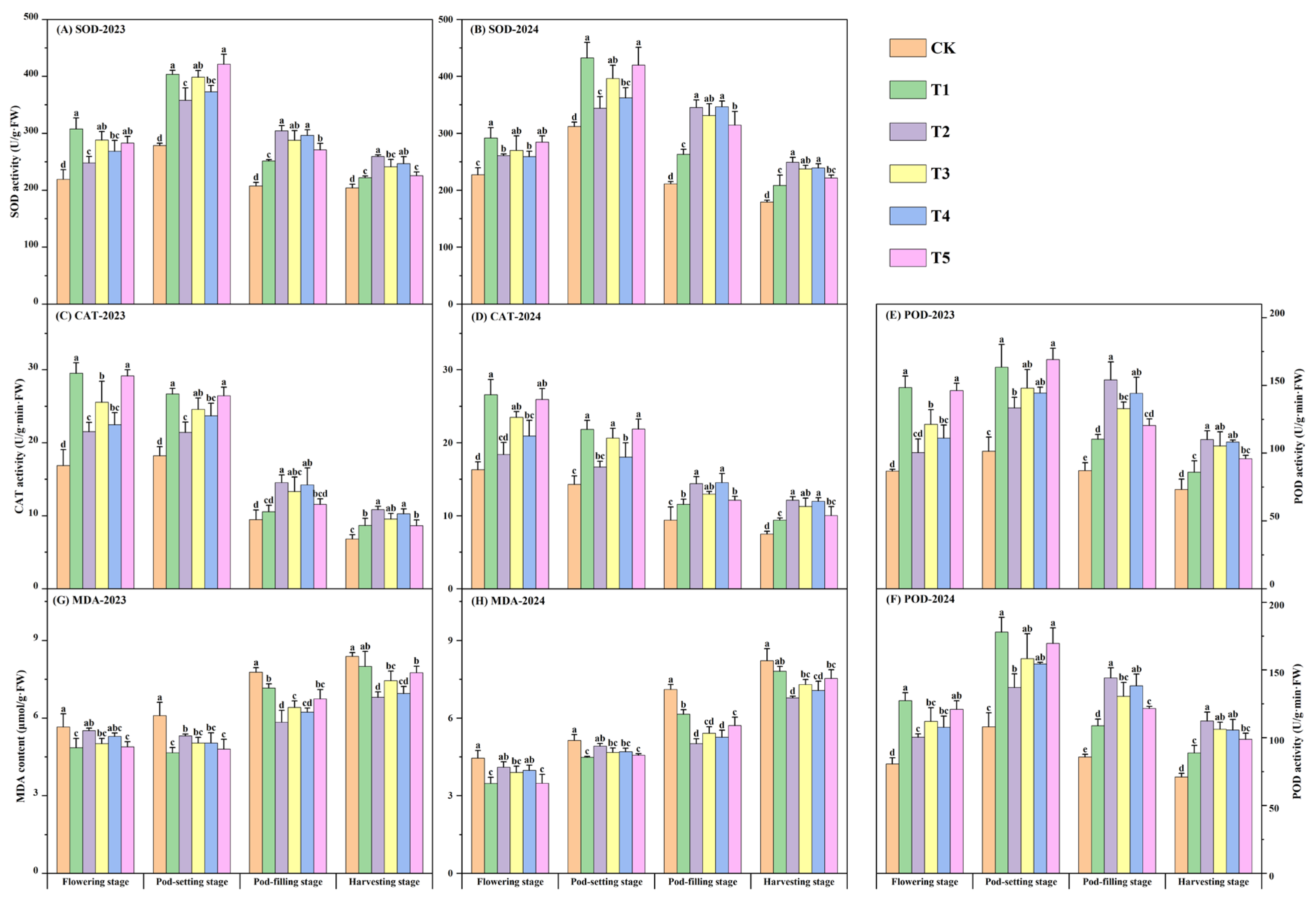

| Year | Organic Matter (g·kg−1) | Available N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) |
|---|---|---|---|---|
| 2023 | 12.14 | 71.73 | 14.36 | 90.20 |
| 2024 | 13.46 | 70.77 | 13.92 | 80.88 |
| Years | Treatments | Yield (kg·ha−1) | Pods Number per Plant | Pods Number per kg | 100-Pods Weight (g) | 100-Kernel Weight (g) | Kernel Percent (%) |
|---|---|---|---|---|---|---|---|
| 2023 | CK | 3666.99 d | 13.44 c | 689 a | 221.40 b | 87.45 c | 59.02 b |
| T1 | 4013.09 c | 14.50 bc | 601 c | 230.87 a | 87.59 bc | 60.46 ab | |
| T2 | 4154.58 bc | 15.53 ab | 621 bc | 227.67 ab | 91.86 a | 61.94 a | |
| T3 | 4397.84 a | 16.45 a | 643 b | 227.47 ab | 89.90 abc | 61.03 ab | |
| T4 | 4514.42 a | 16.80 a | 621 bc | 225.33 ab | 90.45 ab | 61.28 ab | |
| T5 | 4227.62 b | 15.96 ab | 614 c | 230.40 a | 92.50 a | 61.56 ab | |
| 2024 | CK | 3723.88 e | 14.08 d | 664 a | 215.40 b | 88.44 b | 63.28 c |
| T1 | 4063.61 d | 15.40 c | 622 ab | 229.40 a | 90.93 ab | 65.35 b | |
| T2 | 4218.68 c | 16.04 bc | 621 ab | 228.37 a | 91.39 ab | 66.69 a | |
| T3 | 4349.51 ab | 16.58 ab | 630 ab | 225.60 a | 91.15 ab | 65.25 b | |
| T4 | 4440.63 a | 17.20 a | 626 ab | 225.27 a | 91.26 ab | 65.34 b | |
| T5 | 4262.14 bc | 16.44 abc | 614 b | 230.53 a | 92.73 a | 65.70 b |
| Years | Treatments | Crude Protein (%) | Oil Content (%) | Oleic Acid (%) | Linoleic Acid (%) | O/L | Total Amino Acid (%) |
|---|---|---|---|---|---|---|---|
| 2023 | CK | 20.22 b | 55.25 a | 34.59 b | 39.58 a | 0.87 a | 22.04 b |
| T1 | 21.49 a | 53.92 b | 34.84 ab | 39.36 a | 0.89 a | 22.86 ab | |
| T2 | 21.81 a | 53.94 b | 34.98 ab | 38.39 a | 0.91 a | 23.05 ab | |
| T3 | 21.85 a | 53.64 b | 35.08 ab | 38.33 a | 0.92 a | 23.37 a | |
| T4 | 22.3 a | 53.49 b | 36.06 a | 38.10 a | 0.95 a | 23.40 a | |
| T5 | 21.56 a | 53.79 b | 35.24 ab | 37.60 a | 0.94 a | 22.94 ab | |
| 2024 | CK | 18.16 b | 55.60 a | 36.95 c | 38.52 a | 0.96 c | 21.68 b |
| T1 | 19.17 ab | 54.10 a | 37.08 bc | 37.97 ab | 0.98 bc | 22.35 ab | |
| T2 | 19.41 ab | 54.48 a | 37.05 bc | 37.07 b | 1.00 abc | 23.31 ab | |
| T3 | 19.45 ab | 54.99 a | 37.94 ab | 37.84 ab | 1.01 ab | 23.35 ab | |
| T4 | 20.14 a | 54.79 a | 38.30 a | 37.69 ab | 1.02 a | 23.51 a | |
| T5 | 19.28 ab | 54.41 a | 37.61 abc | 36.98 b | 1.02 a | 23.27 ab |
| Treatments | NUE (%) | PFPN (kg·kg−1) | AEN (kg·kg−1) | |||
|---|---|---|---|---|---|---|
| 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | |
| T1 | 31.39 c | 30.03 c | 32.61 c | 33.86 d | 2.05 c | 2.00 d |
| T2 | 39.43 b | 42.50 b | 33.79 bc | 35.16 c | 3.23 bc | 3.29 c |
| T3 | 48.26 a | 49.14 a | 35.82 a | 36.25 ab | 5.26 a | 4.38 ab |
| T4 | 50.20 a | 50.75 a | 36.79 a | 37.01 a | 6.23 a | 5.14 a |
| T5 | 41.84 b | 41.63 b | 34.40 b | 35.52 bc | 3.84 b | 3.65 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Luo, L.; Fang, R.; Liu, F.; Tan, Z.; Wu, Z.; Zheng, M.; Zhang, K.; Wan, Y. Optimizing Controlled-Release Urea and Conventional Urea Ratios Enhances Nitrogen Use Efficiency and Yield in Peanut. Agriculture 2025, 15, 1923. https://doi.org/10.3390/agriculture15181923
Gu M, Luo L, Fang R, Liu F, Tan Z, Wu Z, Zheng M, Zhang K, Wan Y. Optimizing Controlled-Release Urea and Conventional Urea Ratios Enhances Nitrogen Use Efficiency and Yield in Peanut. Agriculture. 2025; 15(18):1923. https://doi.org/10.3390/agriculture15181923
Chicago/Turabian StyleGu, Mingxuan, Lu Luo, Ruiyuan Fang, Fengzhen Liu, Zhen Tan, Zheng Wu, Mengjian Zheng, Kun Zhang, and Yongshan Wan. 2025. "Optimizing Controlled-Release Urea and Conventional Urea Ratios Enhances Nitrogen Use Efficiency and Yield in Peanut" Agriculture 15, no. 18: 1923. https://doi.org/10.3390/agriculture15181923
APA StyleGu, M., Luo, L., Fang, R., Liu, F., Tan, Z., Wu, Z., Zheng, M., Zhang, K., & Wan, Y. (2025). Optimizing Controlled-Release Urea and Conventional Urea Ratios Enhances Nitrogen Use Efficiency and Yield in Peanut. Agriculture, 15(18), 1923. https://doi.org/10.3390/agriculture15181923






