Arbuscular Mycorrhizal Fungi Inoculation Enhances Nutritional Quality of Prickly Pear (Opuntia ficus-indica) Fruits and Cladodes
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Experimental Design
2.3. Plant Material
2.4. Sample Preparation
2.5. Proximal Composition
2.6. Sugar and Ascorbic Acid Analysis
2.7. Polyphenols and Betalains
2.8. Mineral Composition
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fruits
3.1.1. Macroconstituents of Fruits from the Two Prickly Pear Phenotypes and Effect of Mycorrhizal Biofertilization
3.1.2. Effect of Mycorrhizal Biofertilization on the Micronutrient Contents
3.2. Cladodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuel, A.; Eltahir, E.A.B. Why Is the Mediterranean a Climate Change Hot Spot? J. Clim. 2020, 33, 5829–5843. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef]
- Louhaichi, M.; Hamdeni, I.; Slim, S.; Sawsan, H.; Harbegue, L.; Gouhis, F. Economic valuation of cactus pear production in semi-arid regions of Tunisia. In X International Congress on Cactus Pear and Cochineal: Cactus-the New Green Revolution in Drylands 1343; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2022; pp. 97–102. [Google Scholar] [CrossRef]
- Neffar, S.; Beddiar, A.; Menasria, T.; Chenchouni, H. Planting prickly pears as a sustainable alternative and restoration tool for rehabilitating degraded soils in dry steppe rangelands. Arab. J. Geosci. 2022, 15, 287. [Google Scholar] [CrossRef]
- Ávila-Gómez, E.S.; Meléndez-Ramírez, V.; Castellanos, I.; Zuria, I.; Moreno, C.E. Prickly pear crops as bee diversity reservoirs and the role of bees in Opuntia fruit production. Agric. Ecosyst. Environ. 2019, 279, 80–88. [Google Scholar] [CrossRef]
- Inglese, P.; Saenz, C.; Mondragon, C.; Nefzaoui, A.; Louhaichi, M. Crop Ecology, Cultivation and Uses of Cactus Pear; FAO: Rome, Italy, 2017. [Google Scholar]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Rocamora-Montiel, B.; Legua, P.; Hernández, F.; López-Lluch, D. Economic estimation of cactus pear production and its feasibility in Spain. Trends Food Sci. Technol. 2020, 103, 379–385. [Google Scholar] [CrossRef]
- Louppis, A.P.; Constantinou, M.S.; Kontominas, M.G.; Blando, F.; Stamatakos, G. Geographical and botanical differentiation of Mediterranean prickly pear using specific chemical markers. J. Food Compos. Anal. 2023, 119, 105219. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Saenz, C. Processing technologies: An alternative for cactus pear (Opuntia spp.) fruits and cladodes. J. Arid. Environ. 2000, 46, 209–225. [Google Scholar] [CrossRef]
- Barbera, G.; Inglese, P.; Mantia, T.L. Seed content and fruit characteristics in cactus pear (Opuntia ficus-indica Mill.). Sci. Hortic. 1994, 58, 161–165. [Google Scholar] [CrossRef]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Morghese, M.; Santulli, A.; Liguori, G.; Inglese, P. Seasonal characterization of nutritional and antioxidant properties of Opuntia ficus-indica [(L.) Mill.] mucilage. Food Hydrocoll. 2021, 111, 106398. [Google Scholar] [CrossRef]
- Patel, S. Reviewing the prospects of Opuntia pears as low cost functional foods. Rev. Environ. Sci. Bio/Technol. 2013, 12, 223–234. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; González-Laredo, R.F.; Reynoso-Camacho, R.; Medina-Torres, L.; Cervantes-Cardozo, V. Effect of air flow rate on the polyphenols content and antioxidant capacity of convective dried cactus pear cladodes (Opuntia ficus indica). Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S2), 80–87. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Peña, M.P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef]
- Hadj Sadok, T.; Aid, F.; Bellal, M.; Abdul Hussain, M.S. Composition chimique des jeunes cladodes d’Opuntia Ficus Indica et possibilités de valorisation alimentaire. Agric.—ŞtiinŃă şi Pract. 2008, 1, 65–66. [Google Scholar]
- Dubeux, J.C.B.; dos Santos, M.V.F.; da Cunha, M.V.; dos Santos, D.C.; de Almeida Souza, R.T.; de Mello, A.C.L.; de Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed. Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. Available online: https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2019.01068 (accessed on 1 January 2025). [CrossRef]
- Ouallal, I.; Abbas, Y.; ElYacoubi, H.; Imtara, H.; Al Zain, M.N.; Ouajdi, M.; El Goumi, Y.; Alzamel, N.M. Effects of Arbuscular Mycorrhizal Inoculation by Indigenous Fungal Complexes on the Morpho-Physiological Behavior of Argania spinosa Subjected to Water Deficit Stress. Horticulturae 2022, 8, 280. [Google Scholar] [CrossRef]
- Guo, X.; Wang, P.; Wang, X.; Li, Y.; Ji, B. Specific Plant Mycorrhizal Responses Are Linked to Mycorrhizal Fungal Species Interactions. Front. Plant Sci. 2022, 13, 930069. Available online: https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2022.930069 (accessed on 1 January 2025). [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Tahiri, A.I.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the Potential Role of Compost, PGPR, and AMF in Improving Tomato Plant Growth, Yield, Fruit Quality, and Water Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Lahbouki, S.; Fernando, A.L.; Rodrigues, C.; Ben-Laouane, R.; Ait-El-Mokhtar, M.; Outzourhit, A.; Meddich, A. Effects of Humic Substances and Mycorrhizal Fungi on Drought-Stressed Cactus: Focus on Growth, Physiology, and Biochemistry. Plants 2023, 12, 4156. [Google Scholar] [CrossRef] [PubMed]
- Kebede, T.G.; Birhane, E.; Ayimut, K.M.; Egziabher, Y.G. Arbuscular mycorrhizal fungi increased biomass, nutritional value, and cochineal resistance of Opuntia ficus-indica plants. BMC Plant Biol. 2024, 24, 706. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the AOAC. In International; Association of Official Analytical Chemist International: Washington, DC, USA, 1990; Available online: http://www.aoacofficialmethod.org/ (accessed on 1 January 2025).
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Corrales, C.V.; Lebrun, M.; Vaillant, F.; Madec, M.N.; Lortal, S.; Pérez, A.M.; Fliedel, G. Key odor and physicochemical characteristics of raw and roasted jicaro seeds (Crescentia alata K.H.B.). Food Res. Int. 2017, 96, 113–120. [Google Scholar] [CrossRef]
- Tamba, A.; Servent, A.; Mertz, C.; Cissé, M.; Dornier, M. Coupling of pressure-driven membrane technologies for concentrating, purifying and fractionizing betacyanins in cactus pear (Opuntia dillenii Haw.) juice. Innov. Food Sci. Emerg. Technol. 2019, 52, 244–255. [Google Scholar] [CrossRef]
- Thanh, C.; Mith, H.; Peng, C.; Servent, A.; Poss, C.; Laillou, A.; Phal, S.; Avallone, S. Assessment of the nutritional profiles and potentially toxic elements of wild and farmed freshwater fish in Cambodia. J. Food Compos. Anal. 2024, 133, 106357. [Google Scholar] [CrossRef]
- Reis, G.C.M.; Gazarini, C.L.; Ribeiro, M.M. Fruit production from Opuntia ficus-indica ecotypes in comparison to commercial Italian clones. Hortic. Sci. 2018, 45, 92–100. [Google Scholar] [CrossRef]
- Felker, P.; Rodriguez, S.D.C.; Casoliba, R.M.; Filippini, R.; Medina, D.; Zapata, R. Comparison of Opuntia ficus indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J. Arid. Environ. 2005, 60, 405–422. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Ramírez-Pérez, M.; Hidalgo-Martínez, D.; Reyes-López, C.A.; Pinedo-Espinoza, J.M.; Hernández-Fuentes, A.D.; Becerra-Martínez, E. Discrimination of different prickly pear (Opuntia spp.) accessions by NMR-based metabolomics. J. Food Compos. Anal. 2024, 130, 106158. [Google Scholar] [CrossRef]
- Melgar, B.; Pereira, E.; Oliveira, M.B.P.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Sokovic, M.; Barros, L.; Ferreira, I.C. Extensive profiling of three varieties of Opuntia spp. fruit for innovative food ingredients. Food Res. Int. 2017, 101, 259–265. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017. [Google Scholar]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.M.G.; Gouveia, C.; Vitorino, M.C.; Gazarini, L.C.; Ribeiro, M.M.; Peres, F. Bioactive Compounds and Morphology in Opuntia spp. Fruits from Portuguese Ecotypes. Bulg. J. Agric. Sci. 2017, 23, 929–938. [Google Scholar]
- Dehbi, F.; Hasib, A.; Bouaziz, M.; Ouatmane, A.; Elbatal, H.; Jaouad, A.; Sayadi, S. Effect of phenolic compounds and betalain pigments on the antioxidant capacity of Moroccan prickly pear juices. Nat. Technol. 2013, 5, 2–7. [Google Scholar]
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Goreta Ban, S.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Bubola, K.B.; Colla, G.; Rouphael, Y.; et al. Fruit quality and volatile compound composition of processing tomato as affected by fertilisation practices and arbuscular mycorrhizal fungi application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica, L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorganic Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef]
- Soliman, S.; Alebidi, A.; Al-Obeed, R.; Al-Saif, A. Effect of potassium fertilizer on fruit quality and mineral composition of fig (Ficus carica L. cv. brown turky). Pak. J. Bot. 2018, 50, 1753–1758. [Google Scholar]
- Wang, J.; Lu, Y.; Zhang, X.; Hu, W.; Lin, L.; Deng, Q.; Xia, H.; Liang, D.; Lv, X. Effects of Potassium-Containing Fertilizers on Sugar and Organic Acid Metabolism in Grape Fruits. Int. J. Mol. Sci. 2024, 25, 2828. [Google Scholar] [CrossRef]
- Castellano, J.; Marrero, M.D.; Ortega, Z.; Romero, F.; Benitez, A.N.; Ventura, M.R. Opuntia spp. Fibre Characterisation to Obtain Sustainable Materials in the Composites Field. Polymers 2021, 13, 2085. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira Agostini-Costa, T. Genetic and environment effects on bioactive compounds of Opuntia cacti—A review. J. Food Compos. Anal. 2022, 109, 104514. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Huang, Y.M.; Li, Y.; He, X.H. Arbuscular mycorrhizal fungi induce sucrose cleavage for carbon supply of arbuscular mycorrhizas in citrus genotypes. Sci. Hortic. 2013, 160, 320–325. [Google Scholar] [CrossRef]
- Al-Arjani, A.B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, W.; Xu, T.; Tang, M. Effect of arbuscular mycorrhizal fungi and phosphorus on drought-induced oxidative stress and 14-3-3 proteins gene expression of Populus cathayana. Front. Microbiol. 2022, 13, 934964. Available online: https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.934964 (accessed on 1 January 2025). [CrossRef] [PubMed]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef] [PubMed]
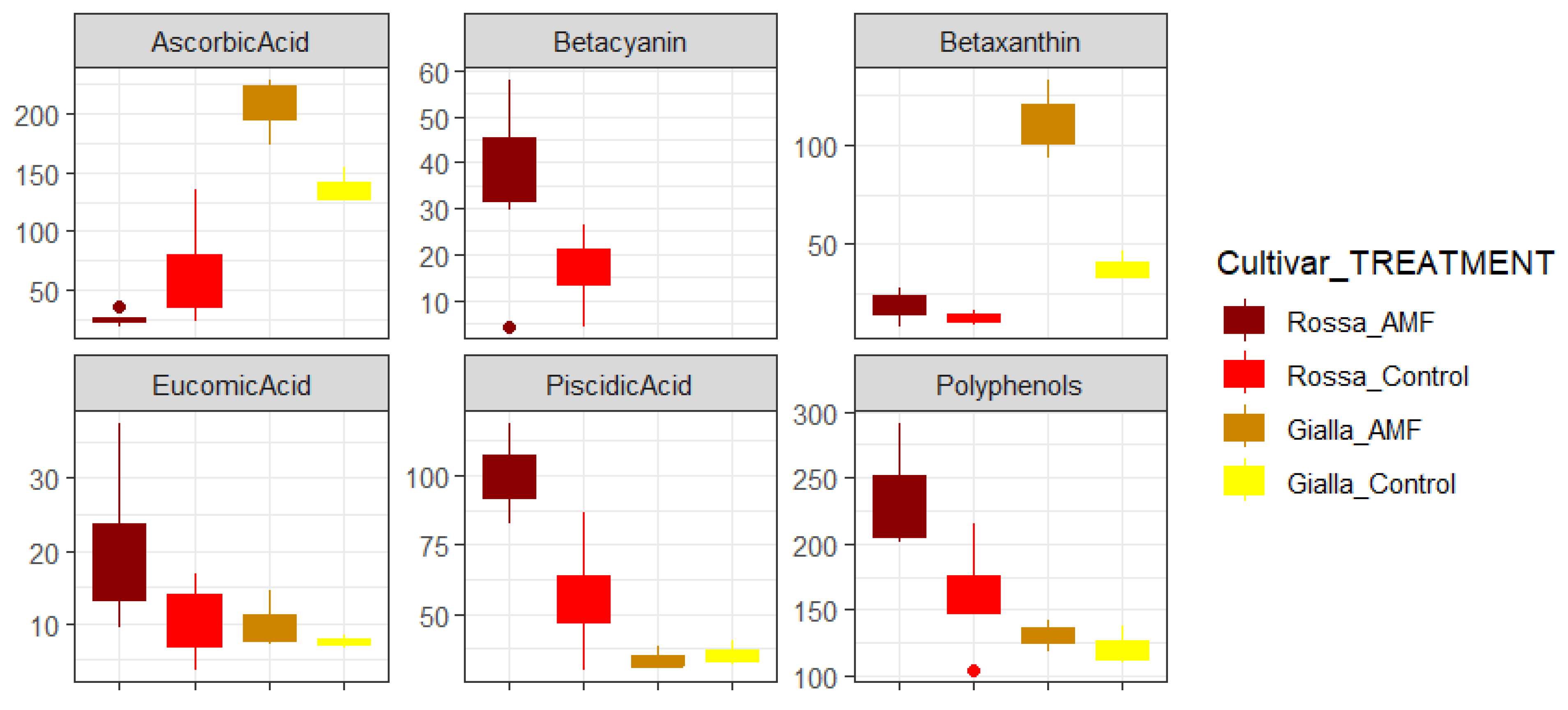
| Treatment | With AMF | Control | ||
|---|---|---|---|---|
| Phenotype | Gialla | Rossa | Gialla | Rossa |
| Juice/Fruit ratio | 0.44 (0.03) a | 0.53 (0.08)a | 0.37 (0.03) b | 0.43 (0.11) a |
| Seeds/Fruit ratio | 0.08 (0.01) | 0.08 (0.02) | 0.08 (0.01) | 0.10 (0.02) |
| pH | 6.01 (0.17) b | 5.63 (0.16) a | 6.37 (0.09) b | 5.74 (0.16) a |
| Total soluble solids (%) | 9.80 (0.75) | 10.27 (2.71) | 8.64 (0.25) | 9.08 (0.91) |
| Dry matter (%) | 9.59 (0.95) | 10.69 (1.64) | 8.23 (0.09) | 9.02 (0.98) |
| Glucose (%) | 4.34 (0.52) | 3.29 (1.06) | 3.70 (0.08) | 3.77 (0.89) |
| Sucrose (%) | 0.02 (0.01) b | 0.10 (0.02) a | 0.01 (0.01) b | 0.08 (0.01) a |
| Fructose (%) | 4.19 (0.61) | 4.76 (1.49) | 3.38 (0.10) | 3.71 (0.79) |
| Titratable Acidity (%) | 0.01 (0.01) b | 0.14 (0.02) a | 0.02 (0.01) b | 0.11 (0.02) a |
| Ash% | 0.81 (0.38) a | 0.80 (0.08) a | 0.44 (0.06) b | 0.64 (0.06) a |
| Protein (%) | 0.70 (0.07) | 0.80 (0.08) | 0.61 (0.06) | 0.57 (0.06) |
| Estimated Fibers (%) | 0.45 (0.29) | 1.22 (0.55) | 0.33 (0.12) | 0.86 (0.50) |
| Treatment | With AMF | Control | ||
|---|---|---|---|---|
| Phenotype | Gialla | Rossa | Gialla | Rossa |
| Ascorbic Acid mg·L−1 | 206 (30) a | 26 (6) b | 136 (15) b | 64 (30) b |
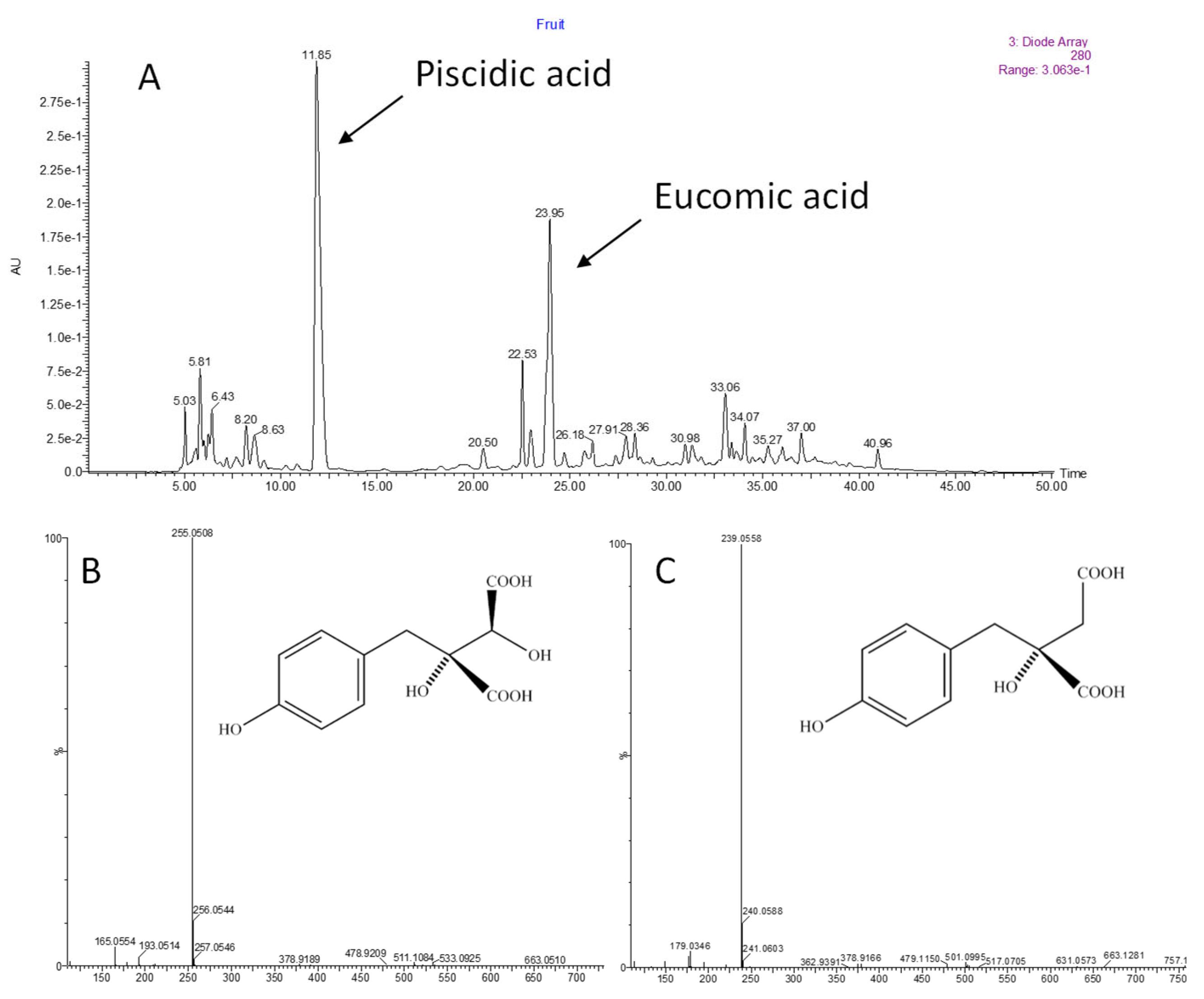
| Piscidic Acid mg·L−1 | 33 (4) b | 99 (13) a | 35 (4) b | 55 (15) b |
| Eucomic Acid mg·L−1 | 10 (4) | 21 (10) | 7 (1) | 10 (5) |
| Polyphenols mg·L−1 | 130 (12) b | 232 (35) a | 121 (14) b | 159 (35) b |
| Betacyanin mg·L−1 | ND | 46 (19) a | ND | 21 (10) b |
| Betaxanthin mg·L−1 | 111 (20) a | 18 (7) b | 38 (8) b | 13 (3) b |
| P (mg·100 mL−1) | 23 (1) | 22 (7) | 22 (1) | 21 (7) |
| K (mg·100 mL−1) | 237 (9) b | 345 (49) a | 250 (16) a,b | 326 (74) a |
| Ca (mg·100 mL−1) | 36 (2) b | 84 (21) a | 51 (3) a,b | 80 (32) a |
| Mg (mg·100 mL−1) | 24 (4) | 28 (8) | 37 (2) | 27 (7) |
| Na (mg·100 mL−1) | 1.00 (0.01) | 0.90 (0.01) | 1.10 (0.01) | 0.80 (0.01) |
| Treatment | With AMF | Control |
|---|---|---|
| pH | 4.21 (0.10) | 4.34 (0.22) |
| Total Soluble Solids (%) | 7.15 (0.42) | 6.75 (0.49) |
| Dry Matter (%) | 11.35 (1.06) a | 9.41 (0.92) b |
| Titrable acidity (%) | 1.21 (0.43) | 1.24 (0.33) |
| Glucose (%) | 0.23 (0.04) a | 0.12 (0.06) b |
| Sucrose (%) | 0.03 (0.02) | 0.01 (0.01) |
| Fructose (%) | 0.31 (0.06) a | 0.23 (0.04) b |
| Hemicellulose (%) | 1.15 (0.24) | 1.07 (0.17) |
| Cellulose (%) | 0.78 (0.06) | 0.76 (0.05) |
| Lignin (%) | 0.07 (0.01) | 0.15 (0.11) |
| Ash (%) | 1.53 (0.19) | 1.53 (0.23) |
| Proteins (%) | 0.93 (0.06) | 0.85 (0.04) |
| Ascorbic Acid mg·kg−1 | 263 (43) a | 213 (25) b |
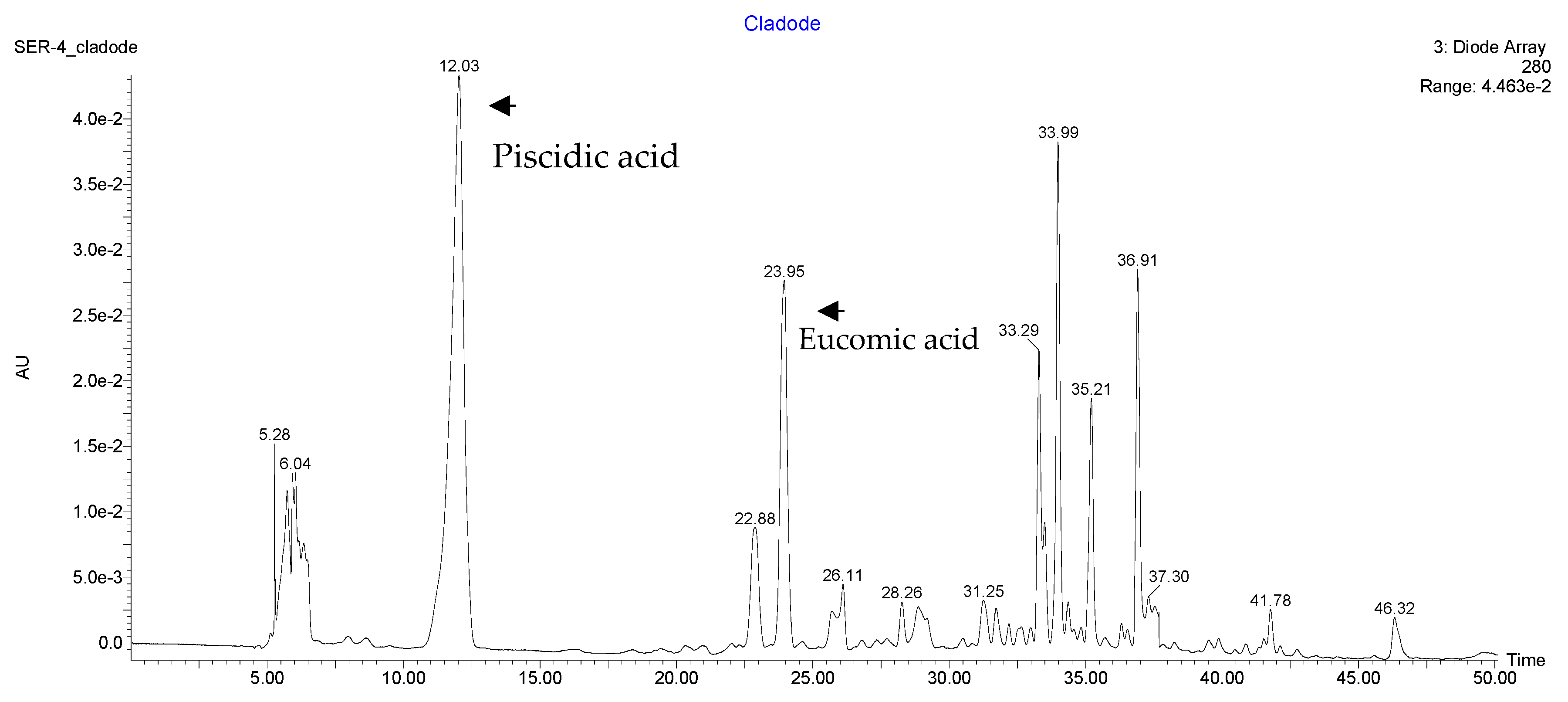
| Piscidic Acid mg·kg−1 | 132 (34) | 111 (24) |
| Eucomic Acid mg·kg−1 | 67 (30) | 73 (12) |
| Polyphenols mg·kg−1 | 450 (87) | 406 (53) |
| P mg·100 g−1 | 29 (10) a | 24 (1) b |
| K mg·100 g−1 | 260 (31) | 272 (17) |
| Ca mg·100 g−1 | 321 (37) | 414 (80) |
| Mg mg·100 g−1 | 85 (13) | 121 (44) |
| Na mg·100 g−1 | 0.80 (0.01) | 0.90 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labidi, S.; Servent, A.; Bouzoumita, G.; Julien, T.; Cazals, G.; Ibrahim, M.; Hammami, S.B.M.; Achir, N. Arbuscular Mycorrhizal Fungi Inoculation Enhances Nutritional Quality of Prickly Pear (Opuntia ficus-indica) Fruits and Cladodes. Agriculture 2025, 15, 1902. https://doi.org/10.3390/agriculture15171902
Labidi S, Servent A, Bouzoumita G, Julien T, Cazals G, Ibrahim M, Hammami SBM, Achir N. Arbuscular Mycorrhizal Fungi Inoculation Enhances Nutritional Quality of Prickly Pear (Opuntia ficus-indica) Fruits and Cladodes. Agriculture. 2025; 15(17):1902. https://doi.org/10.3390/agriculture15171902
Chicago/Turabian StyleLabidi, Sonia, Adrien Servent, Ghofran Bouzoumita, Tina Julien, Guillaume Cazals, Manel Ibrahim, Sofiène B. M. Hammami, and Nawel Achir. 2025. "Arbuscular Mycorrhizal Fungi Inoculation Enhances Nutritional Quality of Prickly Pear (Opuntia ficus-indica) Fruits and Cladodes" Agriculture 15, no. 17: 1902. https://doi.org/10.3390/agriculture15171902
APA StyleLabidi, S., Servent, A., Bouzoumita, G., Julien, T., Cazals, G., Ibrahim, M., Hammami, S. B. M., & Achir, N. (2025). Arbuscular Mycorrhizal Fungi Inoculation Enhances Nutritional Quality of Prickly Pear (Opuntia ficus-indica) Fruits and Cladodes. Agriculture, 15(17), 1902. https://doi.org/10.3390/agriculture15171902






