Djulis (Chenopodium formosanum) Stems as Sustainable Sawdust Alternative for Pleurotus sajor-caju Cultivation: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation and Djulis Stem Processing
2.2. Fungal Strains and Inoculant Preparations
2.3. Small-Scale Mycelial Growth Assessment in Petri Dishes
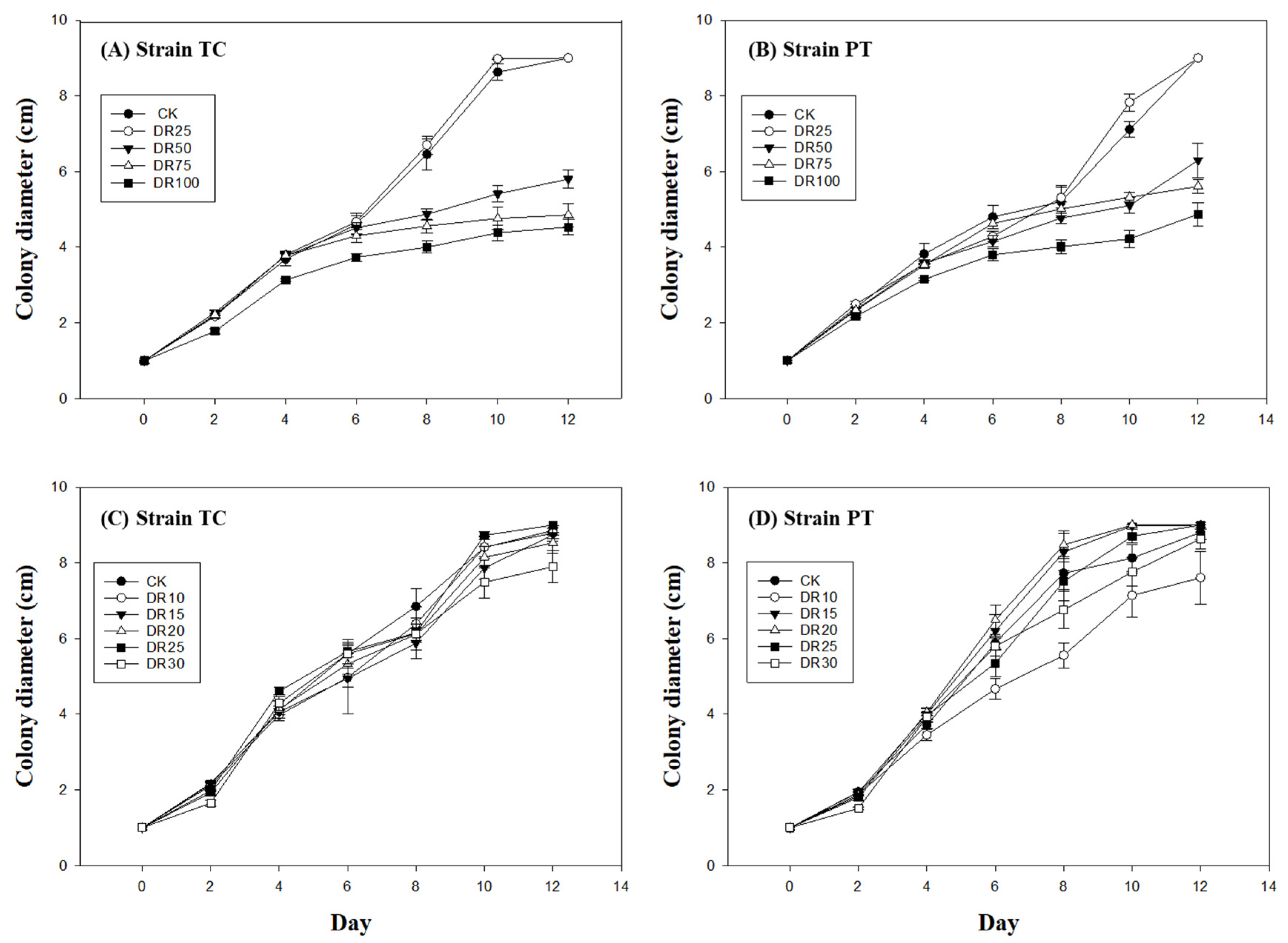
2.3.1. Initial Screening: 0–100% Djulis Stem Replacement
2.3.2. Refined Assessment: 0–30% Djulis Stem Replacement
2.4. Commercial-Scale Evaluation in Mushroom Grow Bags
2.4.1. Substrate Preparation and Inoculation
2.4.2. Control Treatments
2.4.3. Fruiting Body Production and Harvest
2.5. Nutritional and Bioactive Composition Analysis
2.5.1. Sample Preparation
2.5.2. Basic Nutritional Composition
2.5.3. Amino Acid Analysis
2.5.4. Phenolic Compound Analysis
2.5.5. Free Radical Scavenging Activity and Reducing Power Assessment
2.6. Data Analysis
3. Results
3.1. Mycelial Growth Response to Varying Djulis Stem Concentrations in Petri Dish Experiments
3.1.1. Initial Screening (0–100% Djulis Replacement)
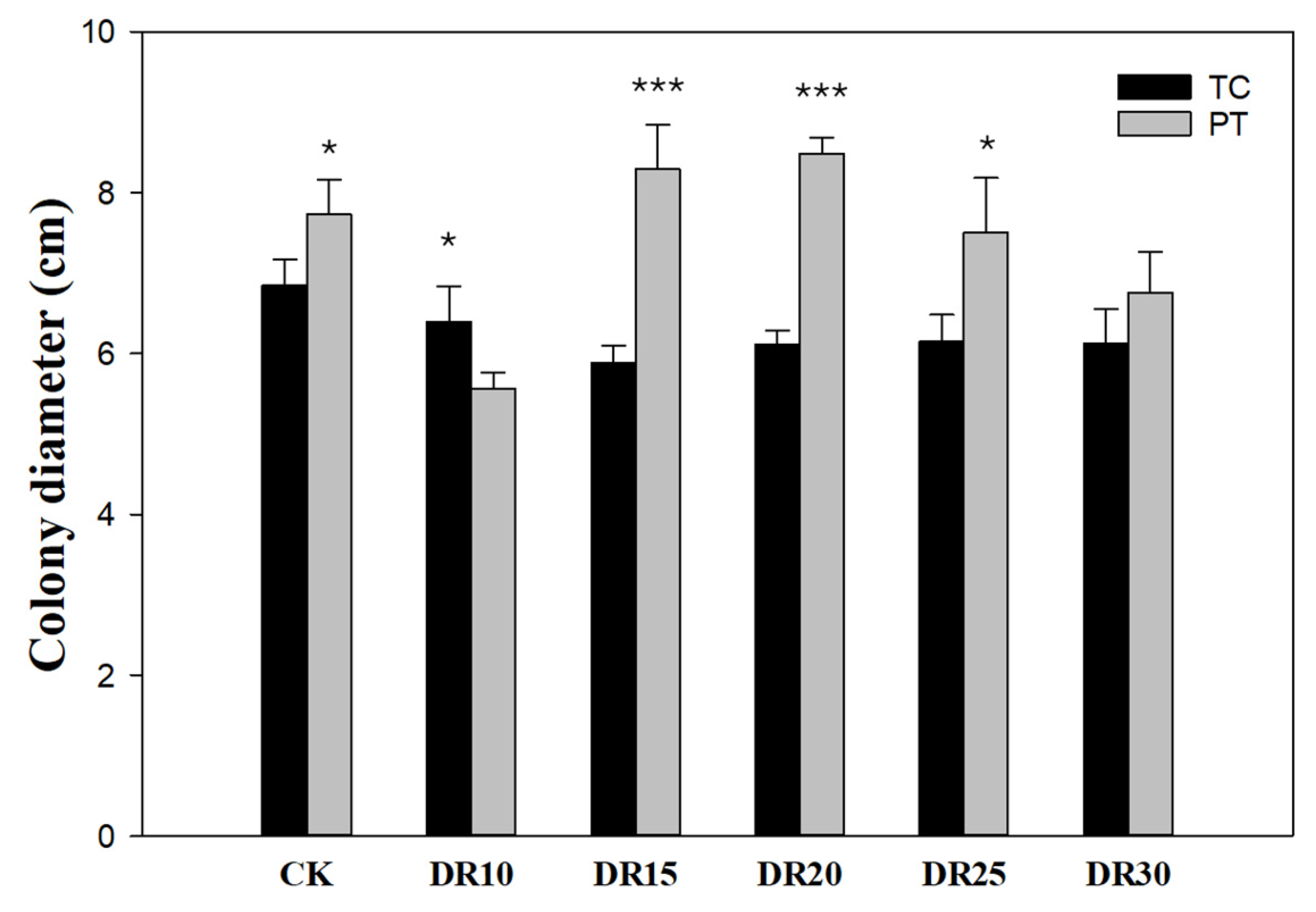
3.1.2. Refined Range Test (0–30% Djulis Replacement)
3.2. Commercial-Scale Mycelial Colonization in Mushroom Grow Bags
3.3. Fruiting Body Productivity and Biological Efficiency Across Harvest Cycles
3.4. Impact of Djulis Incorporation on Mushroom Nutritional and Bioactive Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | Branched-Chain Amino Acids |
| BHT | Butylated Hydroxytoluene |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DR | Djulis Replacement |
| PDA | Potato Dextrose Agar |
| PT | P. sajor-caju strain PT |
| TC | P. sajor-caju strain TC |
| TCA | Trichloroacetic Acid |
| TFAA | Total Free Amino Acids |
References
- Chen, T.M.; Lu, Y.S.; Shih, H.D. Current production status of Taiwan’s mushroom industry. In Biotechnology Conference on Mushrooms; Taiwan Agricultural Research Institute: Taichung, Taiwan, 2016. [Google Scholar]
- Chen, C.T.; Yeh, R.Y. Application of bamboo sawdust in mushroom cultivation. For. Res. Newsl. 2017, 24, 14–18. [Google Scholar]
- Fasehah, S.; Shah, A. Effect of using various substrates on cultivation of Pleurotus sajor-caju. J. Eng. Sci. Technol. 2017, 12, 1104–1110. [Google Scholar]
- Sharma, S.; Yadav, R.K.P.; Pokhrel, C.P. Growth and yield of oyster mushroom (Pleurotus ostreatus) on different substrates. J. New Biol. Rep. 2013, 2, 3–8. [Google Scholar]
- Li, W.S.; Lu, Y.S.; Chen, M.H. Application of rice straw in Pleurotus geesteranus cultivation. J. Taiwan Agric. Res. 2012, 61, 90–99. [Google Scholar]
- Kalmıs, E.; Azbar, N.; Yıldız, H.; Kalyoncu, F. Feasibility of using olive mill effluent (OME) as a wetting agent during the cultivation of oyster mushroom, Pleurotus ostreatus, on wheat straw. Bioresour. Technol. 2008, 99, 164–169. [Google Scholar] [CrossRef]
- Nguyen, B.; Le, V.; Nguyen, H.; Nguyen, H.; Nguyen, L.; Ngo, N. Cotton waste as an optimal substrate for cultivation of the pink oyster mushroom Pleurotus djamor. J. Appl. Biol. Biotechnol. 2025, 13, 184–191. [Google Scholar] [CrossRef]
- Subedi, S.; Kunwar, N.; Pandey, K.R.; Joshi, Y.R. Performance of oyster mushroom (Pleurotus ostreatus) on paddy straw, water hyacinth and their combinations. Heliyon 2023, 9, e19051. [Google Scholar] [CrossRef] [PubMed]
- Klibansky, M.; Mansur, M.; Gutierrez, I.; González, L. Production of Pleurotus ostreatus mushrooms on sugar cane agrowastes. Acta Biotechnol. 1993, 13, 71–78. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Hsieh, C.-C.; Huang, Y.-C.; Kuo, C.-H.; Chen, M.-H.; Hsieh, C.-W.; Cheng, K.-C. Development of functional fermented dairy products containing Taiwan djulis (Chenopodium formosanum Koidz.) in regulating glucose utilization. Fermentation 2022, 8, 423. [Google Scholar] [CrossRef]
- Ye, J.-W.; Ong, W.A.; Chao, Y.-Y. Analysis of antioxidant capacity of different colour strain of djulis (Chenopodium formosanum Koidz.). Int. J. Agric. Innov. Technol. Glob. 2021, 2, 157–172. [Google Scholar] [CrossRef]
- Chen, C.Y. Taiwan djulis: A new star among miscellaneous grains. Taitung Dist. Agric. Res. Ext. Stn. Newsl. 2011, 75, 18–20. [Google Scholar]
- Mulio, A.T.; Chiu, C.S.; Chan, Y.J.; Lu, W.C.; Li, P.H. New perspectives on djulis (Chenopodium formosanum Koidz.) and its potential application in functional food. Food Chem. X 2025, 25, 102135. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xing, X.; Xie, Y.; Sun, Y.; Bian, S.; Liu, L.; Chen, G.; Wang, X.; Yu, X.; Su, Y. Evaluation of preparation and detoxification of hemicellulose hydrolysate for improved xylitol production from quinoa straw. Int. J. Mol. Sci. 2022, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Fourtaka, K.; Melanouri, E.M.; Dedousi, M.; Diamantis, I.; Gardeli, C.; Papanikolaou, S. Examining the impact of substrate composition on the biochemical properties and antioxidant activity of Pleurotus and Agaricus mushrooms. Fermentation 2023, 9, 689. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Sardar, H.; Ali, M.A.; Anjum, M.A.; Nawaz, F.; Hussain, S.; Naz, S.; Karimi, S.M. Agro-industrial residues influence mineral elements accumulation and nutritional composition of king oyster mushroom (Pleurotus eryngii). Sci. Hortic. 2017, 225, 327–334. [Google Scholar] [CrossRef]
- Sato, M.; Ramarathnam, N.; Suzuki, Y.; Ohkubo, T.; Takeuchi, M.; Ochi, H. Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources. J. Agric. Food Chem. 1996, 44, 37–41. [Google Scholar] [CrossRef]
- Van Hung, P.; Morita, N. Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chem. 2008, 109, 325–331. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Zárate-Salazar, J.R.; Santos, M.N.; ECaballero, N.M.; Martins, O.G.; Herrera, Á.A.P. Use of lignocellulosic corn and rice wastes as substrates for oyster mushroom (Pleurotus ostreatus Jacq.) cultivation. Discov. Appl. Sci. 2020, 2, 1904. [Google Scholar] [CrossRef]
- Baysal, E.; Peker, H.; Yalinkiliç, M.K.; Temiz, A. Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour. Technol. 2003, 89, 95–97. [Google Scholar] [CrossRef]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef]
- Adenipekun, C.; Omolaso, P. Comparative study on cultivation, yield performance and proximate composition of Pleurotus pulmonarius Fries. (Quelet) on rice straw and banana leaves. World J. Agric. Sci. 2015, 11, 151–158. [Google Scholar]
- Odunmbaku, O.; Adenipekun, C. Cultivation of Pleurotus ostreatus (Jacq Fr.) Kumm on Gossypium hirsutum Roxb. (Cotton waste) and Gmelina arborea L. sawdust. Int. Food Res. J. 2018, 25, 1140–1145. [Google Scholar]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Penninckx, M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2008, 35, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Zhan, W.X.; Huang, K.R.; Lin, J.C.; Chen, Z.M.; Lu, Y.S.; Chen, Y.H. Estimation of Sawdust Consumption and Sources for Mushroom Cultivation in 2020. For. Res. Newsl. 2022, 29, 44–46. [Google Scholar]
| Substrate | Mycelium Growth Progression (cm) | ||||
|---|---|---|---|---|---|
| DAY7 | DAY14 | DAY21 | DAY28 | DAY35 | |
| M | 4.61 ± 0.22 b | 15.46 ± 0.20 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a |
| CK | 3.18 ± 0.10 c | 8.86 ± 0.14 e | 13.70 ± 0.21 c | 18.29 ± 0.14 b | 20.00 ± 0.00 a |
| DR15 | 4.15 ± 0.13 b | 11.00 ± 0.18 c | 16.70 ± 0.14 b | 20.00 ± 0.00 a | 20.00 ± 0.00 a |
| DR20 | 4.23 ± 0.15 b | 10.08 ± 0.18 d | 17.04 ± 0.14 b | 20.00 ± 0.00 a | 20.00 ± 0.00 a |
| DR25 | 6.83 ± 0.16 a | 14.69 ± 0.12 b | 20.00 ± 0.00 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a |
| Substrate | 1st Flush | 2nd Flush | 3rd Flush | Total Yield | Biological Efficiency |
|---|---|---|---|---|---|
| (g/Bag) | (g/Bag) | (g/Bag) | (g/Bag) | (%) | |
| CK | 85.10 ± 8.87 ab | 92.46 ± 3.13 c | 108.50 ± 17.79 ab | 286.10 ± 3.7 a | 71.53% |
| DR15 | 68.40 ± 14.26 ab | 157.20 ± 7.163 a | 94.30 ± 7.759 b | 319.90 ± 14.5 a | 76.17% |
| DR20 | 73.30 ± 4.57 ab | 108.32 ± 4.79 bc | 124.90 ± 10.23 a | 306.50 ± 17.5 ab | 72.98% |
| DR25 | 91.50 ± 11.6 a | 115.28 ± 3.79 b | 94.50 ± 3.87 b | 301.33 ± 11.9 a | 71.75% |
| M | 52.50 ± 3.63 b | 107.90 ± 4.79 bc | 85.40 ± 2.72 b | 245.78 ± 27.7 b | 76.80% |
| Component | Treatment | ||||
|---|---|---|---|---|---|
| CK | DR15 | DR20 | DR25 | M | |
| Moisture (g/100 g fw) | 90.4 ± 0.52 a | 90.1 ± 0.49 a | 89.1 ± 0.31 a | 89.7 ± 0.19 a | 90.3 ± 0.27 a |
| Crude fat (g/100 g fw) | 2.10 ± 0.57 a | 1.77 ± 0.91 a | 1.44 ± 0.61 a | 1.48 ± 0.65 a | 1.98 ± 0.14 a |
| Crude ash (g/100 g fw) | 0.66 ± 0.04 a | 0.67 ± 0.03 a | 0.74 ± 0.02 a | 0.73 ± 0.01 a | 0.68 ± 0.02 a |
| Crude protein (g/100 g fw) | 4.14 ± 0.22 a | 3.90 ± 0.36 a | 4.32 ± 0.18 a | 3.92 ± 0.12 a | 3.98 ± 0.21 a |
| Total carbohydrates (g/100 g fw) | 2.70 ± 0.36 c | 3.56 ± 0.15 abc | 4.44 ± 0.17 a | 4.20 ± 0.24 ab | 3.06 ± 0.10 c |
| Total free amino acids (TFAA, g/100 g fw) | 0.50 ± 0.07 ab | 0.43 ± 0.09 b | 0.63 ± 0.10 a | 0.69 ± 0.11 a | 0.64 ± 0.12 a |
| Total branched-chain amino acids (BCAA, g/100 g fw) | 0.18 ± 0.01 b | 0.21 ± 0.05 ab | 0.18 ± 0.02 b | 0.21 ± 0.01 ab | 0.22 ± 0.01 a |
| Lysine (g/100 g fw) | 0.06 ± 0.00 a | 0.07 ± 0.009 a | 0.06 ± 0.002 a | 0.07 ± 0.009 a | 0.06 ± 0.008 a |
| Total phenolics (mg/g dw) | 25.57 ± 0.33 c | 27.78 ± 0.51 b | 22.31 ± 0.67 e | 23.41 ± 0.12 d | 32.54 ± 0.98 a |
| Total flavonoids (mg/g dw) | 4.80 ± 0.06 a | 3.20 ± 0.05 d | 4.10 ± 0.05 b | 3.40 ± 0.09 c | 4.10 ± 0.04 b |
| Free radical scavenging activity (% DPPH) | 75.00 ± 3.31 a | 72.03 ± 10.62 ab | 57.10 ± 10.1 b | 68.80 ± 7.90 ab | 59.30 ± 5.29 b |
| Reducing power (OD700nm) | 0.46 ± 0.03 bc | 0.43 ± 0.017 c | 0.41 ± 0.02 c | 0.56 ± 0.03 a | 0.52 ± 0.04 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, T.-H.; Ong, W.-A.; Li, W.-S.; Chao, Y.-Y.; Chang, P.P. Djulis (Chenopodium formosanum) Stems as Sustainable Sawdust Alternative for Pleurotus sajor-caju Cultivation: A Feasibility Study. Agriculture 2025, 15, 1878. https://doi.org/10.3390/agriculture15171878
Hung T-H, Ong W-A, Li W-S, Chao Y-Y, Chang PP. Djulis (Chenopodium formosanum) Stems as Sustainable Sawdust Alternative for Pleurotus sajor-caju Cultivation: A Feasibility Study. Agriculture. 2025; 15(17):1878. https://doi.org/10.3390/agriculture15171878
Chicago/Turabian StyleHung, Tzu-Huan, Wee-Ann Ong, Wei-Sung Li, Yun-Yang Chao, and Pearl Peichun Chang. 2025. "Djulis (Chenopodium formosanum) Stems as Sustainable Sawdust Alternative for Pleurotus sajor-caju Cultivation: A Feasibility Study" Agriculture 15, no. 17: 1878. https://doi.org/10.3390/agriculture15171878
APA StyleHung, T.-H., Ong, W.-A., Li, W.-S., Chao, Y.-Y., & Chang, P. P. (2025). Djulis (Chenopodium formosanum) Stems as Sustainable Sawdust Alternative for Pleurotus sajor-caju Cultivation: A Feasibility Study. Agriculture, 15(17), 1878. https://doi.org/10.3390/agriculture15171878









