Neural Network-Guided Smart Trap for Selective Monitoring of Nocturnal Pest Insects in Agriculture
Abstract
1. Introduction
- Integrated smart trap design and deployment: this study presents the complete design, construction, and field implementation of an automatic trap system for the attraction and capture of pest insects. It responds to the limitations found in traditional methods, such as low selectivity, high manual labor, and insufficient automation.
- Stimuli-driven selectivity (visual and chemical): the trap attracts insects using a combination of a fermented food-based attractant and a light stimulus based on high-luminosity LEDs (green: 500–570 nm, yellow: 570–590 nm). These wavelengths were selected to exploit the visual sensitivity of nocturnal pests, particularly from the Lepidoptera and Diptera orders, as validated by literature and INIFAP field trials.
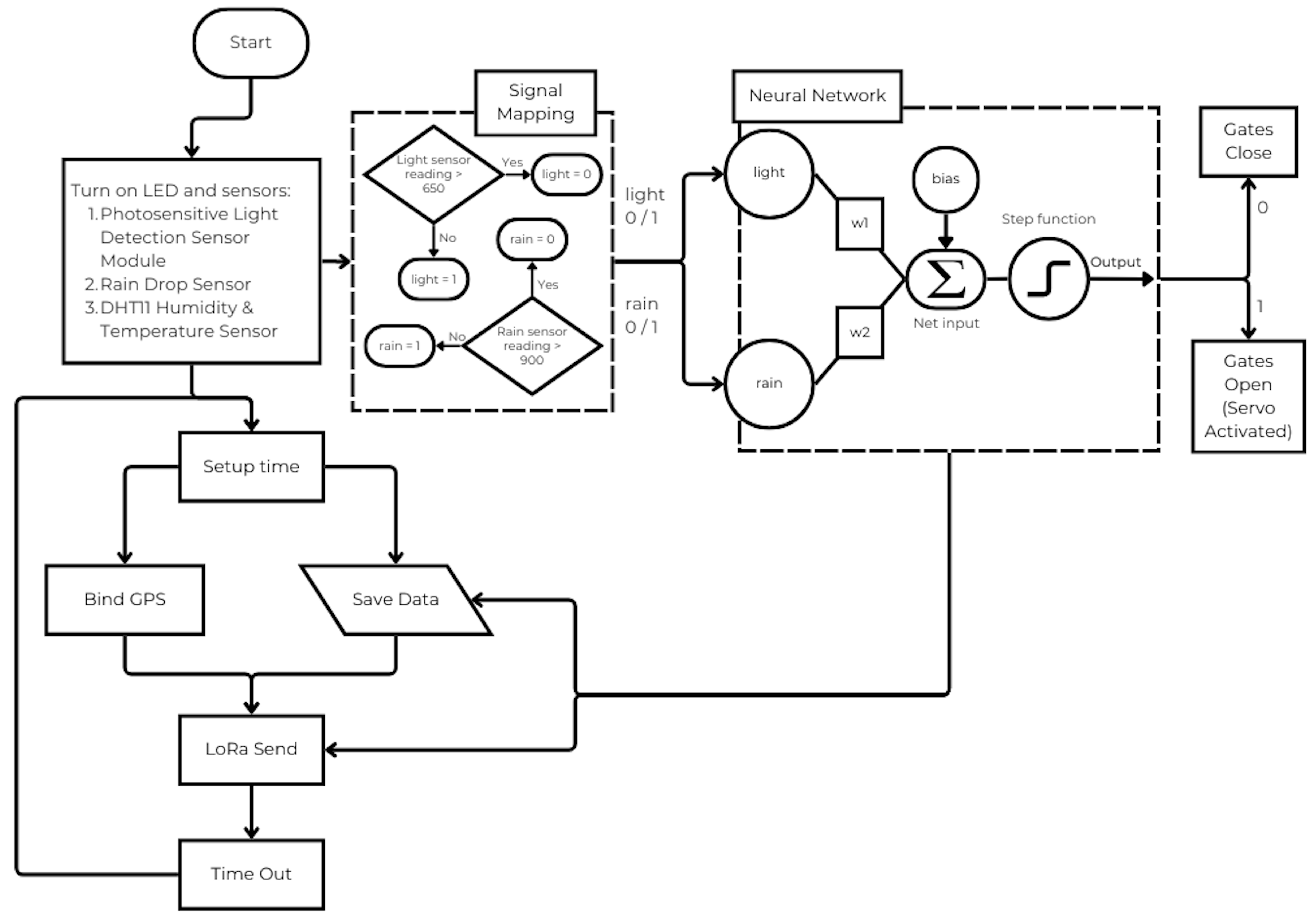
- Autonomous mechanical control based on environmental conditions: the device includes servo-actuated gates that selectively allow insects entry during periods of darkness and dry conditions. This is achieved through an environmental control logic implemented as a perceptron neural network embedded in an ATMEGA-2560 microcontroller, using binary-mapped inputs from light and rain sensors.
- Real-time environmental sensing and system monitoring: the trap continuously collects operational data such as gate state, LED activity, temperature, and humidity, which are stored on an SD card, displayed via LCD/I2C, and optionally transmitted using LoRa. This architecture enables remote status monitoring and supports the long-term, unattended deployment in agricultural environments.
- Structural and electronic integration: a modular mechanical structure houses all components, including a transparent upper dome, removable tray, gate drive system, and sensor platform. The integration of sensing, actuation, and processing ensures a compact and robust solution fabricated using PLA via 3D printing.
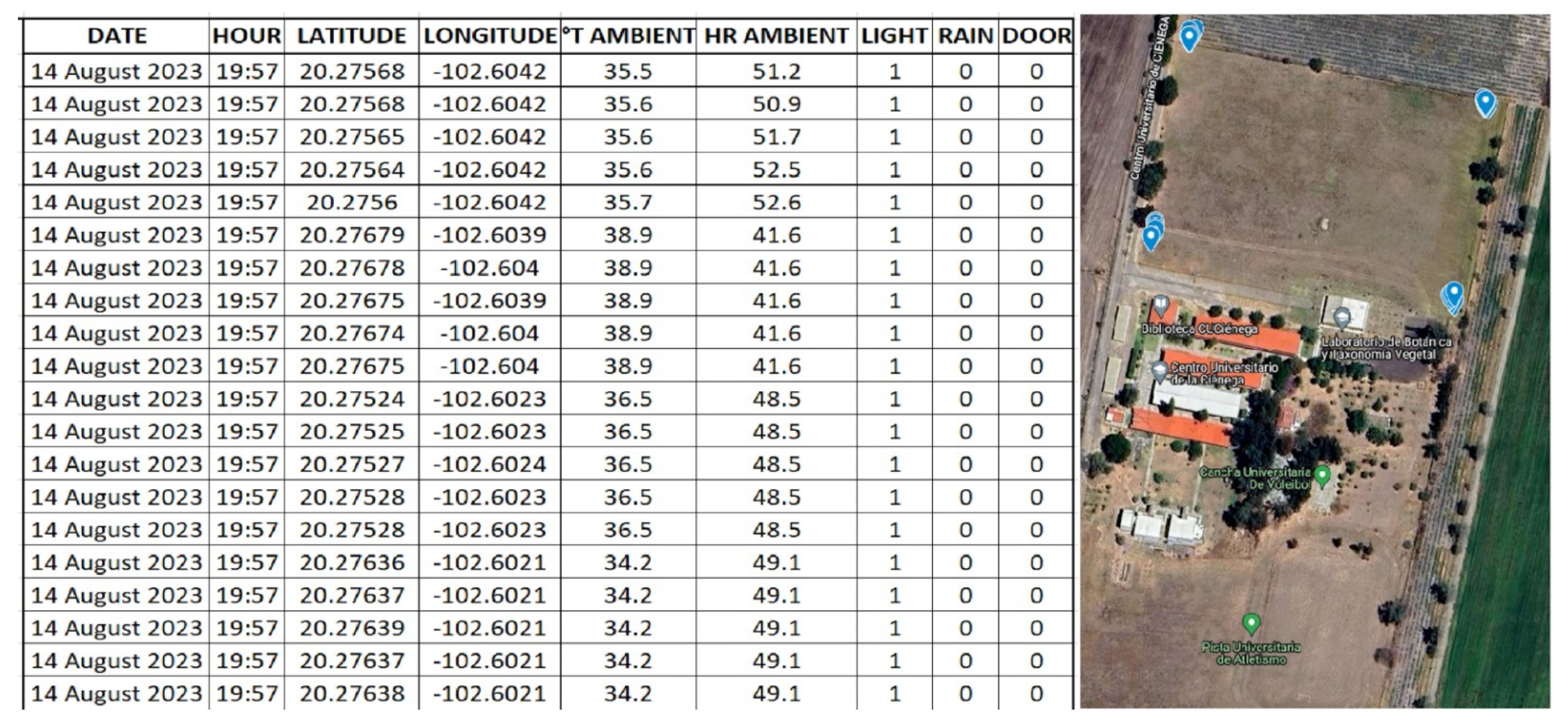
- Validation through experimental field trials: field experiments were conducted over 21 consecutive days at the experimental site of the University of Guadalajara in La Barca, Jalisco, Mexico (20°16’29.0″ N, 102°36’14.4″ W). Multiple treatments were tested to evaluate performance under real environmental conditions using a non-automated baseline for comparison.
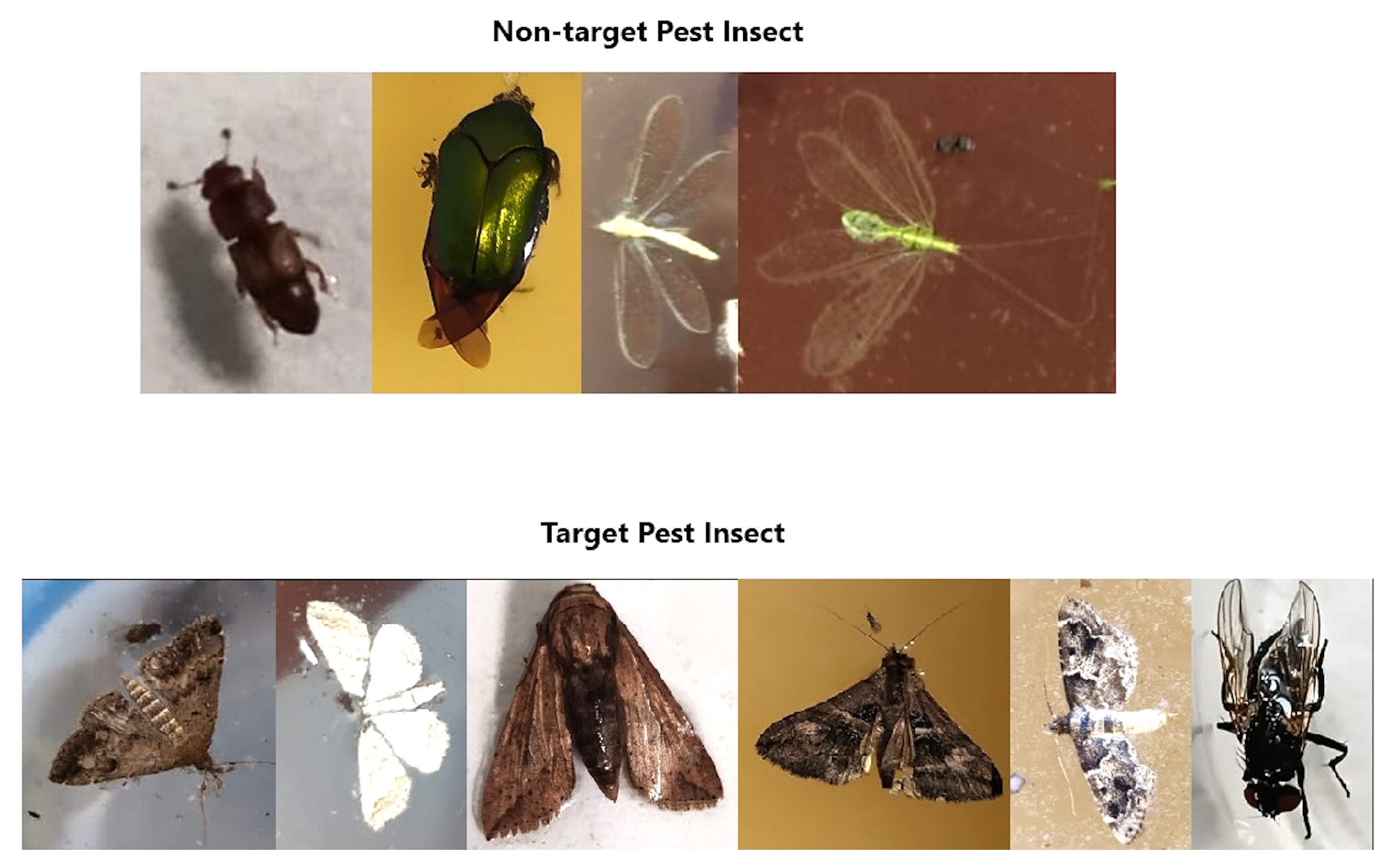
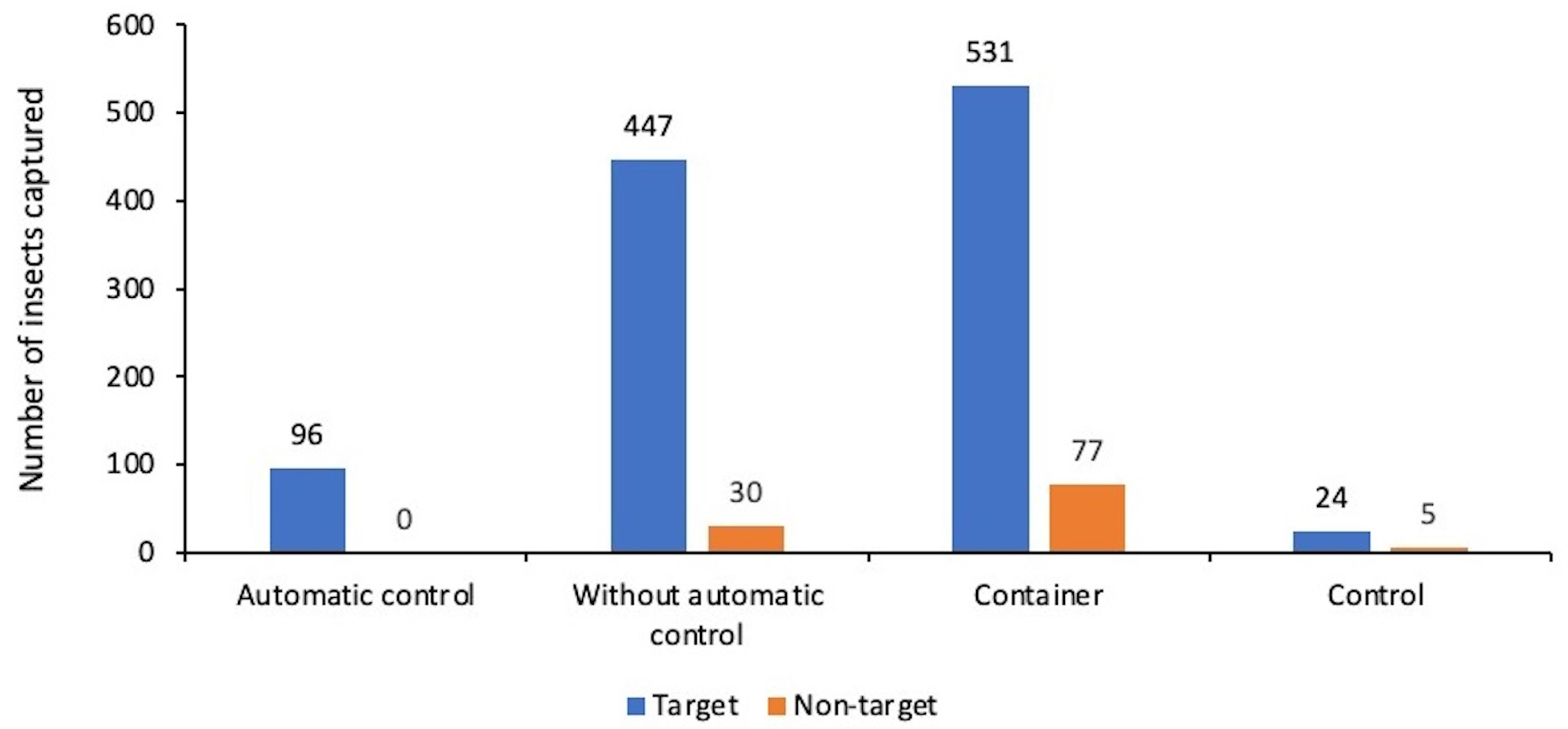
- Biological classification and species identification: Captured insects were analyzed offline through a two-step process: initial morphological assessment, followed by species identification using the Seek application from iNaturalist. Target pests such as Tetanolita floridiana, Synchlora spp., Estigmene acrea, Sphingomorpha chlorea, and Musca domestica were successfully identified, while beneficial and non-target species such as Chrysoperla spp. and Carpophilus spp. were largely excluded.
- Experimental treatments and statistical validation: the methodology included a comparative analysis of four treatments, using different attractant combinations and automation levels. The Kruskal–-Wallis test confirmed significant differences in the effectiveness and selectivity of the smart trap compared to traditional setups.
- Support for integrated pest management (IPM): by enabling selective, data-driven pest detection with minimal human intervention, the proposed trap provides a practical and scalable solution aligned with sustainable agriculture and modern IPM strategies.
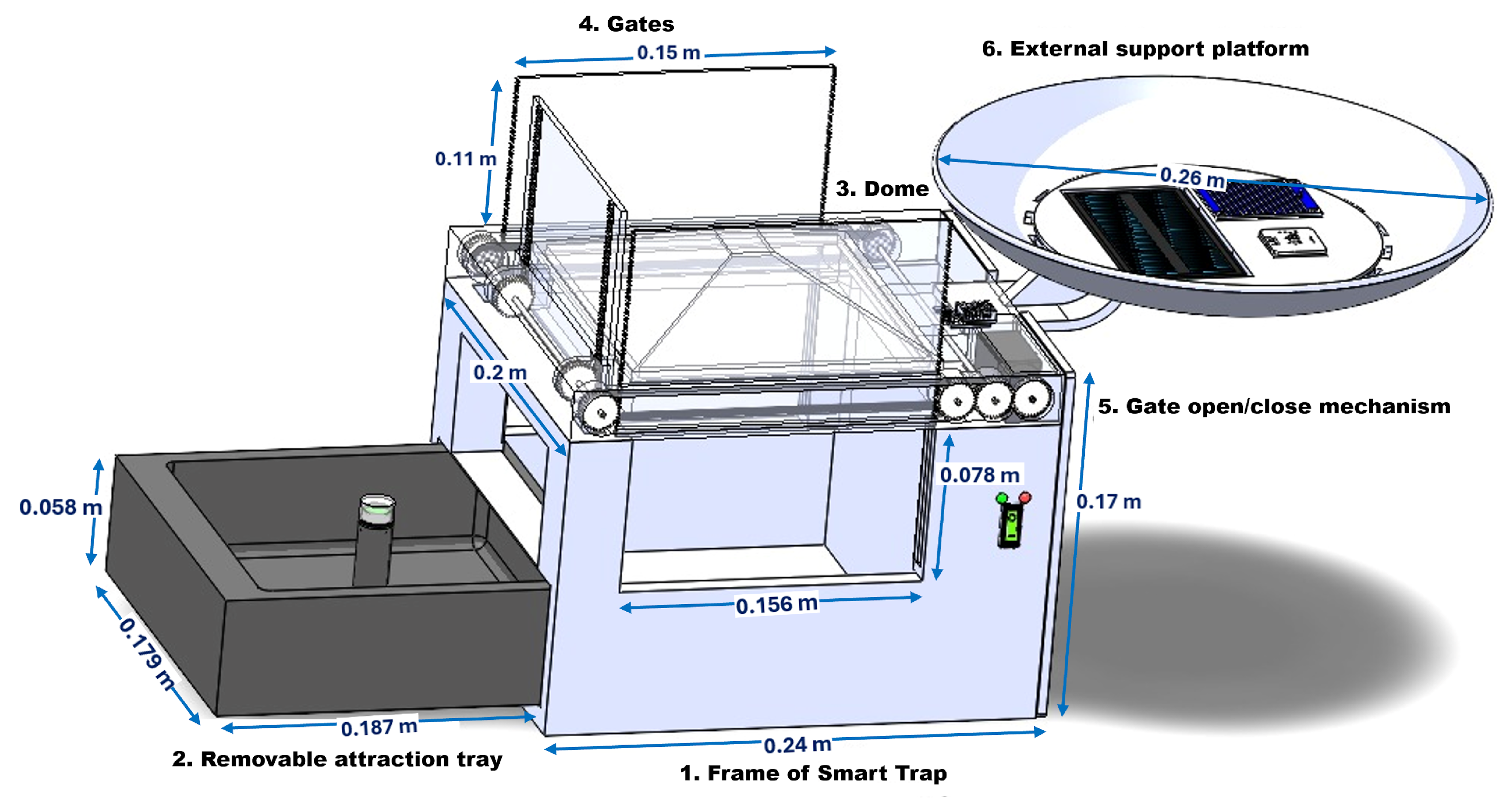
2. Mechanical Design of the Automated Device for the Capture of Adult Arthropod Pests
- 1.
- Frame of the trap: This structural unit houses the mechanical and electronic subsystems. It features side openings for pest entry, a top-mounted dome, and a base cavity for the removable tray. The frame dimensions are 0.24 m (length), 0.20 m (width), and 0.17 m (height). The front aperture provides a usable width of 0.156 m to facilitate tray insertion and retrieval.
- 2.
- Removable attraction tray: Located at the bottom of the trap, this container holds a fermented food attractant and includes a light module with green and yellow LEDs (wavelengths 500–570 nm and 570–590 nm [48]). These visual cues enhance species-specific attraction. Tray dimensions are 0.187 m × 0.179 m × 0.058 m.
- 3.
- Dome: The upper dome is a square-pyramidal shell made of transparent or translucent material. It allows light emitted by the internal LEDs to escape upward, maximizing visual stimulus for flying nocturnal insects while minimizing unwanted illumination of surrounding vegetation.
- 4.
- Gates: Two side gates, each measuring 0.15 m (width) and 0.11 m (height), are mounted to regulate insect access. These gates, made of plastic with serrated edges, are actuated by a motorized transmission mechanism and can be removed for cleaning or replacement.
- 5.
- Gate open/close mechanism: An electric motor is housed within the main frame and is mechanically coupled to a gear assembly. The motor drives a toothed shaft that rotates the first gate and, via a pulley-linked secondary shaft, actuates the second gate in synchronized motion. The vertical clearance for this subsystem is approximately 0.078 m. Under 5 V operation, the complete open-close cycle of the gates is executed in approximately 3.2 s, ensuring rapid adaptation to changing environmental conditions.
- 6.
- External support platform: This lateral circular platform (diameter: 0.26 m) serves as the mount for environmental sensors (e.g., sunlight, rain, humidity) and power modules such as solar panels (not included in this version). A built-in drainage outlet prevents water accumulation and protects the electronics.
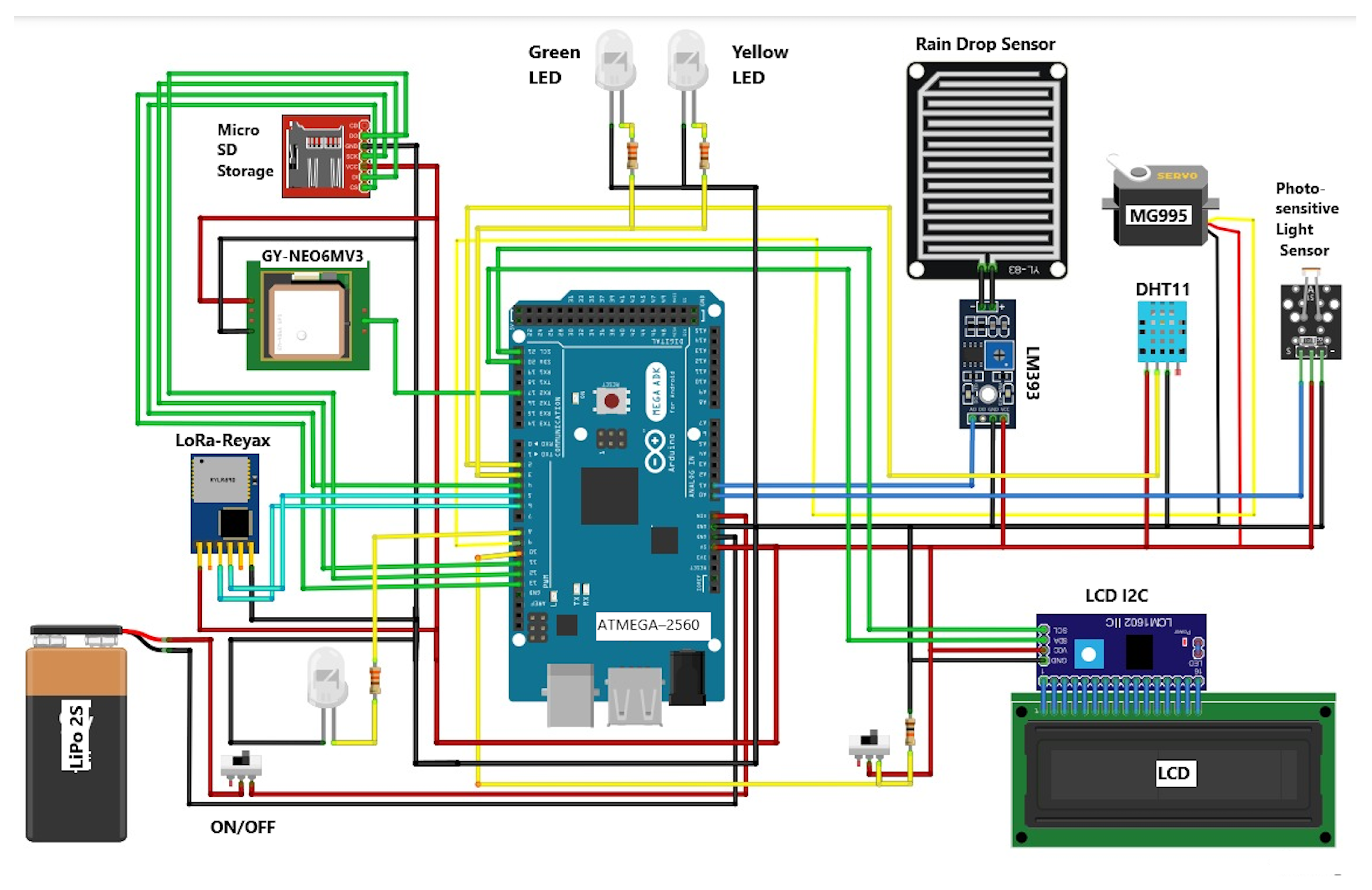
3. Electrical Design of the Automated Device for the Capture of Adult Arthropod Pests
- Actuators: This block includes the high-brightness LEDs and the servo motor used to control the gate mechanisms. Specifically, the system employs two 5 mm LEDs (green and yellow) with wavelengths ranging from 500 to 590 nm [48] to attract nocturnal pests (see Table 3). These components are installed in the removable tray, ensuring visual stimulus from within the trap. The gate movement is achieved by a high-torque Tower Pro MG995 servomotor (see Table 4), selected for its mechanical strength and compact integration within the frame.
- Sensors: The trap continuously acquires environmental data through three key sensor types installed on the external support platform. These include a photosensitive light detection module, a rain drop sensor (YL-83), and a DHT11 module for temperature and humidity measurement. Each sensor is connected to the microcontroller via analog or digital interfaces and is described in detail in Table 5.
- Microcontroller: The central processing unit of the trap is the ATMEGA-2560 microcontroller. It handles sensor input processing, neural decision logic, actuator control, and data communication. Its technical specifications and connectivity options are provided in Table 6.
- Information management: Data generated by the system is stored locally and optionally transmitted wirelessly. The human–machine interface consists of an LCD screen with I2C module for real-time display, a microSD card for local data logging, and a LoRa module for remote data transmission. Technical details are summarized in Table 7.
- Power supply: The system is powered by a 2S (7.4 V) lithium polymer (LiPo) battery, offering sufficient current for uninterrupted nocturnal operation. The battery characteristics and configuration are presented in Table 8.
4. Methodology for the Capture of the Adult Arthropod Pests
- Sensors to measure the abiotic conditions relevant to the target pest insects, such as temperature, environmental humidity, among others.
- An automatic on/off system for green and yellow LED lights, with wavelengths of 500–570 nm and 570–590 nm, respectively, as well as the automatic opening and closing of the gates in the absence of sunlight, controlled by actuators.
- A system for managing the information collected in the trap.
- The use of a smart trap with the feeding attractant solution proposed in [49] to attract target pest insects.
Evaluation of the Performance of the Automatic Trap
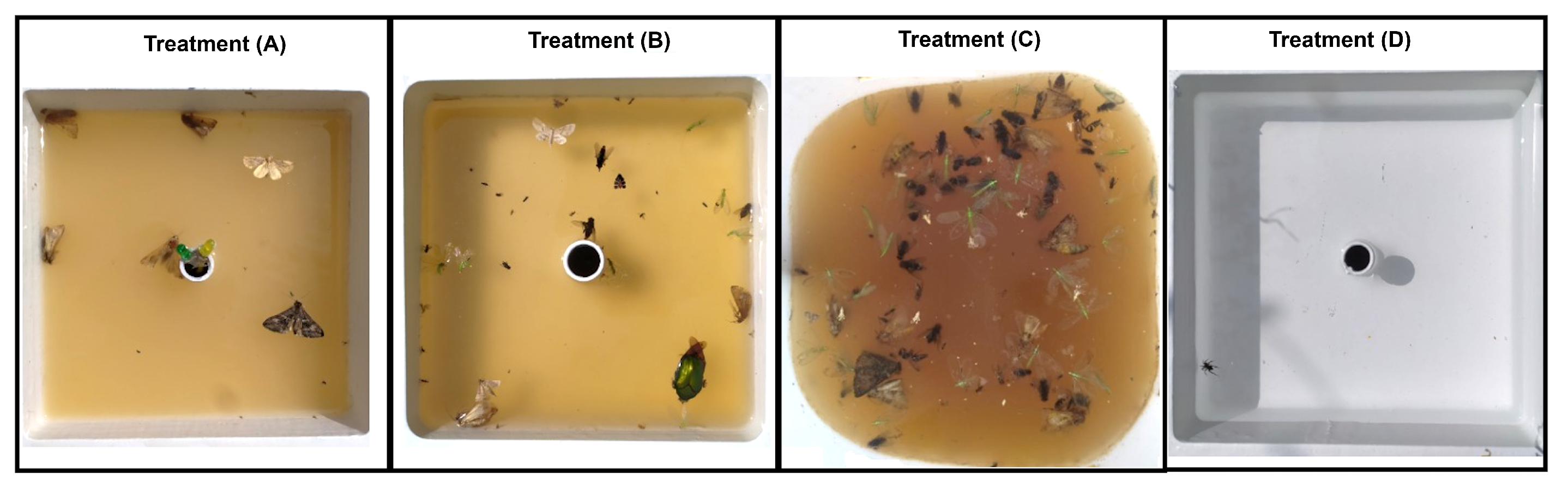
- (A)
- Automatic trap equipped with sensors and an automated system, utilizing the attractant solution described in [49] within its reservoir.
- (B)
- Automatic trap without sensors or automation, using the attractant solution described in [49] in its reservoir.
- (C)
- Non-automated container incorporating the feeding attractant solution described in [39].
- (D)
- Automatic trap without sensors or automation, using distilled water as the attractant solution in its reservoir.
- Operational data from the automatic trap for capturing pest insects in real-time, which was stored on a microSD memory card to create a database.
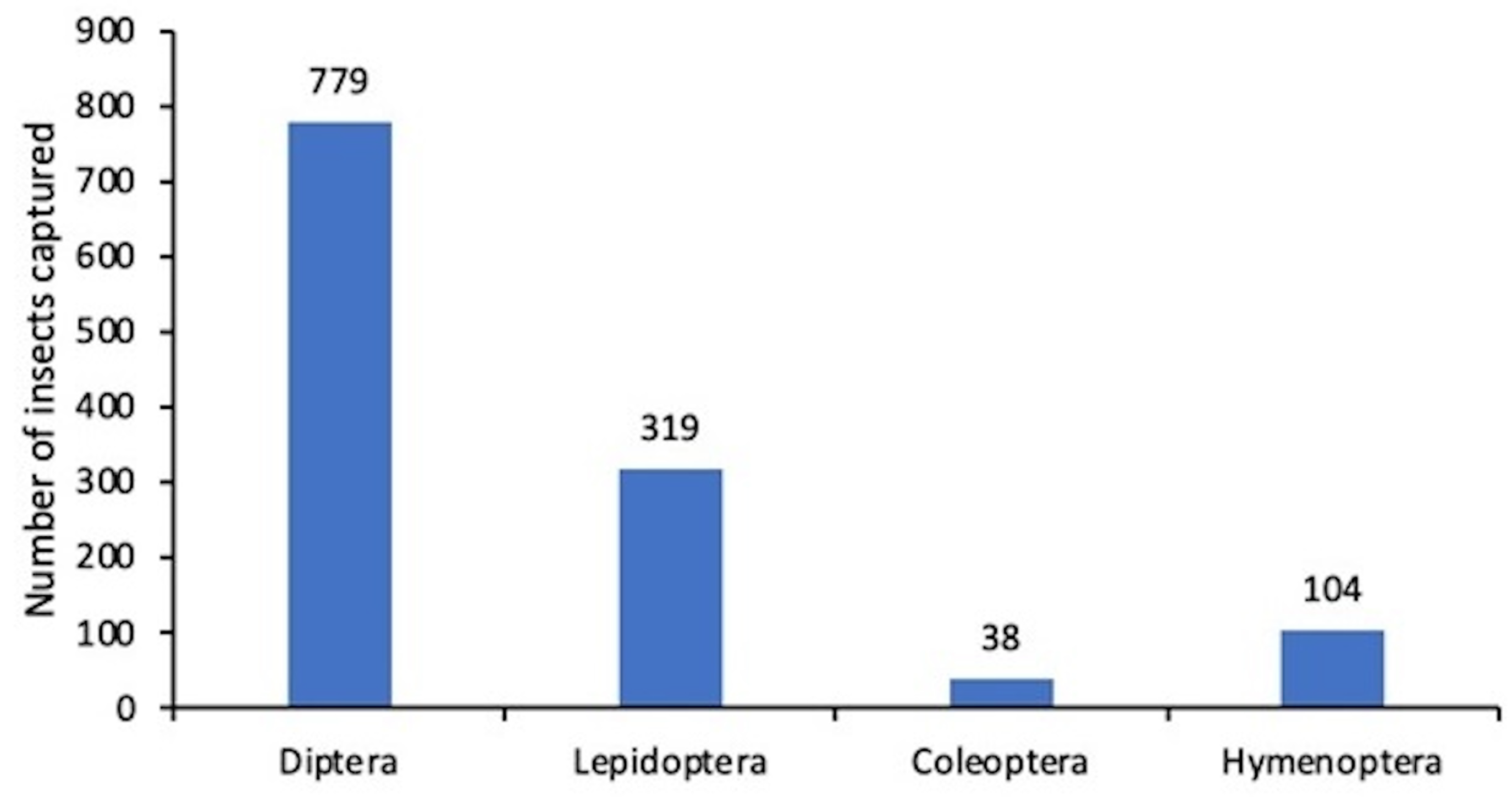
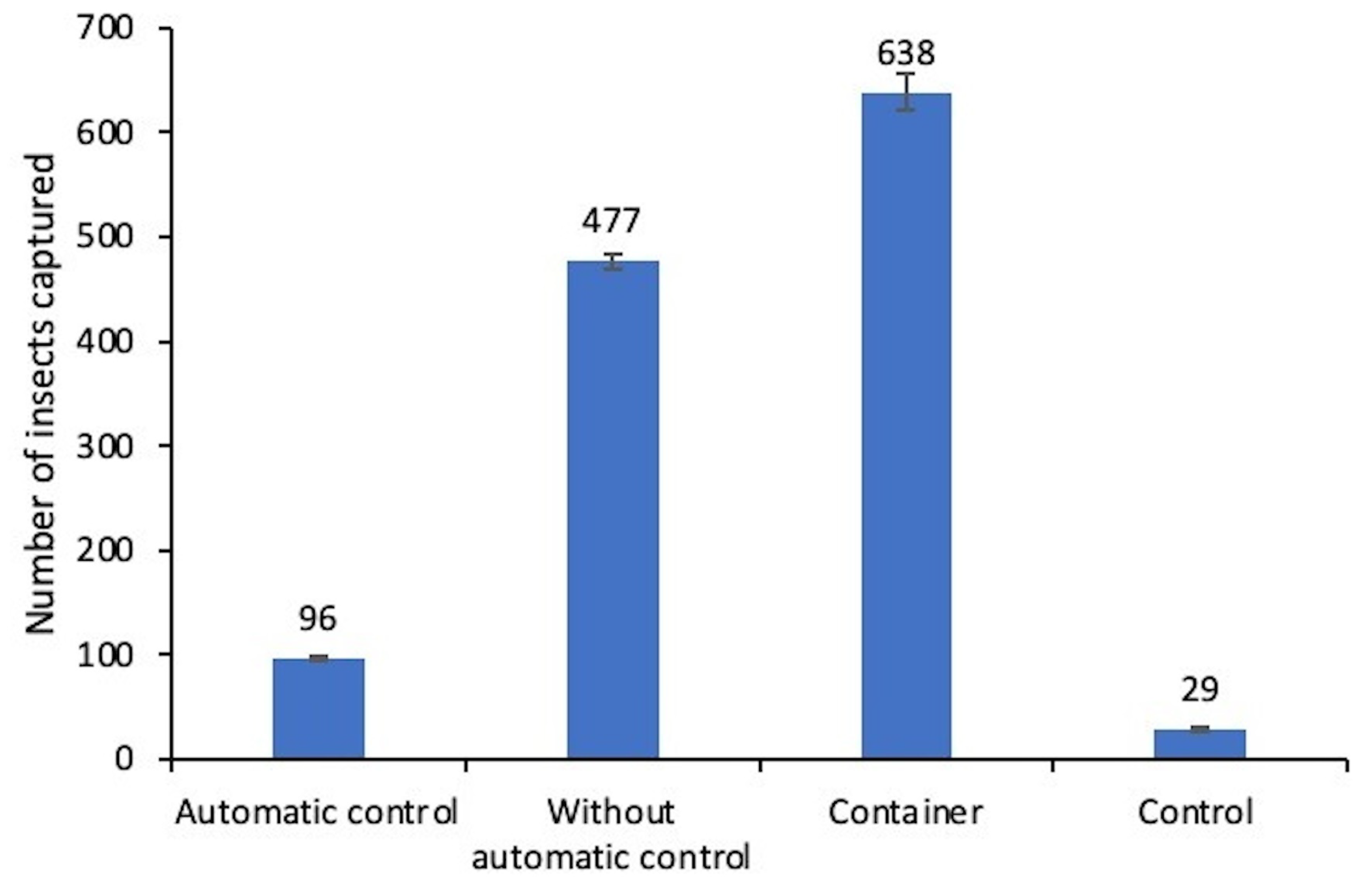
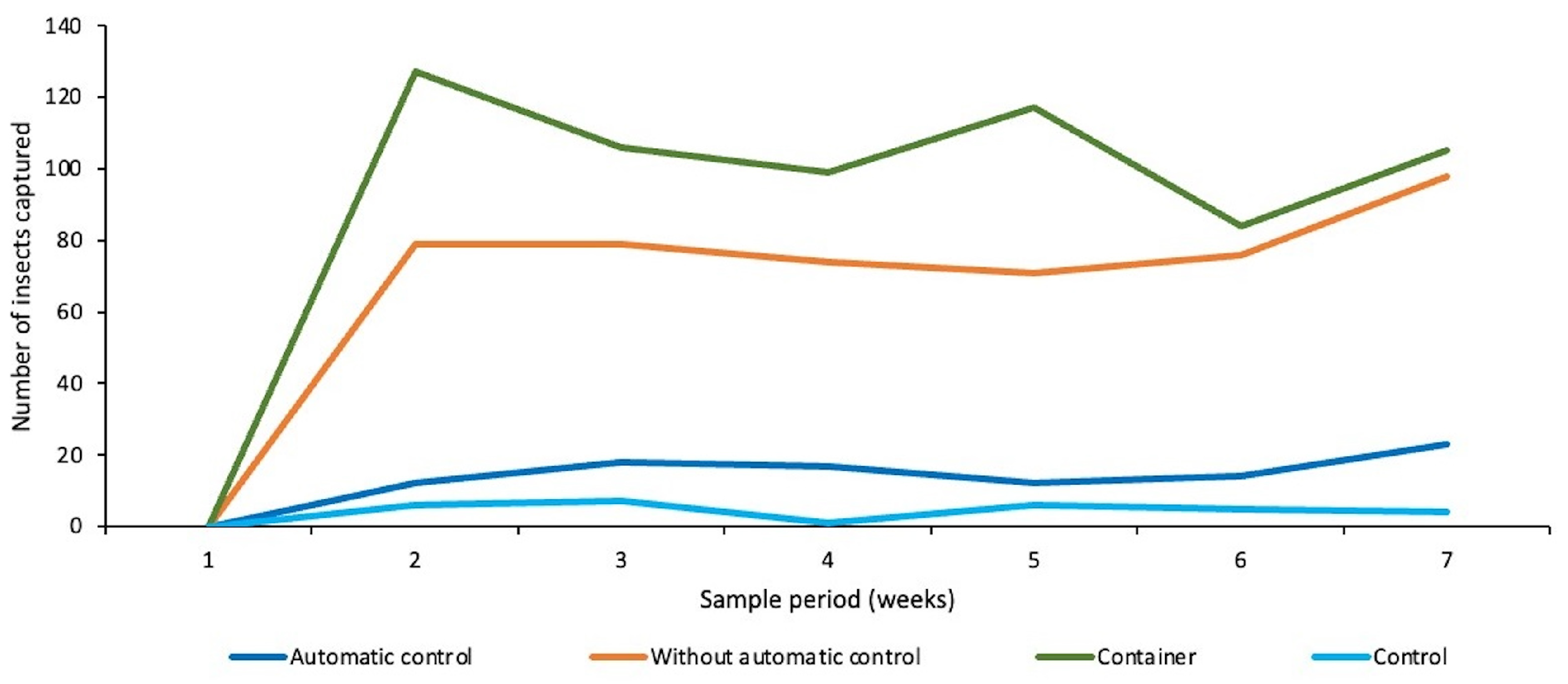
- Manual insect counts conducted over a 21-day period at the trap site, with the reservoir tray being inspected every three days. The insect carcasses, which had drowned in the attractant solution, were retrieved using a strainer or mesh filter. After filtration, the captured insects were classified into their respective orders through visual comparison, based on their morphological characteristics. The number of individuals from each order was then tallied. Qualitative identification of the insect carcasses was performed using the Seek mobile application from iNaturalist. To ensure result reliability, all treatments were conducted in triplicate, and the number of insects caught was used as the primary evaluation metric.
5. Results and Discussions
5.1. Results
5.2. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- FAO. CABI: Community-Based Fall Armyworm (Spodoptera frugiperda) Monitoring, Early Warning and Management, Training of Trainers Manual, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Llorente-Bousquets, J.; Vargas-Fernández, I.; Luis-Martínez, A.; Trujano-Ortega, M.; Hernández-Mejía, B.C.; Warren, A.D. Biodiversity of lepidopterans in México. Rev. Mex. Biodivers. 2014, 85, 353–371. [Google Scholar] [CrossRef]
- Ávila-Martínez, D.; Cervantes-Ortiz, F.; Rodríguez-Pérez, G.; Gámez-Vázquez, A.J.; García-Rodríguez, J.G.; Mendoza-Elos, M. Damage and population dynamics of codling moth (Spodoptera frugiperda) in elite lines of maize. Agron. Mesoam. 2023, 34, 53809–53819. [Google Scholar]
- Ávila García, R.; Cano Ríos, P.; Orona Castillo, I.; Espinoza Arellano, J.J.; Ramírez Segoviano, R. Establishment of the baseline for the evaluation of the technical and socioeconomic impact of the campaign against regulated pests of the cotton crop (Gossypium Hirsutum) in the state of Coahuila, México. Mex. J. Agribus. 2017, 41, 720–731. [Google Scholar] [CrossRef]
- DGSV-CNRF: Gusano Soldado Spodoptera exigua (Hubner, 1808) (Lepidoptera: Noctuidae); SADER-SENASICA, Dirección General de Sanidad Vegetal-Centro Nacional de Referencia Fitosanitaria: Tecámac, Mexico, 2020; pp. 20–30.
- DGSV-DCNRF: Gusano Soldado Mythimna unipuncta; SADER-SENASICA, Dirección General de Sanidad Vegetal-Dirección del Centro Nacional de Referencia Fitosanitaria: Tecámac, Mexico, 2019; pp. 18–28.
- Angon, P.B.; Mondal, S.; Jahan, I.; Datto, M.; Antu, U.B.; Ayshi, F.J.; Islam, M.S. Integrated Pest Management (IPM) in agriculture and its role in maintaining ecological balance and biodiversity. Adv. Agric. 2023, 1, 5546373. [Google Scholar] [CrossRef]
- He, W.; Zhao, X.; Ge, S.; Wu, K. Food attractants for field population monitoring of Spodoptera exigua (Hübner). J. Crop Prot. 2021, 145, 105616. [Google Scholar] [CrossRef]
- Cardim Ferreira Lima, M.; Damascena de Almeida Leandro, M.E.; Valero, C.; Pereira Coronel, L.C.; Gonçalves Bazzo, C.O. Detección y monitoreo automático de plagas de insectos: Una revisión. Agricultura 2020, 10, 161. [Google Scholar]
- Murchie, K.A. Advances in techniques for trapping crop insect pests. In Burleigh Dodds Series in Agricultural Science; Fountain, M., Pope, T., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK; NIAB-EMR: West Malling, UK, 2023; pp. 3–46. [Google Scholar]
- Preti, M.; Verheggen, F.; Angeli, S. Insect pest monitoring with camera equipped traps: Strengths and limitations. J. Pest Sci. 2021, 94, 203–217. [Google Scholar] [CrossRef]
- Sciarretta, A.; Calabrese, P. Development of automated devices for the monitoring of insect pests. Curr. Agric. Res. J. 2019, 7, 19–25. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, J.; Li, R.; Li, J.; Hong, P.; Xia, J.; Chen, P. Automatic classification for field crop insects via multiple-task sparse representation and multiple-kernel learning. Comput. Electron. Agric. 2015, 119, 123–132. [Google Scholar] [CrossRef]
- Rigakis, I.I.; Varikou, K.N.; Nikolakakis, A.E.; Skarakis, Z.D.; Tatlas, N.A.; Potamitis, I.G. The E-Funnel trap: Automatic monitoring of Lepidoptera; a case study of tomato leaf miner. Comput. Electron. Agric. 2021, 185, 106154. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Meagher, R.L.; Winsou, J.K.; Dahoueto, B.T.A.; Tamò, M.; Sæthre, M.G.; Nagoshi, R.N. Monitoring Spodoptera frugiperda in Benin: Assessing the influence of trap type, pheromone blends, and habitat on pheromone trapping. Fla. Entomol. 2022, 105, 71–78. [Google Scholar] [CrossRef]
- Chulu, F.; Nkunika, P.; Chiwamba, S. Developing an automatic identification and early warning and monitoring web-based system of fall army worm based on machine learning in developing countries. Zambia ICT J. 2019, 3, 13–20. [Google Scholar] [CrossRef]
- Jiménez, A.F.; Jiménez, F.R.; Garciía, D.Y.; Guevara, E.J.; Quiñones, A.J.P. Electronic IOT trap for monitoring Spodoptera frugiperda in corn crops. Pistas Educ. 2024, 147, 651–666. (In Spanish) [Google Scholar]
- Li, J.; Zhou, H.; Wang, Z.; Jian, F.; Jayas, D.S.; Cui, M. A method to estimate densities of Cryptolestes pusillus (Schonherr) adults captured in electronic probe traps in paddy based on deep neural networks. Comput. Electron. Agric. 2023, 209, 107819. [Google Scholar] [CrossRef]
- Liu, W.-L.; Wang, Y.; Chen, Y.-X.; Chen, B.-Y.; Lin, A.Y.-C.; Dai, S.-T.; Chen, C.-H.; Liao, L.-D. An IoT-based smart mosquito trap system embedded with real-time mosquito image processing by neural networks for mosquito surveillance. Front. Bioeng. Biotechnol. 2023, 11, 1100968. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, L.; Zeni, V.; Incrocci, L.; Canale, A.; Benelli, G.; Falchi, F.; Amato, G.; Chessa, S. A deep learning-based pipeline for whitefly pest abundance estimation on chromotropic sticky traps. Ecol. Inform. 2023, 78, 102384. [Google Scholar] [CrossRef]
- Saradopoulos, I.; Potamitis, I.; Konstantaras, A.I.; Eliopoulos, P.; Ntalampiras, S.; Rigakis, I. Image-Based Insect Counting Embedded in E-Traps That Learn without Manual Image Annotation and Self-Dispose Captured Insects. Information 2023, 14, 267. [Google Scholar] [CrossRef]
- Tercel, M.P.T.G.; Veronesi, F.; Pope, T.W. Phylogenetic clustering of wingbeat frequency and flight-associated morphometrics across insect orders. Physiol. Entomol. 2018, 43, 149–157. [Google Scholar] [CrossRef]
- Santos, D.A.A.; Rodrigues, J.J.P.C.; Furtado, V.; Saleem, K.; Koroteav, V. Automated electronic approaches for detecting disease vectors mosquitoes through the wing-beat frequency. J. Clean. Prod. 2019, 217, 767–775. [Google Scholar] [CrossRef]
- Meagher, R.L., Jr.; Agboka, K.; Tounou, A.K.; Koffi, D.; Agbevohia, K.A.; Amouze, T.R.; Adjévi, K.M.; Nagoshi, R.N. Comparison of pheromone trap design and lures for Spodoptera frugiperda in Togo and genetic characterization of moths caught. Entomol. Exp. Appl. 2019, 167, 507–516. [Google Scholar] [CrossRef]
- Sun, G.; Liu, H.; Lou, H.; Feng, Z.; Yang, B.; Lou, J.; Tang, J.; Yao, Q.; Xu, J. Intelligent monitoring system of migratory pests based on searchlight trap and machine vision. Front. Plant Sci. 2022, 13, 897739. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, L.; Lv, C.; Ge, S.; Haowen, Z.; Jiang, S.; Chu, B.; Yang, X.; Wyckhuys, K.; Wu, K. Use of food attractants to monitor and forecast Spodoptera frugiperda (J. E. Smith) seasonal abundance in Southern China. J. Pest Sci. 2023, 96, 1509–1521. [Google Scholar] [CrossRef]
- Popescu, D.; Dinca, A.; Ichim, L.; Angelescu, N. New trends in detection of harmful insects and pests in modern agriculture using artificial neural networks. a review. Front. Plant Sci. 2023, 14, 1268167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, B.; Husnain, N.; Wang, Q. Overview of Agricultural Machinery Automation Technology for Sustainable Agriculture. Agronomy 2025, 15, 1471. [Google Scholar] [CrossRef]
- Yang, H.; Lu, J.; Zhu, P.; Sun, Y.; Hu, Z.; Li, D.; Huang, J. Blue light attracts more Spodoptera frugiperda moths and promotes their flight speed. J. Insects 2024, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.J.; Sadler, J.P.; Everett, G.; Grundy, D.; Lowe, N.; Davis, G.; Baker, D.; Bridge, M.; Clifton, J.; Freestone, R.; et al. Assessing the value of the garden moth scheme citizen science dataset: How does light trap type affect catch? Entomol. Exp Appl. 2013, 146, 386–397. [Google Scholar] [CrossRef]
- Awuor Okello, E.; van Tol, R.W.H.M.; Watako, A.; Ariga, E.S. Evaluation of optimal light wavelength for the attraction of Spodoptera exigua, a model insect for mass trapping and control of Spodoptera frugiperda. Int. J. Entomol. Res. 2020, 5, 27–32. [Google Scholar]
- Nascimento, I.; Oliveira, G.; Souza, M.; Nunes, G.; Leite Alves, A.; Araújo, H.; Batista, J. Light-Emitting diodes (LED) as luminous lure for adult Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae). J. Expel. Agric. Int. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Belton, P.; Kempster, R. Some factors affecting the catches of Lepidoptera in light traps. Can. Entomol. 1963, 95, 832–837. [Google Scholar] [CrossRef]
- Singh, R.; Böttger, D.; Brehm, G. Moth light traps perform better with vanes: A comparison of different designs. J. Appl. Entomol. 2022, 146, 1343–1352. [Google Scholar] [CrossRef]
- Haftay, G.; Fissiha, G.G. Night-Time Light-Traps and Push-Pull integrated system enhanced the control of different life stages of fall armyworm, Spodoptera frugiperda, (Lepidoptera: Noctuidae). Cabi Agric. Biosci. 2024, 5, 104. [Google Scholar]
- Batallas, R.E.; Evenden, M.L. Fermented or floral? Developing a generalized food bait lure to monitor cutworm and armyworm moths (Lepidoptera: Noctuidae) in field crops. J. Insects 2023, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Pantoja, A.; Hagerty, A.; Crabo, L.; Green, D. Moths trapped in Alaska with feeding attractant lures and the seasonal flight patterns of potential agricultural pests. Can. Entomol. 2007, 139, 278–291. [Google Scholar] [CrossRef]
- Grocock, N.L.; Batallas, R.E.; McNamara, E.A.; Sturm, A.B.; Manson, J.S.; Evenden, M.L. Bumble bees (Hymenoptera: Apidae) respond to moth (Lepidoptera: Noctuidae) pheromone components, leading to Bee Bycatch in monitoring traps targeting moth Pests. Front. Ecol. Evol. 2020, 8, 576692. [Google Scholar] [CrossRef]
- Mena-Covarrubias, J.; Ramírez-Cabral, N.Y.Z.; Medina-García, G.; Sánchez-Gutiérrez, A. Food Trap for Agro-Ecological Management of Insect Pest in Staple Crops and Vegetables; National Institute for Forestry, Agriculture and Livestock Research (INIFAP): Mexico City, Mexico, 2021. [Google Scholar]
- Rojas, J.; Virgen, A.; Santiesteban-Hernández, A.; Cruz-Esteban, S. Los cebos azucarados no atraen a las hembras se Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) pero si afectan la atracción de los machos a la feromona sexual. Ento. Agricola 2020, 145–151. Available online: https://www.researchgate.net/publication/344180071 (accessed on 25 June 2025).
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 124675. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.T.; Mesaglio, T.; Ascher, J.S.; Brooks, T.M.; Cabras, A.A.; Chandler, M.; Cornwell, W.K.; Cristóbal Ríos-Málaver, I.; Dankowicz, E.; Urfi Dhiya’ulhaq, N.; et al. The benefits of contributing to the citizen science platform iNaturalist as an identifier. PLoS Biol. 2022, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Michonneau, F.; Paulay, G. Using iNaturalist to learn more about Echinoderms. Figshare J. Contrib. 2015. [Google Scholar] [CrossRef]
- Thomas, R.L.; Fellowes, M.D.E. Effectiveness of mobile apps in teaching field-based identification skills. J. Biol. Educ. 2016, 51, 136–143. [Google Scholar] [CrossRef]
- Fraser, D.L.; Ironside, K.; Wayne, R.K. Connectivity of mule deer (Odocoileus hemionus) populations in a highly fragmented urban landscape. Landscape Ecol. 2019, 34, 1097–1115. [Google Scholar] [CrossRef]
- Nugent, J. Citizen Science: Summer Squash Citizen Science. Sci. Scope 2019, 42, 22–24. [Google Scholar] [CrossRef]
- Hinojosa–Dávalos, J.; Acosta Lúa, C. Automated Trap for Capturing Insect Pests. Patent NMX 5425 B, 6 December 2024. (In Spanish). [Google Scholar]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Hinojosa–Dávalos, J.; Acosta Lúa, C. Food Composition Attractant of Pest Insects Affecting Agricultural Crops. Patent No.MX/a/2022/016260, 15 December 2023. (In Spanish). [Google Scholar]
- Hinojosa–Dávalos, J.; Mares Guzman, I.; Varela Hernandez, J.J.; Cardona López, M.A.; Sanchez Morales, M.E.; Acosta Lúa, C. Rotary germination device to produce safe sprouts. Patent No. 4551, 24 March 2021. (In Spanish). [Google Scholar]



| Order | Family | Scientific Name | Crop Affected |
|---|---|---|---|
| Lepidoptera | Noctuidae | Peridroma saucia | Fruit and vegetables |
| Spodoptera frugiperda | Corn, cotton, sorghum, wheat | ||
| Spodoptera ornithogalli | Corn, cotton, sorghum, wheat | ||
| Spodoptera cosmioides | Soybean, corn, rice, alfalfa | ||
| Spodoptera eridania | Soybean | ||
| Dargida albilinea | Wheat | ||
| Estigmene acrea | Tomatoes, peppers, eggplants, beans, soybeans, corn, wheat | ||
| Sphingomorpha chlorea | Corn, sorghum, wheat, beans, soybeans, chickpeas | ||
| Tetanolita floridana | Tomatoes, peppers, eggplants | ||
| Tortricidae | Grapholita molesta | Apple tree, plum tree, pear, quince, peanut, walnut, pine nuts | |
| Phycitidae | Plodia interpunctella | Peanuts, walnuts, pine nuts | |
| Geometridae | Synchlora spp. | Lettuce, pepper, eggplant, beans, soybeans | |
| Gymnoscelis rufifasciata | Tomatoes, peppers, pumpkins, apple trees, pear trees, cherry trees |
| Order | Family | Scientific Name | Crop Affected |
|---|---|---|---|
| Díptera | Tephritidae | Bactrocera dorsalis | Apricot, peach, apple, pear, tomato, aubergine, loquat, and orange |
| Ceratitis capitata | Apricot, peach, apple, pear, tomato, orange, nectarine, and pitahaya | ||
| Bactrocera latrifons | Tomato, potato, pepper, and aubergine | ||
| Drosophilidae | Drosophila suzukii | Cherry, strawberry, raspberry, and grape | |
| Anthomyiidae | Delia radicum | Cauliflower, broccoli, cabbage, and strawberry | |
| Agromyzidae | Liriomyza huidobrensis | Lettuce, tomato, pepper, garlic, aubergine, courgette, pea, kidney bean, chrysanthemum, melon, cucumber, watermelon, beetroot, and spinach | |
| Muscoidea | Musca domestica | Apples, pears, grapes, citrus, banana, mango, papaya |
| Name | Description | Technical Information | Figure |
|---|---|---|---|
| LED 5 mm High luminosity (green) by Epistar Corp. (Hsinchu, Taiwan) | High luminosity green LED. | Operating voltage: 1.8–3.4 V. Operating current: 20 mA. Luminous intensity: 12 cd. Wavelengths: 500–570 nm. |  |
| LED 5 mm High luminosity (yellow) by Epistar Corp. (Hsinchu, Taiwan) | High luminosity yellow LED. | Operating Voltage: 1.8–3.4 V. Operating current: 20 mA. Luminous intensity: 12 cd. Wavelengths: 570–590 nm. |  |
| Name | Description | Technical Information | Figure |
|---|---|---|---|
| Servomotor Tower Pro MG995 by JH Global Trading (Shenzhen, China) | High torque servo up to 11 kg cm. | Operating voltage: 4.6–6.6 V. Torque: 11 kgf.cm. Weight: 55 g. Operating current: 10 mA. Maximum load current: 1200 mA. |  |
| Name | Description | Technical information | Figure |
|---|---|---|---|
| Photosensitive light detection sensor module | Sensor that, through a photoresistor, measures the amount of incident light. | Operating voltage: 5 V. Operating current: 15 mA. Type of signal: digital or analog. Sensitivity can be adjusted using the onboard potentiometer. |  |
| YL-83. Rain drop sensor | A rain sensor designed as a low-cost electronic sensor for detecting rainfall or water drops. | Operating voltage: 5 V. Operating current: 15 mA. Type of signal: digital or analog Sensitivity of rain Detection: minimum wet area: 0.05 cm2. |  |
| DHT11 humidity and temperature sensor by ASAIR Co. (Shenzhen, China) | Basic digital sensor for temperature and humidity measurement. This sensor uses a thermistor to measure the surrounding air (temperature) and implements an internal capacitive sensor for humidity measurement. | Operating voltage: 5 V. Operating current: 2.5 mA. Type of signal: digital. Temperature measurement range: 0–50 °C. Temperature measurement accuracy: ±2.0 °C. Temperature resolution: 0.1 °C. Humidity measurement range: 20–90% RH. Humidity measurement accuracy: 5% Humidity resolution: 1% RH. |  |
| Name | Description | Technical Information | Figure |
|---|---|---|---|
| ATMEGA-2560 by Microchip Technology Inc. | |||
| (Chandler, AZ, USA) | Microcontroller board based on the ATMEGA-2560, featuring 54 digital input/output pins. | Operating voltage: 5 V. Input voltage: 6–20 V. Digital I/O pins: 54. Flash memory: 256 KB. SRAM: 8 KB. EEPROM: 4 KB. Clock speed: 16 Mhz. |  |
| Name | Description | Technical Information | Figure |
|---|---|---|---|
| Micro SD TF card storage memory module | The micro SD Reader module allows connection to a microcontroller or development board via SPI communication. It is ideal it works at a voltage between 4.5 and 5 V. | Operating voltage: 4.5–5 V. Operating current: 200 mA. Memory: MicroSD. Communication protocol: SPI. |  |
| Display LCD | 16 × 2-character alphanumeric LCD display. | Resolution: 2 lines of 16 characters of 8 × 5 pixels each. Operation voltage: 5 V. Operation current: 1.1 mA. |  |
| LCD I2C Module | Device that allows control of a display through the I2C bus, using only two wires. | Operation voltage: 5 V. Operation current: 50 mA. Communication protocol: I2C. |  |
| LoRa Reyax by REYAX Technology Co. (Shenzhen, China.) | Features the Lora long-range modem provides long-range spread spectrum communication and high interference immunity while minimizing power consumption. | Operation voltage: 3.3 V. Operation current: 25 mA. Communication protocol: RX-TX. Frequency accuracy ±2 ppm. |  |
| Name | Description | Technical Information | Figure |
|---|---|---|---|
| 2S LiPo Battery 7.4 Volts | The battery consists of two cells connected in series to provide a voltage of 7.4 V. | Voltage: 7.4 V. Capacity: 2200 mAh. Discharge: 50 C. |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojosa-Dávalos, J.; Robles-García, M.Á.; Gutiérrez-Lomelí, M.; Flores Jiménez, A.B.; Acosta Lúa, C. Neural Network-Guided Smart Trap for Selective Monitoring of Nocturnal Pest Insects in Agriculture. Agriculture 2025, 15, 1562. https://doi.org/10.3390/agriculture15141562
Hinojosa-Dávalos J, Robles-García MÁ, Gutiérrez-Lomelí M, Flores Jiménez AB, Acosta Lúa C. Neural Network-Guided Smart Trap for Selective Monitoring of Nocturnal Pest Insects in Agriculture. Agriculture. 2025; 15(14):1562. https://doi.org/10.3390/agriculture15141562
Chicago/Turabian StyleHinojosa-Dávalos, Joel, Miguel Ángel Robles-García, Melesio Gutiérrez-Lomelí, Ariadna Berenice Flores Jiménez, and Cuauhtémoc Acosta Lúa. 2025. "Neural Network-Guided Smart Trap for Selective Monitoring of Nocturnal Pest Insects in Agriculture" Agriculture 15, no. 14: 1562. https://doi.org/10.3390/agriculture15141562
APA StyleHinojosa-Dávalos, J., Robles-García, M. Á., Gutiérrez-Lomelí, M., Flores Jiménez, A. B., & Acosta Lúa, C. (2025). Neural Network-Guided Smart Trap for Selective Monitoring of Nocturnal Pest Insects in Agriculture. Agriculture, 15(14), 1562. https://doi.org/10.3390/agriculture15141562









