Combined High Irradiance and Water Deficit Alters the Anatomy and Physiology of Photomorphogenic Mutant Micro-Tom Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Conditions, and Experimental Design
2.2. Evaluations
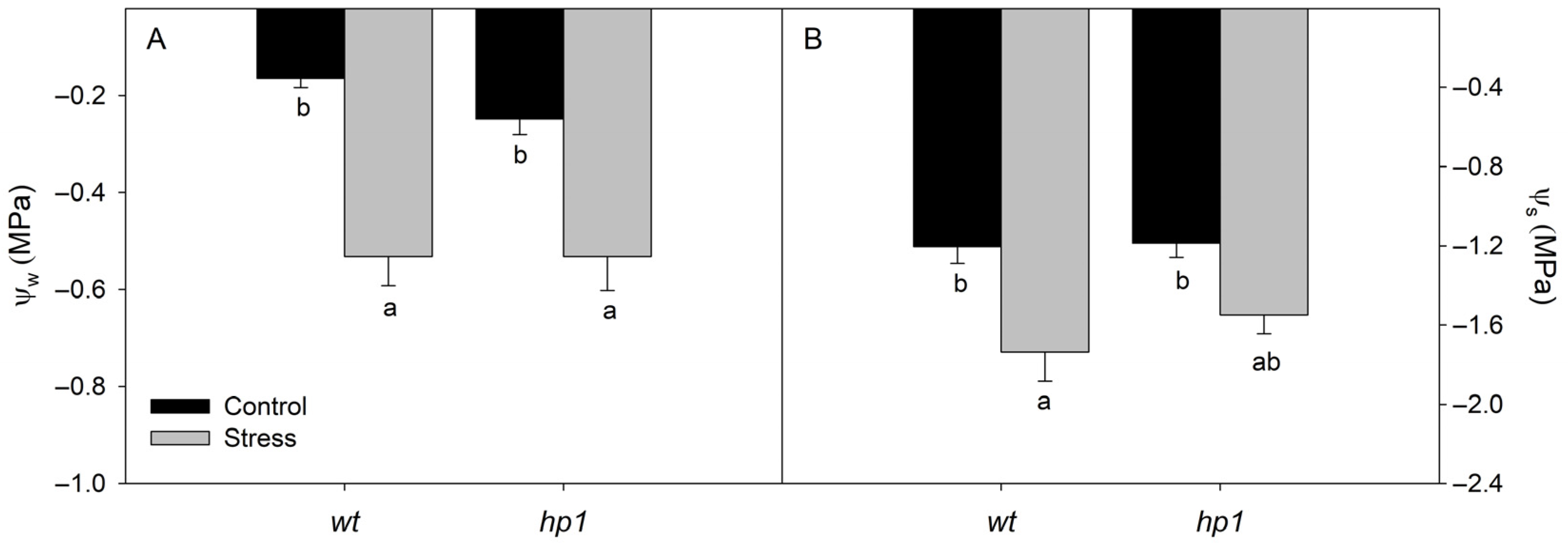
2.2.1. Leaf Water and Osmotic Potential
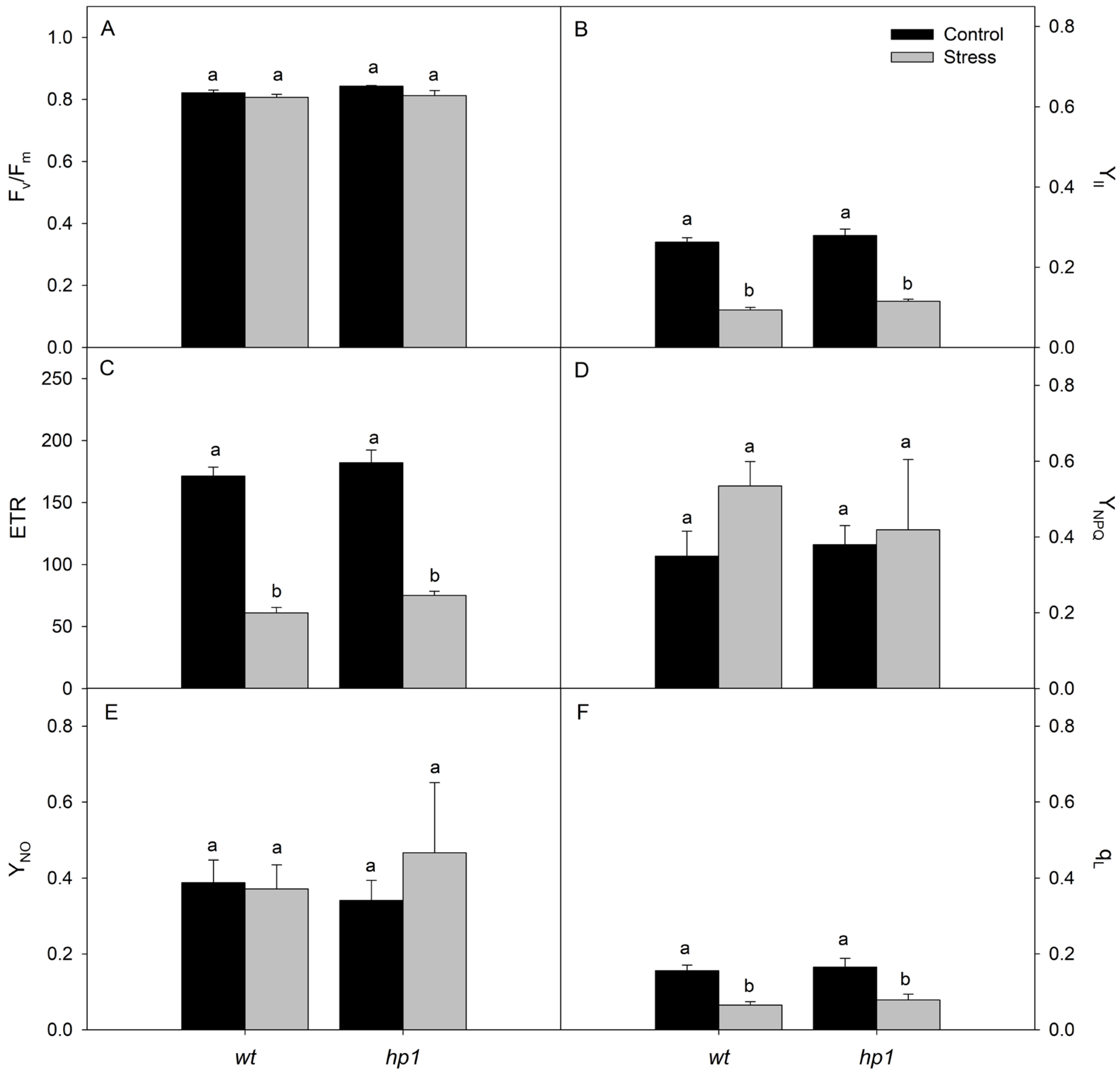
2.2.2. Gas Exchange and Chlorophyll Fluorescence
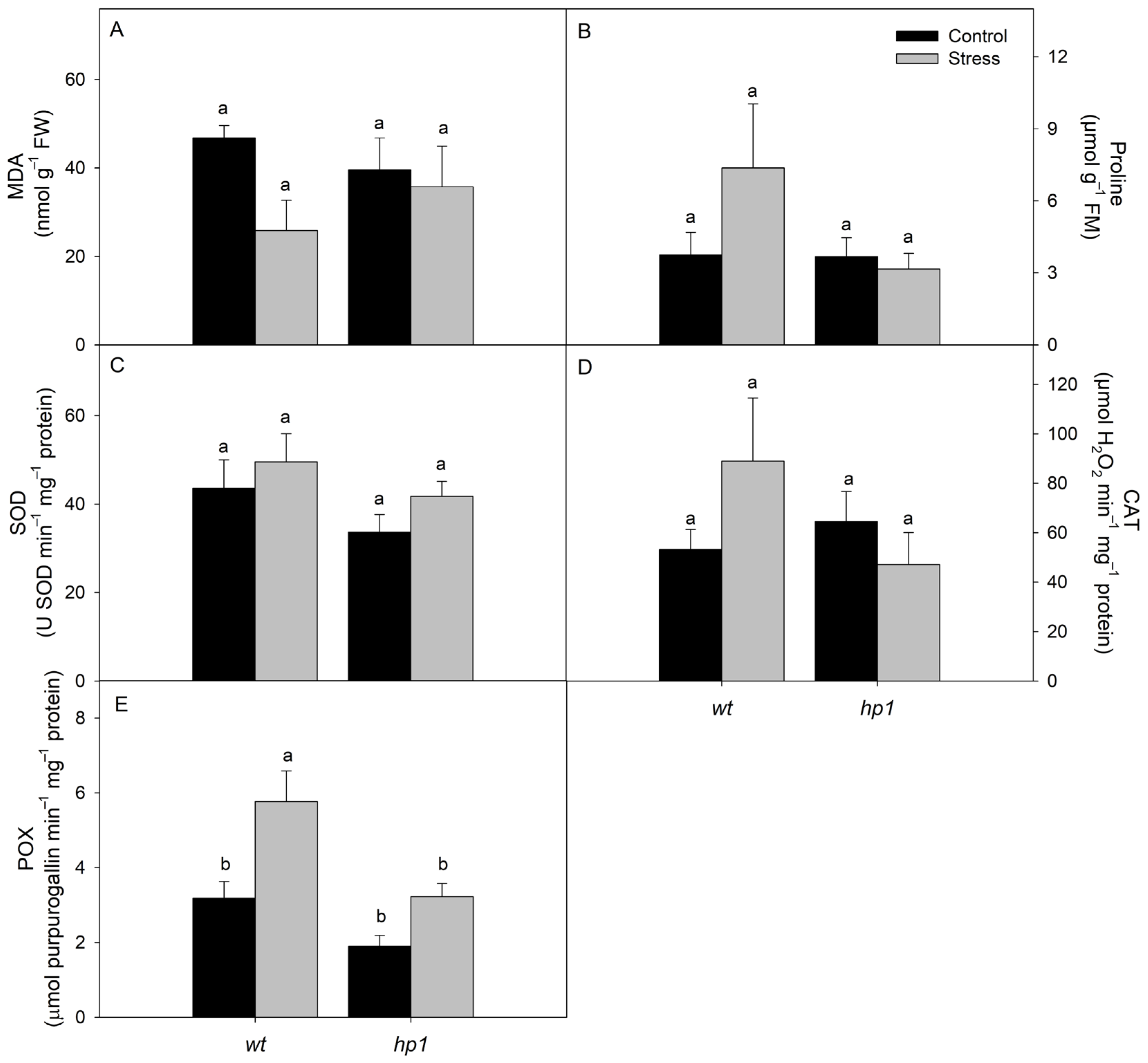
2.2.3. Malondialdehyde (MDA) Concentration
2.2.4. Proline Concentration
2.2.5. Antioxidant Enzyme Activity
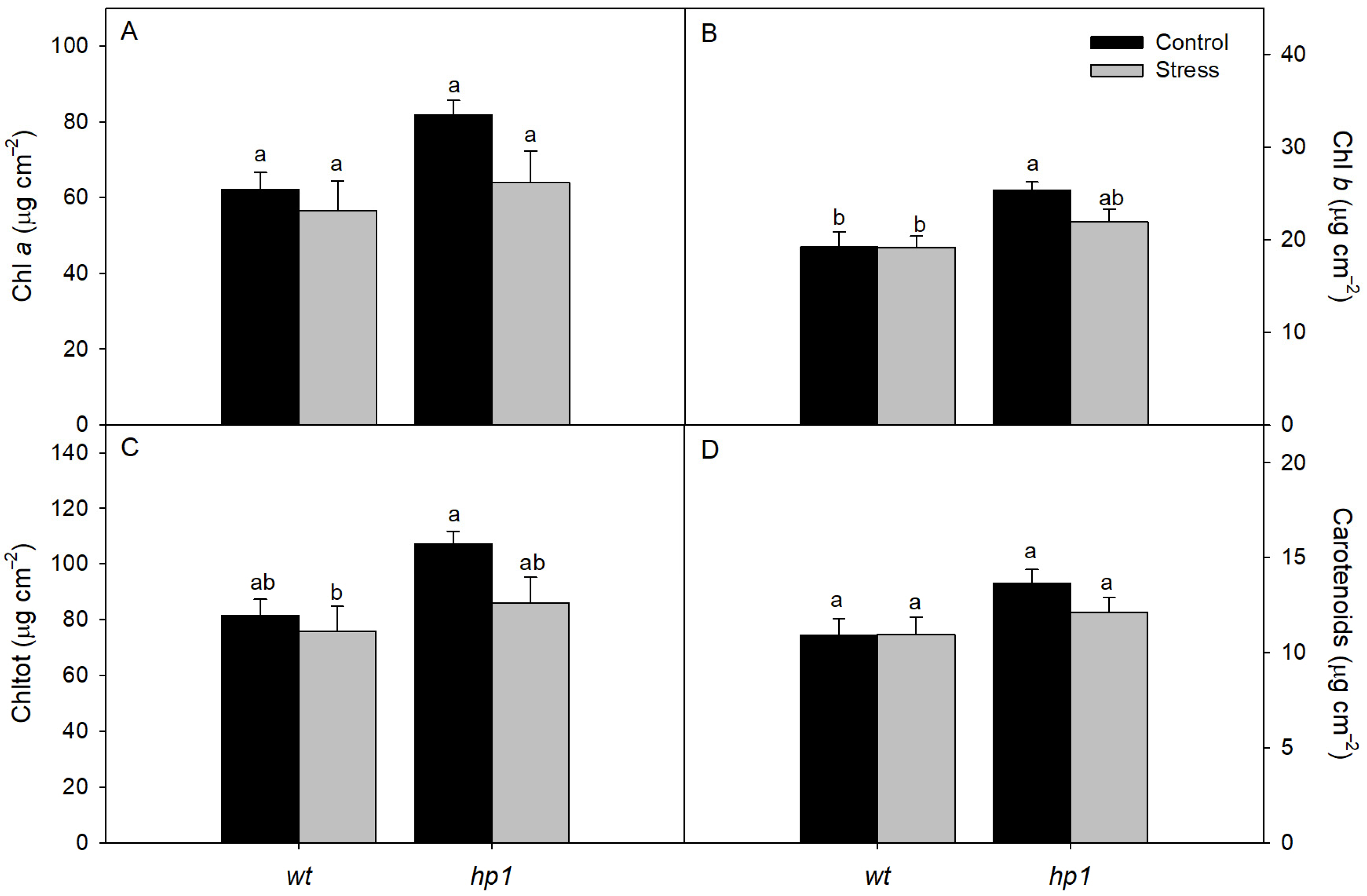
2.2.6. Leaf Pigment Concentration
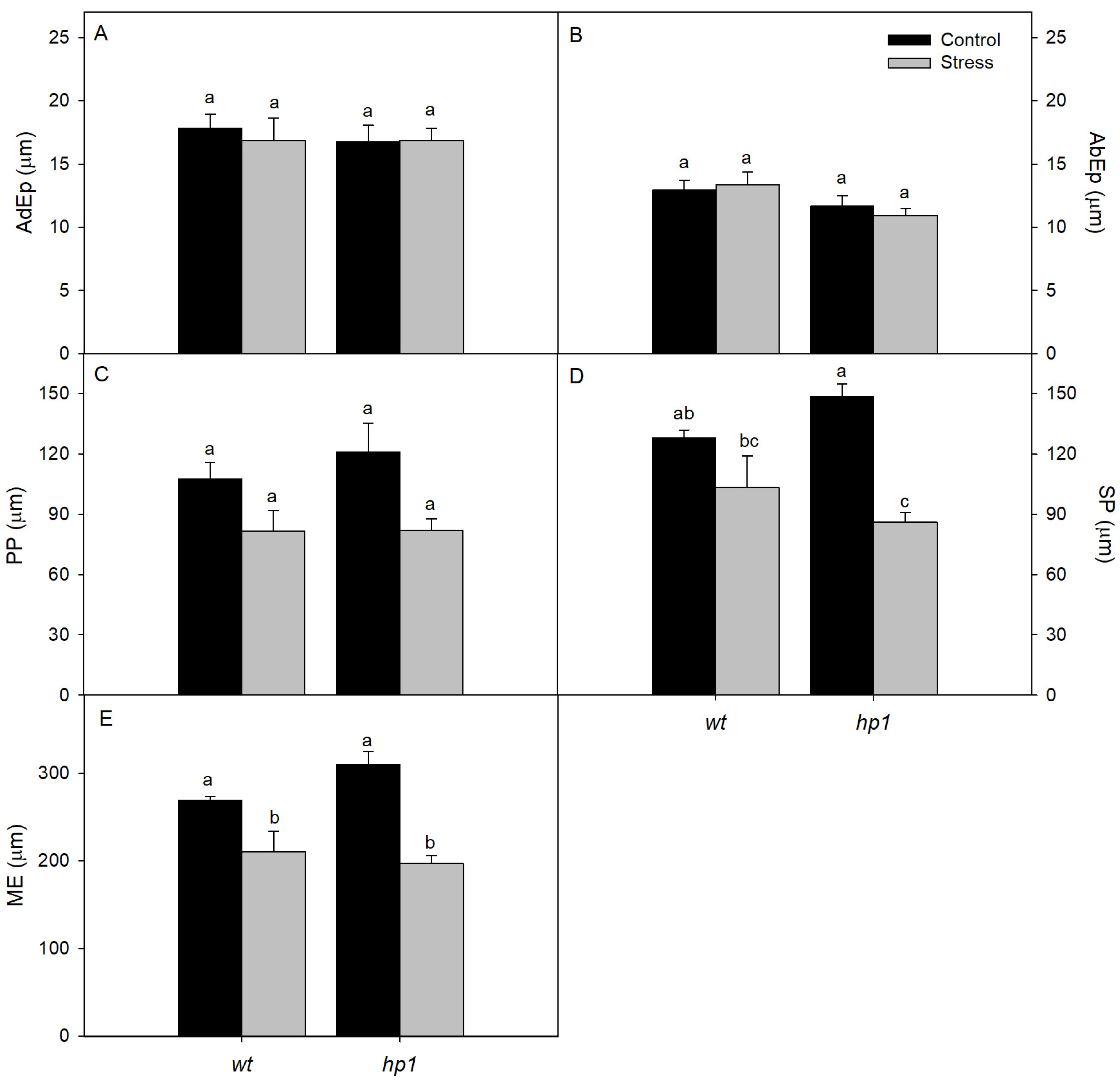
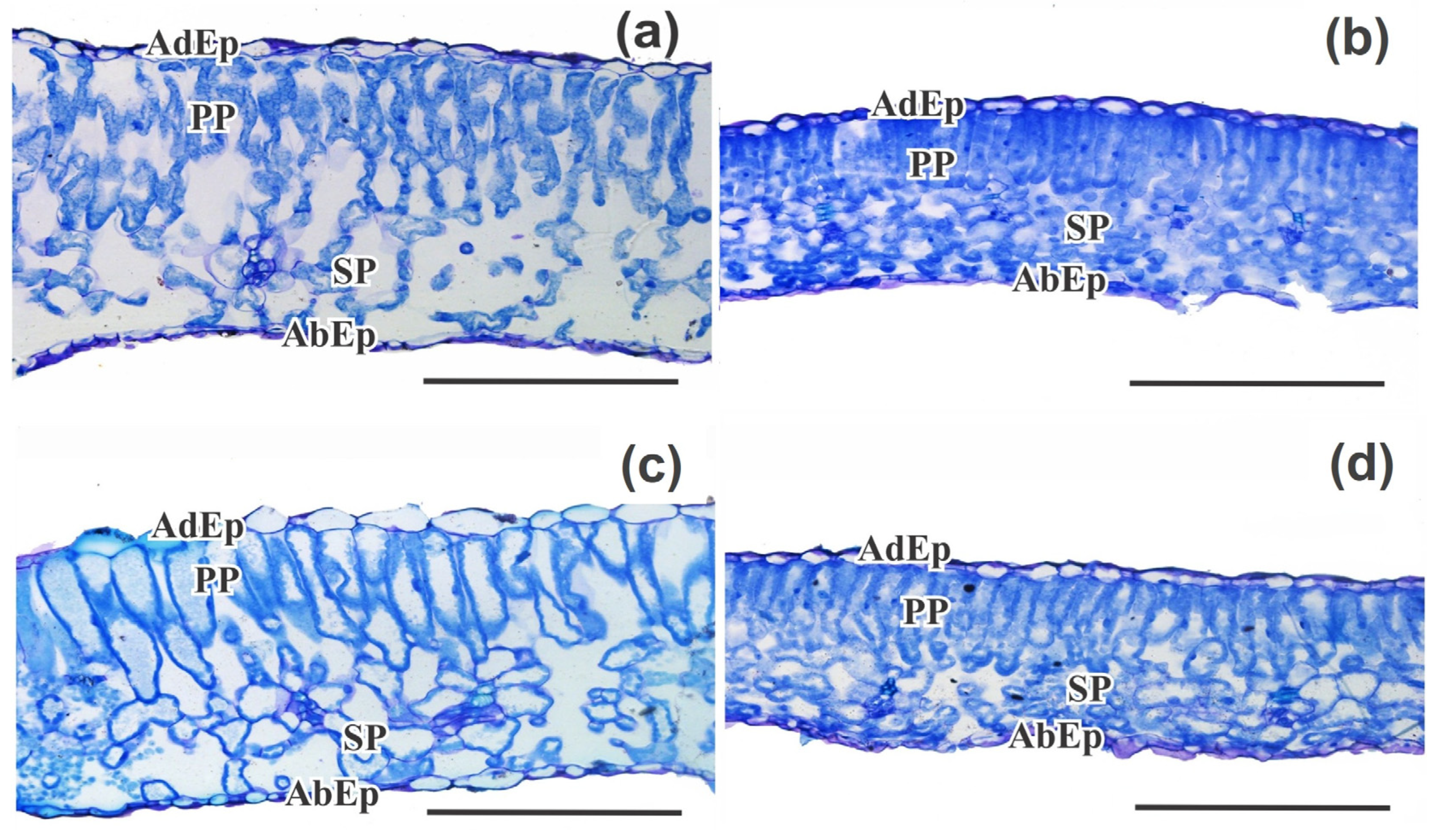
2.2.7. Leaf Micromorphometry
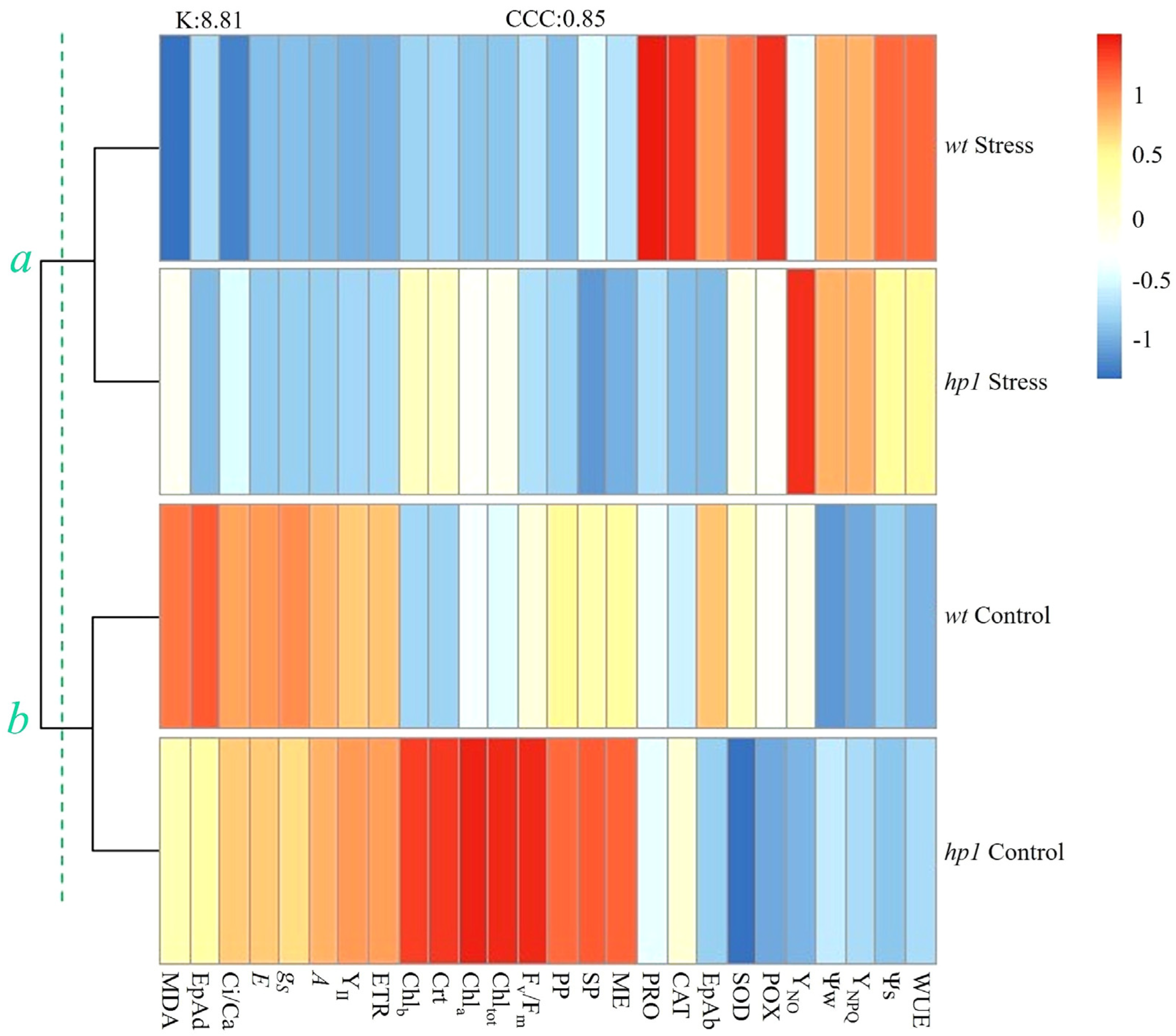
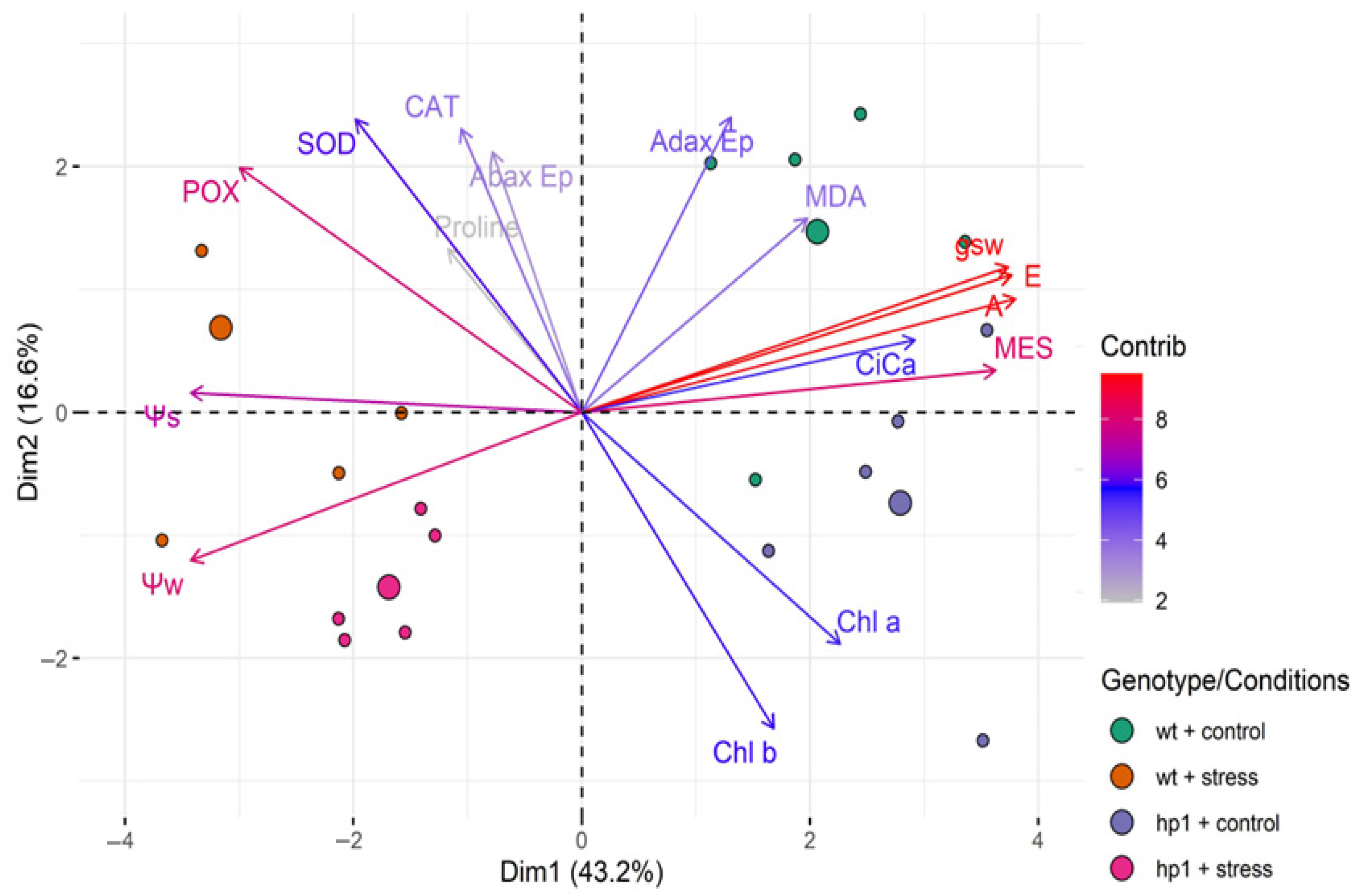
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derbyshire, M.C.; Batley, J.; Edwards, D. Use of multiple ‘omics techniques to accelerate the breeding of abiotic stress tolerant crops. Curr. Plant Biol. 2022, 32, 100262. [Google Scholar] [CrossRef]
- Mesa, T.; Polo, J.; Arabia, A.; Caselles, V.; Munné-Bosch, S. Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J. Plant Physiol. 2022, 268, 153581. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.I.A.; Guimarães, J.J.; Costa, J.V.; Cantuário, F.S.; Salomão, L.C.; Oliveira, R.C.; Luz, J.M.Q. Growth of sweet pepper plants submitted to water tensions in soil and potassium silicate doses. Hortic. Bras. 2019, 37, 82–88. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Luz, J.M.Q. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Becklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining plant physiological responses to climate change through an evolutionary lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar] [CrossRef]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura, L.M.F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenaea stigonocarpa) plants. Photosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Combined effect of high temperature and water-deficit stress imposed at vegetative and reproductive stages on seed quality in soybean. Indian J. Plant Physiol. 2018, 23, 227–244. [Google Scholar] [CrossRef]
- Castro, J.N.; Müller, C.; Almeida, G.M.; Costa, A.C. Physiological tolerance to drought under high temperature in soybean cultivars. Aust. J. Crop Sci. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Batista, P.F.; Costa, A.C.; Müller, C.; Silva-Filho, R.O.; Barbosa da Silva, F.; Merchant, A.; Mendes, G.C.; Nascimento, K.J.T. Nitric oxide mitigates the effect of water deficit in Crambe abyssinica. Plant Physiol. Biochem. 2018, 129, 310–322. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Kaiser, E. Photosynthetic acclimation to fluctuating irradiance in plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Marti, E. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef]
- Alves, F.R.R.; de Melo, H.C.; Crispim-Filho, A.J.; Costa, A.C.; Nascimento, K.J.T.; Carvalho, R.F. Physiological and biochemical responses of photomorphogenic tomato mutants (cv. Micro-Tom) under water withholding. Acta Physiol. Plant. 2016, 38, 155. [Google Scholar] [CrossRef]
- Huther, C.M.; Martinazzo, E.G.; Schock, A.A.; Rombaldi, C.V.; Bacarin, M.A. Production components in transformed and untransformed ‘Micro-Tom’ tomato plants. Rev. Ciênc. Agron. 2018, 49, e20180010. [Google Scholar] [CrossRef]
- Parrotta, L.; Aloisi, I.; Faleri, C.; Romi, M.; Del Duca, S.; Cai, G. Chronic heat stress affects the photosynthetic apparatus of Solanum lycopersicum L. cv Micro-Tom. Plant Physiol. Biochem. 2020, 154, 463–475. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Monteiro, C.C.; Campos, M.L.; Melo, H.C.; Carvalho, R.F. Phytochromes are key regulators of abiotic stress responses in tomato. Sci. Hortic. 2017, 222, 126–135. [Google Scholar] [CrossRef]
- D’Amico-Damião, V.; Cruz, F.J.R.; Gavassi, M.A.; Santos, D.M.M.; Melo, H.C.; Carvalho, R.F. Photomorphogenic modulation of water stress in tomato (Solanum lycopersicum L.): The role of phytochromes A, B1, and B2. J. Hortic. Sci. Biotechnol. 2015, 90, 25–30. [Google Scholar] [CrossRef]
- Klose, C.; Nagy, F.; Schäfer, E. Thermal reversion of plant phytochromes. Mol. Plant 2020, 13, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Crispim Filho, A.J.; Costa, A.C.; Alves, F.R.R.; Batista, P.F.; Rodrigues, A.A.; Vasconcelos Filho, S.C.; Nascimento, K.J.T. Deficiency in phytochromobilin biosynthesis enhances heat-stress-induced impairments to the photosynthetic apparatus in tomato. Biol. Plant. 2019, 63, 134–144. [Google Scholar] [CrossRef]
- Pino-Nunes, L.E.; Lattarulo, M.; Peres, L.E.P. Capítulo 2: Plantio, Irrigação e Adubação nas Canaletas/Vasos e Cultivo no Canteiro. In Manual do Modelo Vegetal Micro-Tom. Available online: https://www.esalq.usp.br/docentes/lazaropp/MMTCap2Cultivo.pdf (accessed on 12 February 2025).
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]
- Laisk, A.; Loreto, F. Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence (ribulose-1,5-bisphosphate carboxylase/oxygenase specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron flow). Plant Physiol. 1996, 110, 903–912. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Rao, R.C. Advanced Statistical Methods in Biometric Research; John Wiley & Sons: New York, NY, USA, 1952. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Melo, H.C.; Castro, E.M.; Alves, E.; Perina, F.J. Anatomia foliar de microtomateiros fitocromo-mutantes e ultra-estrutura de cloroplastos. Ciênc. Agrotec. 2011, 35, 11–18. [Google Scholar] [CrossRef]
- Cookson, P.J.; Kiano, J.W.; Shipton, C.A.; Fraser, P.D.; Romer, S.; Schuch, W.; Bramley, P.M.; Pyke, K.A. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta 2003, 217, 896–903. [Google Scholar] [CrossRef]
- Pereira, A.M.; Martins, A.O.; Batista-Silva, W.; Condori-Apfata, J.A.; Nascimento, V.L.; Silva, V.F.; Oliveira, L.A.; Medeiros, D.B.; Martins, S.C.V.; Fernie, A.R.; et al. Elevated carbon assimilation and metabolic reprogramming in tomato high pigment mutants support the increased production of pigments. Plant Cell Rep. 2022, 41, 1907–1929. [Google Scholar] [CrossRef]
- Kunderlikova, K.; Brestic, M.; Živčák, M.; Kusniarova, P. Photosynthetic responses of sun- and shade-grown chlorophyll b deficient mutant of wheat. J. Cent. Eur. Agric. 2016, 17, 950–956. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef]
- Gollan, P.J.; Aro, E.-M. Photosynthetic signalling during high light stress and recovery: Targets and dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190406. [Google Scholar] [CrossRef]
- Wang, A.; Chen, D.; Ma, Q.; Rose, J.K.C.; Fei, Z.; Liu, Y.; Giovannoni, J.J. The tomato HIGH PIGMENT1/DAMAGED DNA BINDING PROTEIN 1 gene contributes to regulation of fruit ripening. Hortic. Res. 2019, 6, 15. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.; Khudyakova, A.; Ashikhmin, A.; Bolshakov, M.; Kozhevnikova, A.; Kosobryukhov, A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Effect of high-intensity light on the photosynthetic activity, pigment content and expression of light-dependent genes of photomorphogenetic Solanum lycopersicum hp mutants. Plant Physiol. Biochem. 2021, 167, 91–100. [Google Scholar] [CrossRef]
- Menconi, J.; La Monaca, M.; Cataldo, I.; Niccolini, P.M.; Perata, P.; Gonzali, S. Loss of DET1 in High pigment 2 tomato prevents high temperature repression of anthocyanin biosynthesis in fruit through HY5 stabilization. Plant Cell Environ. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 2018, 178, 1461–1472. [Google Scholar] [CrossRef]
- He, Z.; Zhang, P.; Jia, H.; Zhang, S.; Nishawy, E.; Sun, X.; Dai, M. Regulatory mechanisms and breeding strategies for crop drought resistance. New Crops 2024, 1, 100029. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Zhang, Y.; Kreidler, N.; Sun, S.; Ardy, R.; Cao, K.; Sack, L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014, 17, 1580–1590. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnevale, A.B.; Costa, A.C.d.; Santos, E.C.D.; Silva, A.A.d.; Batista, P.F.; Silva, F.B.d.; Moura, L.M.d.F.; Müller, C. Combined High Irradiance and Water Deficit Alters the Anatomy and Physiology of Photomorphogenic Mutant Micro-Tom Plants. Agriculture 2025, 15, 1518. https://doi.org/10.3390/agriculture15141518
Carnevale AB, Costa ACd, Santos ECD, Silva AAd, Batista PF, Silva FBd, Moura LMdF, Müller C. Combined High Irradiance and Water Deficit Alters the Anatomy and Physiology of Photomorphogenic Mutant Micro-Tom Plants. Agriculture. 2025; 15(14):1518. https://doi.org/10.3390/agriculture15141518
Chicago/Turabian StyleCarnevale, Ariana Bertola, Alan Carlos da Costa, Emily Carolina Duarte Santos, Adinan Alves da Silva, Priscila Ferreira Batista, Fábia Barbosa da Silva, Luciana Minervina de Freitas Moura, and Caroline Müller. 2025. "Combined High Irradiance and Water Deficit Alters the Anatomy and Physiology of Photomorphogenic Mutant Micro-Tom Plants" Agriculture 15, no. 14: 1518. https://doi.org/10.3390/agriculture15141518
APA StyleCarnevale, A. B., Costa, A. C. d., Santos, E. C. D., Silva, A. A. d., Batista, P. F., Silva, F. B. d., Moura, L. M. d. F., & Müller, C. (2025). Combined High Irradiance and Water Deficit Alters the Anatomy and Physiology of Photomorphogenic Mutant Micro-Tom Plants. Agriculture, 15(14), 1518. https://doi.org/10.3390/agriculture15141518







