Innovative Protocols for Blackberry Propagation: In Vitro Cultivation in Temporary Immersion Systems with Ex Vitro Acclimatization
Abstract
1. Introduction
2. Materials and Methods
2.1. Initiation and Stabilization of In Vitro Cultures
2.1.1. Selection of Plant Material and Media Preparation
2.1.2. Disinfection of Plant Material
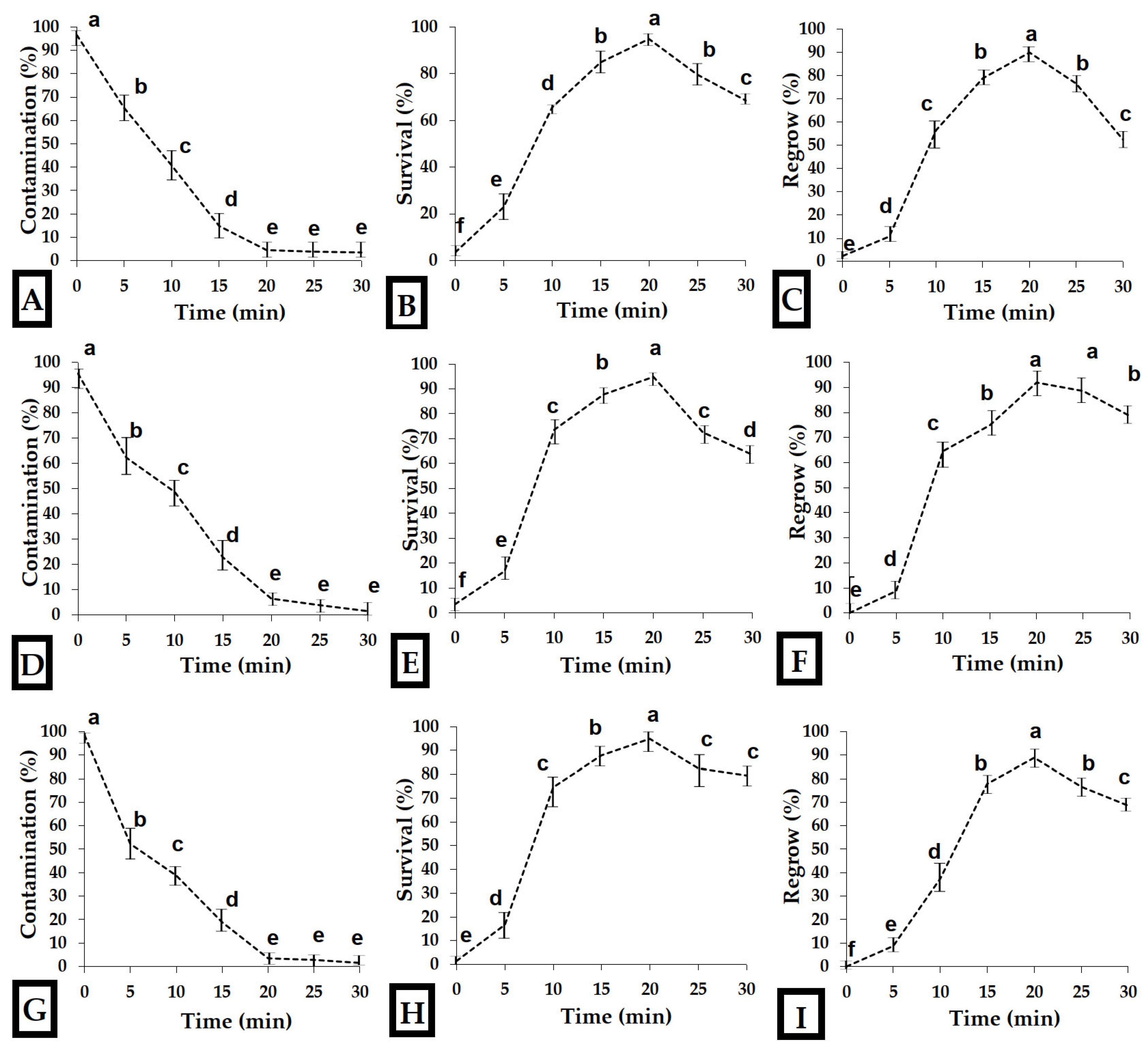
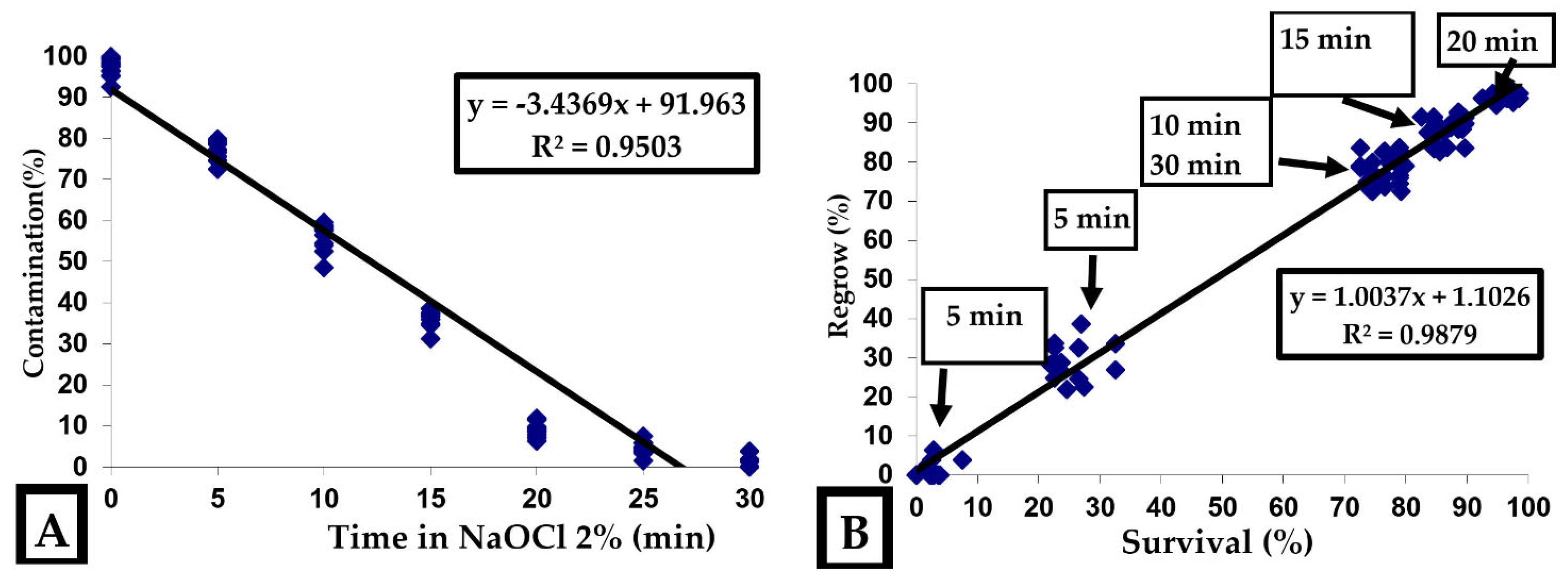
2.1.3. Evaluation of Contamination, Survival, and Regeneration
2.2. In Vitro Multiplication Stage
2.2.1. Multiplication of Plant Material
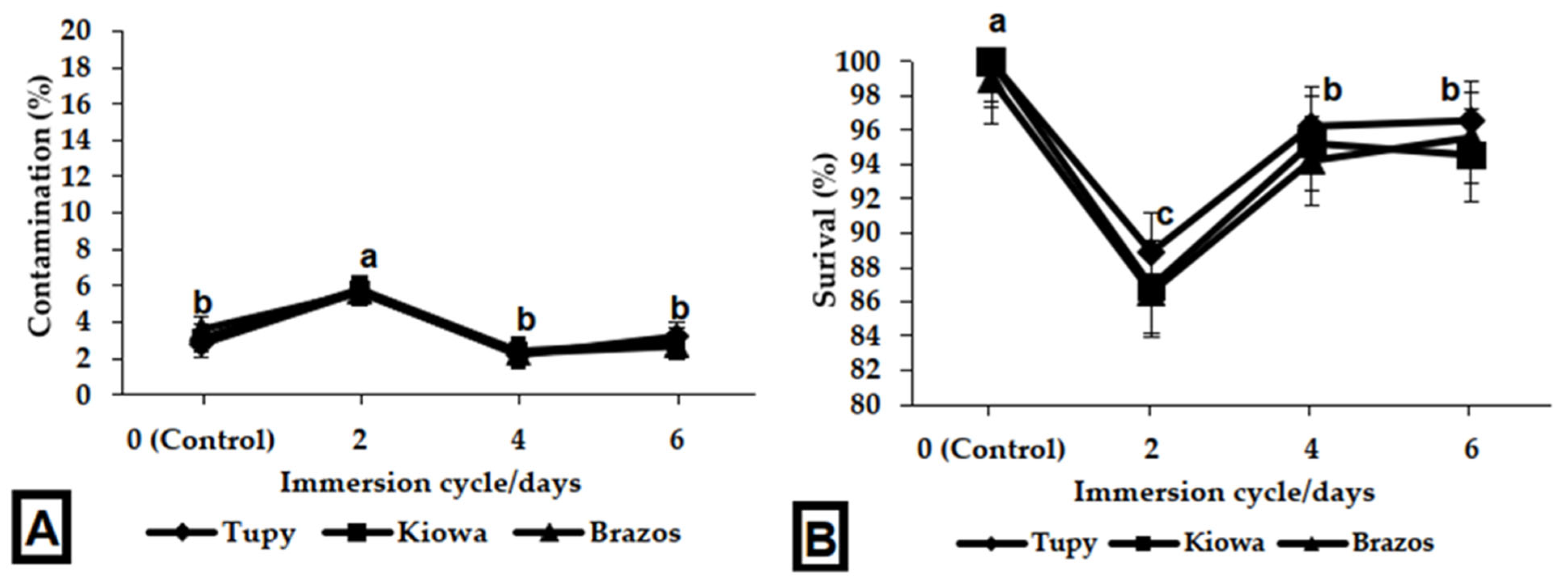
2.2.2. Establishment of TIS
2.2.3. Effect of 6-Benzylaminopurine (6-BAP) on TIS
2.3. Rooting Stage
2.4. Acclimatization and Field Performance
2.4.1. Ex Vitro Acclimatization
2.4.2. Field Performance
2.5. Statistical Analysis
3. Results
3.1. Initiation and Stabilization of In Vitro Cultures
3.2. In Vitro Multiplication Stage
3.3. Rooting Stage
3.4. Acclimatization and Field Performance
3.4.1. Effect of Acclimatization Conditions on Survival Rates
3.4.2. Optimization of In Vitro Propagation Protocol
3.4.3. Vegetative Growth Under Field Conditions
3.4.4. Reproductive Growth and Yield Performance
3.4.5. Final Field Establishment and Morphological Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correia, S.; Matos, M.; Leal, F. Advances in Blueberry (Vaccinium spp.) In Vitro Culture: A Review. Horticulturae 2024, 10, 533. [Google Scholar] [CrossRef]
- Torres, A.I.Z.; Figueroa, I.B.; Leyva, R.A.M. La producción de la zarzamora en México: Un análisis de rentabilidad y ventaja comparativa. Inquietud Empres. 2023, 23, e15333. [Google Scholar] [CrossRef]
- FAOSTAT. Cultivos y Productos de Ganadería. Producción de Zarzamora a Nivel Mundial y en Mexico. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 January 2025).
- Vélez-Torres, M.; Cruz-Gutiérrez, E.J.; Calderón-Zavala, G.; Arellano-Ostoa, G. Conservación in vitro de recursos genéticos de zarzamora (Rubus sp.) y fresa (Fragaria × ananassa) mediante crecimiento mínimo. Agro-Divulgacion 2023, 3, 8–9. [Google Scholar] [CrossRef]
- Corral Melgoza, J.J.; García-Saucedo, P.A.; Aguirre-Paleo, S.; Vargas-Sandoval, M.; Guzmán-de Casa, A.; Ávila-Val, T.d.C. Microorganismos antagonistas como manejo del marchitamiento de la zarzamora por Fusarium oxysporum. Rev. Mex. Cienc. Agric. 2024, 15, 1–13. [Google Scholar] [CrossRef]
- Lara-Chávez, M.B.N.; Raya-Montaño, Y.A.; Apáez-Barrios, P. Identificación del agente causal de la pudrición de raíz y corona en zarzamora cv. Tupy y su control in vitro: Identification of the causal agent of the root and crown rot in blackberry cv. Tupy and its in vitro control. e-CUCBA 2023, 20, 121–131. [Google Scholar] [CrossRef]
- Shchelkunova, S.E.; Popov, Y.G. Poluchenie svobodnykh ot virusa rasteny maliny putem kultury izolirovanykh apeksov Fiziol Rast. Sov. Plant Physiol. 1970, 17, 618–622. [Google Scholar]
- Broome, O.C.; Zimmerman, R.H. In vitro propagation of blackberry. HortScience 1978, 13, 151–153. [Google Scholar] [CrossRef]
- Skirvin, R.M.; Chu, M.C.; Gomez, E. In vitro propagation of thornless trailing blackberries. HortScience 1981, 16, 310–312. [Google Scholar] [CrossRef]
- Babić, V.; Nesković, M. Propagation of three blackberry cultivars from small apical buds in vitro. J. Hortic. Sci. 1984, 59, 183–185. [Google Scholar] [CrossRef]
- Fernandez, G.E.; Clark, J. In vitro propagation of the erect thornless “Navaho” blackberry. HortScience 1991, 26, 1219. [Google Scholar] [CrossRef]
- Badjakov, I.; Georgiev, V.; Georgieva, M.; Dincheva, I.; Vrancheva, R.; Ivanov, I.; Georgiev, D.; Hristova, D.; Kondakova, V.; Pavlov, A. Bioreactor Technology for In Vitro Berry Plant Cultivation. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2021; pp. 383–431. [Google Scholar] [CrossRef]
- Debnath, S. Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Ayub, R.A.; dos Santos, J.N.; Zanlorensi Junior, L.A.; da Silva, D.M.; de Carvalho, T.C.; Grimaldi, F. Sucrose concentration and volume of liquid medium on the in vitro growth and development of blackberry cv. Tupy in temporary immersion systems. Ciênc. Agrotec. 2019, 43, 007219. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary immersion systems (TISs): A comprehensive review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef]
- da Silva, I.A.O.; Biasi, L.A. Double-phase culture medium and plant growth regulators in the micropropagation of blackberries. Comun. Sci. 2022, 13, e3613. [Google Scholar]
- Georgieva, L.; Tsvetkov, I.; Georgieva, M.; Kondakova, V. New protocol for in vitro propagation of berry plants by TIS bioreactor. Bulg. J. Agric. Sci. 2016, 22, 745–751. [Google Scholar]
- Regni, L.; Cesarini, A.; Micheli, M.; Proietti, P. A bibliometric analysis of research on blackberry micropropagation. Plant Cell Tissue Organ Cult. (PCTOC) 2025, 160, 33. [Google Scholar] [CrossRef]
- Sorcia-Morales, M.; Mancilla-Álvarez, E.; Baltazar-Bernal, O.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. In vitro physiological and biochemical response of Stevia rebaudiana exposure to carbon nanotubes: Hormetic and photomixotrophic effect. Ind. Crops Prod. 2024, 220, 119168. [Google Scholar] [CrossRef]
- Jabín, B.J.; Luis, S.J.; Eucario, M.Á. Hormesis in plant tissue culture. Plant Cell Tiss Organ Cult. 2024, 159, 16. [Google Scholar] [CrossRef]
- Montoya-Jasso, V.M.; Arreola-Tostado, J.M.; Castillo-Valdez, X.; Báez-Pérez, A. Organic-based fertilization in blackberry (Rubus fructicosus L.) production: Effect on yield variables and fruit quality. Suelos Ecuat. 2023, 53, 12–17. [Google Scholar]
- Samaan, M.S.F.; El-Hamed Nasser, M.A. Micropropagation of Blackberry (Rubus fruticosus) cv. Karaka Black. Egypt. J. Hortic. 2022, 49, 187–198. [Google Scholar] [CrossRef]
- Wójcik-Seliga, J.; Wójcik-Gront, E. Evaluation of blackberry and hybrid berry cultivars new to Polish climate-Short communication. Hortic. Sci. 2013, 40, 88–91. [Google Scholar] [CrossRef]
- Badr-Elden, A.M.; Nower, A.A.; Abdallah, A.A.; Albeah, H.A. In vitro direct and indirect propagation of blackberry (Rubus sp.). Res. J. Appl. Biotechnol. 2016, 2, 89–100. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Barrios, E.A.M.; Vilca, L.C.; Huamani, O.M. Efecto de dos enraizantes naturales y uno sintético en la propagación de zarzamora (Rubus robustus C. Presl). Aporte Santiaguino 2022, 15, 72–86. [Google Scholar] [CrossRef]
- Kefayetı, N.; Kafkas, E.; Ercişli, S. Micropropagation of ‘chester thornless’ blackberry cultivar using axillary bud explants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 162–168. [Google Scholar] [CrossRef]
- Stefanova, M.; Nacheva, L.; Ganeva, T.; Dimitrova, N. LED lighting affects the biomass accumulation and leaf stomatal characteristics of raspberry (Rubus idaeus L.) in vitro. J. Cent. Eur. Agric. 2024, 25, 492–501. [Google Scholar] [CrossRef]
- AbdAlla, M.M.; Mostafa, R.A.A. In vitro propagation of Blackberry (Rubus fruticosus L.). Assiut J. Agric. Sci. 2015, 46, 88–99. [Google Scholar]
- Ismaini, L.; Surya, M.I. In vitro plant regeneration from hypocotyl of Arben (Rubus fraxinifolius Poir.). Aust. J. Crop Sci. 2017, 11, 474–478. [Google Scholar] [CrossRef]
- Hunková, J.; Libiakova, G.; Fejér, J.; Vujović, T.; Gajdosova, A. Testing of different iron sources and concentrations on shoot multiplication of blackberry (Rubus fruticosus L.). Genet.-Belgrade 2018, 50, 351–356. [Google Scholar] [CrossRef]
- Martínez Rivero, A.; Ramírez-Mosqueda, M.A.; Mosqueda Frómeta, O.; Escalona Morgado, M.M.; Rivas Paneca, M.; Rodríguez Escriba, R.C.; Daquinta Gradaille, M.A.; Bello-Bello, J.J. Influence of Vitrofural® on sugarcane micropropagation using temporary immersion system. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 447–453. [Google Scholar] [CrossRef]
- Dönmez, B.A.; Polat, Ş.; Hamakhan, A.M.; Kafkas, N.E. Methods of Blackberry Propagation in vitro Condition. BIO Web Conf. 2024, 85, 01009. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Zhang, C.; Lyu, L.; Li, W. Breeding and Growth Performance of ‘Ningzhi 4’, a New Blackberry Cultivar with High Yield Potential and Good Quality in China. Plants 2023, 12, 1661. [Google Scholar] [CrossRef]
- Muratalla-Lúa, A.; Jaen-Contreras, D.; Arévalo-Galarza, L. La producción de frambuesa y zarzamora en México. Agro Product. 2013, 6. [Google Scholar]
- Sotelo Ruiz, E.D.; Cruz Bello, G.; González Hernández, A.; Flores López, R. Actualización de la cartografía edafológica del Estado de México: Una herramienta para la planeación. Rev. Mex. Cienc. Agríc. 2020, 11, 1775–1788. [Google Scholar] [CrossRef]
- Moller, A.d.C.M.; Figueroa, M.G.R.; Ochoa, V.R.D.; Aguilar, R.N. La zarzamora (Rubus sp.), cultivo alternativo para el estado de Sonora. Rev. Mex. Agronegocios 2013, 33, 600–608. [Google Scholar] [CrossRef]
- Cárdenas, D.M.M.; Cardona, W.A.; Muñoz, M.C.G.; Benavides, M.M.B. Relationship between variable doses of N, P, K and Ca and the physicochemical and proximal characteristics of andean blackberry (Rubus glaucus Benth.). Sci. Hortic. 2019, 256, 108528. [Google Scholar] [CrossRef]
- Quezada, R.A.P.; Ontiveros, J.L.R.; Hernández, V.A.G. Transpiración, potencial hídrico y prolina en zarzamora bajo déficit hídrico. Terra Latinoam. 1999, 17, 125–130. [Google Scholar]
- Mukundan, D.; Jeevanandan, G. Cytotoxic effect of two different concentrations of Sodium Hypochlorite: An In-Vitro Study. Cureus 2024, 16, e66999. [Google Scholar] [CrossRef]
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Bañados, M.P. Worldwide Blackberry Production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef]
- Fathy, H.; El-Leel, O.; Amin, M.; AbuEl-Leel, O. Micropropagation and biomass production of Rubus fruticosus L. (Blackberry) plant. Middle East J. Appl. Sci. 2020, 8, 1215. [Google Scholar]
- Muñoz-Concha, D.; Quintero, J.; Ercişli, S. Media and hormones influence in micropropagation success of blackberry cv ‘Chester’. Res. J. Biotechnol. 2021, 16, 103–108. [Google Scholar]
- Ahmed, M.E.A.E.; Abd Elaziem, T.M.A.E. In vitro regeneration and improving kaempferol accumulation in blackberry (Rubus fruticosus L.) callus and suspension cultures. Egypt. J. Chem. 2022, 65, 369–383. [Google Scholar] [CrossRef]
- Nunes, N.B.; Reis, J.O.d.; Castro, V.S.; Machado, M.A.M.; Cunha-Neto, A.d.; Figueiredo, E.E.d.S. Optimizing the Antimicrobial Activity of Sodium Hypochlorite (NaClO) over Exposure Time for the Control of Salmonella spp. In Vitro. Antibiotics 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Spinoso-Castillo, J.; Chavez-Santoscoy, R.; Bogdanchikova, N.; Pérez-Sato, J.; Morales-Ramos, V.; Bello-Bello, J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Yildiz, M.; Fatih Ozcan, S.; T. Kahramanogullari, C.; Tuna, E. The effect of sodium hypochlorite solutions on the viability and in vitro regeneration capacity of the tissue. Nat. Prod. J. 2012, 2, 328–331. [Google Scholar] [CrossRef]
- Mori-Vásquez, J.A.; Tenazoa, N.M.P.; Fernandez, V.J.M.; Herrera-Saavedra, M.A.; de Setta, N.; de Oliveira Ferreira, G.; Avalos-Díaz, A.G.; Gonzales-Alvarado, A.C. Efecto de la desinfección, el desarrollo foliar y las fitohormonas en la inducción de callos de Simarouba amara Aubl. Cienc. Y Tecnol. Agropecu. 2024, 25. [Google Scholar] [CrossRef]
- Kwok, C.S.; Dashti, M.; Tafuro, J.; Nasiri, M.; Muntean, E.A.; Wong, N.; Kemp, T.; Hills, G.; Mallen, C.D. Methods to disinfect and decontaminate SARS-CoV-2: A systematic review of in vitro studies. Ther. Adv. Infect. Dis. 2021, 8. [Google Scholar] [CrossRef]
- Rodboot, N.; Yenchon, S.; Te-chato, S. Optimization of explant sterilization and plant growth regulators for enhancing the in vitro propagation of Nymphaea colorata Peter. Plant Cell Tiss Organ Cult. 2024, 159, 59. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Desouky, W.; El-Leel, O.F.A. Micropropagation, phytochemical content and antioxidant activity of gamma-irradiated blackberry (Rubus fruticosus L.) plantlets. In Vitr. Cell. Dev. Biol.-Plant 2022, 58, 457–469. [Google Scholar] [CrossRef]
- Zunazri, N.H.; Kemat, N.; Ariffin, N.; Rineksane, I.A. Effect of media components on hyperhydricity in horticultural crops: A review. J. Plant Biotechnol. 2024, 51, 307–319. [Google Scholar] [CrossRef]
- Sanchéz, J.; Daquinta, M.; Capote, I.; Da Silva, J.T. Shoot propagation of Zantedeschia spp. in a temporary immersion system-Effect of culture parameters on plant proliferation and quality. Floric. Ornam. Biotechnol. 2011, 5, 78–80. [Google Scholar]
- Damiano, C.; Arias, P.M.D.; La Starza, S.R.; Frattarelli, A. Temporary immersion system for temperate fruit trees. Acta Hortic. 2004, 748, 87–90. [Google Scholar] [CrossRef]
- Aguilar, M.E.; Wang, X.-Y.; Escalona, M.; Yan, L.; Huang, L.-F. Somatic embryogenesis of Arabica coffee in temporary immersion culture: Advances, limitations, and perspectives for mass propagation of selected genotypes. Front. Plant Sci. 2022, 13, 994578. [Google Scholar] [CrossRef]
- Vujović, T.; Ružić, Đ.; Cerović, R.; Šurlan Momirović, G. Adventitious regeneration in blackberry (Rubus fruticosus L.) and assessment of genetic stability in regenerants. Plant Growth Regul. 2010, 61, 265–275. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Al-Ali, A.M.; Rihan, H.Z.; Alshahrani, T.; Alwahibi, M.S.; Almutairi, K.F.; Naidoo, Y.; Fuller, M.P. Effects of artificial light spectra and sucrose on the leaf pigments, growth, and rooting of blackberry (Rubus fruticosus) microshoots. Agronomy 2023, 13, 89. [Google Scholar] [CrossRef]
- Reed, B.; Poothong, S.; Hall, H.K. Propagation of blackberries and related Rubus species. In Blackberries and Their Hybrids; CABI: Wallingford, UK, 2017; pp. 101–112. [Google Scholar]
- Long, Z.; Xue, Y.; Ning, Z.; Sun, J.; Li, J.; Su, Z.; Liu, Q.; Xu, C.; Yan, J.-K. Production, characterization, and bioactivities of exopolysaccharides from the submerged culture of Ganoderma cantharelloideum M. H. Liu. 3 Biotech 2021, 11, 145. [Google Scholar] [CrossRef]
- Titenkov, A.V.; Lushpin, M.N.; Lushpina, T.N.; Kotsareva, N.V.; Kryukov, A.N. Adaptation of microclones of blackberries to in vivo conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012022. [Google Scholar] [CrossRef]
- Bravo-Ruíz, I.N.; González-Arnao, M.T.; Hernández-Ramírez, F.; López-Domínguez, J.; Cruz-Cruz, C.A. Types of Temporary Immersion Systems Used in Commercial Plant Micropropagation. In Micropropagation Methods in Temporary Immersion Systems; Ramírez-Mosqueda, M.A., Cruz-Cruz, C.A., Eds.; Methods in Molecular Biology Humana; Springer: New York, NY, USA, 2024; Volume 2759, pp. 9–24. [Google Scholar] [CrossRef]
- Sözmen, E.U.; Karaköy, T.; Aasim, M. Optimizing benzylaminopurine (BAP) and naphthalene acetic acid (NAA) concentration for in vitro micropropagation of Goji Berry (Lycium barbarum L.) using various optimizing tools. Pak. J. Agric. Sci. 2024, 61, 85–91. [Google Scholar]
- Lodi, E.; Bello, Y.D.; Duarte, K.B.d.P.; Montagner, F.; Cecchin, D. Antimicrobial Efficacy and Dentin Collagen Damage Caused by Calcium Hypochlorite and Sodium Hypochlorite. Braz. Dent. J. 2024, 35, e245771. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Y.; Wu, W.; Li, W.; Jin, Y. Screening and evaluation of excellent blackberry cultivars and strains based on nutritional quality, antioxidant properties, and genetic diversity. Plants 2023, 12, 2982. [Google Scholar] [CrossRef]
- Ricci, A.; Iocoli, L.; D’Aloiso, D.; Mezzetti, B.; Savini, G.; Sabbadini, S. Assessment of different factors to induce the adventitious regeneration in two blackberry cultivars starting from leaf and petiole explants. Acta Hortic. 2024, 1388, 79–84. [Google Scholar] [CrossRef]
- Regni, L.; Micheli, M.; Del Pino, A.M.; Facchin, S.L.; Rabica, E.; Camilloni, L.; Cesarini, A. Blackberry synthetic seeds storage: Effects of temperature, time, and sowing substrate. Plant Cell Tiss Organ Cult. 2024, 158, 17. [Google Scholar] [CrossRef]
- Villa, F.; Pasqual, M.; Assis, F.A.D.; Pio, L.A.S.; Assis, G.A.D. In vitro blackberry growing: Effect of growth regulators and cultivar. Cienc. Agrotecnologia 2008, 32, 1754–1759. [Google Scholar] [CrossRef]
- Dziedzic, E.; Jagła, J. Micropropagation of rubus and Ribes spp. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 149–160. [Google Scholar] [CrossRef]
- Sabooni, N.; Shekafandeh, A.; Gharaghani, A.; Teixeira Da Silva, J.A. Tissue culture of Rubus sp. by different methods and assessment of genetic fidelity of regenerated plants using RAPD. Agric. Conspec. Sci. 2022, 87, 223–230. [Google Scholar]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation hormesis in plants. Curr. Opin. Toxicology. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Eucario, M.Á.; Luis, S.C.J.; Arturo, M.M.T.R.; Francisco, P.P.K.; Jabín, B.B.J. Temporary immersion bioreactor as an efficient method for in vitro propagation of Agave marmorata. South Afr. J. Bot. 2024, 169, 6–11. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Freire-Seijo, M.; Rodríguez, R.B.; Garcia, S.T. Comparative assessment between liquid culture in static and temporary immersion systems on multiplication, morpho-physiological and biochemical characteristics of Bambusa vulgaris Schrad. ex Wendl. shoots. Acta Physiol. Plant 2024, 46, 11. [Google Scholar] [CrossRef]
- Trofim, M.; Chiorchina, N.; Mîrza, A.; Tabăra, G.M. The influence of external factors on the development of in vitro cul-tured blackberry pants under ex vitro conditions. Rev. Bot. 2018, 17, 14–20. [Google Scholar]




| Chemical Property | Value for a Vertisol |
|---|---|
| pH in H2O | 6.12 |
| Organic Matter (OM) | 3.5% |
| Cation Exchange Capacity (CEC) | 35.22 cmol kg−1 |
| CEC at Base Saturation | 43.18 cmol kg−1 |
| Exchangeable Aluminum (Al) | 0.8 cmol kg−1 |
| Calcium (Ca) | 32.43 cmol kg−1 |
| Magnesium (Mg) | 16.22 cmol kg−1 |
| Potassium (K) | 0.38 cmol kg−1 |
| Sodium (Na) | 2.85 cmol kg−1 |
| Base Saturation | 73.62% |
| Electrical Conductivity (EC) | 1.86 dS m−1 |
| Available Phosphorus (P) | 15.22 mg kg−1 |
| Total Nitrogen (N) | 0.25% |
| C:N Ratio (Carbon:Nitrogen) | 12:1 |
| Soil Texture | 60% clay, 30% silt, 20% sand |
| Cultivars | Immersion Cycles per Day | Number of Necrotic Explants | Number of Leaves with Hyperhydricity | Total Fresh Mass of Shoots (g/per Culture Vessel) | Total Dry Mass of Shoots (g/per Culture Vessel) |
|---|---|---|---|---|---|
| Tupy | 0 (Control) | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 2.33 ± 1.20 c | 0.065 ± 0.001 c |
| 2 | 5.5 ± 0.6 a | 0.0 ± 0.0 c | 6.22 ± 1.70 b | 0.186 ± 0.003 b | |
| 4 | 2.2 ± 0.5 b | 1.0 ± 0.5 b | 9.34 ± 0.60 a | 0.280 ± 0.001 a | |
| 6 | 3.1 ± 0.5 b | 2.8 ± 0.8 a | 9.40 ± 0.60 a | 0.281 ± 0.002 a | |
| Kiowa | 0 (Control) | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 1.88 ± 1.22 c | 0.042 ± 0.001 b |
| 2 | 6.0 ± 0.7 a | 0.3 ± 0.5 c | 7.22 ± 0.40 b | 0.194 ± 0.001 b | |
| 4 | 3.0 ± 0.5 b | 1.3 ± 0.3 b | 10.28 ± 0.70 a | 0.282 ± 0.002 a | |
| 6 | 4.0 ± 0.8 b | 3.0 ± 1.0 a | 10.35 ± 0.70 a | 0.281 ± 0.001 a | |
| Brazos | 0 (Control) | 0.2 ± 0.1 c | 0.0 ± 0.0 c | 2.07 ± 1.25 c | 0.067 ± 0.001 b |
| 2 | 5.8 ± 0.8 a | 1.3 ± 0.5 b | 5.55 ± 0.72 b | 0.184 ± 0.001 b | |
| 4 | 2.8 ± 0.5 b | 1.3 ± 0.5 b | 8.97 ± 0.05 a | 0.311 ± 0.002 a | |
| 6 | 3.5 ± 0.7 b | 3.8 ± 1.0 a | 9.12 ± 0.05 a | 0.312 ± 0.001 a |
| Cultivars | Immersion Time (min) | Contamination (%) | Survival (%) | Total Fresh Mass of Shoots (g/ per Culture Vessel) | Total Dry Mass of Shoots (g/per Culture Vessel) |
|---|---|---|---|---|---|
| Tupy | 0 (Control) | 1.64 ± 0.00 a | 96.80 ± 0.38 a | 2.37 ± 0.03 c | 0.069 ± 0.001 c |
| 5 | 1.88 ± 0.01 a | 97.26 ± 0.42 a | 9.82 ± 0.05 a | 0.186 ± 0.002 a | |
| 10 | 1.44 ± 0.00 a | 97.88 ± 0.43 a | 8.46 ± 0.06 b | 0.080 ± 0.010 b | |
| 15 | 1.32 ± 0.00 a | 98.02 ± 0.52 a | 8.40 ± 0.05 b | 0.081 ± 0.020 b | |
| Kiowa | 0 (Control) | 1.32 ± 0.01 a | 97.83 ± 0.46 a | 1.88 ± 0.02 c | 0.042 ± 0.020 c |
| 5 | 1.39 ± 0.00 a | 97.45 ± 0.45 a | 9.94 ± 0.04 a | 0.194 ± 0.002 a | |
| 10 | 1.45 ± 0.00 a | 98.03 ± 0.47 a | 7.88 ± 0.07 b | 0.082 ± 0.010 b | |
| 15 | 1.36 ± 0.01 a | 98.01 ± 0.48 a | 7.35 ± 0.06 b | 0.281 ± 0.010 b | |
| Brazos | 0 (Control) | 1.58 ± 0.02 a | 98.21 ± 0.42 a | 2.07 ± 0.01 c | 0.067 ± 0.010 c |
| 5 | 1.65 ± 0.03 a | 98.03 ± 0.39 a | 9.85 ± 0.04 b | 0.184 ± 0.030 c | |
| 10 | 1.73 ± 0.01 a | 97.88 ± 0.38 a | 8.97 ± 0.03 a | 0.078 ± 0.020 b | |
| 15 | 1.84 ± 0.02 a | 97.92 ± 0.42 a | 8.12 ± 0.04 a | 0.082 ± 0.010 b |
| Cultivars | Concentration BAP (mg L−1) | Shoots per Initial Bud | Shoot Length (cm) | Buds per New Shoot | Total Fresh Mass of the Shoots per Culture Vessel (g) |
|---|---|---|---|---|---|
| Tupy | 0 (Control) | 2.90 ± 0.90 d | 3.0 ± 0.6 c | 2.4 ± 0.8 c | 9.34 ± 0.40 c |
| 1 | 23.30 ± 1.30 b | 5.5 ± 1.5 a | 5.2 ± 0.5 a | 10.22 ± 0.80 b | |
| 2 | 34.01 ± 1.20 a | 5.4 ± 1.2 a | 5.1 ± 0.7 a | 11.15 ± 0.60 a | |
| 3 | 9.53 ± 0.50 c | 3.0 ± 0.8 b | 3.0 ± 0.8 b | 6.34 ± 1.70 c | |
| 4 | 8.42 ± 0.24 d | 2.3 ± 0.6 c | 2.3 ± 0.7 c | 4.42 ± 1.02 d | |
| 5 | 6.87 ± 0.18 e | 1.7 ± 0.4 d | 1.9 ± 0.5 d | 3.22 ± 0.87 e | |
| Kiowa | 0 (Control) | 1.80 ± 0.60 d | 3.5 ± 0.4 c | 2.9 ± 0.5 c | 10.28 ± 0.70 c |
| 1 | 20.70 ± 1.50 a | 8.4 ± 2.5 a | 6.3 ± 1.1 a | 11.92 ± 0.90 b | |
| 2 | 20.60 ± 1.26 a | 5.4 ± 1.9 b | 5.6 ± 0.5 a | 12.22 ± 0.50 a | |
| 3 | 7.00 ± 1.80 b | 2.7 ± 0.5 c | 4.4 ± 0.6 b | 7.35 ± 0.90 d | |
| 4 | 5.45 ± 1.20 c | 2.6 ± 0.5 c | 3.12 ± 0.4 b | 5.54 ± 0.71 e | |
| 5 | 5.57 ± 1.20 c | 1.8 ± 0.3 d | 2.88 ± 0.3 b | 4.75 ± 0.40 f | |
| Brazos | 0 (Control) | 2.30 ± 1.00 c | 4.4 ± 0.9 b | 3.2 ± 0.4 b | 9.17 ± 0.04 c |
| 1 | 18.50 ± 0.80 b | 6.9 ± 0.7 a | 5.8 ± 0.6 a | 10.38 ± 0.80 b | |
| 2 | 20.00 ± 1.30 a | 6.5 ± 0.5 a | 5.3 ± 0.6 a | 11.42 ± 0.41 a | |
| 3 | 8.30 ± 0.70 d | 3.6 ± 0.4 c | 3.5 ± 0.5 b | 5.22 ± 1.40 d | |
| 4 | 6.22 ± 0.40 e | 3.1 ± 0.2 d | 3.5 ± 0.5 b | 4.18 ± 1.11 e | |
| 5 | 6.18 ± 0.40 e | 2.7 ± 0.2 e | 3.5 ± 0.5 b | 3.82 ± 1.41 f |
| Rooting Method | Cultivar | Root System Length (cm) | Number of Roots per Plant | Root Emission (%) |
|---|---|---|---|---|
| TIS * | Tupy | 1.14 ± 0.08 | 3.97 ± 0.20 | 96.25 ± 0.81 |
| Kiowa | 1.16 ± 0.09 | 3.98 ± 0.18 | 97.22 ± 0.78 | |
| Brazos | 1.18 ± 0.07 | 4.01 ± 0.21 | 96.28 ± 0.76 | |
| On solid Medium * | Tupy | 1.17 ± 0.08 | 3.92 ± 0.17 | 96.12 ± 0.80 |
| Kiowa | 1.14 ± 0.07 | 3.96 ± 0.18 | 96.18 ± 0.88 | |
| Brazos | 1.15 ± 0.09 | 3.95 ± 0.19 | 96.32 ± 0.87 |
| Indicator | Cultivar | ||
|---|---|---|---|
| Tupy | Kiowa | Brazos | |
| Survival (%) | 98.42 ± 0.82 a | 97.52 ± 0.83 a | 97.55 ± 0.81 a |
| Plant height (cm) | 1.48 ± 0.58 a | 1.32 ± 0.58 b | 1.26 ± 0.58 b |
| Stem diameter (mm) | 2.89 ± 0.48 a | 2.53 ± 0.47 b | 2.58 ± 0.46 b |
| Fresh plant mass (kg) | 2.96 ± 0.67 a | 2.22 ± 0.68 b | 2.15 ± 0.67 b |
| Dry plant mass (kg) | 0.089 ± 0.006 a | 0.064 ± 0.005 b | 0.058 ± 0.007 b |
| Indicator | Cultivar | ||
|---|---|---|---|
| Tupy | Kiowa | Brazos | |
| Anthesis stage (days) | 187 ± 1.25 c | 192 ± 1.27 b | 202 ± 1.26 a |
| Number of flowers per plant | 292 ± 4.22 a | 262 ± 4.20 b | 268 ± 4.21 b |
| Flower fertilization (%) | 89.93 ± 1.12 a | 86.52 ± 1.11 b | 87.57 ± 1.12 b |
| Number of fruits per plant | 268 ± 3.87 a | 227 ± 3.88 b | 232 ± 3.88 b |
| Fruit mass per plant (g) | 3.96 ± 0.13 b | 4.32 ± 0.14 a | 3.82 ± 0.14 b |
| Yield (t ha−1) | 12.88 ± 0.34 a | 10.38 ± 0.33 b | 10.65 ± 0.34 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivia-Rojas, G.; Aguirre-Mancilla, C.L.; Ramírez-Pimentel, J.G.; Joaquín-Ramos, A.d.J.; Martinez-Montero, M.E.; Villalobos-Olivera, A.; de La Cruz-Torres, E. Innovative Protocols for Blackberry Propagation: In Vitro Cultivation in Temporary Immersion Systems with Ex Vitro Acclimatization. Agriculture 2025, 15, 1505. https://doi.org/10.3390/agriculture15141505
Valdivia-Rojas G, Aguirre-Mancilla CL, Ramírez-Pimentel JG, Joaquín-Ramos AdJ, Martinez-Montero ME, Villalobos-Olivera A, de La Cruz-Torres E. Innovative Protocols for Blackberry Propagation: In Vitro Cultivation in Temporary Immersion Systems with Ex Vitro Acclimatization. Agriculture. 2025; 15(14):1505. https://doi.org/10.3390/agriculture15141505
Chicago/Turabian StyleValdivia-Rojas, Gamaliel, Cesar Leobardo Aguirre-Mancilla, Juan Gabriel Ramírez-Pimentel, Ahuitzolt de Jesús Joaquín-Ramos, Marcos Edel Martinez-Montero, Ariel Villalobos-Olivera, and Eulogio de La Cruz-Torres. 2025. "Innovative Protocols for Blackberry Propagation: In Vitro Cultivation in Temporary Immersion Systems with Ex Vitro Acclimatization" Agriculture 15, no. 14: 1505. https://doi.org/10.3390/agriculture15141505
APA StyleValdivia-Rojas, G., Aguirre-Mancilla, C. L., Ramírez-Pimentel, J. G., Joaquín-Ramos, A. d. J., Martinez-Montero, M. E., Villalobos-Olivera, A., & de La Cruz-Torres, E. (2025). Innovative Protocols for Blackberry Propagation: In Vitro Cultivation in Temporary Immersion Systems with Ex Vitro Acclimatization. Agriculture, 15(14), 1505. https://doi.org/10.3390/agriculture15141505







