Mycorrhizal Fungi Modulate the Development and Composition of Purslane (Portulaca oleracea L.) Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
- (1)
- NM—Control non-mycorrhizal P. oleracea plants grown on soil substrate without added mycorrhizal inoculum;
- (2)
- AM1—P. oleracea plants grown on soil substrate with added mycorrhizal inoculum strain Claroideoglomus claroideum;
- (3)
- AM2—P. oleracea plants grown on soil substrate with mycorrhizal inoculum strain Funneliformis mosseae.
2.2. Quantification of Mycorrhizal Colonization
2.3. Easily Extracted and Total Extracted Glomalin-Related Soil Proteins (EE-GRSP and TE-GRSP)
2.4. Determination of Plant Growth and Physiological Development
2.5. Evaluation of the Antioxidant (AO) Status
2.6. Statistics
3. Results
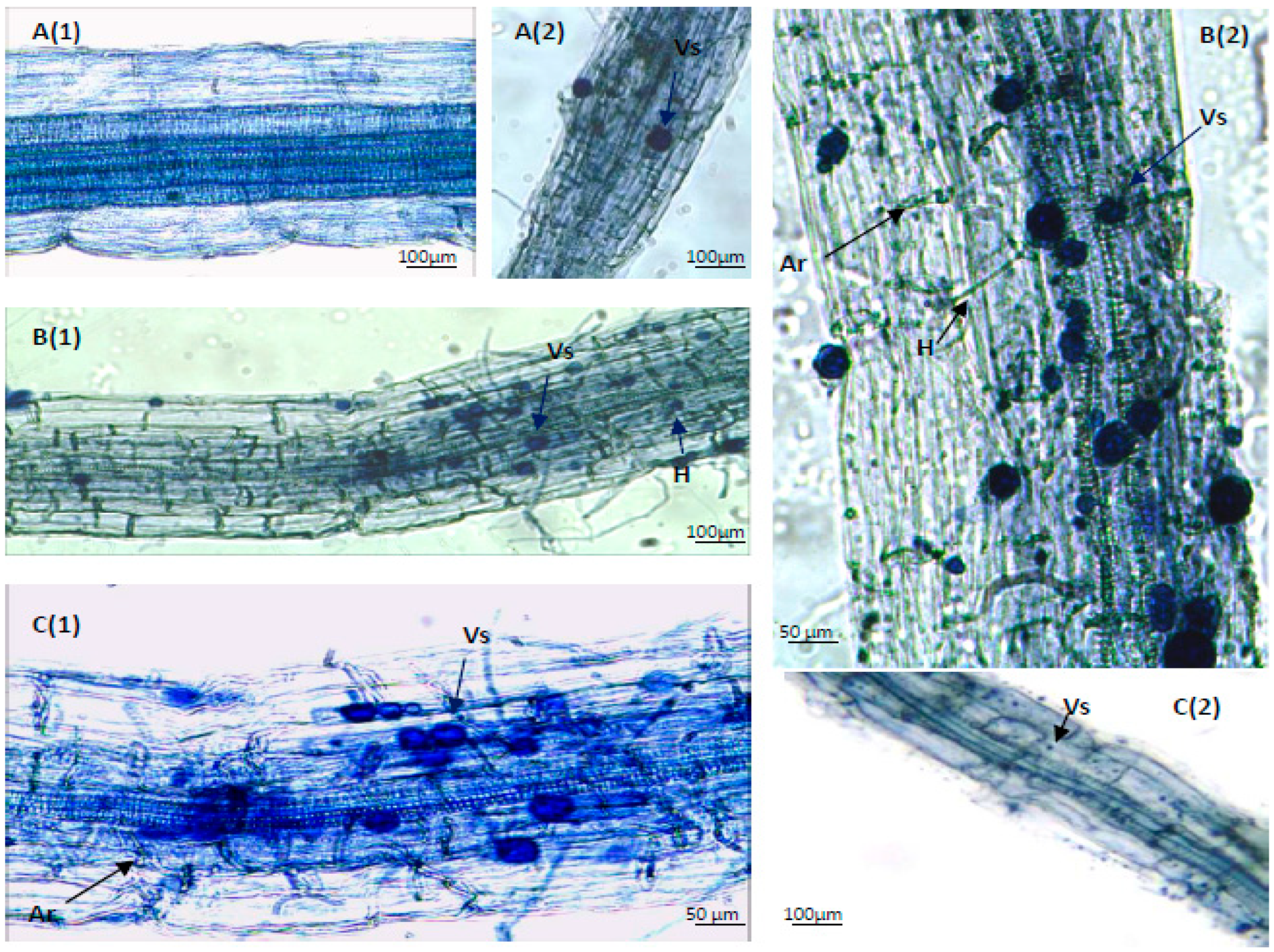
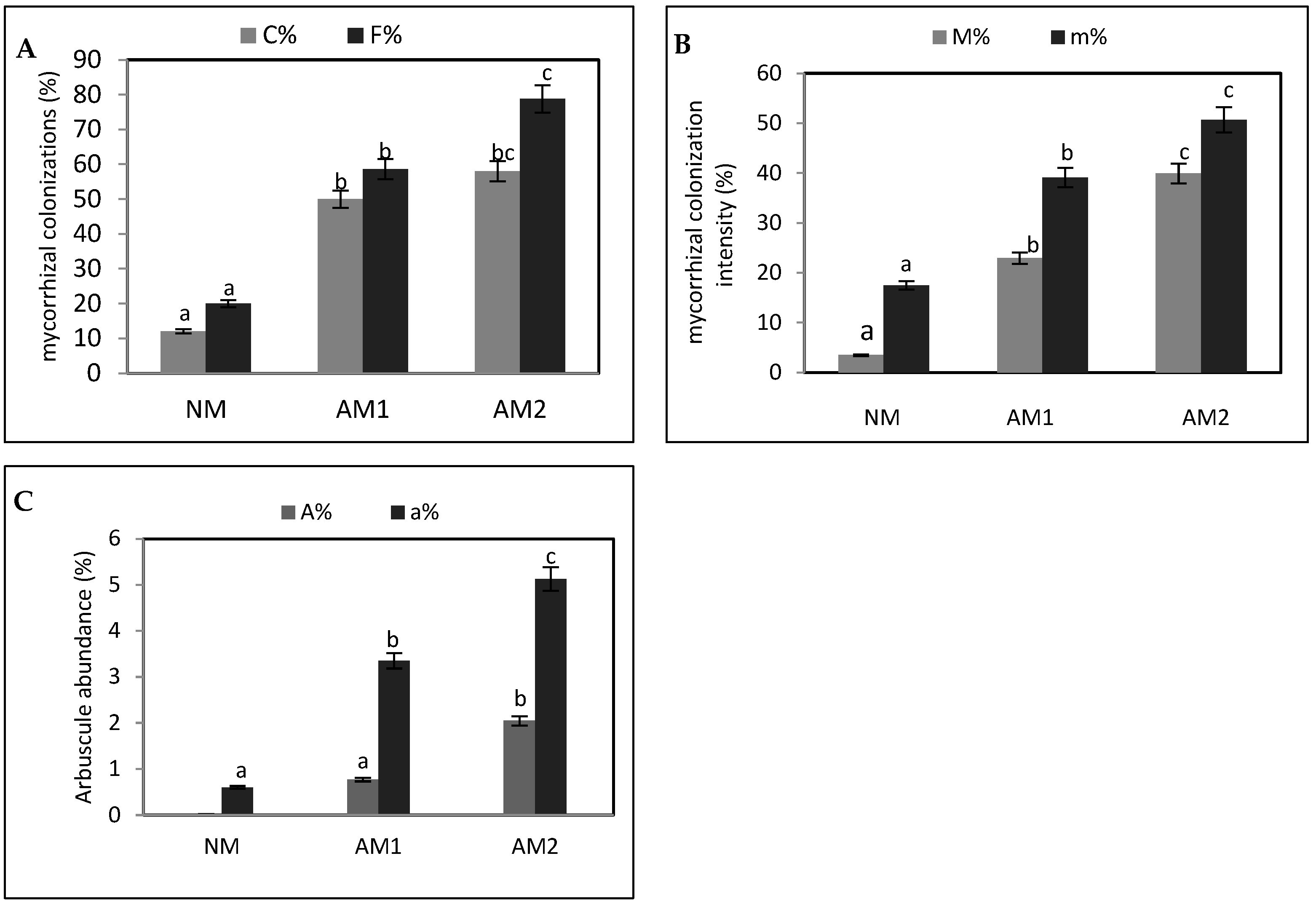
3.1. Comparative Analysis of the Arbuscular Mycorrhizal Association in the Roots of Portulaca oleracea
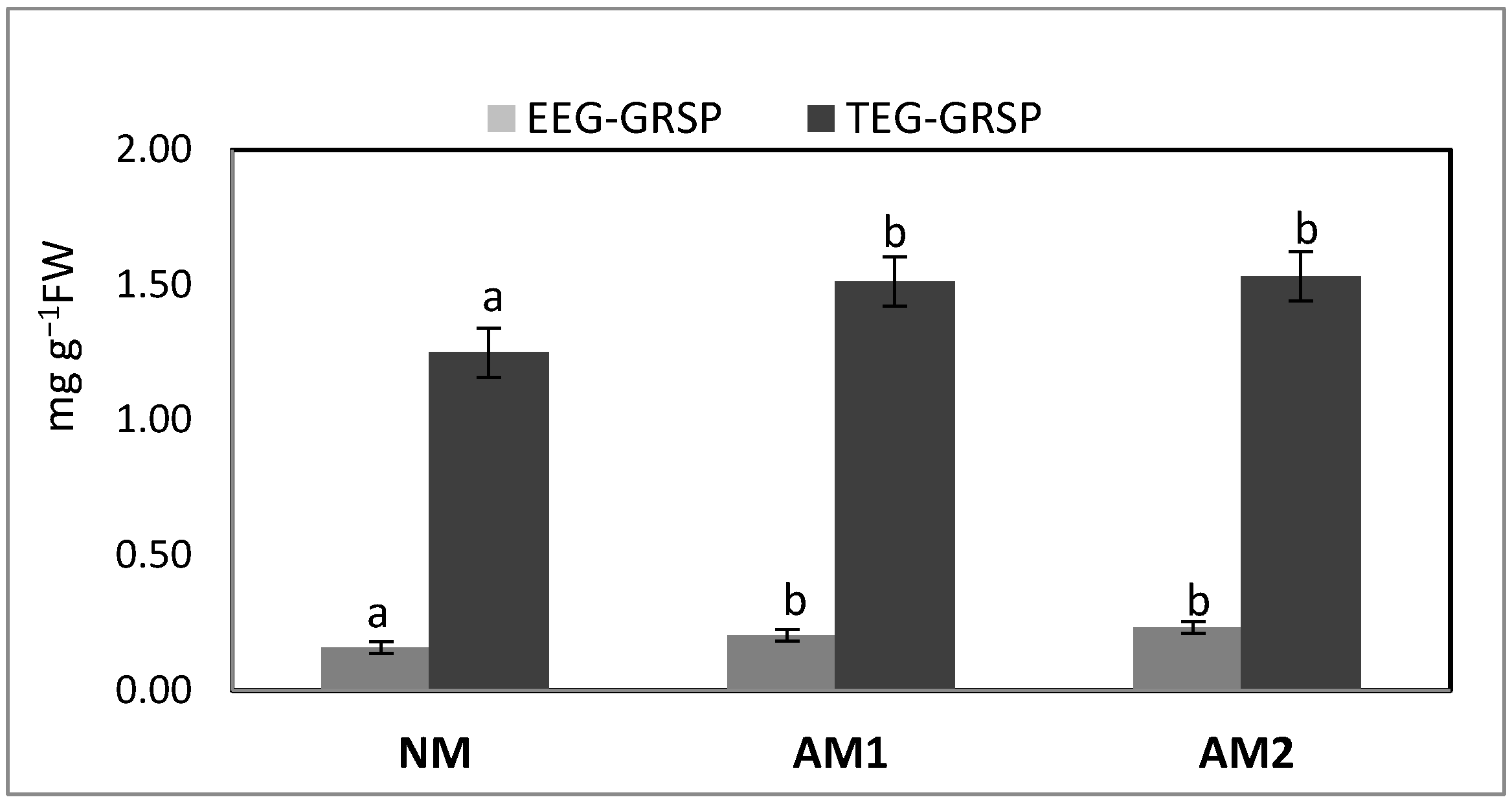
3.2. Amount of the Specific Mycorrhizal Protein Glomalin (EE-GRSP and TE-GRSP) in the Rhizosphere of P. oleracea
3.3. Growth and Physiological Development of P. oleracea Plants Depended on Mycorrhizal Association Efficiency
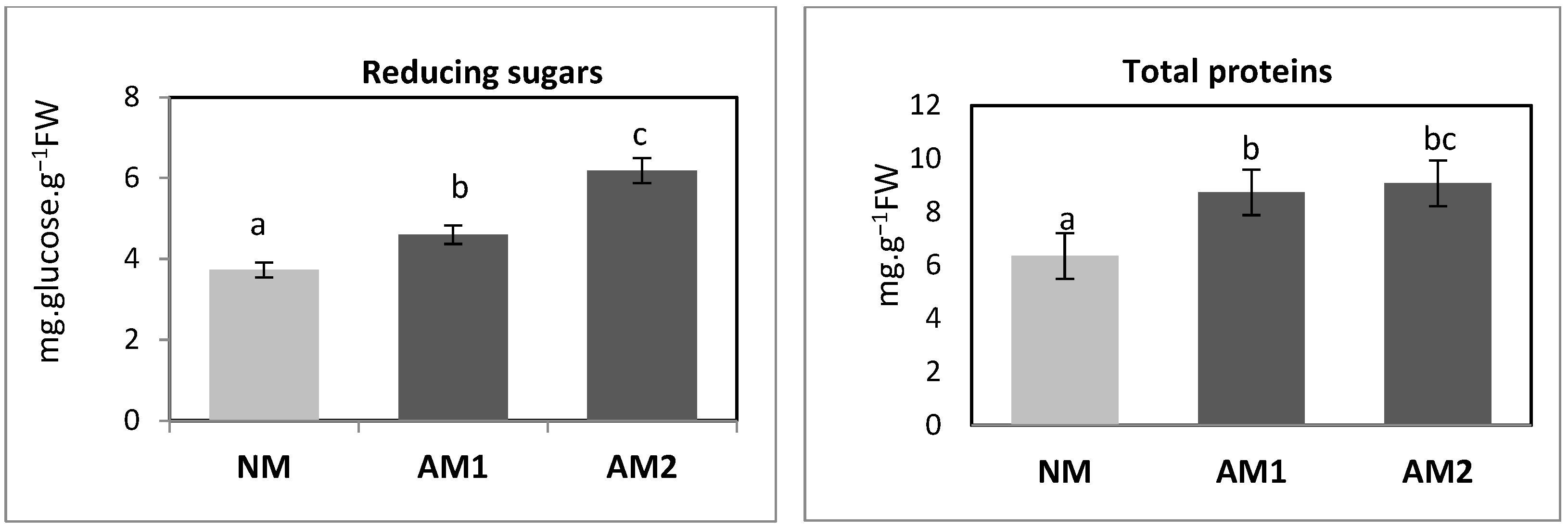
3.4. Determining the Content of Total Proteins and Reducing Sugars in the Above-Ground Parts
3.5. Content of Plastid Pigments: Chlorophylls and Carotenoids (Carotenes and Xanthophylls)
3.6. Content of Anthocyanins and Betalains
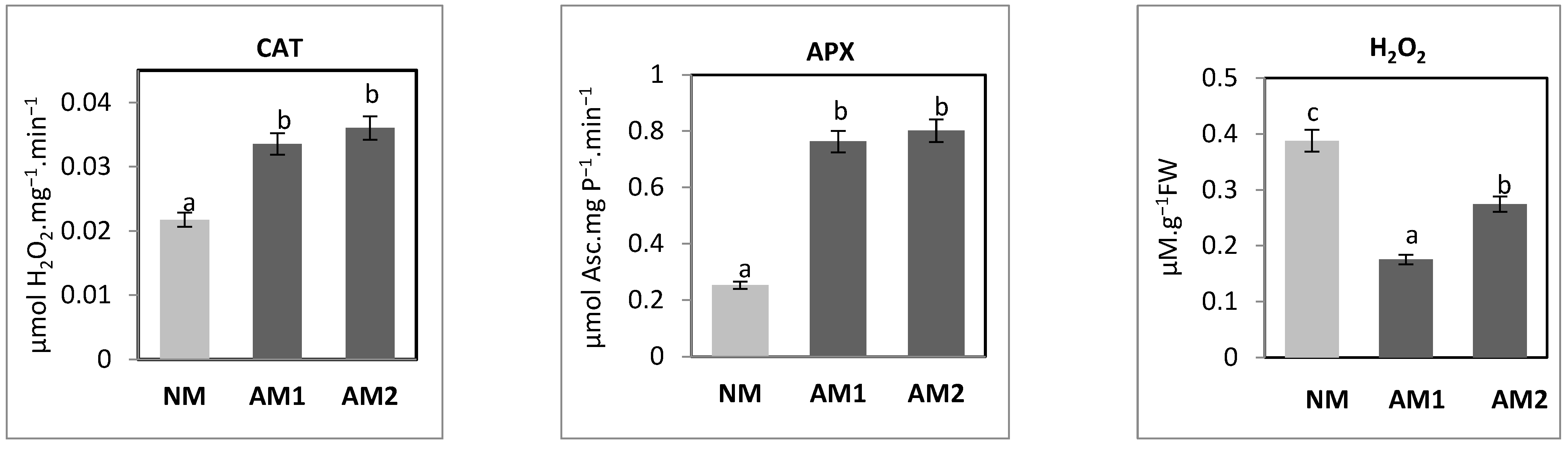
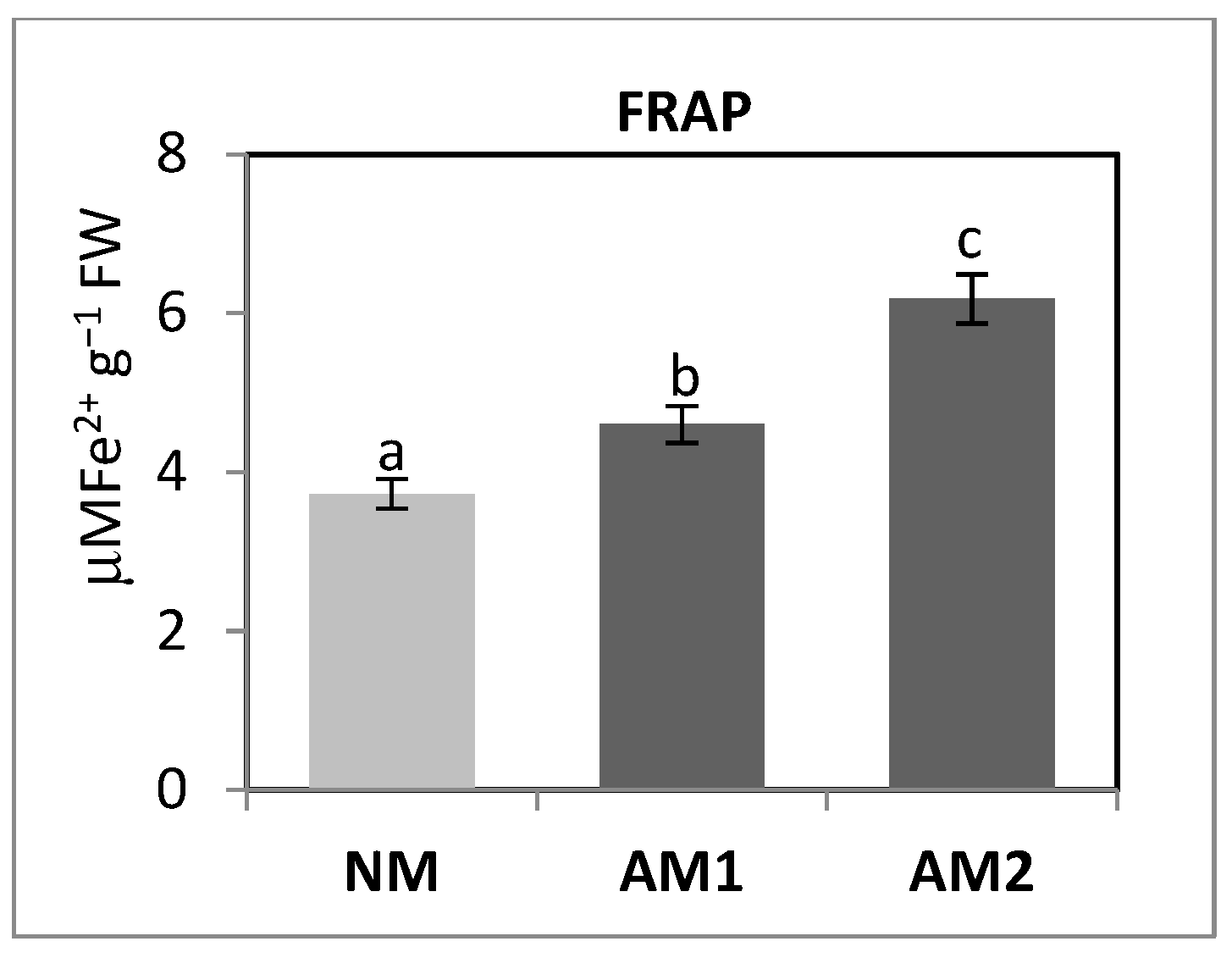
3.7. Evaluation of P. oleracea L. Antioxidant Status Modified by the Mycorrhizal Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| EE-GRSP | Easilyextractedglomalin-related soil protein |
| TE-GRSP | Totalextracted glomalin-related soil proteins |
| MD | Mycorrhizal dependence |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| WS-AO | Water-soluble antioxidantcapacity |
| LS-AO | Lipid-soluble antioxidant capacity |
| FRAP | Ferric-reducing antioxidant power |
References
- Zhang, X.J.; Ji, Y.B.; Qu, Z.Y.; Xia, J.C.; Wang, L. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro. Chin. J. Microbiol. 2002, 14, 277–280. [Google Scholar]
- Karimi, G.; Hosseinzadeh, H.; Ettehad, N. Evaluation of the gastric antiulcerogenic effects of Portulaca oleracea L. extracts in mice. Phytother. Res. 2004, 18, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Islam, M.W.; Kamil, M.; Radhakrishnan, R.; Zakaria, M.N.; Habibullah, M.; Attas, A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp Sativa (Haw.) Celak. J. Ethnopharmacol. 2000, 73, 445–451. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, H.; Zhao, W.; Zhou, W.; Yuan, Q.; Yang, G. Effects of aqueous extract of Portulaca oleracea L. on oxidative stress and liver, spleen leptin, PARα and FAS mRNA expression in high-fat diet induced mice. Mol. Biol. Rep. 2012, 39, 7981–7988. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Tech. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef] [PubMed]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.; Mahyari, S.; Hassani, F.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Ying, X.; Stien, D. New flavonoids from Portulaca oleracea L. and their activities. Fitoterapia 2018, 127, 257–262. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 1, 925631. [Google Scholar]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Moreno-Villena, J.; Zhou, H.; Gilman, S.; Tausta, L.; Cheung, M.; Edwards, J. Spatial resolution of an integrated C4+CAM photosynthetic metabolism. Sci. Adv. 2022, 8, eabn2349. [Google Scholar] [CrossRef] [PubMed]
- Yazici, I.; Türkan, I.; Sekmen, A.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Koch, K.; Kennedy, R. Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiol. 1980, 65, 193–197. [Google Scholar] [CrossRef]
- Dighton, J. Mycorrhizae. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 153–162. [Google Scholar]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.; Horz, D.; Bortolato, L.; Mazzafera, P. Organic plant biostimulants and fruit quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Machiani, A.; Javanmard, A.; Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Kamble, V.; Pal, A. Assessment of three representative species of Portulacaceae with ambiguity about mycorhizal status. IOSR-JESTFT 2016, 10, 2319–2399. [Google Scholar]
- Kanade, A.; Bhosale, R. Effect of VA Mycorrhizae inoculation on vegetative growth in Portulaca oleracea L. (Purslane, Gholu). IJRAR 2019, 6, 710–712. [Google Scholar]
- Sinegani, A.; Yeganeh, M. The occurrence of arbuscular mycorrhizal fungi in soil and root of medicinal plants in Bu-Ali Sinagarden in Hamadan. Iran Biol. J. Microorg. 2017, 5, 43–59. [Google Scholar]
- McGee, P. Variation in propagule numbers of vesicular- arbuscular mycorrhizal fungi in a semi-arid soil. Mycol. Res. 1989, 92, 28–33. [Google Scholar] [CrossRef]
- Schüßler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera URL. Published in December 2010 in Libraries at The Royal Botanic Garden Edinburgh, The Royal Botanic Garden Kew, Botanische Staats Sammlung Munich, and Oregon State University Arthur Schüßler and Christopher Walker, Gloucester. 2010. Available online: http://www.amf-phylogeny.com (accessed on 10 April 2024).
- Jackson, N.; Franklin, R.; Miller, R. Effects of vesicular-arbuscular mycorrhizae on growth and phosphorus content of three agronomic crops. Soil Sci. Soc. Am. Proc. 1972, 36, 64–67. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular- arbuscular mycorrhizal infection in roots. New Phytopatol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Wright, S.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the estimation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Plenchette, C.; Fortm, J.; Furlan, V. Growthresponseofseveralplantspeciestomycorrhizaein a soil ofmoderate Pfertility.I.Mycorrhizal dependency under field conditions. Plant Soil 1983, 70, 199–209. [Google Scholar] [CrossRef]
- Plummer, D. An introduction to practical biochemistry. In Biochemical Education; McGraw Hill: New York, NY, USA, 1988; Volume 16, pp. 98–100. [Google Scholar]
- Asare-Brown, E.; Bullock, C. Simple practical investigation using invertase. Biochem. Educ. 1988, 16, 98–100. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Zin, M.; Márki, E.; Bánvölgyi, S. Conventional extraction of betalain compounds from beetroot peels with aqueous ethanol solvent. Acta Aliment. 2020, 49, 163–169. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.; Mohdaly, A.; Gabr, A.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J. Agric. Food Chem. 2002, 50, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.; Wrolstad, R. Flavonoids determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. JAOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventys, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.; Szeto, Y. Total antioxidant capacity of teas by ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts: Its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; Band, VCH, Verlagsgessellschaft mbH: Weinheim, Germany, 1986; pp. 273–286. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain—Treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulantsinhorticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–9. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture. In ACIAR Monograph Series, Pirie, Canberra; Australian Centre for International Agricultural Research: Canberra, Australia, 1996; Volume 374. [Google Scholar]
- Burrows, R. Glomalin production and infectivity of arbuscular-mycorrhizal fungi in response to grassland plant diversity. Am. J. Plant Sci. 2014, 5, 103–111. [Google Scholar] [CrossRef]
- Hammer, E.; Rillig, M. The influence of different stresses on glomalin levels in an arbuscular mycorrhizal fungus-salinity increases glomalin content. PLoS ONE 2011, 6, e28426. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Ghalavand, A.; Mashhadi-Akbar-Boojar, M.; Modarres-Sanavy, S.; Mokhtassi-Bidgoli, A. Increased medicinal contents of purslane by nitrogen and arbuscular mycorrhiza under drought stress. Commun. Soil Sci. Plant 2020, 51, 118–135. [Google Scholar] [CrossRef]
- Luginbuehl, L.; Menard, G.; Kurup, S.; Erp, H.; Radhakrishnan, G.; Breakspear, A. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Li, D.W.; Wang, D.M.; Yu, Z.Z. Advances on physiological and biochemistry effects of the symbiosis between AM fungi and plants. Acta Bot Boreali-Occident Sin. 2002, 22, 1255–1262. [Google Scholar]
- De Andrade, S.; Domingues, A.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.U.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food. Agric. 2018, 99, 2275–2284. [Google Scholar] [CrossRef]
- Florian, C.S.; Reinhold, C. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar]
- D’Andrea, R.M.; Andreo, C.S.; Lara, M.V.; Andrea, R.M.D.; Andreo, C.S.; Lara, M.V. Deciphering the mechanisms involved in Portulaca oleracea (C4) response to drought: Metabolic changes including crassulacean acid-like metabolism induction and reversal upon re-watering. Physiol. Plant 2014, 152, 414–430. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Sbrana, C.; Avio, L.; Giovanetti, M. Beneficial mycorrhizal symbionts affecting the production of health-promoting phytochemicals. Electrophoresis 2014, 35, 1535–1546. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Blilou, I.; Ocampo, J.A.; Garcia, G.J. Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J. Exp. Bot. 2000, 51, 1969–1977. [Google Scholar] [CrossRef]
- Bonanomi, A.; Oetiker, J.H.; Guggenheim, R.; Boller, T.; Wiemken, A.; Vogeli-Lange, R. Arbuscular mycorrhiza in mini-mycorrhizotrons: First contact of Medicago truncatula roots with Glomus intraradices induces chalcone synthase. New Phytol. 2001, 150, 573–582. [Google Scholar] [CrossRef]
- Strack, D.; Fester, T. Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytol. 2006, 172, 22–34. [Google Scholar] [CrossRef]
- Larose, G.; Chenevert, R.; Moutoglis, P.; Gagne, S.; Piche, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolic profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef]
- Lohse, S.; Schliemann, W.; Ammer, C.; Kopk, J.; Strack, D.; Fester, T. Organisation and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol. 2005, 139, 329–340. [Google Scholar] [CrossRef][Green Version]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 202, 42–51. [Google Scholar] [CrossRef]
- Hristozkova, M.; Gigova, L.; Geneva, M.; Stancheva, I.; Velikova, V.; Marinova, G. Influence of mycorrhizal fungi and microalgae dual inoculation on basil plants performance. Gesunde Pflanz. 2018, 70, 99–107. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Iliev, I.; Azcon-Aguilar, C. Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P.; Alyemeni, M.N.; Alsahli, A.A.; Ahmad, P. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi J. Biol. Sci. 2021, 28, 1465–1476. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Sun, D.; Shang, X.; Cao, H.; Lee, S.-J.; Wang, L.; Gan, Y.; Feng, S. Physio-biochemical mechanisms of arbuscular mycorrhizal fungi enhancing plant resistance to abiotic stress. Agriculture 2024, 14, 2361. [Google Scholar] [CrossRef]
- Guo, X.; Wang, P.; Wang, X.; Li, Y.; Ji, B. Specific plant mycorrhizal responses are linked to mycorrhizal fungal species interactions. Front. Plant Sci. 2022, 13, 930069. [Google Scholar] [CrossRef]
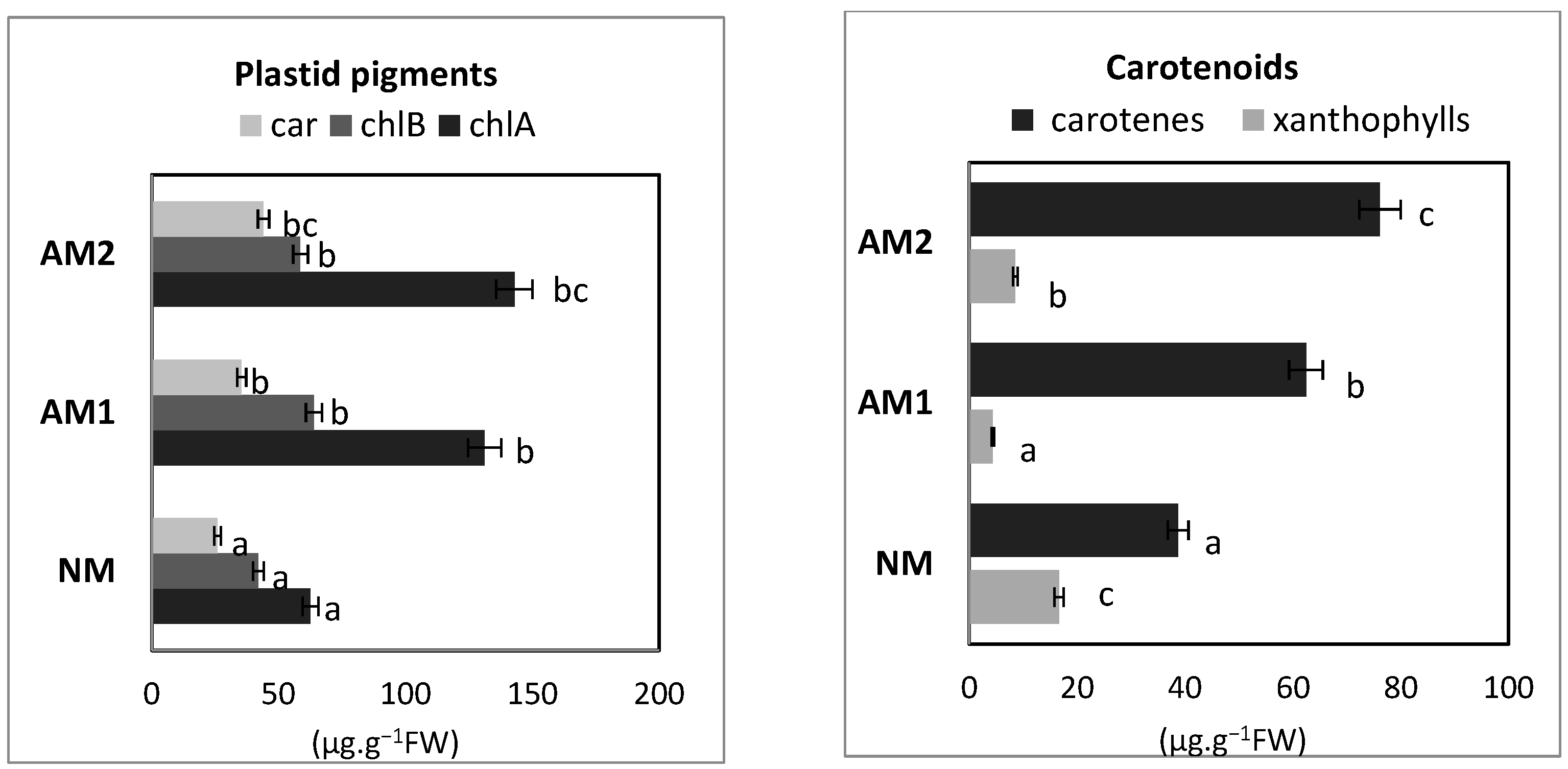
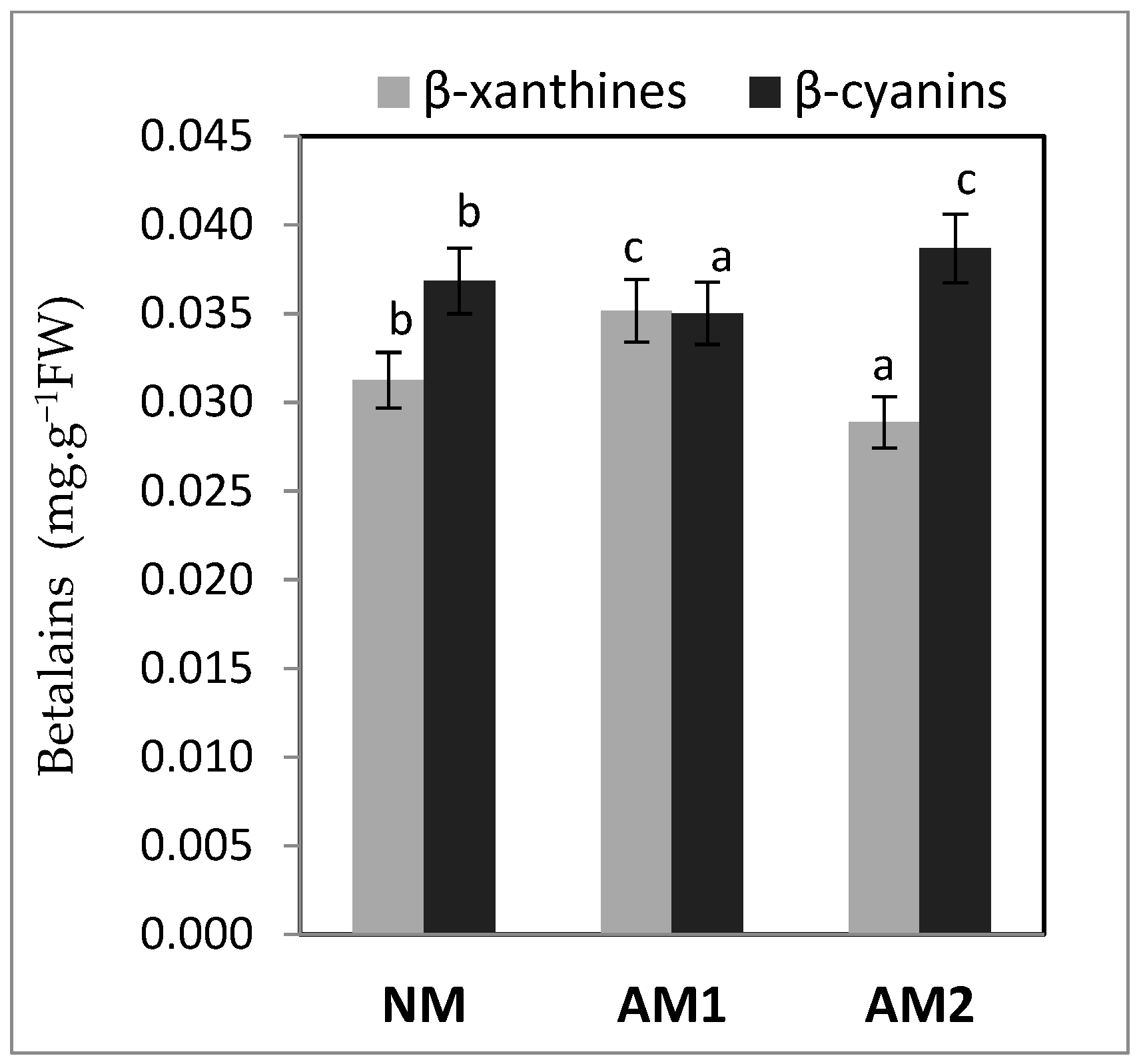
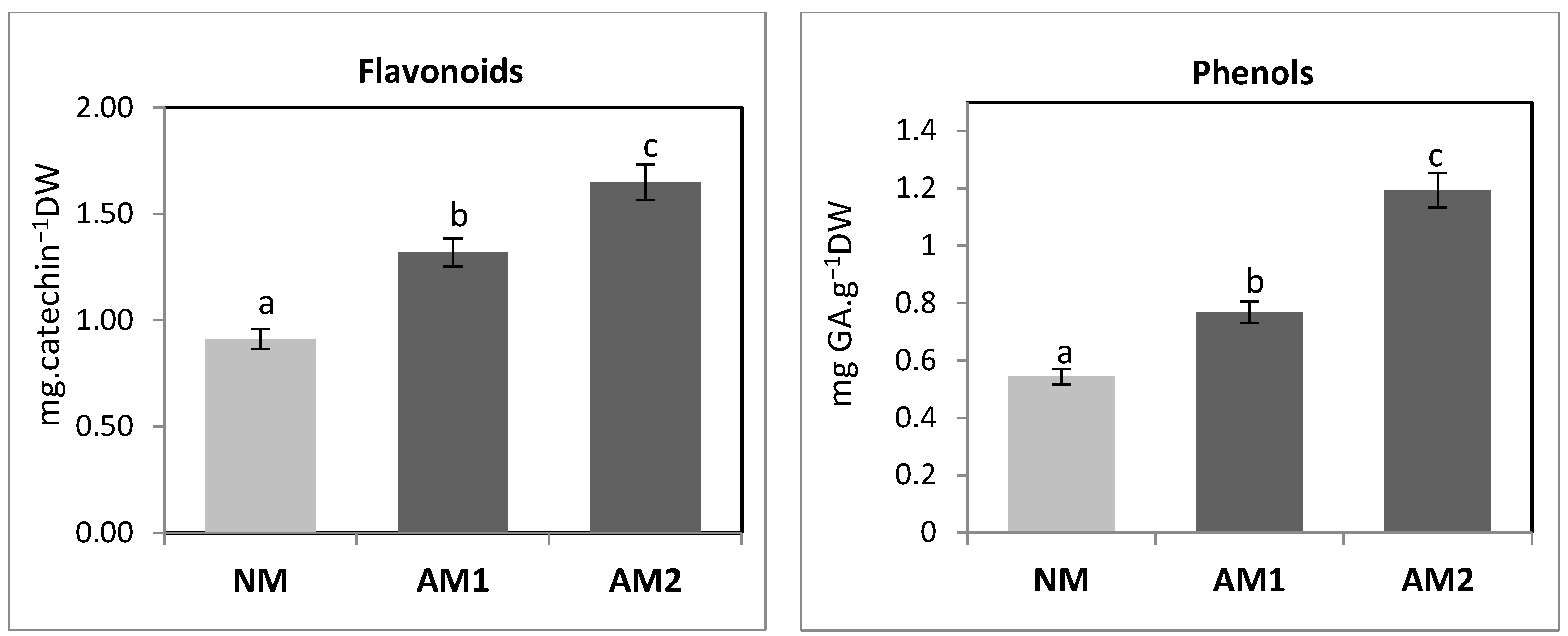
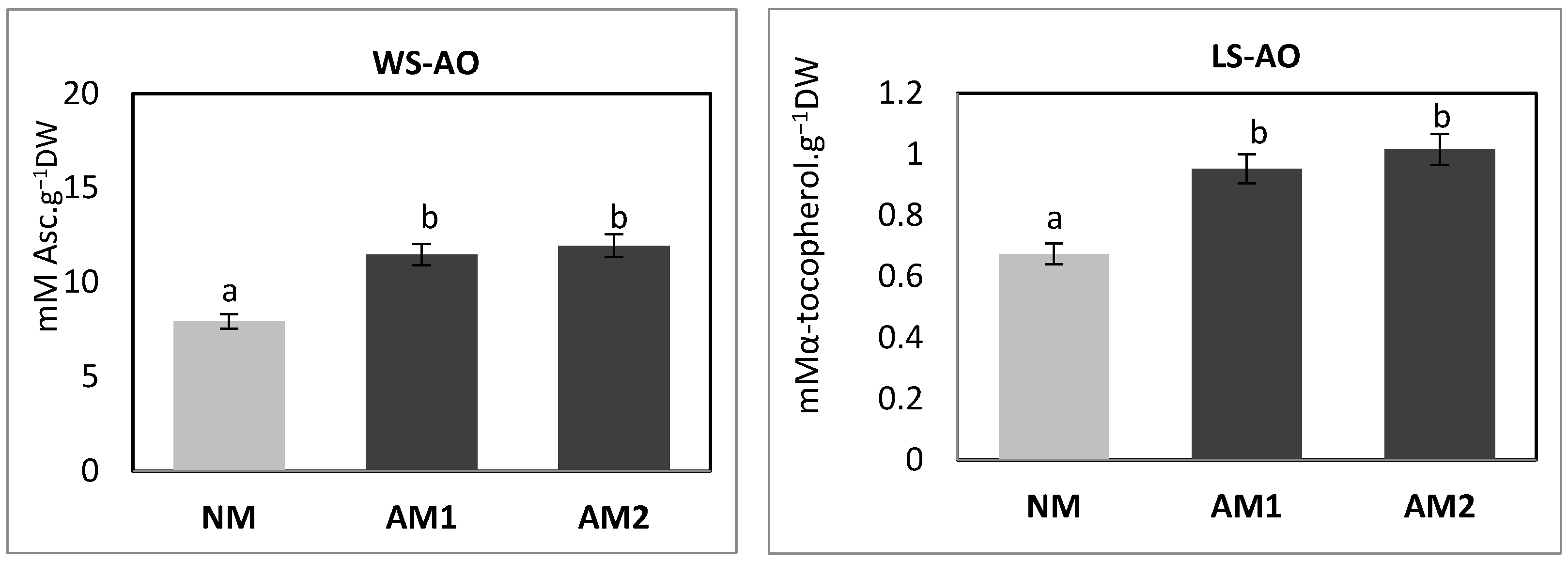
| Variants | Shoot FW (g−1 FW) | Shoot DW (g−1 DW) | Root FW (g−1 FW) | Root DW (g−1 DW) | MD (%) |
|---|---|---|---|---|---|
| NM | 1.001 a* | 0.042 a | 0.050 a | 0.007 a | - |
| AM1 | 1.749 b | 0.074 b | 0.070 b | 0.011 b | 53.593 a |
| AM2 | 1.894 c | 0.081 c | 0.098 c | 0.013 c | 57.381 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hristozkova, M.; Valkova, K.; Geneva, M. Mycorrhizal Fungi Modulate the Development and Composition of Purslane (Portulaca oleracea L.) Bioactive Compounds. Agriculture 2025, 15, 1458. https://doi.org/10.3390/agriculture15131458
Hristozkova M, Valkova K, Geneva M. Mycorrhizal Fungi Modulate the Development and Composition of Purslane (Portulaca oleracea L.) Bioactive Compounds. Agriculture. 2025; 15(13):1458. https://doi.org/10.3390/agriculture15131458
Chicago/Turabian StyleHristozkova, Marieta, Katrin Valkova, and Maria Geneva. 2025. "Mycorrhizal Fungi Modulate the Development and Composition of Purslane (Portulaca oleracea L.) Bioactive Compounds" Agriculture 15, no. 13: 1458. https://doi.org/10.3390/agriculture15131458
APA StyleHristozkova, M., Valkova, K., & Geneva, M. (2025). Mycorrhizal Fungi Modulate the Development and Composition of Purslane (Portulaca oleracea L.) Bioactive Compounds. Agriculture, 15(13), 1458. https://doi.org/10.3390/agriculture15131458






