Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Plant Material
2.2. Extraction by PLE
2.3. Application of Extracts in the Pre- and Post-Germination of Weeds
2.4. Chromatographic Analysis
2.5. Statistical Analysis
3. Results
3.1. Germination
3.2. Shoot Length
3.3. Root Length
3.4. Fresh and Dry Biomass
3.5. Live Plants
3.6. Bioactive Compounds in the Aqueous Extract of Baccharis trimera (Less) DC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leguizamón, E.S. The Unsung Champions of Evolution: Weeds and Their Management in Agricultural Systems. Agriculture 2024, 14, 2368. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy of the invasive species Ludwigia decurrens against rice and rice weeds. Agriculture 2024, 14, 1297. [Google Scholar] [CrossRef]
- Jiao, J.; Hu, L.; Chen, G.; Chen, C.; Zang, Y. Development and Experimentation of Intra-Row Weeding Device for Organic Rice. Agriculture 2024, 14, 146. [Google Scholar] [CrossRef]
- Jiao, J.; Zang, Y.; Chen, C. Key smart weeding technologies for vegetables: A review. Agriculture 2024, 14, 1378. [Google Scholar] [CrossRef]
- Shi, J.; Bai, Y.; Zhou, J.; Zhang, B. Multicrop navigation line extraction based on enhanced YOLO-v8 and Threshold-DBSCAN in complex agricultural environments. Agriculture 2023, 14, 45. [Google Scholar] [CrossRef]
- dos Santos, L.L.C.; de Souza, R.C.; de Sousa Junior, B.S.; da Silva Souza, R.; de Oliveira Farias, A.R.; da Silva Júnior, A.B.; Cunha, J.L.X.L.; Bulhões, L.E.L.; de Araújo Neto, V.F. Efficiency of reduced rates of 2,4-D in mixture with wood vinegar in weed control. Pedagog. Noteb. 2024, 21, e5093. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Mia, M.D.; Murri, G.; Neri, D. Sustainable alternatives to chemicals for weed control in the orchard-a Review. Hortic. Sci. 2020, 47, 1–12. [Google Scholar] [CrossRef]
- Jabran, K. Manipulation of Allelopathic Crops for Weed Control; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Flach, K.A.; Da Rosa, G.M.; Volpi, G.B.; Wastowski, A.D. Allelopathic effect and chemical analysis of hydroalcoholic extracts of Baccharis dracunculifolia, Baccharis trimera and Baccharis gaudichaudiana on Lactuca sativa L. cultivar. Res. Soc. Dev. 2021, 11, e251101119487. [Google Scholar] [CrossRef]
- Schoffel, A.; Koefender, J.; Camera, J.N. Allelopathy effect of Baccharis articulata extract on luffa cylindrica seeds and seedlings. Holos 2021, 6, e10563. [Google Scholar] [CrossRef]
- Pismarović, L.; Šoštarčić, V.; Kljak, K.; Lazarević, B.; Šćepanović, M. Three inhibitory phenolic acids against common ragweed (Ambrosia artemisiifolia L.) had a minimal effect on maize growth in vitro and in vivo. PLoS ONE 2024, 19, e0308825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Z.; Zou, J.; Li, Q. Allelopathic effects of sesame extracts on seed germination of moso bamboo and identification of potential allelochemicals. Sci. Rep. 2022, 12, 6661. [Google Scholar] [CrossRef] [PubMed]
- Semerdjieva, I.; Atanasova, D.; Maneva, V.; Zheljazkov, V.; Radoukova, T.; Astatkie, T.; Dincheva, I. Allelopathic effects of Juniper essential oils on seed germination and seedling growth of some weed seeds. Ind. Crops Prod. 2022, 180, 114768. [Google Scholar] [CrossRef]
- Dias, L.B.; Bittencourt, V.B.; Tormen, L.; da Silva, L.T.B.; Giovanetti, L.K. Chemical characterization and bioactivity of extracts and essential oil from guabirobeira (Campomanesia xanthocarpa) leaves in alfalfa and wheat seedlings. Principia J. 2025, 62, 1–14. [Google Scholar] [CrossRef]
- Tran, N.Y.T.; Le, T.D.; Dao, P.T.; Bach, G.L.; Huynh, P.X.; Tran, Q.N. Evaluation of different extraction methods on the polyphenols yield, flavonoids yield, and antioxidant activity of the pomelo flavedo extract from Da Xanh (Citrus maxima [burm] merr.) variety. Food Sci. Technol. 2022, 42, e97021. [Google Scholar] [CrossRef]
- Anchieta, M.G.; Pigatto, G.; Baisch, J.S.; Dolianitis, B.M.; Coradi, P.C.; Guedes, J.V.C.; Mazutti, M.A.; Tres, M.V.; Zabot, G.L. Pre-and post-emergence control of Hovenia dulcis with extracts obtained from pepper (Capsicum baccatum). Eng. Agric. Mag.-REVENG 2022, 30, 371–382. [Google Scholar] [CrossRef]
- Pereira, G.L. Extraction of compounds of interest in tropical and subtropical plants using supercritical fluid, pressurized liquid and ultrasound: A brief review. Contrib. Las Cienc. Soc. 2023, 6, 3051–3062. [Google Scholar]
- Dolianitis, B.M.; Pfeifenberg, R.; Frescura, V.D.-S.; Tres, M.V.; Zabot, G.L. Phytochemicals from Eucalyptus camaldulensis and Coleus barbatus Control Eragrostis plana in Horticulture. Horticulturae 2025, 11, 291. [Google Scholar] [CrossRef]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Brun, T.; Luft, L.; Ugalde, G.A.; Prá, V.D.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extraction and composition of extracts obtained from Lupinus albescens using supercritical carbon dioxide and compressed liquefied petroleum gas. J. Supercrit. Fluids 2017, 128, 395–403. [Google Scholar] [CrossRef]
- Brazil Ministry of Agriculture, Livestock and Food Supply. Rules for Seed Analysis/Ministry of Agriculture, Livestock and Food Supply; Secretariat of Agricultural Defense: Brasília, Brazil, 2009; 399p. [Google Scholar]
- Zadra, M.; Menezes, B.B.D.; Frescura, L.M.; Essi, L.; Amaro de Carvalho, C.; Barcellos da Rosa, M. Ruellia angustiflora (Nees) Lindau ex Rambo: Extraction and characterization of phenolic compounds and evaluation of antiradical, photoprotective and antimicrobial activities. Nat. Prod. Res. 2024, 38, 2082–2090. [Google Scholar] [CrossRef]
- Soin, K.; Klemencic, M.E.; Koce, J.D. Plant cell responses to allelopathy: From oxidative stress to programmed cell death. Protoplasma 2022, 259, 1111–1124. [Google Scholar]
- Karim, K.A.; Subkhan, M.A.; Leonard, M.E. Effect of Allelopathy from Methanolic Extract of Broadleaf Weeds (Ageratum conyzoides and Borreria alata) on the Viability of Soybean Seeds (Glycine max L.). J. Trop. Crop Sci. 2023, 10, 229–237. [Google Scholar] [CrossRef]
- Gusmão, V.M.D.; Carreira, R.C. Ricinus communis L. (castor bean): Toxic Seeds with Allelopathic Potential. Sci. Gen. 2023, 4, 1–22. [Google Scholar] [CrossRef]
- Shadab, M.O.; Parveen, U.; Ain, Q.; Akhtar, N.; Khan, M.; Siddiqui, M.B. Allelopathic effects of Stem Aqueous Extract of Lepidium didymum L. against Lens culinaris and Melilotus alba. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Konig, F.; Gonçalves, C.E.P.; Aguiar, A.R.; Silva, A.C.F. Bioma Pampa: Interações entre micro-organismos e espécies vegetais nativas. Rev. Ciências Agrárias 2014, 37, 3–9. [Google Scholar]
- Pinheiro, C.G.; Amaral, L.D.P.; Rolim, J.M.; Longhi, S.J.; Machado, S.L.D.O.; Heinzmann, B.M. Essential oil of the Brazilian native species Hesperozygis ringens: A potential alternative to control weeds. J. Essent. Oil Bear. Plants 2017, 20, 701–711. [Google Scholar] [CrossRef]
- Akter, P.; Siddiqua, T.; Begum, R.; Ahmed, A.A. Evaluation of the Allelopathic Activity of Aqueous and Methanol Extracts of Heliotropium indicum Leaves and Roots on Eight Cucurbit Crops. Horticulturae 2024, 10, 135. [Google Scholar] [CrossRef]
- Rashedy, A.A. Impact of some natural extracts on rooting performance of Coratina olive cuttings. Rev. Bras. Frutic. 2022, 44, e-972. [Google Scholar] [CrossRef]
- Martes, I.N.; Amashita, O.M.; De Carvalho, M.A.C.; Dallacort, R.; Da Silva, I.V. Allelopathic effect of Setaria faberi on lettuce seed germination. Nativa 2024, 12, 725–731. [Google Scholar]
- Rodenburg, J.; Tippe, D.E.; Touré, A.; Irakiza, R.; Kayeke, J.; Bastiaans, L. From rice-like plants to plants liking rice: A review of research on weeds and their management in African rice systems. Field Crops Res. 2022, 276, e108397. [Google Scholar] [CrossRef]
- Procópio, S.O.; Barizon, R.R.M.; Pazianotto, R.A.A.; Morandi, M.A.B.; Braz, G.B.P. Impacts of Weed Resistance to Glyphosate on Herbicide Commercialization in Brazil. Agriculture 2024, 14, 2315. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major wheat weeds—A way forward to reduce the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef] [PubMed]
- Kaab, S.B.; Rebey, I.B.; Hanafi, M.; Hammi, K.M.; Smaoui, A.; Fauconnier, M.L.; Ksouri, R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Seifu, A.; Lulekal, E.; Demissew, S.; Woldu, Z. Allelopathic potential of root and leaf aqueous extracts of invasive alien plant species, Cryptostegia grandiflora, on germination and seedling growth of Linum usitatissimum and Guizotia abyssinica. Front. For. Glob. Change 2023, 6, 1131815. [Google Scholar] [CrossRef]
- Tucugh-Pérez, M.A.; Mendo, G.E.I.; Ledezma-Pérez, A.; Iliná, A.; Hernández-Castillo, F.D.; Barrera-Martinez, C.L.; Arredondo-Valdés, R. The Herbicidal Activity of Nano-and MicroEncapsulated Plant Extracts on the Development of the Indicator Plants Sorghum bicolor and Phaseolus vulgaris and Their Potential for Weed Control. Agriculture 2023, 13, 2041. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy of the medicinal plant Dregea volubilis (Lf) Benth. exHook. f. and its phytotoxic substances with allelopathic activity. Agronomy 2022, 12, 303. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.D.; Tennakoon, K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Elizalde-Romero, C.A.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Plants Fungal Diseases and Phenolics Response. In Plant Phenolics in Biotic Stress Management; Springer Nature: Singapore, 2024; pp. 325–337. [Google Scholar]
- de Almeida, N.S.; Ferraz, A.D.B.F.; Pedron, C.; Correa, D.S.; Vieira, L.B.; Antunes, F.T.T.; de Souza, A.H. Baccharis trimera aqueous extract modulates inflammation and nociception in mice. Clin. Phytosci. 2021, 7, 82. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; Araújo, G.R.D.; Lúcio, K.D.P.; Araújo, C.M.; Miranda, P.H.D.A.; Silva, B.D.M.; Carneiro, A.C.A.; Ribeiro, É.M.D.C.; Lima, W.G.D.; Souza, G.H.D.; et al. Aqueous extract of Baccharis trimera. improves redox status and decreases the severity of alcoholic hepatotoxicity. Rev. Bras. Farmacogn. 2017, 27, 729–738. [Google Scholar] [CrossRef]
- da Silva, A.R.H.; Lopes, L.Q.S.; Cassanego, G.B.; de Jesus, P.R.; Figueredo, K.C.; Santos, R.C.V.; Lopes, G.H.H.; de Freitas Bauermann, L. Acute toxicity and antimicrobial activity of leaf tincture Baccharis trimera. (Less). Biomed. J. 2018, 41, 194–201. [Google Scholar] [CrossRef]
- Barik, R.; Sugunan, S.; Shafri, M.A.B.M. Pressurized Liquid Extraction for the Isolation of Bioactive Compounds. In Bioactive Extraction and Application in Food and Nutraceutical Industries; Springer: New York, NY, USA, 2024; pp. 275–298. [Google Scholar]

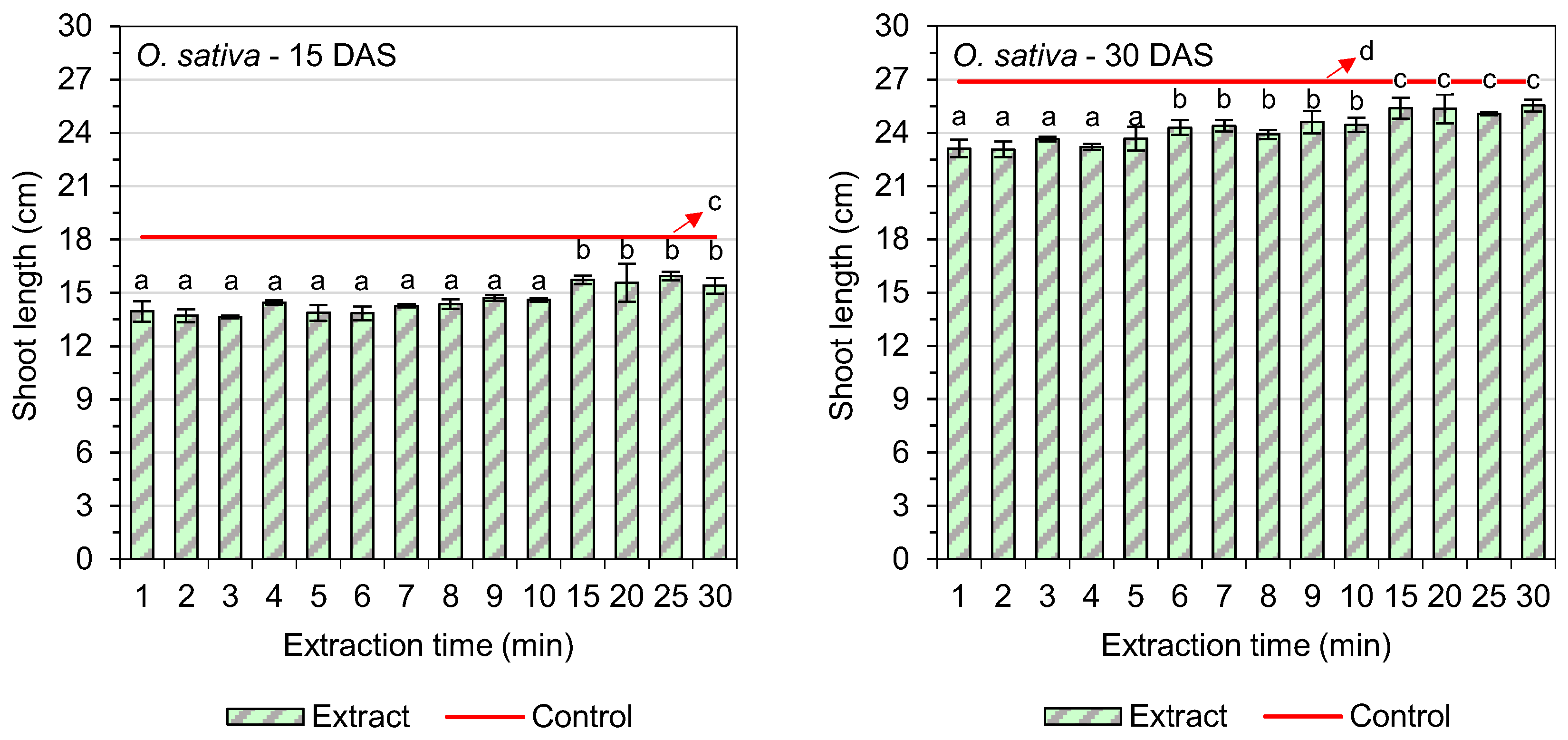
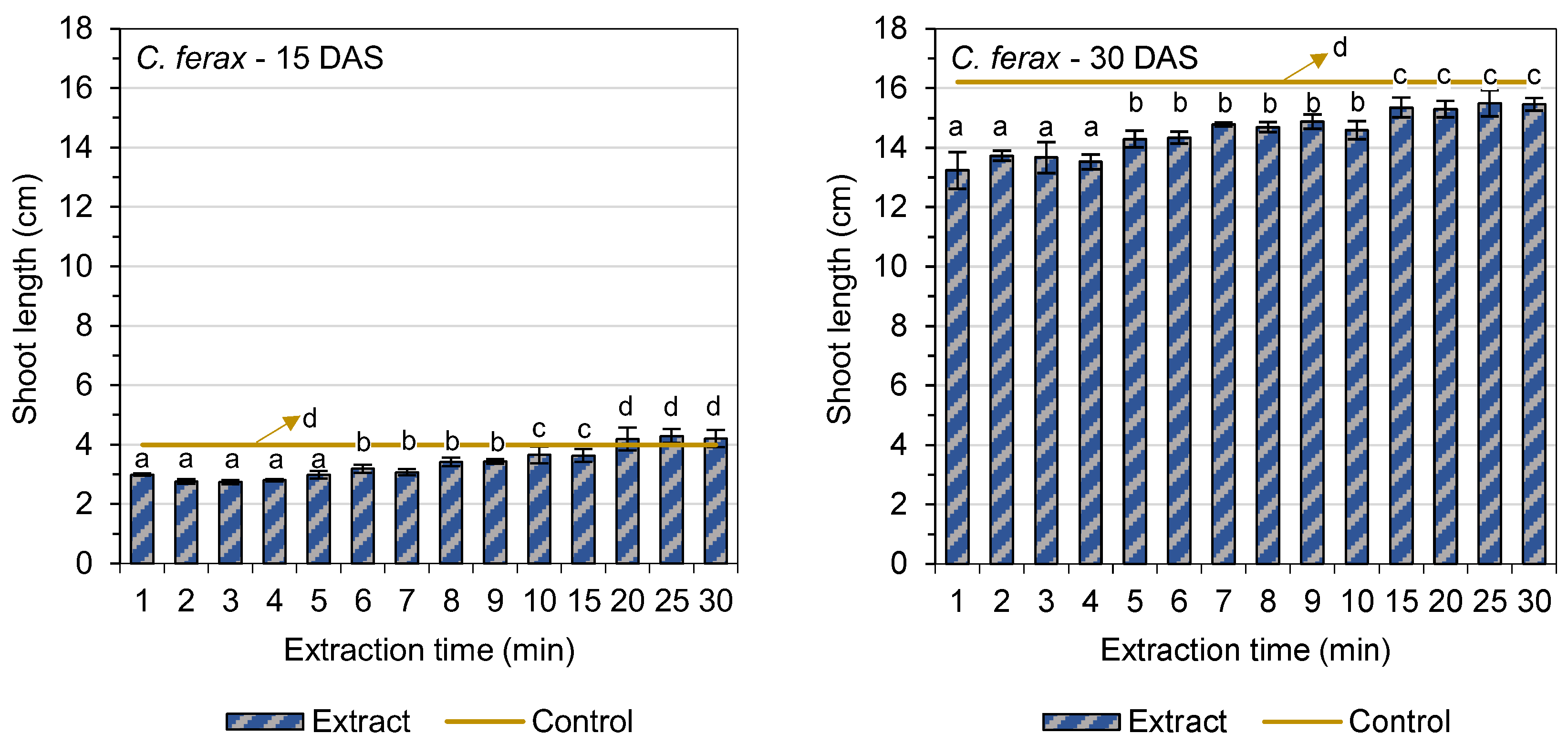
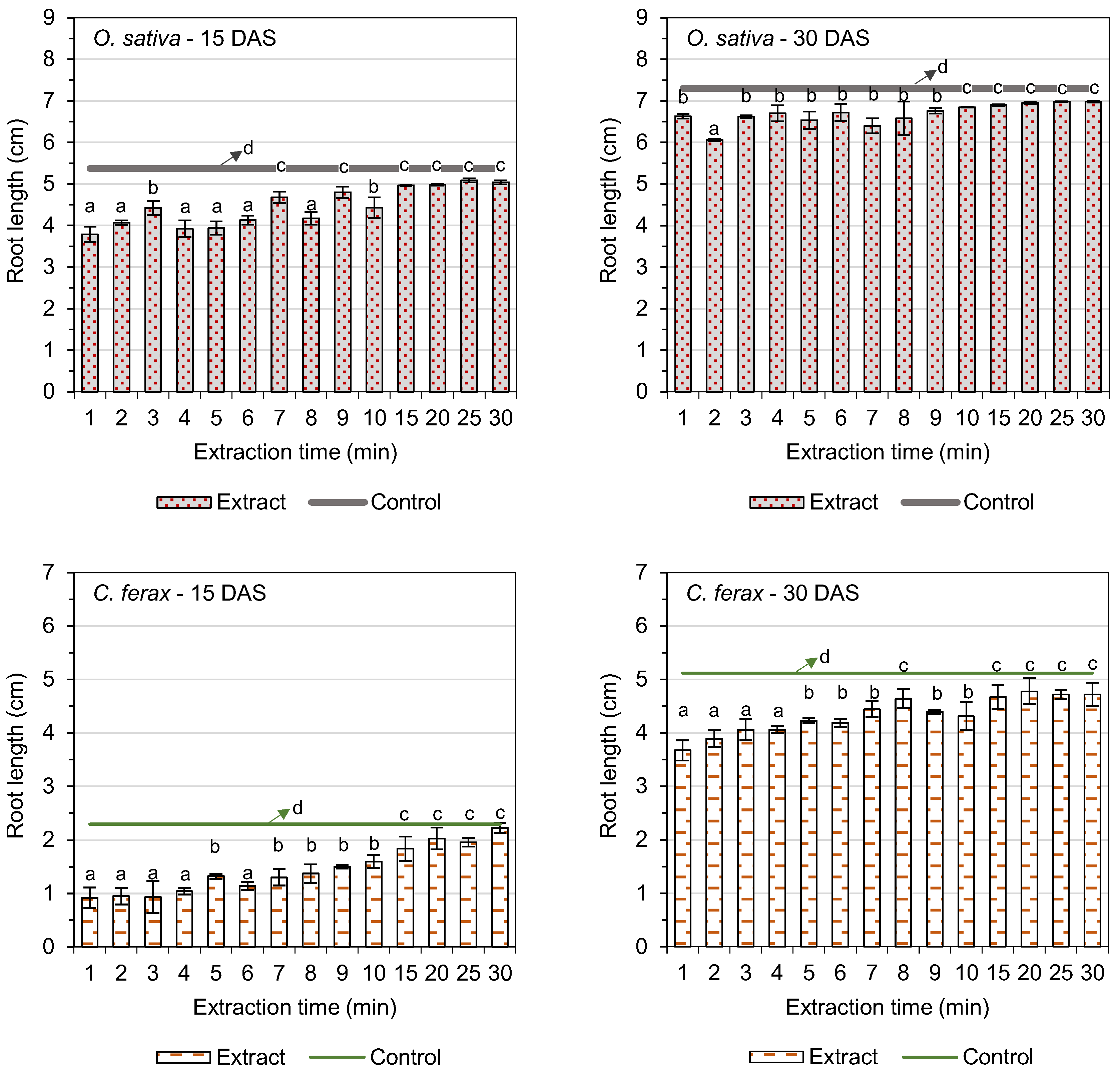
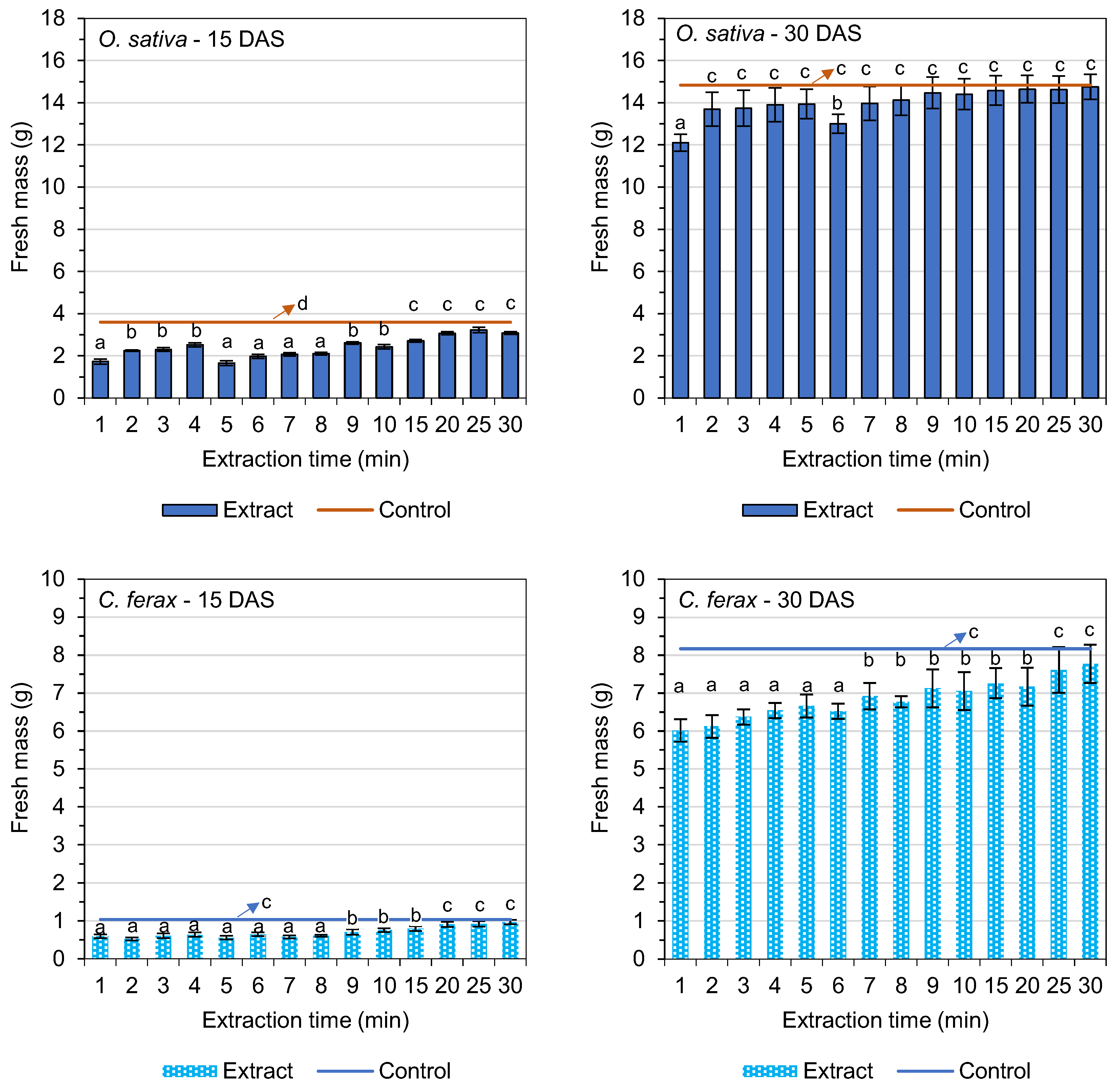
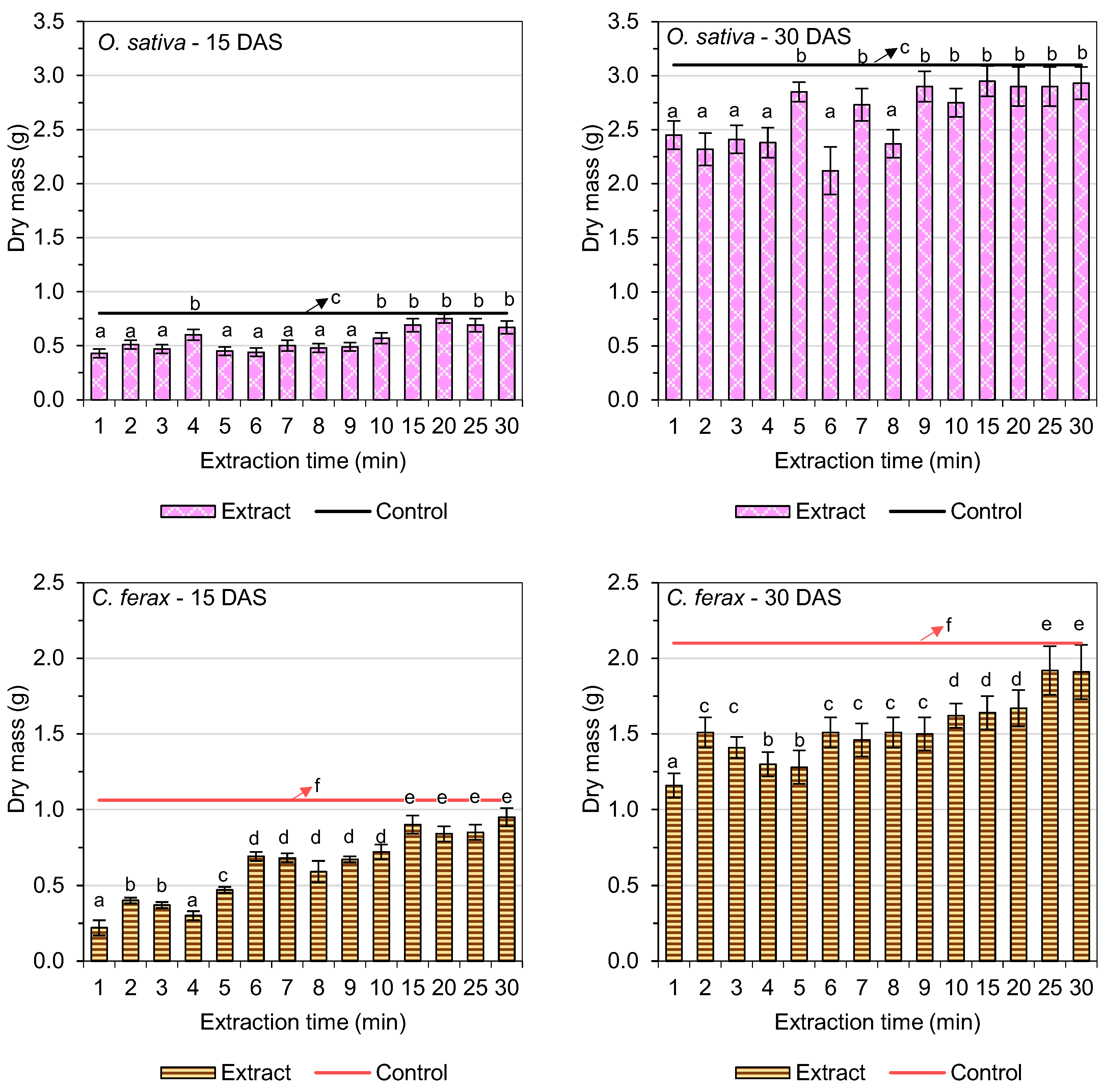
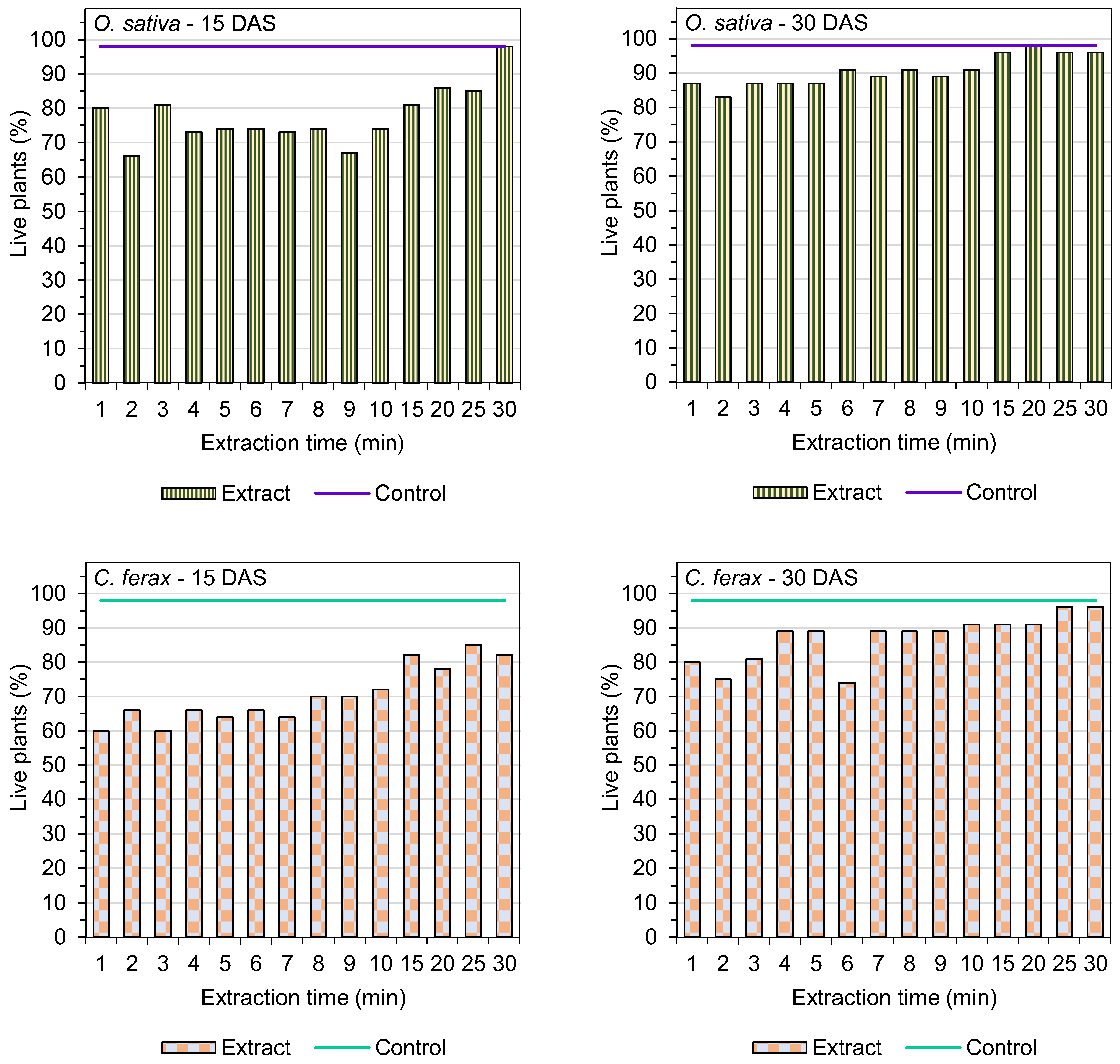
| Extraction Time (min) | Germination of O. sativa L. (%) | GSI (O. sativa L.) | Germination of C. ferax Rich. (%) | GSI (C. ferax Rich.) |
|---|---|---|---|---|
| 1 | 24.67 ± 1.88 a | 1.20 a | 19.33 ± 1.70 a | 1.00 a |
| 2 | 24.33 ± 0.94 a | 1.33 a | 14.66 ± 1.89 a | 0.86 a |
| 3 | 21.33 ± 1.24 a | 1.14 a | 17.67 ± 1.89 a | 0.94 a |
| 4 | 26.34 ± 0.94 a | 1.42 a | 20.66 ± 3.30 a | 1.02 a |
| 5 | 27.00 ± 2.82 a | 1.36 a | 19.00 ± 1.41 a | 1.03 a |
| 6 | 23.00 ± 1.41 a | 1.34 a | 19.00 ± 2.83 a | 1.02 a |
| 7 | 26.33 ± 2.49 a | 1.36 a | 21.34 ± 3.09 a | 1.04 a |
| 8 | 27.00 ± 1.41 a | 1.51 a | 20.67 ± 0.47 a | 1.17 a |
| 9 | 27.00 ± 2.16 a | 1.39 a | 23.00 ± 1.41 b | 1.25 a |
| 10 | 27.67 ± 3.39 a | 1.47 a | 24.00 ± 2.94 b | 1.30 a |
| 15 | 35.33 ± 4.64 b | 1.87 b | 30.00 ± 4.55 c | 1.54 a |
| 20 | 38.33 ± 7.31 b | 1.97 b | 34.66 ± 4.99 d | 1.80 b |
| 25 | 40.00 ± 5.65 b | 2.09 b | 39.00 ± 4.55 d | 2.06 b |
| 30 | 41.66 ± 6.18 b | 2.19 b | 42.33 ± 4.11 d | 2.19 b |
| Control | 58.67 ± 0.02 c | 3.15 c | 57.00 ± 0.17 e | 3.30 c |
| Coefficient of variation (%) | 13.84 | 26.15 | 13.67 | 28.47 |
| Compound | Composition in the Extract (%) | ||
|---|---|---|---|
| Extract from 1 min | Extract from 15 min | Extract from 30 min | |
| Ferulic acid | 8.69 | 7.99 | 1.27 |
| Rutin | 9.58 | 8.78 | 1.66 |
| Quercitrin | 8.51 | 8.16 | 1.76 |
| Quercitin | 2.75 | 9.10 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.M.; Ribeiro, L.K.; Cogo, M.R.d.M.; Frescura, L.M.; da Rosa, M.B.; Schulz, A.; Abaide, E.R.; Tres, M.V.; Zabot, G.L. Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax. Agriculture 2025, 15, 1431. https://doi.org/10.3390/agriculture15131431
Lopes AM, Ribeiro LK, Cogo MRdM, Frescura LM, da Rosa MB, Schulz A, Abaide ER, Tres MV, Zabot GL. Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax. Agriculture. 2025; 15(13):1431. https://doi.org/10.3390/agriculture15131431
Chicago/Turabian StyleLopes, Aline Mazoy, Lucas Kila Ribeiro, Maurício Ricardo de Melo Cogo, Lucas Mironuk Frescura, Marcelo Barcellos da Rosa, Alex Schulz, Ederson Rossi Abaide, Marcus Vinícius Tres, and Giovani Leone Zabot. 2025. "Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax" Agriculture 15, no. 13: 1431. https://doi.org/10.3390/agriculture15131431
APA StyleLopes, A. M., Ribeiro, L. K., Cogo, M. R. d. M., Frescura, L. M., da Rosa, M. B., Schulz, A., Abaide, E. R., Tres, M. V., & Zabot, G. L. (2025). Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax. Agriculture, 15(13), 1431. https://doi.org/10.3390/agriculture15131431







