Dual Role of Iron Oxides in Stabilizing Particulate and Mineral-Associated Organic Carbon Under Field Management in Paddies
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Soil Analyses
2.4. Data Analyses
3. Results
3.1. Contents of SOC in Bulk Soil, POM, and MAOM Fractions
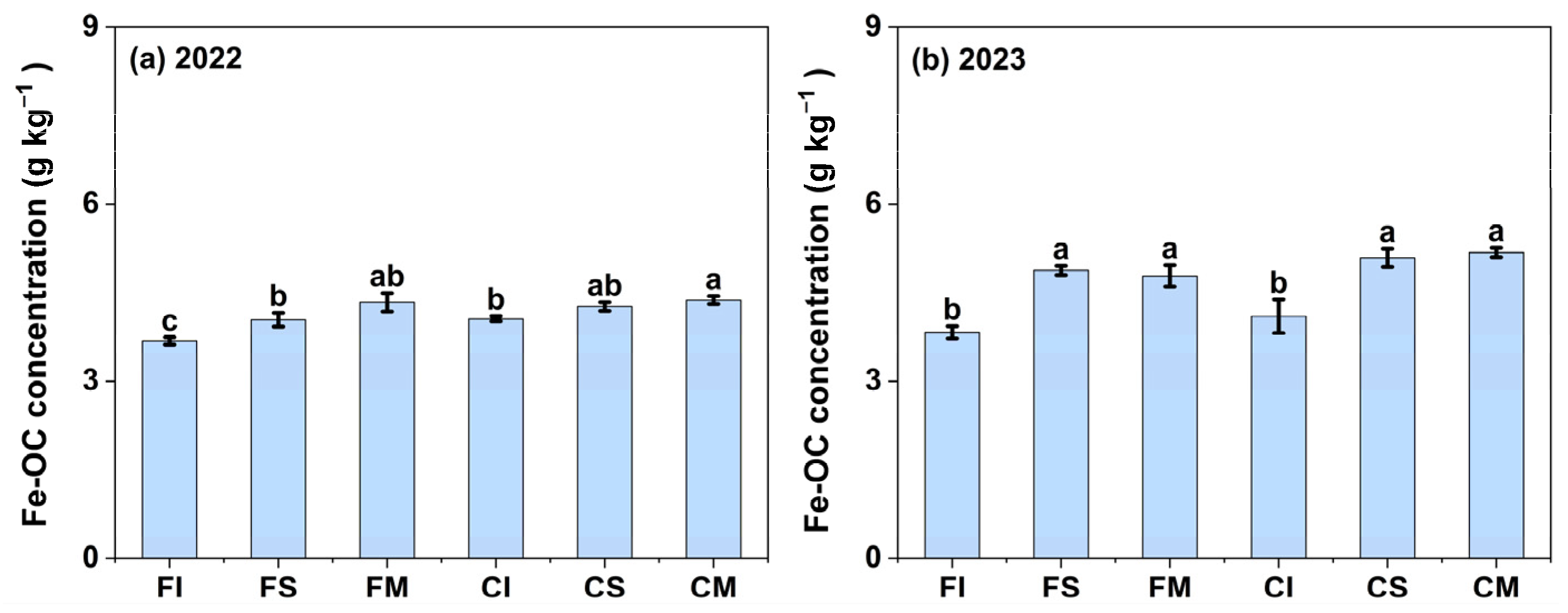
3.2. Contents of Fe-OC in MAOM
3.3. Characteristics of Iron Oxide Distribution in MAOM Under Different Management
3.3.1. Contents of Iron Oxides
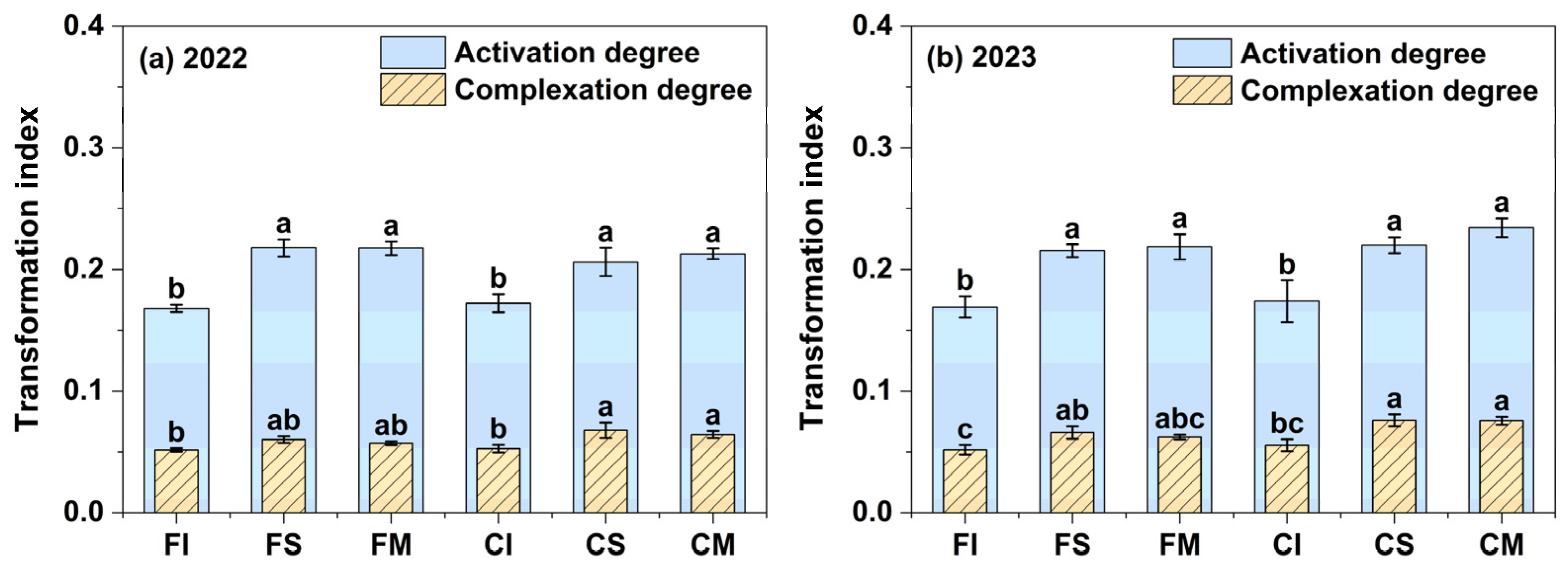
3.3.2. Transformation Indices of Iron Oxides
3.3.3. C:Fe Molar Ratio
3.4. Relationship Between Soil Iron Oxides and Soil Organic Carbon
4. Discussion
4.1. Effects of Irrigation and Organic Amendment Management on Soil Organic Carbon
4.2. Effects of Irrigation and Organic Amendment Management on Iron Oxides
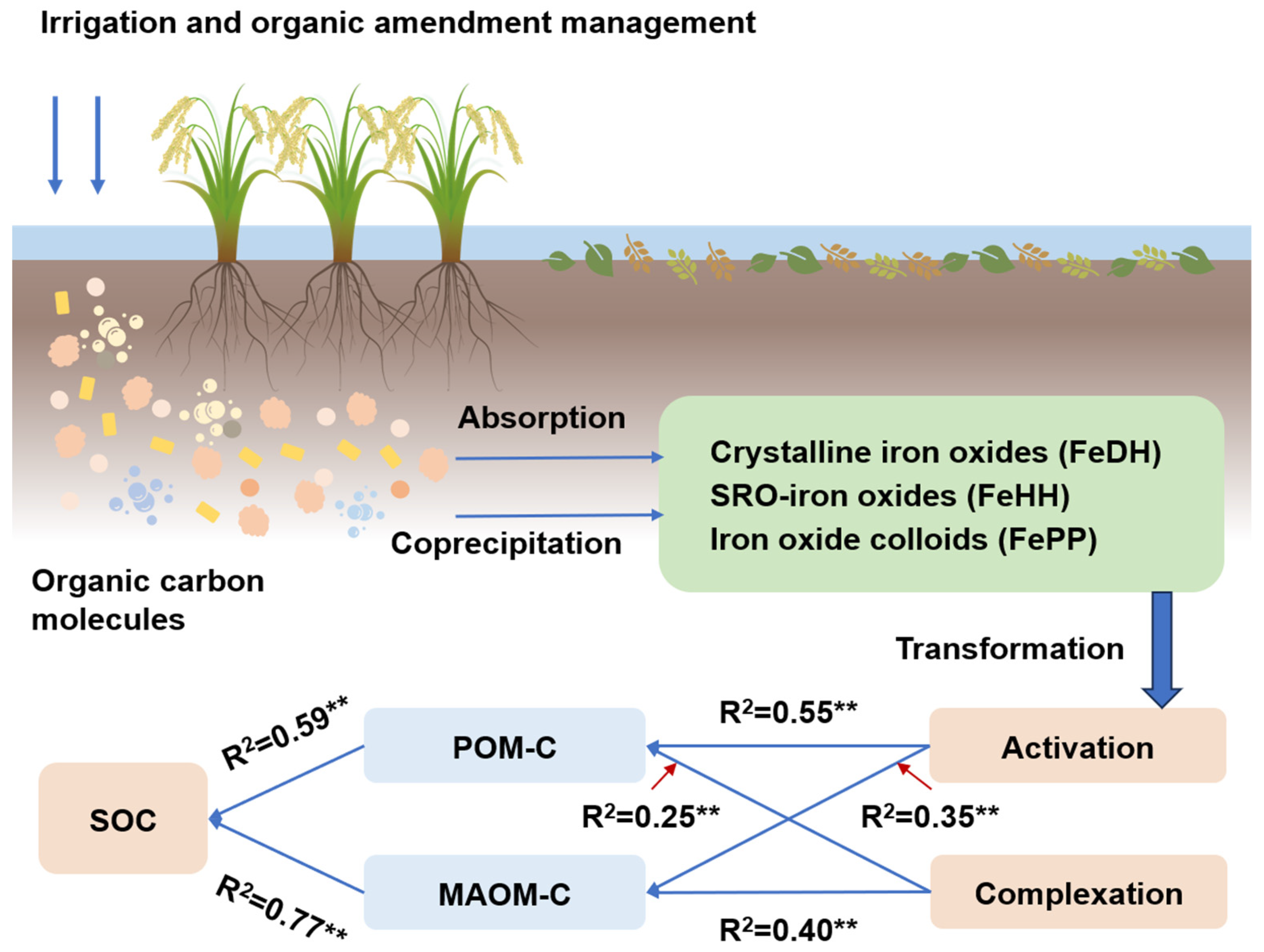
4.3. Relationship Between Iron Oxides and Soil Organic Carbon Accumulation
4.4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, G.; Li, L.; Wu, L.; Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob. Change Biol. 2004, 10, 79–92. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon Storage and Fluxes within Freshwater Wetlands: A Critical Review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Niu, C.Y.; Weng, L.P.; Lian, W.L.; Zhang, R.; Ma, J.; Chen, Y.L. Carbon sequestration in paddy soils: Contribution and mechanisms of mineral-associated SOC formation. Chemosphere 2023, 333, 138927. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Hemamali Peduruhewa, J.; Van Zwieten, L.; Gong, L.; Tan, B.; Zhang, G. The coupling between iron and carbon and iron reducing bacteria control carbon sequestration in paddy soils. CATENA 2023, 223, 106937. [Google Scholar] [CrossRef]
- Alam, M.K.; Bell, R.W.; Biswas, W.K. Increases in soil sequestered carbon under conservation agriculture cropping decrease the estimated greenhouse gas emissions of wetland rice using life cycle assessment. J. Clean. Prod. 2019, 224, 72–87. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.H.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Wissing, L.; Kölbl, A.; Häusler, W.; Schad, P.; Cao, Z.H.; Kögel-Knabner, I. Management-induced organic carbon accumulation in paddy soils: The role of organo-mineral associations. Soil Tillage Res. 2013, 126, 60–71. [Google Scholar] [CrossRef]
- Huang, X.L.; Tang, H.Y.; Kang, W.J.; Yu, G.H.; Ran, W.; Hong, J.P.; Shen, Q.R. Redox interface-associated organo-mineral interactions: A mechanism for C sequestration under a rice-wheat cropping system. Soil Biol. Biochem. 2018, 120, 12–23. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Osenberg, C.W.; Hungate, B.A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 2011, 475, 214–216. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K.; Fiedler, S.; Kölbl, A.; Amelung, W.; Bräuer, T.; Cao, Z.H.; Don, A.; Grootes, P.; Jahn, R.; et al. The carbon count of 2000years of rice cultivation. Glob. Change Biol. 2013, 19, 1107–1113. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ge, T.D.; Zhu, Z.K.; Liu, S.L.; Luo, Y.; Li, Y.; Wang, P.; Gavrichkova, O.; Xu, X.L.; Xu, J.K.; et al. Carbon input and allocation by rice into paddy soils: A review. Soil Biol. Biochem. 2019, 133, 97–107. [Google Scholar] [CrossRef]
- Huang, W.J.; Hall, S.J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter. Nat. Commun. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Kaiser, K.; Mikutta, R.; Guggenberger, G. Increased stability of organic matter sorbed to ferrihydrite and goethite on aging. Soil Sci. Soc. Am. J. 2007, 71, 711–719. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Chen, C.M.; Hall, S.J.; Coward, E.; Thompson, A. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 2020, 11, 2255. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Ding, L.J.; Su, J.Q.; Xu, H.J.; Jia, Z.J.; Zhu, Y.G. Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. Isme J. 2015, 9, 721–734. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Li, X.M.; Sun, G.X.; Cui, L.; Ding, L.J.; He, C.; Li, L.G.; Shi, Q.; Smets, B.F.; Zhu, Y.G. Fate of Labile Organic Carbon in Paddy Soil Is Regulated by Microbial Ferric Iron Reduction. Environ. Sci. Technol. 2019, 53, 8533–8542. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.A.; Possinger, A.R.; Badgley, B.D.; Bowman, M.M.; Gallo, A.C.; Hatten, J.A.; Nave, L.E.; SanClements, M.D.; Swanston, C.W.; Weiglein, T.L.; et al. Moisture-driven divergence in mineral-associated soil carbon persistence. Proc. Natl. Acad. Sci. USA 2023, 120, e2210044120. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.T.; Arias, M.; Guerif, J. Effects of iron and organic matter on the porosity and structural stability of soil aggregates. Soil Tillage Res. 1998, 46, 261–272. [Google Scholar] [CrossRef]
- Goldberg, S.; Glaubig, R.A. Effect of Saturating Cation, pH, and Aluminum and Iron Oxide on the Flocculation of Kaolinite and Montmorillonite. Clays Clay Miner. 1987, 35, 220–227. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Gunina, A.; Tao, L.; Zhu, Z.K.; Kuzyakov, Y.; Van Zwieten, L.; Guggenberger, G.; Shen, C.C.; Yu, G.H.; Singh, B.P.; et al. Rusty sink of rhizodeposits and associated keystone microbiomes. Soil Biol. Biochem. 2020, 147, 107840. [Google Scholar] [CrossRef]
- Wen, Y.L.; Xiao, J.; Liu, F.F.; Goodman, B.A.; Li, W.; Jia, Z.J.; Ran, W.; Zhang, R.F.; Shen, Q.R.; Yu, G.H. Contrasting effects of inorganic and organic fertilisation regimes on shifts in Fe redox bacterial communities in red soils. Soil Biol. Biochem. 2018, 117, 56–67. [Google Scholar] [CrossRef]
- Chen, X.B.; Hu, Y.J.; Xia, Y.H.; Zheng, S.M.; Ma, C.; Rui, Y.C.; He, H.B.; Huang, D.Y.; Zhang, Z.H.; Ge, T.D.; et al. Contrasting pathways of carbon sequestration in paddy and upland soils. Glob. Change Biol. 2021, 27, 2478–2490. [Google Scholar] [CrossRef]
- Wei, L.; Ge, T.D.; Zhu, Z.K.; Luo, Y.; Yang, Y.H.; Xiao, M.L.; Yan, Z.F.; Li, Y.H.; Wu, J.S.; Kuzyakov, Y. Comparing carbon and nitrogen stocks in paddy and upland soils: Accumulation, stabilization mechanisms, and environmental drivers. Geoderma 2021, 398, 115121. [Google Scholar] [CrossRef]
- Longman, J.; Faust, J.C.; Bryce, C.; Homoky, W.B.; Maerz, C. Organic Carbon Burial With Reactive Iron Across Global Environments. Glob. Biogeochem. Cycles 2022, 36, e2022GB007447. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, H.; Zhu, Q.H.; Wang, X.F.; Zhang, Y.Z.; Yu, X.C.; Peng, X. Carbon sequestration efficiency in paddy soil and upland soil under long-term fertilization in southern China. Soil Tillage Res. 2013, 130, 42–51. [Google Scholar] [CrossRef]
- Yu, G.H.; Chen, C.M.; He, X.H.; Zhang, X.Z.; Li, L.N. Unexpected bulk density and microstructures response to long-term pig manure application in a Ferralic Cambisol Soil: Implications for rebuilding a healthy soil. Soil Tillage Res. 2020, 203, 104668. [Google Scholar] [CrossRef]
- Xu, J.Z.; Peng, S.Z.; Yang, S.H.; Wang, W.G. Ammonia volatilization losses from a rice paddy with different irrigation and nitrogen managements. Agric. Water Manag. 2012, 104, 184–192. [Google Scholar] [CrossRef]
- Wang, K.C.; Xu, J.Z.; Guo, H.; Min, Z.H.; Wei, Q.; Chen, P.; Sleutel, S. Reuse of straw in the form of hydrochar: Balancing the carbon budget and rice production under different irrigation management. Waste Manag. 2024, 189, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.W.; Kramer, M.G.; Chadwick, O.A.; Thompson, A. Mobilization of colloidal carbon during iron reduction in basaltic soils. Geoderma 2014, 221, 139–145. [Google Scholar] [CrossRef]
- Ginn, B.; Meile, C.; Wilmoth, J.; Tang, Y.Z.; Thompson, A. Rapid Iron Reduction Rates Are Stimulated by High-Amplitude Redox Fluctuations in a Tropical Forest Soil. Environ. Sci. Technol. 2017, 51, 3250–3259. [Google Scholar] [CrossRef]
- Possinger, A.R.; Bailey, S.W.; Inagaki, T.M.; Kögel-Knabner, I.; Dynes, J.J.; Arthur, Z.A.; Lehmann, J. Organo-mineral interactions and soil carbon mineralizability with variable saturation cycle frequency. Geoderma 2020, 375, 114483. [Google Scholar] [CrossRef]
- Kramer, M.G.; Sanderman, J.; Chadwick, O.A.; Chorover, J.; Vitousek, P.M. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Change Biol. 2012, 18, 2594–2605. [Google Scholar] [CrossRef]
- Giannetta, B.; de Souza, D.O.; Aquilanti, G.; Celi, L.; Said-Pullicino, D. Redox-driven changes in organic C stabilization and Fe mineral transformations in temperate hydromorphic soils. Geoderma 2022, 406, 115532. [Google Scholar] [CrossRef]
- Meijide, A.; Gruening, C.; Goded, I.; Seufert, G.; Cescatti, A. Water management reduces greenhouse gas emissions in a Mediterranean rice paddy field. Agric. Ecosyst. Environ. 2017, 238, 168–178. [Google Scholar] [CrossRef]
- Chen, P.; Xu, J.Z.; Wang, K.C.; Zhang, Z.X.; Zhou, Z.Q.; Li, Y.W.; Li, T.C.; Nie, T.Z.; Wei, Q.; Liao, L.X. Straw return combined with water-saving irrigation increases microbial necromass accumulation by accelerating microbial growth-turnover in Mollisols of paddy fields. Geoderma 2025, 454, 117211. [Google Scholar] [CrossRef]
- Han, Y.; Qi, Z.J.; Chen, P.; Zhang, Z.X.; Zhou, X.; Li, T.C.; Du, S.C.; Xue, L. Water-saving irrigation mitigates methane emissions from paddy fields: The role of iron. Agric. Water Manag. 2024, 298, 108839. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Campbell, A.N.; Tfaily, M.M.; Lin, Y.; Kukkadapu, R.K.; Silver, W.L.; Nico, P.S.; Pett-Ridge, J. Redox Fluctuations Control the Coupled Cycling of Iron and Carbon in Tropical Forest Soils. Environ. Sci. Technol. 2018, 52, 14129–14139. [Google Scholar] [CrossRef]
- Wen, Y.L.; Guo, X.Y.; Cheng, L.; Yu, G.H.; Xiao, J.; He, X.H.; Goodman, B.A. Organic amendments stimulate co-precipitation of ferrihydrite and dissolved organic matter in soils. Geoderma 2021, 402, 115352. [Google Scholar] [CrossRef]
- Yu, G.H.; Sun, F.S.; Yang, L.; He, X.H.; Polizzotto, M.L. Influence of biodiversity aroxide: Implications for soil carbon stabilization and storage. Land Degrad. Dev. 2020, 31, 463–472. [Google Scholar] [CrossRef]
- Ye, C.L.; Huang, W.J.; Hall, S.J.; Hu, S.J. Association of Organic Carbon With Reactive Iron Oxides Driven by Soil pH at the Global Scale. Glob. Biogeochem. Cycles 2022, 36, e2021GB007128. [Google Scholar] [CrossRef]
- Haddix, M.L.; Gregorich, E.G.; Helgason, B.L.; Janzen, H.; Ellert, B.H.; Cotrufo, M.F. Climate, carbon content, and soil texture control the independent formation and persistence of particulate and mineral-associated organic matter in soil. Geoderma 2020, 363, 114160. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Vischetti, C.; Cotrufo, M.F.; Zaccone, C. Distribution and thermal stability of physically and chemically protected organic matter fractions in soils across different ecosystems. Biol. Fertil. Soils 2018, 54, 671–681. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Jilling, A.; Kane, D.; Williams, A.; Yannarell, A.C.; Davis, A.; Jordan, N.R.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; et al. Rapid and distinct responses of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma 2020, 359, 114001. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Coward, E.K.; Thompson, A.T.; Plante, A.F. Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 2017, 306, 206–216. [Google Scholar] [CrossRef]
- Meng, X.T.; Zhang, X.C.; Li, Y.N.; Jiao, Y.P.; Fan, L.C.; Jiang, Y.J.; Qu, C.Y.; Filimonenko, E.; Jiang, Y.H.; Tian, X.H.; et al. Nitrogen fertilizer builds soil organic carbon under straw return mainly via microbial necromass formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, J.C.; Maniruzzaman, M.; Hossain, M.B.; Islam, M.R. Water management and soil amendment for reducing emission factor and global warming potential but improving rice yield. Paddy Water Environ. 2021, 19, 515–527. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z.X.; Li, T.C.; Chen, P.; Nie, T.Z.; Zhang, Z.H.; Du, S.C. Straw return alleviates the greenhouse effect of paddy fields by increasing soil organic carbon sequestration under water-saving irrigation. Agric. Water Manag. 2023, 287, 108434. [Google Scholar] [CrossRef]
- Hao, X.H.; Liu, S.L.; Wu, J.S.; Hu, R.G.; Tong, C.L.; Su, Y.Y. Effect of long-term application of inorganic fertilizer and organic amendments on soil organic matter and microbial biomass in three subtropical paddy soils. Nutr. Cycl. Agroecosyst. 2008, 81, 17–24. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light-Fraction Organic Matter in Soils from Long-Term Crop Rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef]
- Yan, D.Z.; Wang, D.J.; Yang, L.Z. Long-term effect of chemical fertilizer, straw, and manure on labile organic matter fractions in a paddy soil. Biol. Fertil. Soils 2007, 44, 93–101. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, L.Z.; Zhu, O.Y. Organic carbon and its fractions in paddy soil as affected by different nutrient and water regimes. Geoderma 2005, 124, 133–142. [Google Scholar] [CrossRef]
- Tanji, K.K.; Gao, S.; Scardaci, S.C.; Chow, A.T. Characterizing redox status of paddy soils with incorporated rice straw. Geoderma 2003, 114, 333–353. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, H.; Li, S.; He, P.; Wu, X. The need to update and refine concepts relating to mineral-associated organic matter saturation in soil. Soil Biol. Biochem. 2025, 202, 109672. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Organic matter accumulation in submerged soils. Adv. Agron. 2004, 81, 169–201. [Google Scholar]
- Tian, J.; Pausch, J.; Fan, M.S.; Li, X.L.; Tang, Q.Y.; Kuzyakov, Y. Allocation and dynamics of assimilated carbon in rice-soil system depending on water management. Plant Soil 2013, 363, 273–285. [Google Scholar] [CrossRef]
- Yao, H.Y.; Thornton, B.; Paterson, E. Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol. Biochem. 2012, 53, 72–77. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, L.; Mei, Y.R.; Jia, Z.X.; Li, T.L.; Yu, G.H.; Ran, W. Towards a mechanistic understanding of microbial and nonmicrobial mediated topsoil organic carbon sequestration efficiency in a rice-wheat cropping system. Appl. Soil Ecol. 2022, 170, 104259. [Google Scholar] [CrossRef]
- Higashi, T. Characterization of Al/Fe—Humus complexes in dystrandepts through comparison with synthetic forms. Geoderma 1983, 31, 277–288. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M.; Kitayama, K.; Shirato, Y. Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis. Biogeochemistry 2013, 112, 95–109. [Google Scholar] [CrossRef]
- Ren, S.Y.; Wang, C.K.; Zhou, Z.H. Global Distributions of Reactive Iron and Aluminum Influence the Spatial Variation of Soil Organic Carbon. Glob. Change Biol. 2024, 30, e17576. [Google Scholar] [CrossRef]
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J.; Macdonald, L.M. Effects of clay mineralogy and hydrous iron oxides on labile organic carbon stabilisation. Geoderma 2012, 173, 104–110. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Huang, X.L.; Jiang, H.; Li, Y.; Ma, Y.C.; Tang, H.Y.; Ran, W.; Shen, Q.R. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice-wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Henneberry, Y.K.; Kraus, T.E.C.; Nico, P.S.; Horwath, W.R. Structural stability of coprecipitated natural organic matter and ferric iron under reducing conditions. Org. Geochem. 2012, 48, 81–89. [Google Scholar] [CrossRef]
- Porras, R.C.; Pries, C.E.H.; Torn, M.S.; Nico, P.S. Synthetic iron (hydr) oxide-glucose associations in subsurface soil: Effects on decomposability of mineral associated carbon. Sci. Total Environ. 2018, 613, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.D.; Wang, R.D.; Yi, L.K. Drivers of Organic Carbon Preservation Within Soil Aggregates from An Ultisol Treated with Twenty-Year Fertilizations. J. Soil Sci. Plant Nutr. 2024, 24, 5602–5612. [Google Scholar] [CrossRef]
- von Lützow, M.; Kögel-Knabner, I.; Ekschmittb, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods:: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Angers, D.A.; Recous, S.; Aita, C. Fate of carbon and nitrogen in water-stable aggregates during decomposition of (CN)-C-13-N-15-labelled wheat straw in situ. Eur. J. Soil Sci. 1997, 48, 295–300. [Google Scholar] [CrossRef]



| Stage | Re-Greening | Tillering | Jointing–Booting | Heading–Flowering | Milk-Ripe | Yellow-Ripe | |||
|---|---|---|---|---|---|---|---|---|---|
| Early | Middle | Late | Early | Late | |||||
| Moisture thresholds | 5–25 mm | 0.7θs1-θs1 | 0.65θs1-θs1 | 0.65θs1-θs1 | 0.7θs2-θs2 | 0.75θs2-θs2 | 0.8θs3-θs3 | 0.7θs3-θs3 | Naturally drying |
| Root zone depth (cm) | — | 0–20 | 0–20 | 0–20 | 0–30 | 0–30 | 0–40 | 0–40 | — |
| Fertilizer | FI and CI | FS and CS | FM and CM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | |
| Compound fertilizer | 525 | — | — | 525 | — | — | 525 | — | — |
| Urea | 225 | 150 | 120 | 225 | 150 | 120 | — | 150 | 120 |
| Straw | — | — | — | 3000 | — | — | — | — | — |
| Manure | — | — | — | — | — | — | 7500 | — | — |
| ANOVA | 2022 | 2023 | ||||
|---|---|---|---|---|---|---|
| Bulk Soil | POM | MAOM | Bulk Soil | POM | MAOM | |
| I | ** | ns | * | ns | ns | ns |
| C | ** | ** | ** | ** | ** | ** |
| I × C | ns | ns | ns | ns | ns | ns |
| Year | Iron Oxides | FI | FS | FM | CI | CS | CM |
|---|---|---|---|---|---|---|---|
| 2022 | FeDH | 12.18 ± 0.30 a | 11.30 ± 0.35 a | 12.01 ± 0.24 a | 12.21 ± 0.38 a | 11.19 ± 0.73 a | 11.06 ± 0.12 a |
| FeHH | 2.04 ± 0.03 c | 2.45 ± 0.03 ab | 2.61 ± 0.08 a | 2.09 ± 0.05 c | 2.29 ± 0.05 b | 2.35 ± 0.02 b | |
| FePP | 0.62 ± 0.00 c | 0.67 ± 0.01 bc | 0.68 ± 0.02 abc | 0.64 ± 0.02 c | 0.74 ± 0.02 a | 0.71 ± 0.02 ab | |
| 2023 | FeDH | 12.04 ± 0.69 a | 11.02 ± 0.18 a | 11.17 ± 0.32 a | 11.93 ± 0.62 a | 11.26 ± 0.53 a | 10.76 ± 0.27 a |
| FeHH | 2.02 ± 0.00 b | 2.37 ± 0.02 a | 2.43 ± 0.05 a | 2.05 ± 0.10 b | 2.47 ± 0.04 a | 2.52 ± 0.07 a | |
| FePP | 0.62 ± 0.03 c | 0.72 ± 0.06 abc | 0.69 ± 0.03 bc | 0.65 ± 0.02 c | 0.85 ± 0.05 a | 0.81 ± 0.01 ab |
| ANOVA | 2022 | 2023 | ||
|---|---|---|---|---|
| Activation Degree | Complexation Degree | Activation Degree | Complexation Degree | |
| I | ns | ns | ns | * |
| C | ** | * | ** | ** |
| I × C | ns | ns | ns | ns |
| Year | C:Fe | FI | FS | FM | CI | CS | CM |
|---|---|---|---|---|---|---|---|
| 2022 | DH | 0.25 ± 0.00 d | 0.30 ± 0.00 bc | 0.32 ± 0.01 b | 0.27 ± 0.00 cd | 0.29 ± 0.01 bc | 0.36 ± 0.01 a |
| HH | 2.57 ± 0.09 b | 2.68 ± 0.07 ab | 2.58 ± 0.16 b | 2.86 ± 0.09 ab | 2.81 ± 0.04 ab | 2.93 ± 0.10 a | |
| PP | 14.02 ± 0.28 a | 13.02 ± 0.68 a | 14.13 ± 1.08 a | 14.98 ± 0.24 a | 13.53 ± 0.32 a | 13.37 ± 0.60 a | |
| 2023 | DH | 0.26 ± 0.02 c | 0.33 ± 0.00 bc | 0.36 ± 0.02 b | 0.25 ± 0.00 c | 0.33 ± 0.02 bc | 0.44 ± 0.04 a |
| HH | 2.66 ± 0.32 a | 3.11 ± 0.23 a | 3.21 ± 0.12 a | 3.03 ± 0.30 a | 3.20 ± 0.21 a | 3.18 ± 0.23 a | |
| PP | 15.15 ± 1.13 a | 16.18 ± 1.32 a | 15.09 ± 0.56 a | 15.31 ± 2.24 a | 14.18 ± 0.56 a | 13.94 ± 0.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Liao, L.; Xu, J.; Wang, W.; Chen, P.; Min, Z.; Luan, Y.; Han, Y.; Bao, K. Dual Role of Iron Oxides in Stabilizing Particulate and Mineral-Associated Organic Carbon Under Field Management in Paddies. Agriculture 2025, 15, 1385. https://doi.org/10.3390/agriculture15131385
Guo H, Liao L, Xu J, Wang W, Chen P, Min Z, Luan Y, Han Y, Bao K. Dual Role of Iron Oxides in Stabilizing Particulate and Mineral-Associated Organic Carbon Under Field Management in Paddies. Agriculture. 2025; 15(13):1385. https://doi.org/10.3390/agriculture15131385
Chicago/Turabian StyleGuo, Hang, Linxian Liao, Junzeng Xu, Wenyi Wang, Peng Chen, Zhihui Min, Yajun Luan, Yu Han, and Keke Bao. 2025. "Dual Role of Iron Oxides in Stabilizing Particulate and Mineral-Associated Organic Carbon Under Field Management in Paddies" Agriculture 15, no. 13: 1385. https://doi.org/10.3390/agriculture15131385
APA StyleGuo, H., Liao, L., Xu, J., Wang, W., Chen, P., Min, Z., Luan, Y., Han, Y., & Bao, K. (2025). Dual Role of Iron Oxides in Stabilizing Particulate and Mineral-Associated Organic Carbon Under Field Management in Paddies. Agriculture, 15(13), 1385. https://doi.org/10.3390/agriculture15131385







