Stomatal–Hydraulic Coordination Mechanisms of Wheat in Response to Atmospheric–Soil Drought and Rewatering
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement Method
2.2.1. Leaf Gas Exchange Parameters
2.2.2. Midday Leaf Water Potential
2.2.3. Leaf Hydraulic Conductance
2.2.4. Leaf Hydraulic Vulnerability Curves and Stomatal Closure Thresholds
2.3. Data Analysis
3. Results
3.1. Leaf Gas Exchange
3.2. Midday Leaf Water Potential, Leaf Hydraulic Conductance, and Water Transport Efficiency per Unit Photosynthetic Rate
3.3. Comparison of Leaf Vulnerability Curves
3.4. Variation in Stomatal Closure Thresholds
4. Discussion
4.1. The Impact of Combined Drought Stress and Soil Rewatering on Water Transport and Gas Exchange
4.2. Adaptive Changes in Hydraulic Vulnerability and Stomatal Regulation Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zander, A.; Bersier, L.-F.; Gray, S.M. Effects of Temperature Variability on Community Structure in a Natural Microbial Food Web. Glob. Change Biol. 2017, 23, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lu, X.; Liu, Z.; Tang, X.; Yu, Q.; Wang, Y. Atmospheric Drought Dominates Changes in Global Water Use Efficiency. Sci. Total Environ. 2024, 934, 173084. [Google Scholar] [CrossRef] [PubMed]
- Huynh, A.; Aguirre, B.A.; English, J.; Guzman, D.; Wright, A.J. Atmospheric Drying and Soil Drying: Differential Effects on Grass Community Composition. Glob. Change Biol. 2024, 30, e17106. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Park Williams, A.; Gentine, P. Projected Increases in Intensity, Frequency, and Terrestrial Carbon Costs of Compound Drought and Aridity Events. Sci. Adv. 2019, 5, eaau5740. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Akter, N.; Brishty, T.A.; Karim, M.A.; Ahmed, M.J.U.; Islam, M.R. Leaf Water Status and Biochemical Adjustments as a Mechanism of Drought Tolerance in Two Contrasting Wheat (Triticum aestivum L.) Varieties. Acta Physiol. Plant 2023, 45, 50. [Google Scholar] [CrossRef]
- Wang, X.; Du, T.; Huang, J.; Peng, S.; Xiong, D. Leaf Hydraulic Vulnerability Triggers the Decline in Stomatal and Mesophyll Conductance during Drought in Rice. J. Exp. Bot. 2018, 69, 4033–4045. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shangguan, Z. Leaf Gas Exchange and Fluorescence of Two Winter Wheat Varieties in Response to Drought Stress and Nitrogen Supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf Hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef]
- North, G.; Brinton, E.; Browne, M.; Gillman, M.; Roddy, A.; Kho, T.; Wang, E.; Fung, V.; Brodersen, C. Hydraulic Conductance, Resistance, and Resilience: How Leaves of a Tropical Epiphyte Respond to Drought. Am. J. Bot. 2019, 106, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Sack, L. The Causes and Consequences of Leaf Hydraulic Decline with Dehydration. J. Exp. Bot. 2017, 68, 4479–4496. [Google Scholar] [CrossRef]
- Baird, A.S.; Taylor, S.H.; Pasquet-Kok, J.; Vuong, C.; Zhang, Y.; Watcharamongkol, T.; Cochard, H.; Scoffoni, C.; Edwards, E.J.; Osborne, C.P.; et al. Resolving the Contrasting Leaf Hydraulic Adaptation of C3 and C4 Grasses. New Phytol. 2025, 245, 1924–1939. [Google Scholar] [CrossRef]
- Devi, M.J.; Reddy, V.R.; Timlin, D. Drought-Induced Responses in Maize under Different Vapor Pressure Deficit Conditions. Plants 2022, 11, 2771. [Google Scholar] [CrossRef]
- Unce, J.A. How Do Leaf Hydraulics Limit Stomatal Conductance at High Water Vapour Pressure Deficits? Plant Cell Environ. 2006, 29, 1644–1650. [Google Scholar] [CrossRef]
- Schönbeck, L.C.; Schuler, P.; Lehmann, M.M.; Mas, E.; Mekarni, L.; Pivovaroff, A.L.; Turberg, P.; Grossiord, C. Increasing Temperature and Vapour Pressure Deficit Lead to Hydraulic Damages in the Absence of Soil Drought. Plant Cell Environ. 2022, 45, 3275–3289. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among Species of Stomatal Control under Fluctuating Soil Water Status and Evaporative Demand: Modelling Isohydric and Anisohydric Behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic Signals in Long-Distance Signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef]
- Fu, X.; Meinzer, F.C.; Woodruff, D.R.; Liu, Y.; Smith, D.D.; McCulloh, K.A.; Howard, A.R. Coordination and Trade-offs between Leaf and Stem Hydraulic Traits and Stomatal Regulation along a Spectrum of Isohydry to Anisohydry. Plant Cell Environ. 2019, 42, 2245–2258. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Luo, D.-D.; Weng, C.-K.; Jin, Y. Plant Water-Regulation Strategies: Isohydric versus Anisohydric Behavior. Chin. J. Plant Ecol. 2017, 41, 1020–1032. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Langensiepen, M.; Gaiser, T.; Webber, H.; Ahrends, H.; Hueging, H.; Ewert, F. Responses of Winter Wheat and Maize to Varying Soil Moisture: From Leaf to Canopy. Agric. For. Meteorol. 2022, 314, 108803. [Google Scholar] [CrossRef]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting Plant Vulnerability to Drought in Biodiverse Regions Using Functional Traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Creek, D.; Blackman, C.J.; Brodribb, T.J.; Choat, B.; Tissue, D.T. Coordination between Leaf, Stem, and Root Hydraulics and Gas Exchange in Three Arid-Zone Angiosperms during Severe Drought and Recovery. Plant Cell Environ. 2018, 41, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Martorell, S.; Díaz-Espejo, A.; Medrano, H.; Ball, M.C.; Choat, B. Rapid Hydraulic Recovery in Eucalyptus pauciflora after Drought: Linkages between Stem Hydraulics and Leaf Gas Exchange. Plant Cell Environ. 2014, 37, 617–626. [Google Scholar] [CrossRef]
- Brodribb, T.; Brodersen, C.R.; Carriqui, M.; Tonet, V.; Rodriguez Dominguez, C.; McAdam, S. Linking Xylem Network Failure with Leaf Tissue Death. New Phytol. 2021, 232, 68–79. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Chirino, E.; Peguero-Pina, J.J.; Barigah, T.S.; Cochard, H.; Gil-Pelegrín, E. Xylem Cavitation and Embolism in Plants Living in Water-Limited Ecosystems. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin, Heidelberg, 2012; pp. 63–109. ISBN 978-3-642-32653-0. [Google Scholar]
- Tulik, M.K.; Marciszewska, K.D. Hydraulic Efficiency and Safety of Xylem Sap Flow in Relation to Water Stress in Woody Plants. In Hydraulic Conductivity; Silva, V.R.D., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Tonet, V.; Brodribb, T.; Bourbia, I. Variation in Xylem Vulnerability to Cavitation Shapes the Photosynthetic Legacy of Drought. Plant Cell Environ. 2024, 47, 1160–1170. [Google Scholar] [CrossRef]
- Skelton, R.P.; Brodribb, T.J.; McAdam, S.A.M.; Mitchell, P.J. Gas Exchange Recovery Following Natural Drought Is Rapid Unless Limited by Loss of Leaf Hydraulic Conductance: Evidence from an Evergreen Woodland. New Phytol. 2017, 215, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M. Abscisic Acid Mediates a Divergence in the Drought Response of Two Conifers. Plant Physiol. 2013, 162, 1370–1377. [Google Scholar] [CrossRef]
- Rehschuh, R.; Cecilia, A.; Zuber, M.; Faragó, T.; Baumbach, T.; Hartmann, H.; Jansen, S.; Mayr, S.; Ruehr, N. Drought-Induced Xylem Embolism Limits the Recovery of Leaf Gas Exchange in Scots Pine. Plant Physiol. 2020, 184, 852–864. [Google Scholar] [CrossRef]
- Choat, B.; Brodersen, C.R.; McElrone, A.J. Synchrotron X-Ray Microtomography of Xylem Embolism in Sequoia Sempervirens Saplings during Cycles of Drought and Recovery. New Phytol. 2015, 205, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Liu, X.; Li, Y.; Song, H.; Yin, Z.; Zhang, F.; He, Q.; Xu, Z.; Zhou, G. Photosynthetic Resistance and Resilience under Drought, Flooding and Rewatering in Maize Plants. Photosynth. Res. 2021, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Jordan, G.J.; Brodribb, T.J. Wheat Leaves Embolized by Water Stress Do Not Recover Function upon Rewatering. Plant Cell Environ. 2018, 41, 2704–2714. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Chen, Y.; Wei, Z.; Liu, F. Dissecting the Combined Effects of Air Temperature and Relative Humidity on Water-Use Efficiency of Barley under Drought Stress. J. Agron. Crop Sci. 2020, 207, 606–617. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and Biochemical Changes during Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Luo, D.; Hu, X.; Feng, P.; Mo, Y.; Li, H.; Gong, S. Integrated Effects of Soil Moisture on Wheat Hydraulic Properties and Stomatal Regulation. Plants 2024, 13, 2263. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Measurement of Leaf Hydraulic Conductance and Stomatal Conductance and Their Responses to Irradiance and Dehydration Using the Evaporative Flux Method (EFM). J. Vis. Exp. 2012, 70, 4179. [Google Scholar] [CrossRef]
- Sack, L. The Hydraulic Conductance of the Angiosperm Leaf Lamina: A Comparison of Three Measurement Methods. J. Exp. Bot. 2002, 53, 2177–2184. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential Coordination of Stomatal Conductance, Mesophyll Conductance, and Leaf Hydraulic Conductance in Response to Changing Light across Species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef]
- Pammenter, N.; Willigen, C. A Mathematical and Statistical Analysis of the Curves Illustrating Vulnerability of Xylem to Cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef]
- Corso, D.; Delzon, S.; Lamarque, L.J.; Cochard, H.; Torres-Ruiz, J.M.; King, A.; Brodribb, T. Neither Xylem Collapse, Cavitation, or Changing Leaf Conductance Drive Stomatal Closure in Wheat. Plant Cell Environ. 2020, 43, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Duursma, R.; Choat, B. Fitplc - an R Package to Fit Hydraulic Vulnerability Curves. J. Plant Hydraul. 2017, 4, 002. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte Accumulation Plays Important Roles in the Drought Priming Induced Tolerance to Post-Anthesis Drought Stress in Winter Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Wu, C.; Liu, B.; Zhang, X.; Wang, M.; Liang, H. Phytohormone Response of Drought-Acclimated Illicium Difengpi (Schisandraceae). Int. J. Mol. Sci. 2023, 24, 16443. [Google Scholar] [CrossRef]
- Berauer, B.J.; Steppuhn, A.; Schweiger, A.H. The Multidimensionality of Plant Drought Stress: The RelativeImportance of Edaphic and Atmospheric Drought. Plant Cell Environ. 2024, 47, 3528–3540. [Google Scholar] [CrossRef]
- Schönbeck, L.; Grossiord, C.; Gessler, A.; Gisler, J.; Meusburger, K.; D’Odorico, P.; Rigling, A.; Salmon, Y.; Stocker, B.D.; Zweifel, R.; et al. Photosynthetic Acclimation and Sensitivity to Short- and Long-Term Environmental Changes in a Drought-Prone Forest. J. Exp. Bot. 2022, 73, 2576–2588. [Google Scholar] [CrossRef]
- Binks, O.; Cernusak, L.A.; Liddell, M.; Bradford, M.; Coughlin, I.; Bryant, C.; Palma, A.C.; Hoffmann, L.; Alam, I.; Carle, H.J.; et al. Vapour Pressure Deficit Modulates Hydraulic Function and Structure of Tropical Rainforests under Nonlimiting Soil Water Supply. New Phytol. 2023, 240, 1405–1420. [Google Scholar] [CrossRef]
- Backhaus, S.; Kreyling, J.; Grant, K.; Beierkuhnlein, C.; Walter, J.; Jentsch, A. Recurrent Mild Drought Events Increase Resistance Toward Extreme Drought Stress. Ecosystems 2014, 17, 1068–1081. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal Closure during Leaf Dehydration, Correlation with Other Leaf Physiological Traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef]
- Yang, M.; He, J.; Sun, Z.; Li, Q.; Cai, J.; Zhou, Q.; Wollenweber, B.; Jiang, D.; Wang, X. Drought Priming Mechanisms in Wheat Elucidated by In-Situ Determination of Dynamic Stomatal Behavior. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
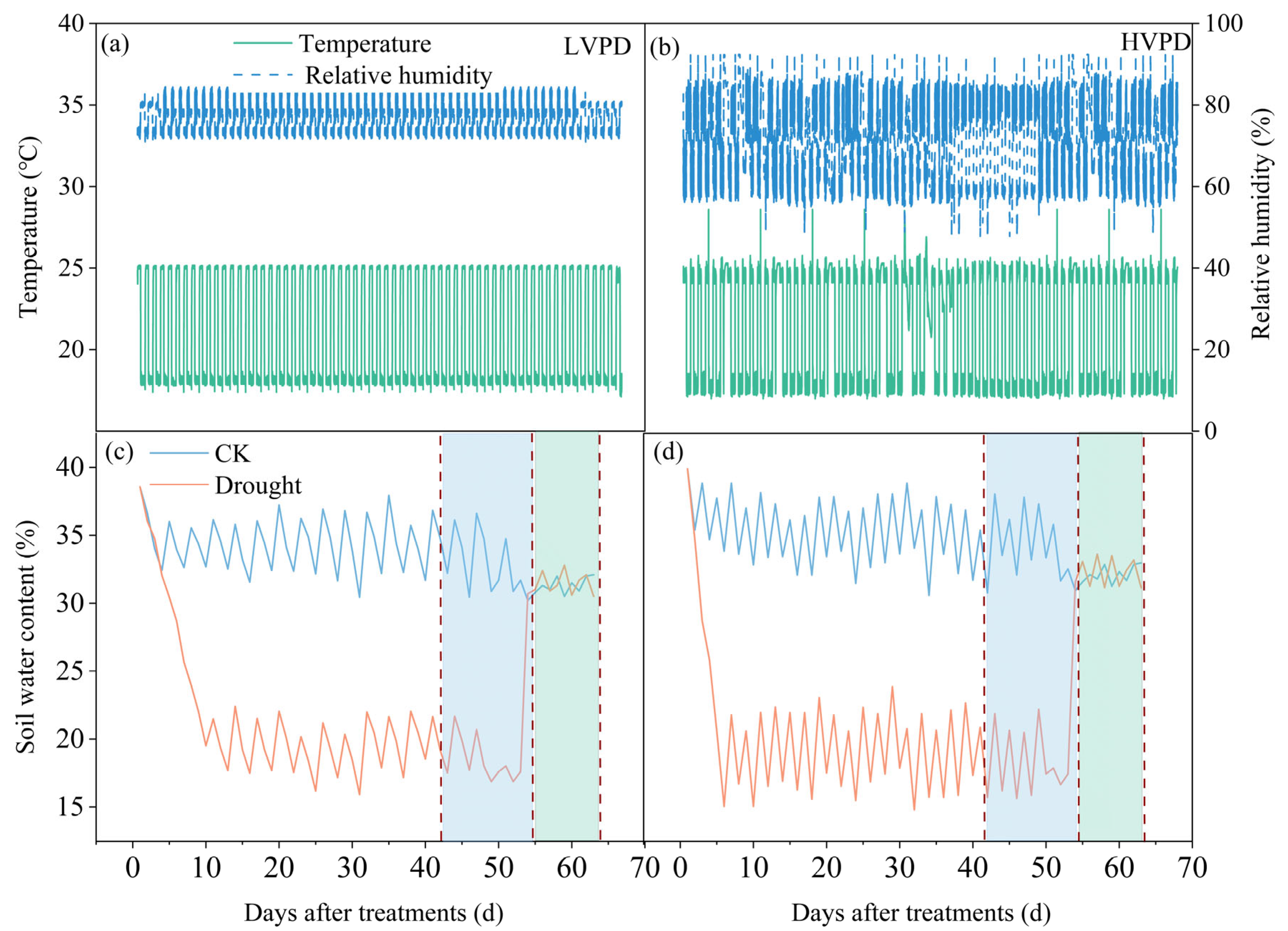
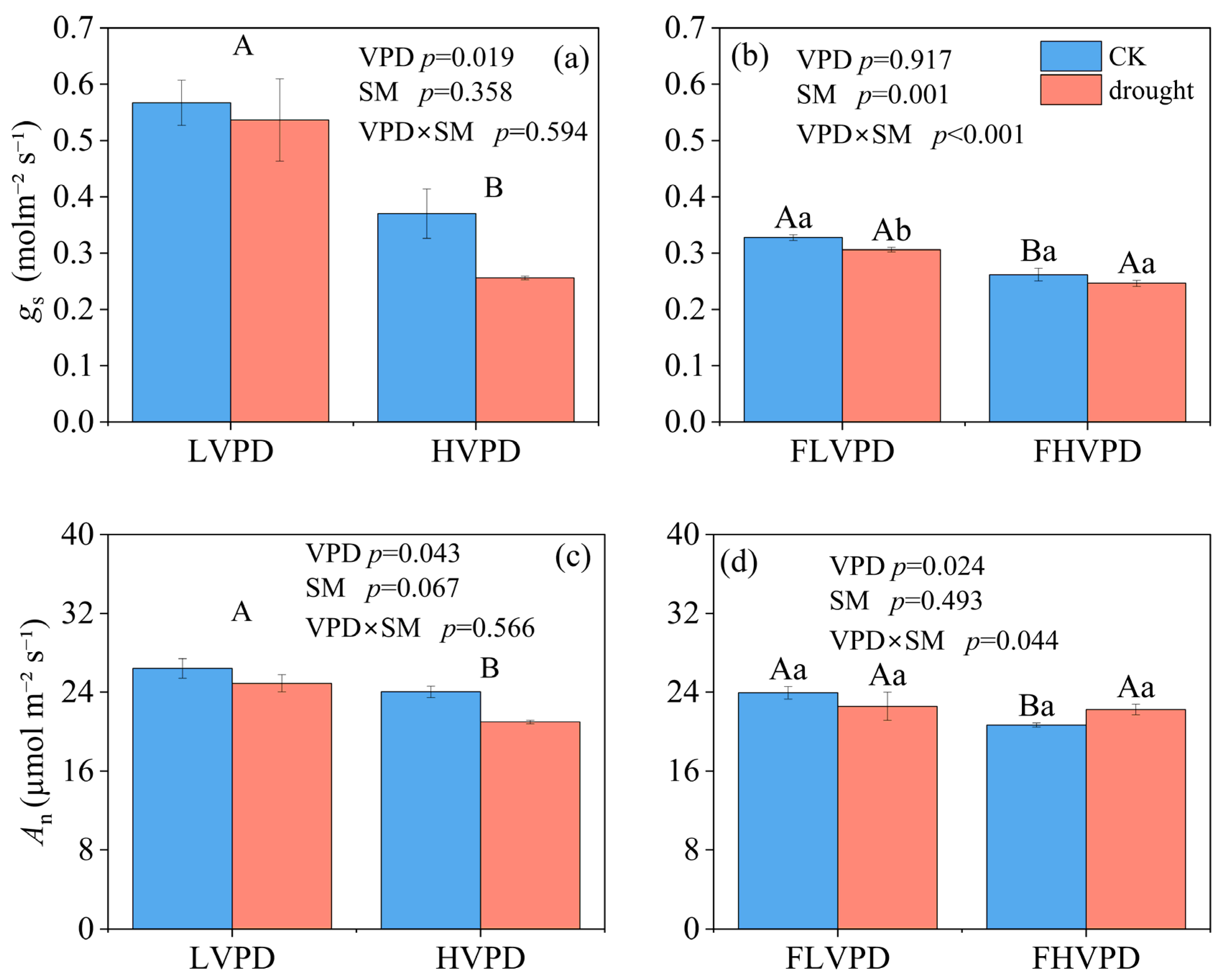

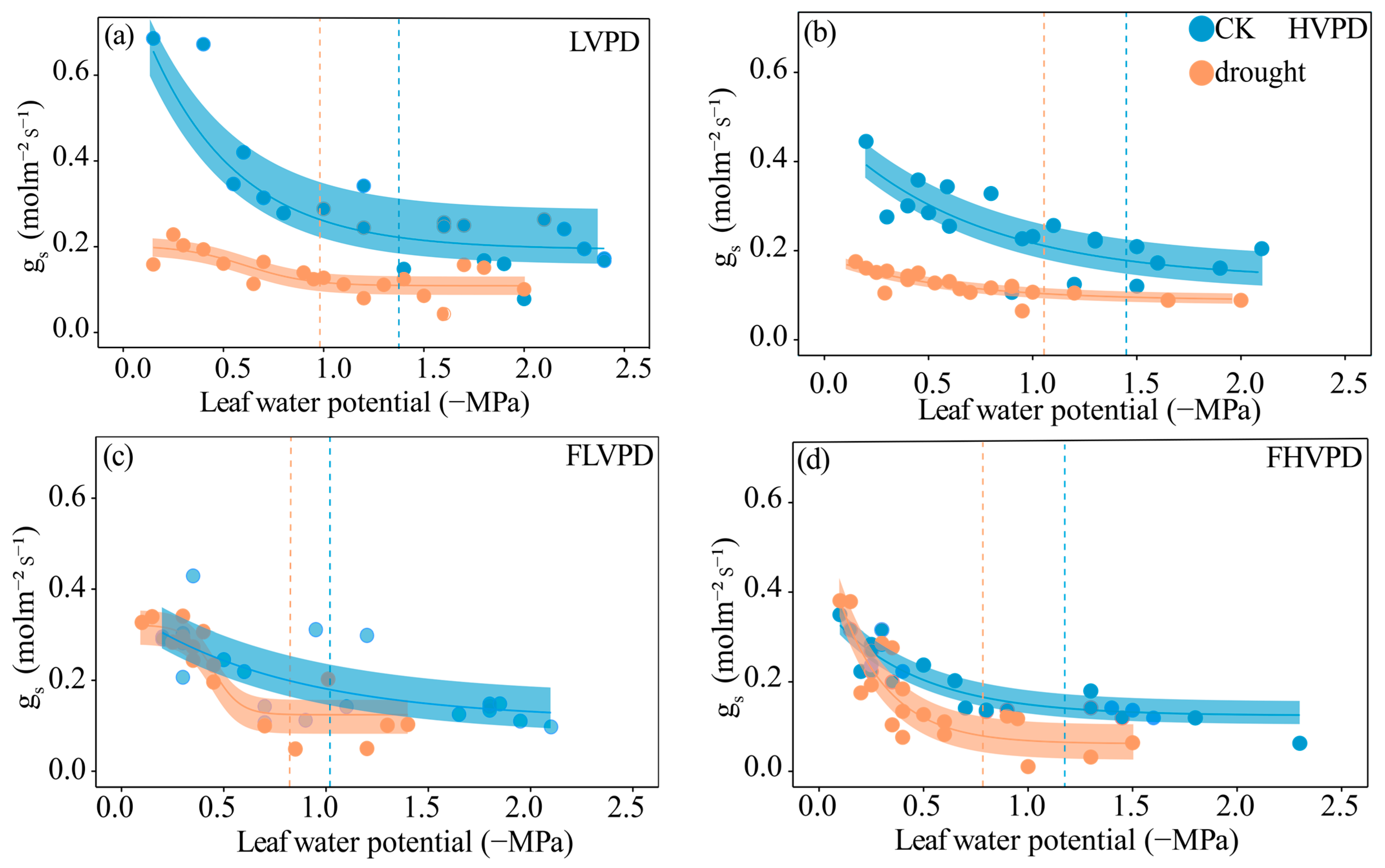
| Measured Parameters | Quantity/Description | Notes |
|---|---|---|
| Midday leaf water potential | Randomly 5 pots, one plant each pot selected | The remaining plants were equally subjected during the subsequent samplings |
| Leaf gas exchange parameters | Randomly 5 pots, one plant each pot selected | Non-destructive measurements |
| Leaf hydraulic conductance | The same flag leaves as above leaf gas exchange measured | In total 5 flag leaves |
| Leaf hydraulic vulnerability curves | Randomly 4 pots selected, 5 plants/pot In total 20 plants | Destructive measurements |
| Measured Parameters | ΨMD | Kleaf | Kleaf:An | ||||
|---|---|---|---|---|---|---|---|
| Stress | Rewatering | Stress | Rewatering | Stress | Rewatering | ||
| SM | CK | −1.39 ± 0.09 a | −1.20 ± 0.06 | 12.07 ± 0.28 a | 8.75 ± 0.14 a | 0.50 ± 0.05 a | 0.40 ± 0.12 a |
| drought | −1.87 ± 0.04 b | −1.25 ± 0.09 | 8.06 ± 0.24 b | 7.18 ± 0.37 b | 0.34 ± 0.01 b | 0.32 ± 0.02 b | |
| p | <0.001 | 0.259 | <0.001 | <0.001 | 0.002 | 0.005 | |
| VPD | LVPD | −1.59 ± 0.09 | −1.26 ± 0.09 | 11.95 ± 0.21 a | 10.09 ± 0.29 a | 0.49 ± 0.03 a | 0.43 ± 0.02 a |
| HVPD | −1.66 ± 0.05 | −1.20 ± 0.06 | 6.34 ± 0.07 b | 5.83 ± 0.21 b | 0.36 ± 0.02 b | 0.28 ± 0.02 b | |
| p | 0.41 | 0.867 | <0.001 | <0.001 | 0.004 | <0.001 | |
| VPD × SM | p | 0.89 | 0.054 | 0.09 | 0.76 | 0.335 | 0.07 |
| VPD | SM | Ψgs88 | P50 | SSM50 | S |
|---|---|---|---|---|---|
| LVPD | CK | −1.38 | −0.74 | −0.64 | 62.65 |
| Drought | −0.97 | −0.82 | −0.15 | 35.11 | |
| HVPD | CK | −1.42 | −0.93 | −0.49 | 59.51 |
| Drought | −1.05 | −1.16 | 0.11 | 49.65 | |
| FLVPD | CK | −1.08 | −0.63 | −0.45 | 114.36 |
| Drought | −0.85 | −0.75 | −0.10 | 101.91 | |
| FHVPD | CK | −1.22 | −0.69 | −0.53 | 85.03 |
| Drought | −0.89 | −1.15 | 0.26 | 112.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, Y.; Li, H.; Hu, X.; Feng, P.; Mo, Y.; Gong, S. Stomatal–Hydraulic Coordination Mechanisms of Wheat in Response to Atmospheric–Soil Drought and Rewatering. Agriculture 2025, 15, 1375. https://doi.org/10.3390/agriculture15131375
Wang L, Zhang Y, Li H, Hu X, Feng P, Mo Y, Gong S. Stomatal–Hydraulic Coordination Mechanisms of Wheat in Response to Atmospheric–Soil Drought and Rewatering. Agriculture. 2025; 15(13):1375. https://doi.org/10.3390/agriculture15131375
Chicago/Turabian StyleWang, Lijuan, Yanqun Zhang, Hao Li, Xinlong Hu, Pancen Feng, Yan Mo, and Shihong Gong. 2025. "Stomatal–Hydraulic Coordination Mechanisms of Wheat in Response to Atmospheric–Soil Drought and Rewatering" Agriculture 15, no. 13: 1375. https://doi.org/10.3390/agriculture15131375
APA StyleWang, L., Zhang, Y., Li, H., Hu, X., Feng, P., Mo, Y., & Gong, S. (2025). Stomatal–Hydraulic Coordination Mechanisms of Wheat in Response to Atmospheric–Soil Drought and Rewatering. Agriculture, 15(13), 1375. https://doi.org/10.3390/agriculture15131375






