Estimation of Canopy Chlorophyll Content of Apple Trees Based on UAV Multispectral Remote Sensing Images
Abstract
1. Introduction
2. Materials and Methods
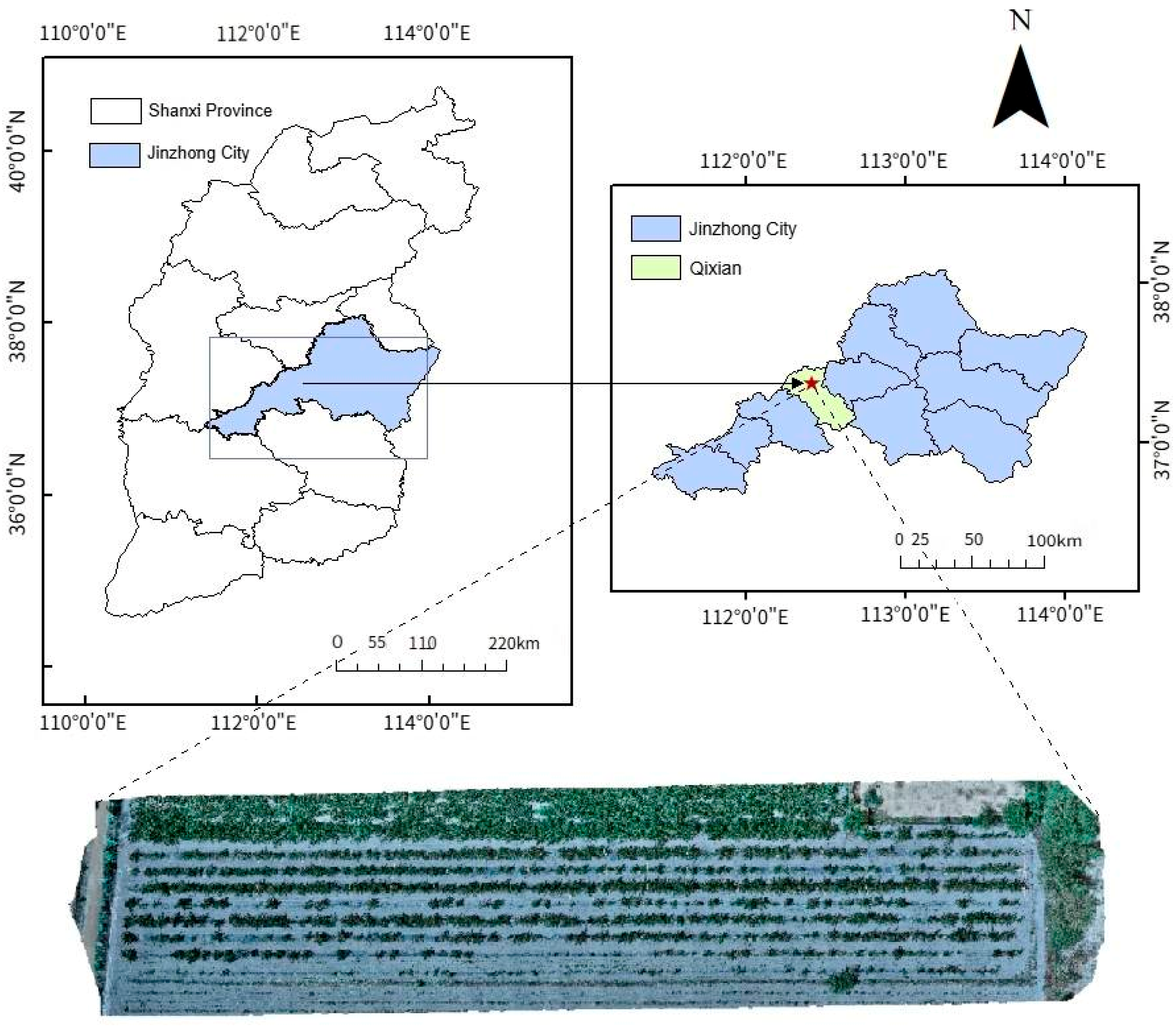
2.1. Study Area and Experimental Design
2.2. Data Acquisition and Processing
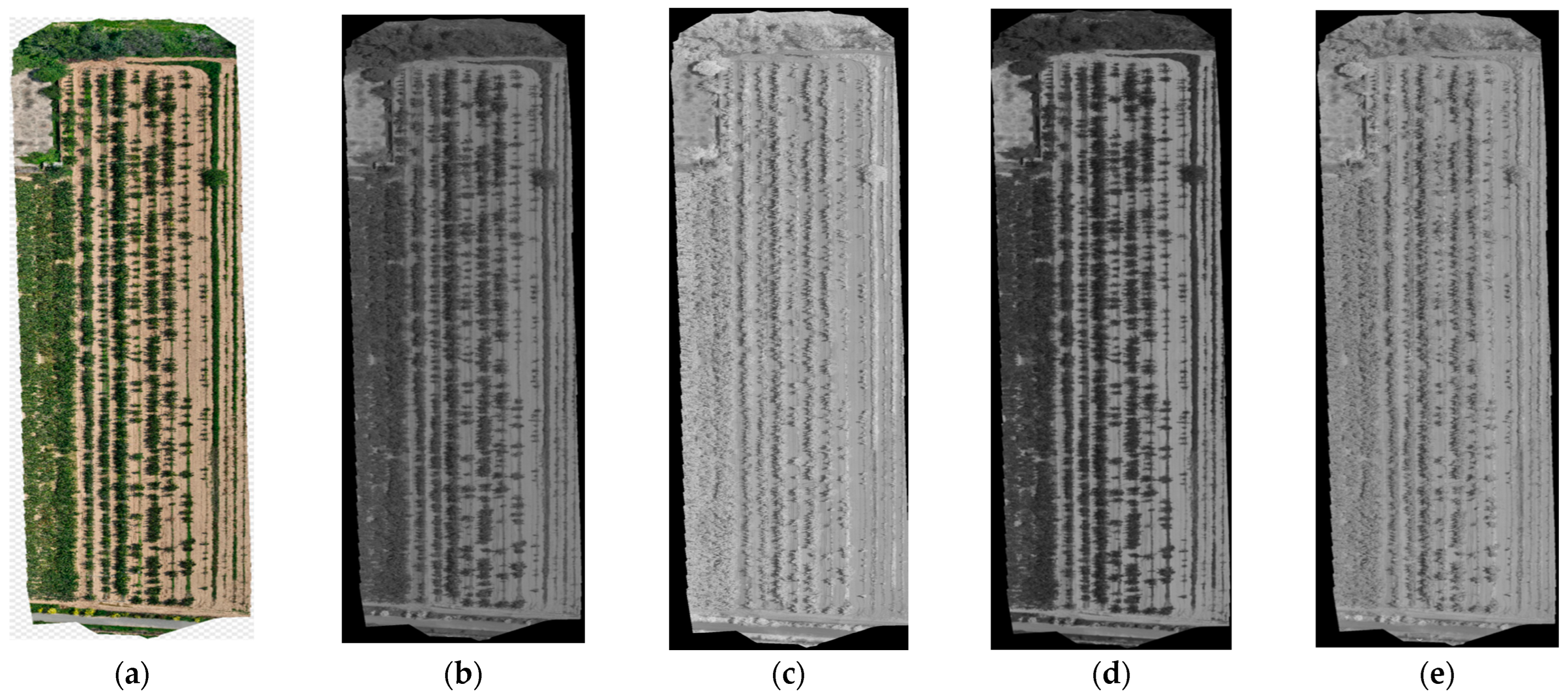
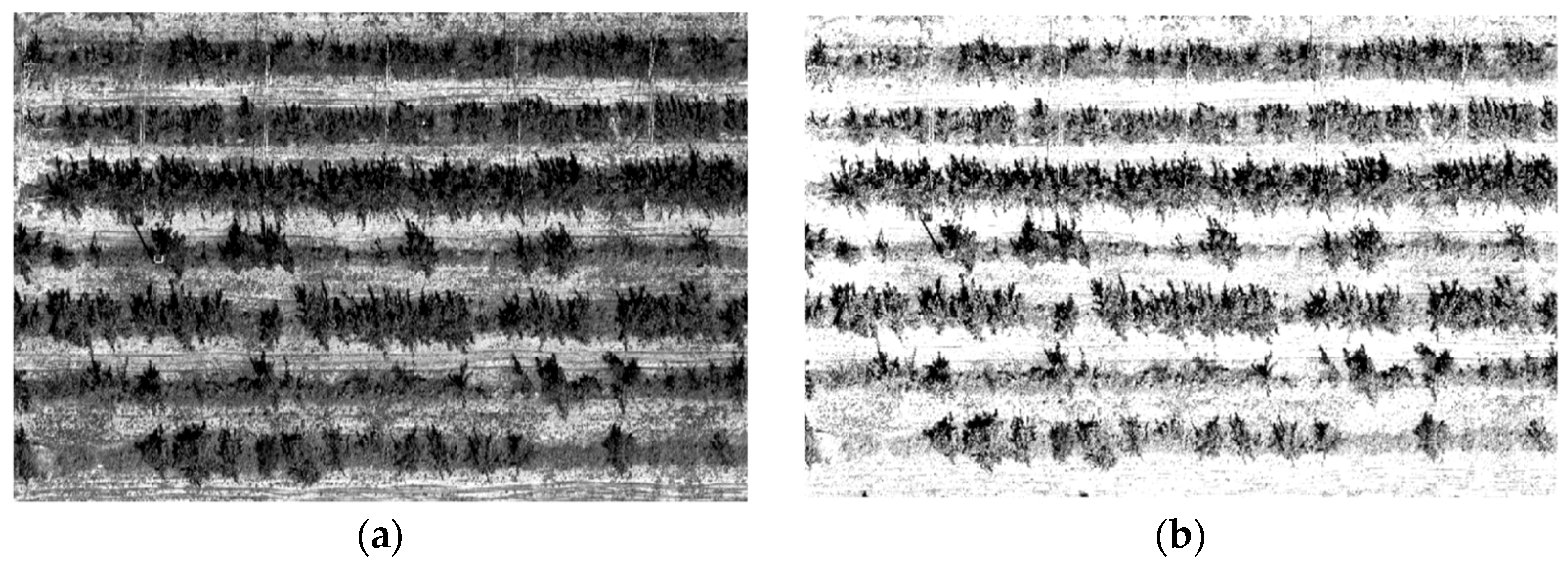
2.2.1. Multispectral Data Acquisition and Processing
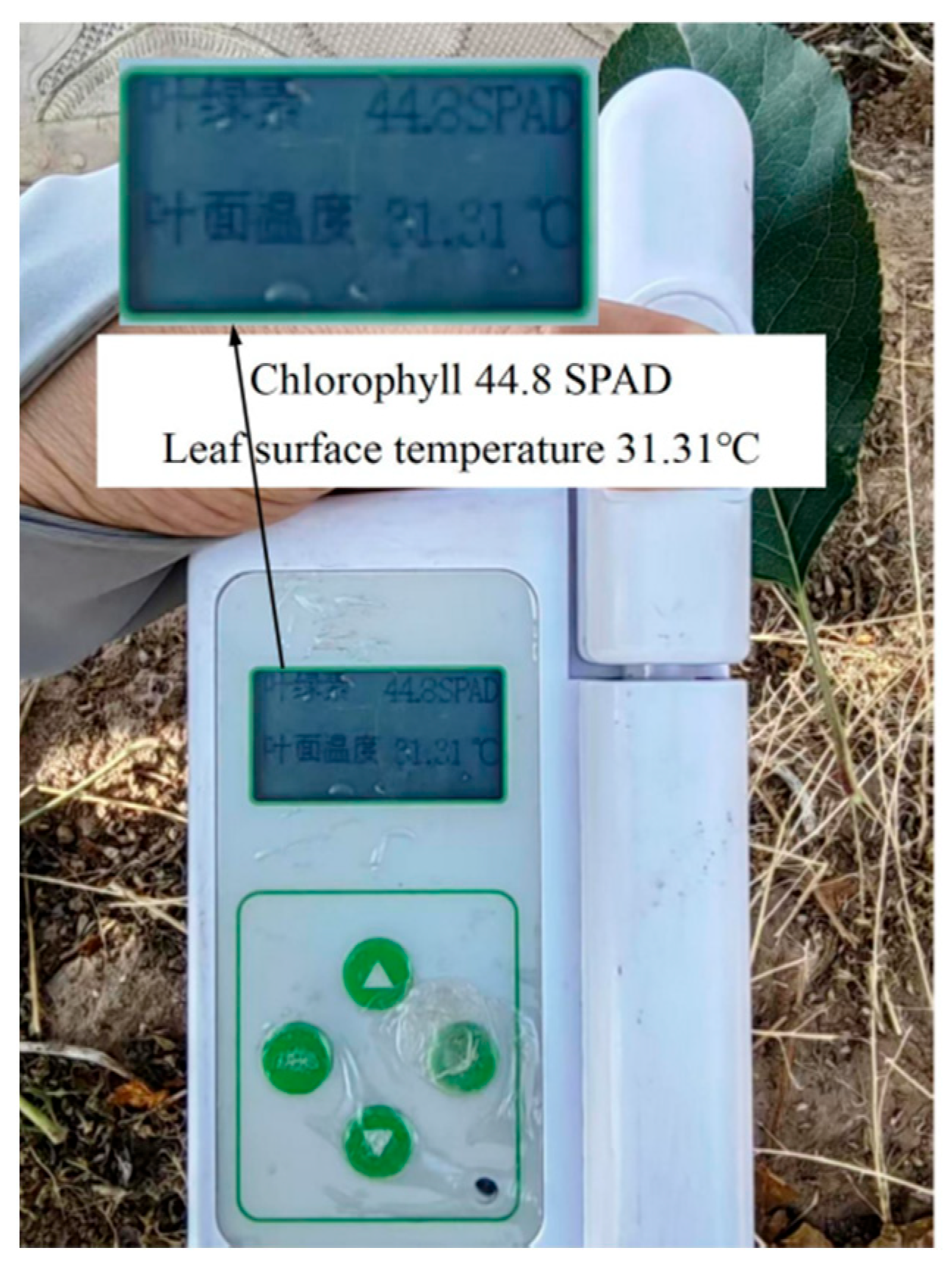
2.2.2. Determination of SPAD Value
2.3. Selection and Calculation of Vegetation Indexes
2.4. Modeling
2.5. Evaluation Indicators
2.6. Data Processing
3. Results
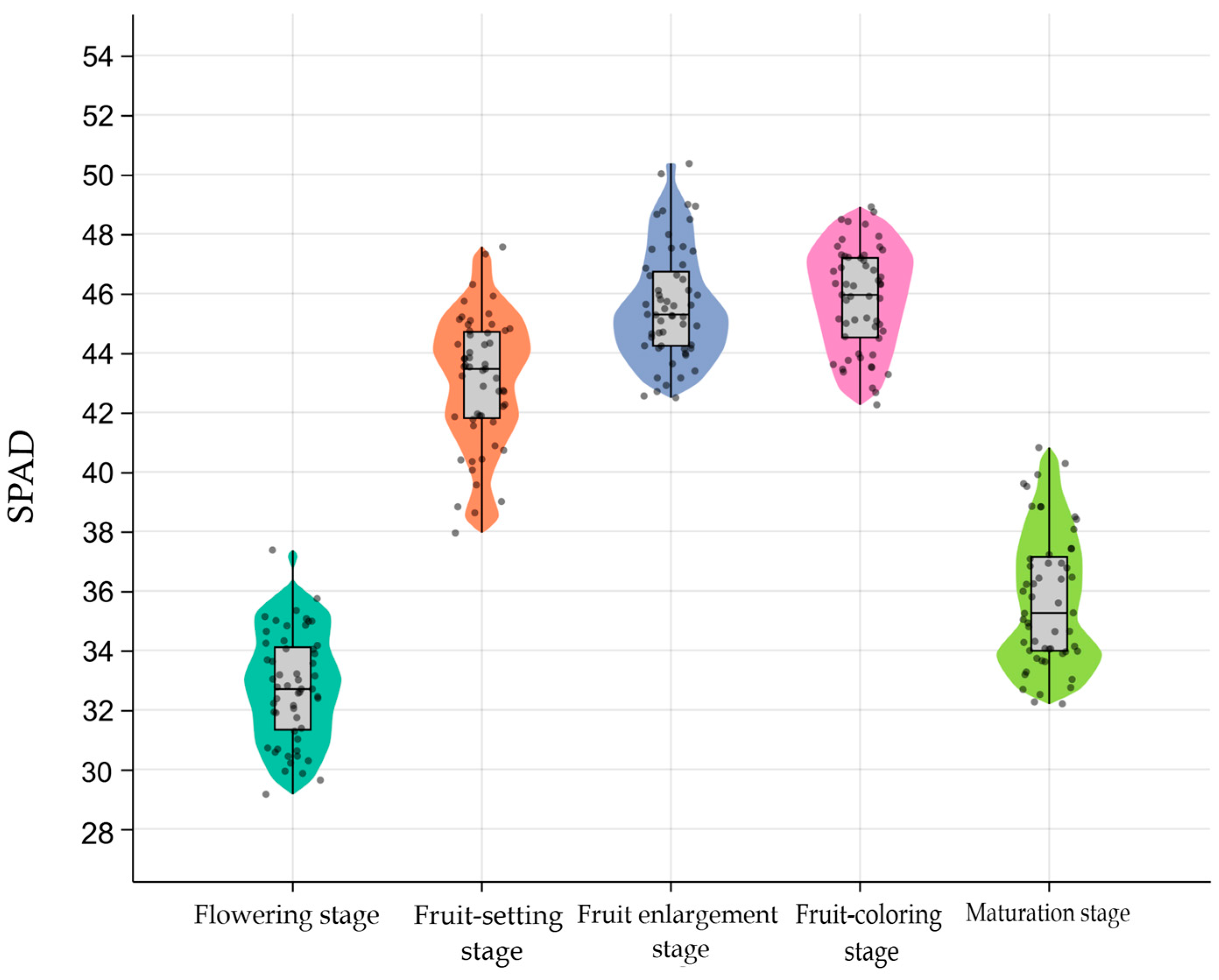
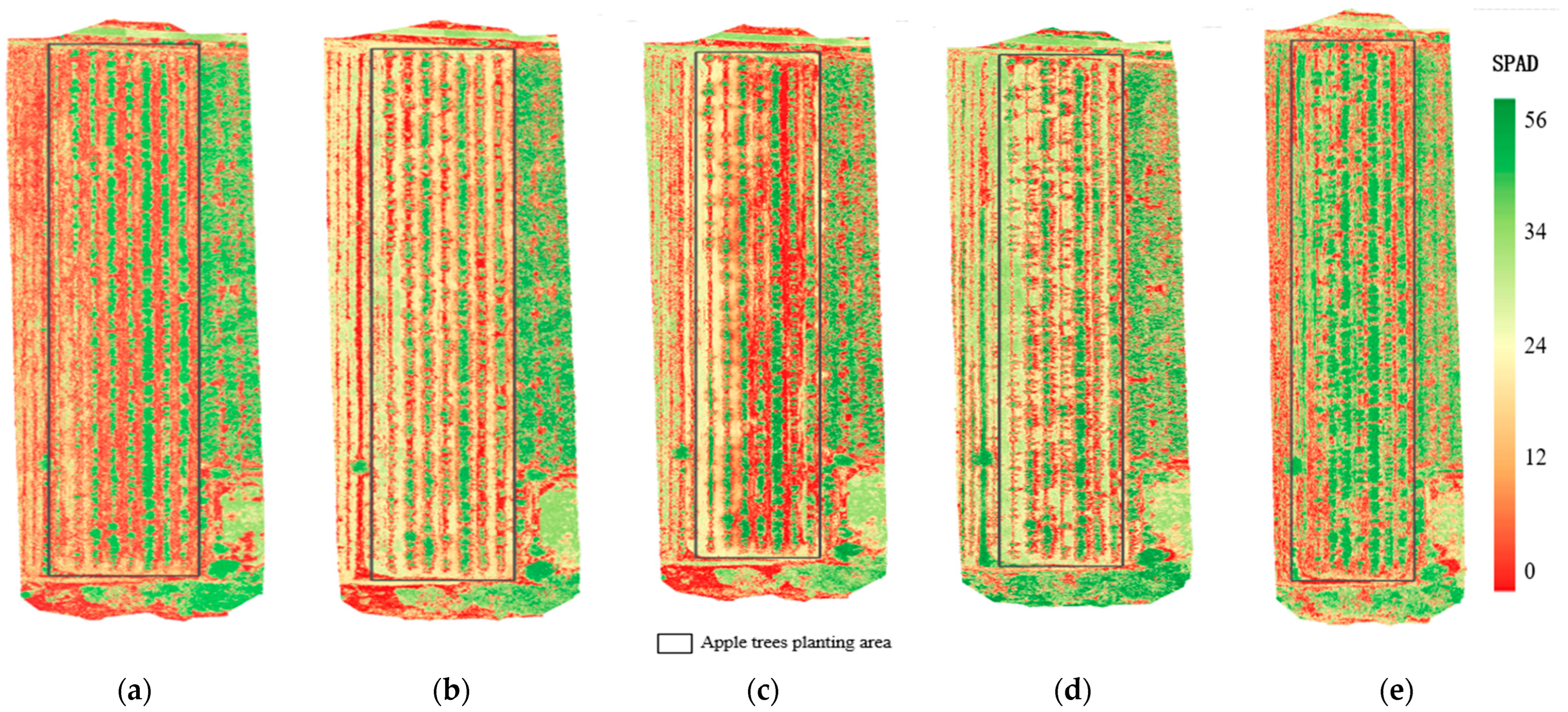
3.1. Measured SPAD Values in Canopy Leaves of Apple Trees
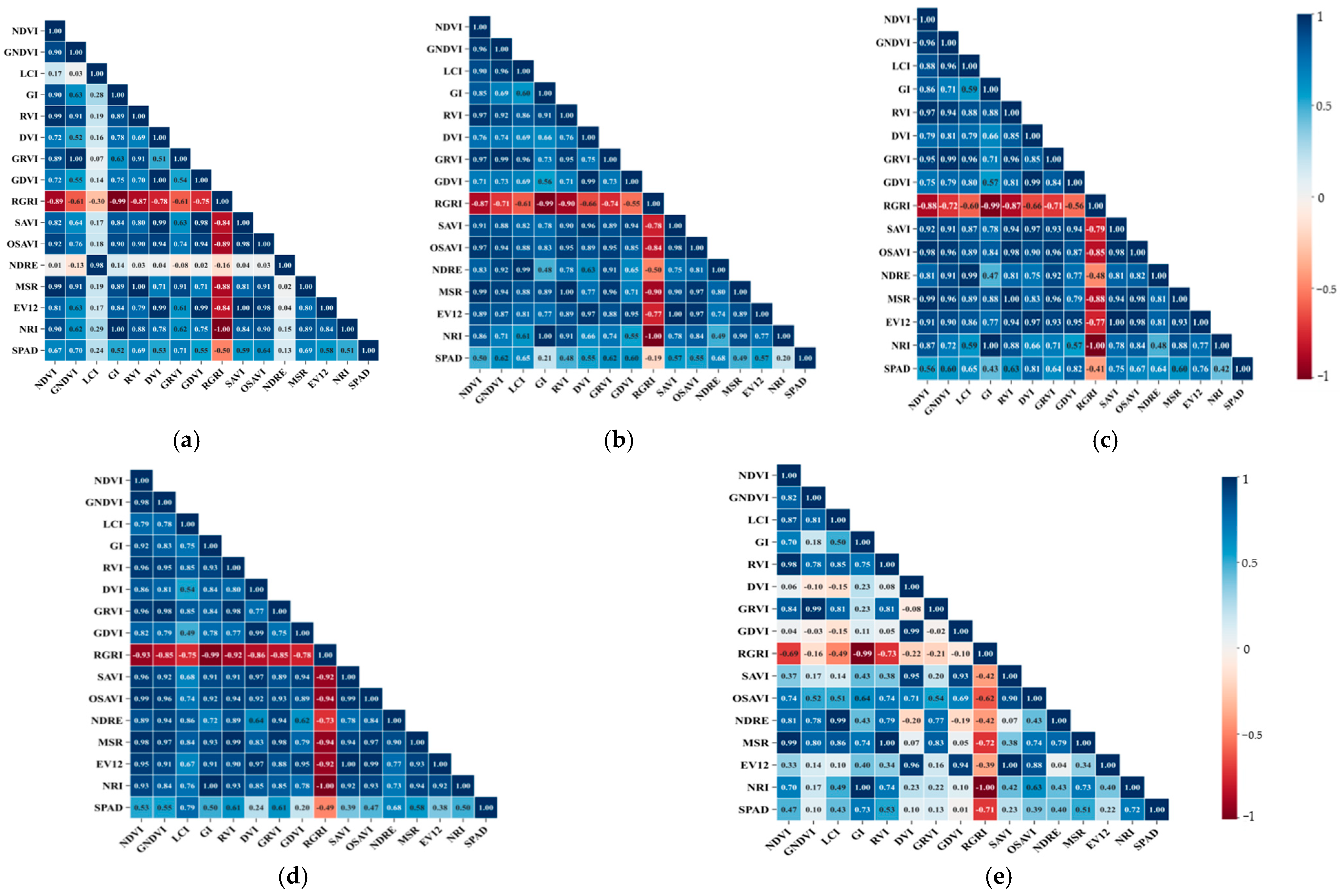
3.2. Correlation Analysis Between Vegetation Index and SPAD Value
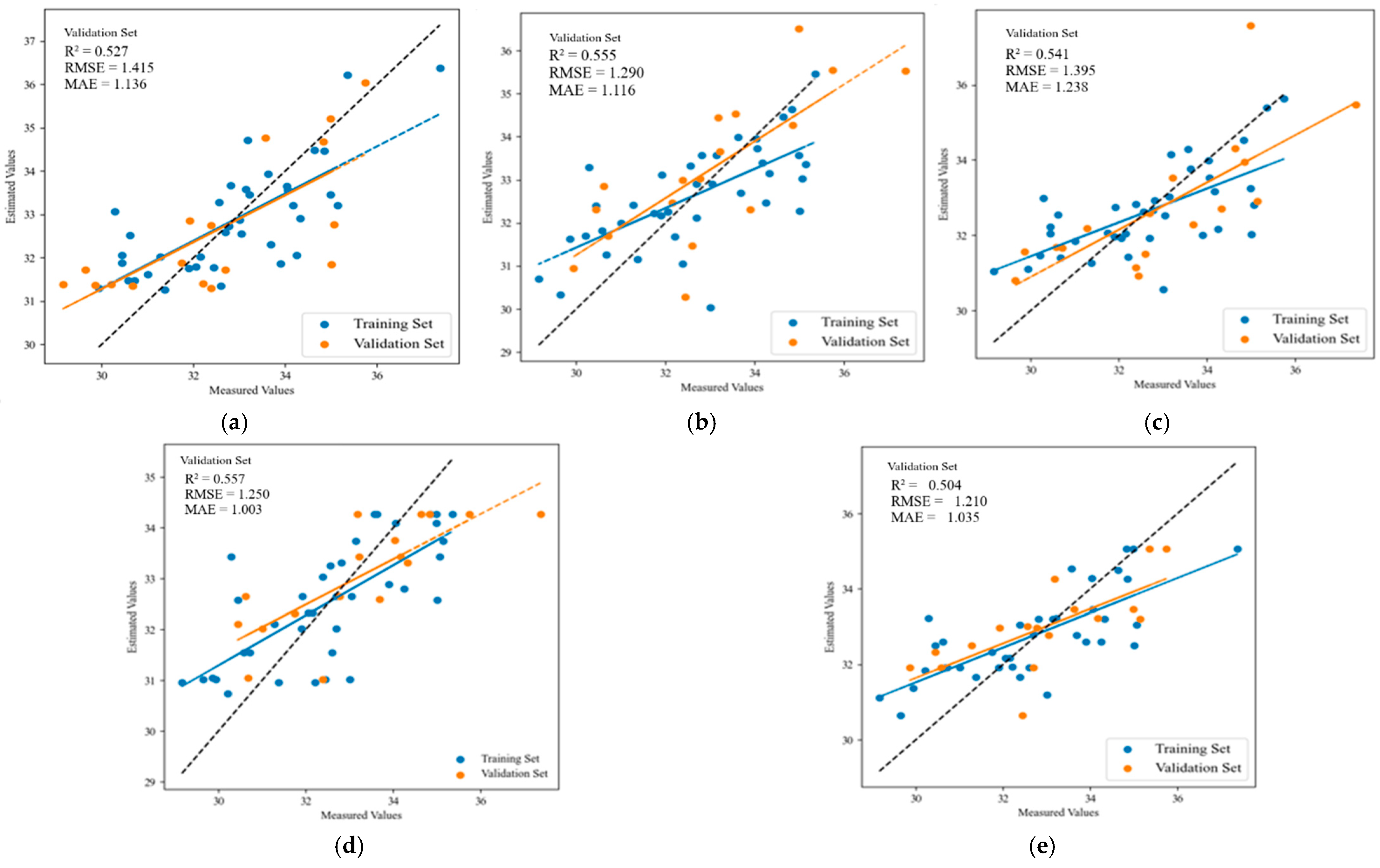
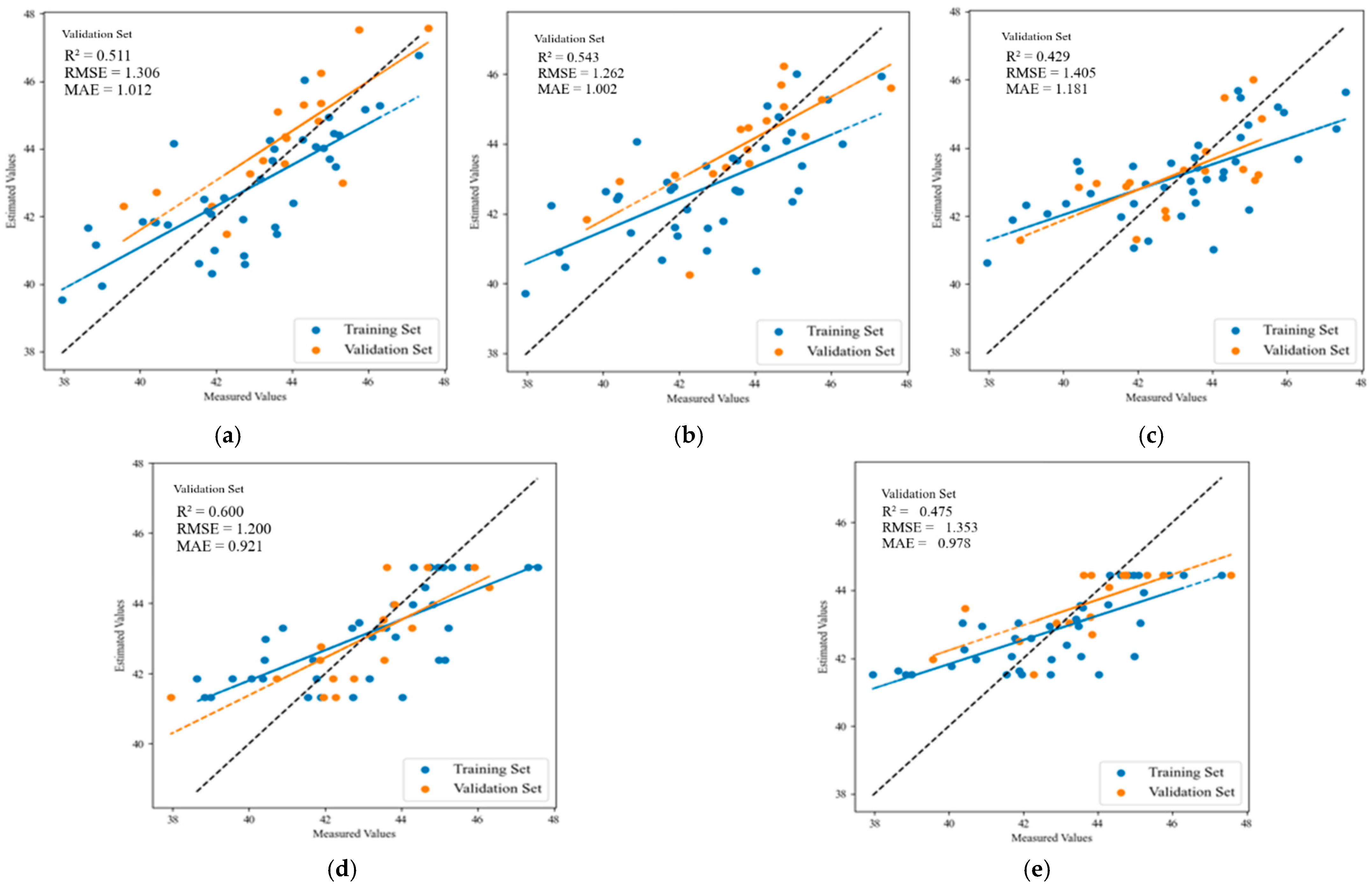
3.3. Establishment and Verification of Prediction Model for Leaf SPAD Value in Apple Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argenta, C.L.; Amarante, D.T.V.C.; Betinelli, S.K.; Brancher, L.T.; Nesi, N.C.; Vieira, J.M. Comparison of fruit attributes of ‘Fuji’ apple strains at harvest and after storage. Sci. Hortic. 2020, 272, 109585. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Development of an optical smart portable instrument for fruit quality detection. IEEE Trans. Instrum. Meas. 2021, 70, 7000109. [Google Scholar] [CrossRef]
- Wang, S.; He, P.; Wang, X.; Wang, H.; Chang, S.; Zheng, W.; Li, L.; He, X. Correlation analysis between soil acidity and fruit nutrient elements in apple orchards in Shandong Province. China Fruits 2024, 3, 21–25. [Google Scholar]
- Barman, U.; Choudhury, R.D. Smartphone image based digital chlorophyll meter to estimate the value of citrus leaves chlorophyll using linear regression, LMBP–ANN and SCGBP–ANN. J. King Saud Univ. Comput. Inf. Sci. 2022, 34, 2938–2950. [Google Scholar] [CrossRef]
- Liu, Y.; Hatou, K.; Aihara, T.; Kurose, S.; Akiyama, T.; Kohno, Y.; Lu, S.; Omasa, K. A robust vegetation index based on different UAV RGB images to estimate SPAD values of naked barley leaves. Remote Sens. 2021, 13, 686. [Google Scholar] [CrossRef]
- Liu, Y. Exploring the Application of UAV-Based Remote Sensing Technology in Agricultural Pest and Disease Monitoring. Hebei Agric. Mach. 2025, 4, 21–23. [Google Scholar]
- Deng, S.; Zhao, Y.; Bai, X.; Li, X.; Sun, Z.; Liang, J.; Li, Z.; Cheng, S. Inversion of chlorophyll and leaf area index for winter wheat based on UAV image segmentation. Trans. Chin. Soc. Agric. Eng. 2022, 38, 136–145. [Google Scholar]
- Liu, Y. Correlation between Detection of Chlorophyll Content in Hami Melon Canopy and Crop Phenotype Based on Digital Images. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2023. [Google Scholar]
- Yin, H.; Li, F.; Yang, H.; Li, Y. Estimation of canopy chlorophyll in potato based on UAV hyperspectral images. J. Plant Nutr. Fertil. 2021, 27, 2184–2195. [Google Scholar]
- Liu, B.; Yuan, D.; Ding, L.; Li, Y.; Xu, Y.; Wang, W. Estimation of SPAD and nitrogen values of winter wheat based on unmanned aerial vehicle multispectral images. J. Chin. Agric. Mech. 2025, 46, 144–150. [Google Scholar] [CrossRef]
- Ma, W.; Han, W.; Zhang, H.; Cui, X.; Zhai, X.; Zhang, L.; Shao, G.; Niu, Y.; Huang, S. UAV multispectral remote sensing for the estimation of SPAD values at various growth stages of maize under different irrigation levels. Comput. Electron. Agric. 2024, 227, 109566. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhu, Y.; Wang, A.; Zhang, L. Accurate Inversion of Rice Chlorophyll Content by Integrating MultIspectral and Textture Features Derived from UAV Multispectral Imagery. Trans. Chin. Soc. Agric. Mach. 2024, 55, 287–293. [Google Scholar]
- Gu, H.; Xue, C.; Wang, G.; Lan, Y.; Wang, H.; Song, C. UAV-Based Multispectral Inversion of Integrated Cotton Growth. Agronomy 2024, 14, 2903. [Google Scholar] [CrossRef]
- Ma, L.; Hu, N.; Li, W.; Qin, W.; Huang, S.; Wang, Z.; Li, F.; Yu, K. Using multispectral drone data to monitor maize’s response to various irrigation modes. J. Plant Nutr. Fertil. 2022, 28, 743–753. [Google Scholar]
- Li, Z.; Su, J.; Fei, C.; Li, Y.; Liu, N.; Fan, H.; Chen, B. Estimation of chlorophyll content in sugar beet under drip irrigation based on hyperspectral data. J. Agric. Resour. Environ. 2020, 37, 761–769. [Google Scholar]
- Zhou, M.; Shao, G.; Zhang, L.; Liu, Z.; Han, W. Multi-spectral Inversion of SPAD Value of Winter Wheat Based on Unmanned Aerial Vehicle Remote Sensing. Water Sav. Irrig. 2019, 9, 40–45. [Google Scholar]
- Huo, Y.; Zhao, S.; Zhao, G.; Sun, J.; Hu, S. Detection Method of Chlorophyll Content in Canopy of Kiwifruit Orchard Based on UAV. Trans. Chin. Soc. Agric. Mach. 2024, 55, 297–307. [Google Scholar]
- Samarakoon, S.; Rupasinghe, C.; Seneweera, S. Coconut leaf nitrogen measurement using different vegetative indices and multispectral images. Smart Agric. Technol. 2025, 10, 100672. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.; Huo, Z.; Liu, C.; Wang, W.; Ding, L. UAV Multi-Spectral Remote Sensing Estimation of Wheat Canopy SPAD Value in Different Growth Periods. Spectrosc. Spectr. Anal. 2023, 43, 1912–1920. [Google Scholar]
- Zhou, H.; Huang, F.; Lou, W.; Gu, Q.; Ye, Z.; Hu, H.; Zhang, X. Yield prediction through UAV-based multispectral imaging and deep learning in rice breeding trials. Agric. Syst. 2025, 223, 104214. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Y.; Li, W.; Wang, J.; Wang, W.; Ahmad, I.; Huo, Z. Estimation of Winter Wheat SPAD Values Based on UAV Multispectral Remote Sensing. Remote Sens. 2023, 15, 3595. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Guo, L.; Wu, Y. Wheat leaf area index prediction using data fusion based on high-resolution unmanned aerial vehicle imagery. Plant Methods 2022, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, Y. Chlorophyll Content Determination and Its Relationship with SPAD Value in Wheat. Hubei Agric. Sci. 2010, 49, 2701–2702+2751. [Google Scholar]
- Boiarskii, B.; Hasegawa, H. Comparison of NDVI and NDRE indices to detect differences in vegetation and chlorophyll content. J. Mech. Contin. Math. Sci. 2019, 4, 20–29. [Google Scholar] [CrossRef]
- Lelong, C.C.D.; Burger, P.; Jubelin, G.; Roux, B.; Labbé, S.; Baret, F. Assessment of Unmanned Aerial Vehicles Imagery for Quantitative Monitoring of Wheat Crop in Small Plots. Sensors 2008, 8, 3557. [Google Scholar] [CrossRef]
- Zurita-Milla, R.; Kaiser, G.; Clevers, J.G.P.W.; Schneider, W.; Schaepman, M.E. Downscaling time series of MERIS full resolution data to monitor vegetation seasonal dynamics. Remote Sens. Environ. 2009, 113, 1874–1885. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Chen, J.; Zhang, Z.; Fu, Q.; Bian, J.; Cui, T.; Ma, Y. Retrieval of cotton plant water content by UAV-based vegetation supply water index (VSWI). Int. J. Remote Sens. Environ. 2020, 41, 4389–4407. [Google Scholar] [CrossRef]
- Liu, H.; Bruning, B.; Garnett, T.; Berger, B. The Performances of Hyperspectral Sensors for Proximal Sensing of Nitrogen Levels in Wheat. Sensors 2020, 20, 4550. [Google Scholar] [CrossRef]
- Li, D.; Ruan, S.; Hu, Q.; Zhang, J.; Zhang, Y.; Dian, Y.; Hu, C.; Liu, Y.; Lei, H.; Zhou, J. Nitrogen estimation and spatial analysis of orchard canopy based on UAV remote sensing. J. Huazhong Agric. Univ. 2023, 42, 158–166. [Google Scholar]
- Ren, H.; Zhou, G.; Zhang, F. Using negative soil adjustment factor in soil-adjusted vegetation index (SAVI) for aboveground living biomass estimation in arid grasslands. Remote Sens. Environ. 2018, 209, 439–445. [Google Scholar] [CrossRef]
- Radočaj, D.; Šiljeg, A.; Marinović, R.; Jurišić, M. State of Major Vegetation Indices in Precision Agriculture Studies Indexed in Web of Science: A Review. Agriculture 2023, 13, 707. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawamura, K.; Maki, M.; Muramoto, Y.; Yoshida, K.; Akiyama, T. Spectral Index for Quantifying Leaf Area Index of Winter Wheat by Field Hyperspectral Measurements: A Case Study in Gifu Prefecture, Central Japan. Remote Sens. 2015, 7, 5329–5346. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, N.; Wang, K.; Sun, H.; Wang, D.; Qiao, J. Effect of Mechanical Compaction on Soybean Yield Based on Machine Learning. Trans. Chin. Soc. Agric. Mach. 2023, 54, 139–147. [Google Scholar]
- Zhang, C.; Xue, Y. Estimation of Biochemical Pigment Content in Poplar Leaves Using Proximal Multispectral Imaging and Regression Modeling Combined with Feature Selection. Sensors 2023, 24, 217. [Google Scholar] [CrossRef]
- Zhou, Y. Monitoring Wheat Growth and Yield Estimation Using UAV Image Data. Mater’s Thesis, Yangzhou University, Yangzhou, China, 2023. [Google Scholar]
- Sarkar, T.K.; Roy, D.K.; Kang, Y.S.; Jun, S.R.; Park, J.W.; Ryu, C.S. Ensemble of Machine Learning Algorithms for Rice Grain Yield Prediction Using UAV-Based Remote Sensing. J. Biosyst. Eng. 2023, 49, 1–19. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, Z.; Hu, Q.; Bao, C.; Zhang, R. Construction and Verification of Pulmonary Nodules Invasion Prediction Model Based on XGBoost Machine Learning Algorithm: A Two-center Study. Chin. J. CT MRI 2024, 22, 166–169. [Google Scholar]
- Wang, W.; Cheng, Y.; Ren, Y.; Zhang, Z.; Geng, H. Prediction of Chlorophyll Content in Multi-Temporal Winter Wheat Based on Multispectral and Machine Learning. Front. Plant Sci. 2022, 13, 896408. [Google Scholar] [CrossRef]
- Huang, Y. Key Technology Research on Chlorophyll Content Monitoring of Fruit Trees. Ph.D. Thesis, Hebei Agricultural University, Baoding, China, 2022. [Google Scholar]
- Guo, K.; Ye, Y.; Yang, Z.; Fang, X. Adaptability Evaluation of Winter Wheat Yield Estimation Based on LSTM. Resour. Guide 2025, 2, 39–43. [Google Scholar]
- Peng, Y.; Fan, M.; Song, J.Y.; Cui, T.; Li, R. Assessment of plant species diversity based on hyperspectral indices at a fine scale. Sci. Rep. 2018, 8, 4776. [Google Scholar] [CrossRef]
- López-Calderón, M.J.; Estrada-Ávalos, J.; Rodríguez-Moreno, V.M.; Mauricio-Ruvalcaba, J.E.; Martínez-Sifuentes, A.R.; Delgado-Ramírez, G.; Miguel-Valle, E. Estimation of total nitrogen content in forage maize (Zea mays L.) using spectral indices: Analysis by random forest. Agriculture 2020, 10, 451. [Google Scholar] [CrossRef]
- Qi, H.; Sun, H.; Lü, L.; Li, S.; Min, J.; Hou, L. Wheat Leaf Area Index Estimation Based on Fusion of UAV Multispectral Information and Texture Features. Trans. Chin. Soc. Agric. Mach. 2025, 56, 334–344. [Google Scholar]
- Ma, R.; Chen, Y.; Hou, P.; Ren, X. Progress on Hyperspectral Remote Sensing Inversion Method for Vegetation Chlorophyll Content. For. Grassl. Resour. Res. 2024, 5, 166–178. [Google Scholar]
- Jiang, H.; Yang, H.; Chen, X.; Wang, S.; Li, X.; Liu, K.; Cen, Y. Research on Accuracy and Stability of Inversing Vegetation Chlorophyll Content by Spectral Index Method. Spectrosc. Spectr. Anal. 2015, 35, 975–981. [Google Scholar]


| Image Acquisition Time | Growth Stage | Number of Images |
|---|---|---|
| 11 May 2024 | Flowering stage | 865 |
| 28 June 2024 | Fruit-setting stage | 865 |
| 28 July 2024 | Fruit enlargement stage | 865 |
| 30 August 2024 | Fruit-coloring stage | 865 |
| 7 October 2024 | Maturation stage | 865 |
| Band Number | Band Name | Center Wavelength (nm) | Bandwidth (nm) | Gray Panel Reflectance |
|---|---|---|---|---|
| 1 | Green | 560 | 32 | 0.5102 |
| 2 | Red | 650 | 32 | 0.5071 |
| 3 | Red Edge | 730 | 32 | 0.5086 |
| 4 | NIR | 840 | 52 | 0.4945 |
| Vegetation Index | Value | References |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (RNIR − RRED)/(RNIR + RRED) | [24] |
| Green Normalized Difference Vegetation Index (GNDVI) | (RNIR − RGREEN)/(RNIR + RGREEN) | [25] |
| Leaf Chlorophyll Index (LCI) | (RNIR − RREDEDGE)/(RNIR + RRED) | [26] |
| Greenness Index (GI) | RGREEN/RRED | [27] |
| Ratio Vegetation Index (RVI) | RNIR/RRED | [28] |
| Difference vegetation index (DVI) | RNIR − RRED | [28] |
| Green Ratio Vegetation Index (GRVI) | RNIR/RGREEN | [29] |
| Green Difference Vegetation Index (GDVI) | RNIR − RGREEN | [29] |
| Red–Green Ratio Index (RGRI) | RRED/RGREEN | [29] |
| Soil-Adjusted Vegetation Index (SAVI) | 1.5 × (RNIR − RRED)/(RNIR + RRED + 0.5) | [30] |
| Optimized Soil-Adjusted Vegetation Index (OSAVI) | 1.16 × (RNIR − RRED)/(RNIR + RRED + 0.16) | [31] |
| Normalized Difference Red Edge Index (NDRE) | (RNIR − RRED)/(RNIR + RRED) | [24] |
| Modified Simple Ratio (MSR) | (RNIR − RGREEN)/(RNIR + RGREEN) | [32] |
| Enhanced Vegetation Index 2(EVI2) | (RNIR − RREDEDGE)/(RNIR + RRED) | [32] |
| Normalized Difference Red Edge Index(NRI) | RGREEN/RRED | [32] |
| Growth Stage | Sample Size | Minimum | Maximum | Mean ± Standard Deviation | Coefficient of Variation |
|---|---|---|---|---|---|
| Flowering stage | 55 | 29.163 | 37.369 | 32.739 ± 1.827 | 5.6% |
| Fruit-setting stage | 55 | 37.950 | 47.562 | 43.051 ± 2.185 | 5.1% |
| Fruit enlargement stage | 55 | 42.492 | 50.367 | 45.645 ± 1.913 | 4.2% |
| Fruit-coloring stage | 55 | 42.253 | 48.908 | 45.796 ± 1.737 | 3.8% |
| Maturation stage | 55 | 32.200 | 40.817 | 35.734 ± 2.267 | 6.3% |
| Growth Stage | Model | Training Set | Validation Set | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE | MAE | R2 | RMSE | MAE | ||
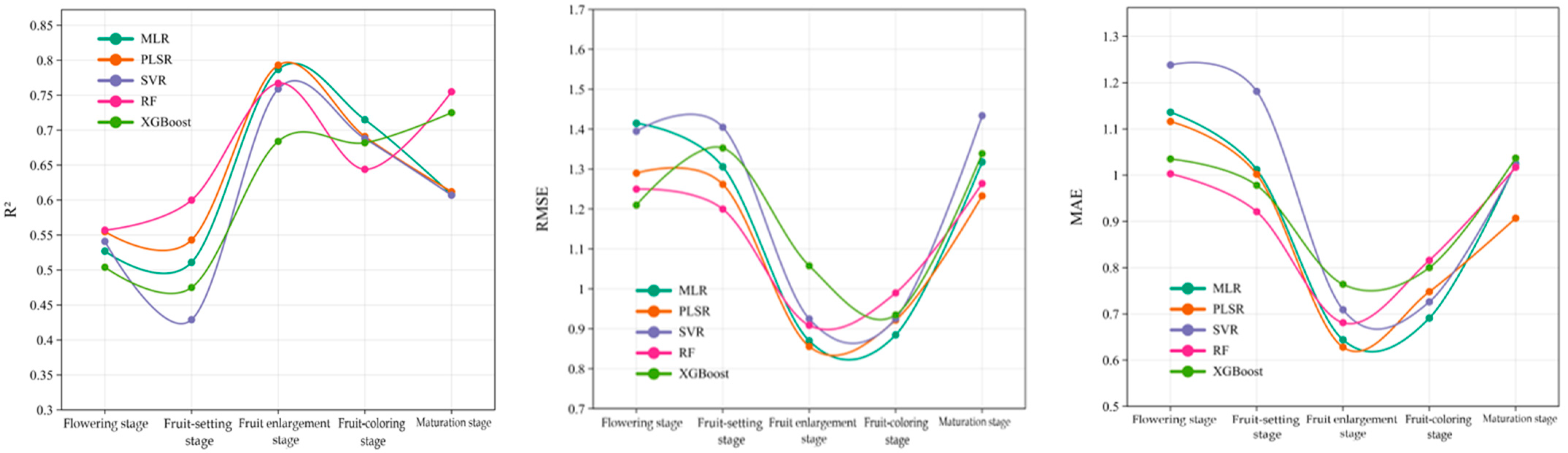
| Flowering stage | MLR | 0.545 | 1.130 | 0.900 | 0.527 | 1.415 | 1.136 |
| PLSR | 0.457 | 1.283 | 1.010 | 0.555 | 1.290 | 1.116 | |
| SVR | 0.434 | 1.263 | 0.935 | 0.541 | 1.395 | 1.238 | |
| RF | 0.532 | 1.188 | 0.984 | 0.557 | 1.250 | 1.003 | |
| XGBoost | 0.541 | 1.249 | 0.966 | 0.504 | 1.210 | 1.035 | |
| Fruit-setting stage | MLR | 0.610 | 1.393 | 1.152 | 0.511 | 1.306 | 1.012 |
| PLSR | 0.461 | 1.639 | 1.336 | 0.543 | 1.262 | 1.002 | |
| SVR | 0.427 | 1.733 | 1.398 | 0.429 | 1.405 | 1.181 | |
| RF | 0.522 | 1.573 | 1.213 | 0.600 | 1.200 | 0.921 | |
| XGBoost | 0.480 | 1.610 | 1.254 | 0.475 | 1.353 | 0.978 | |
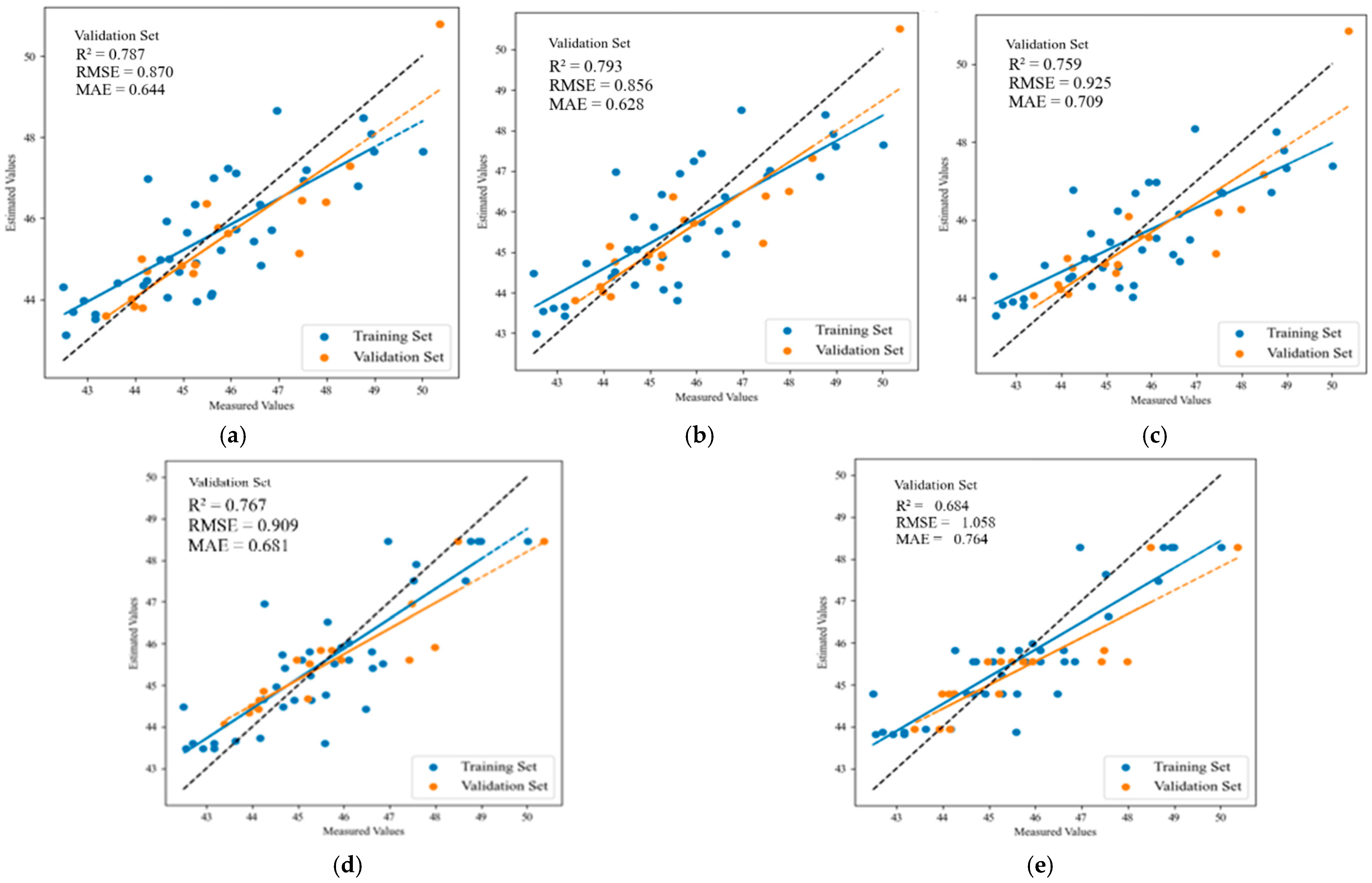
| Fruit enlargement stage | MLR | 0.635 | 1.148 | 0.964 | 0.787 | 0.870 | 0.644 |
| PLSR | 0.630 | 1.155 | 0.970 | 0.793 | 0.856 | 0.628 | |
| SVR | 0.612 | 1.182 | 1.022 | 0.759 | 0.925 | 0.709 | |
| RF | 0.727 | 0.991 | 0.765 | 0.767 | 0.909 | 0.681 | |
| XGBoost | 0.752 | 0.945 | 0.772 | 0.684 | 1.058 | 0.764 | |
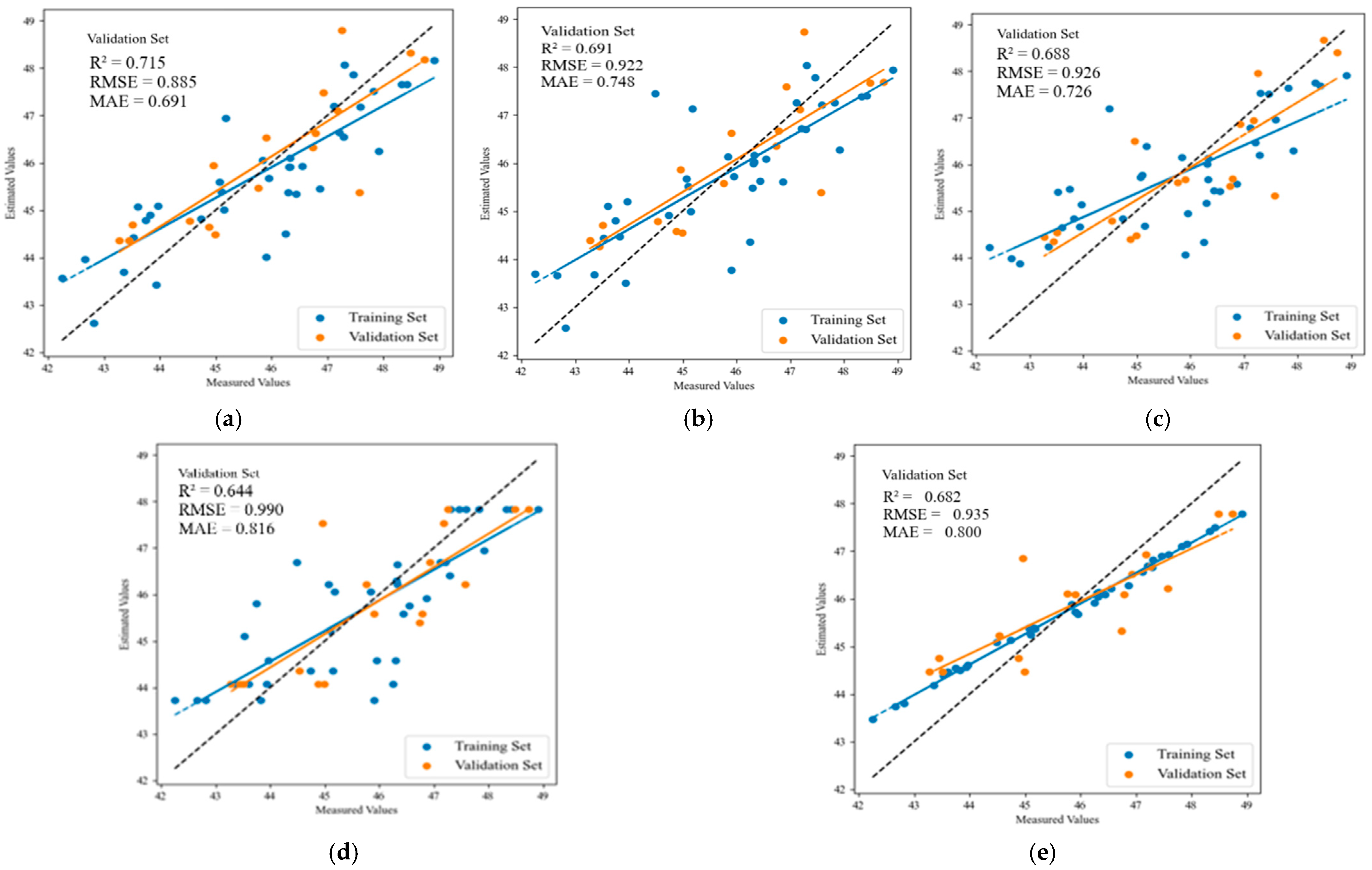
| Fruit-coloring stage | MLR | 0.648 | 1.034 | 0.826 | 0.715 | 0.885 | 0.691 |
| PLSR | 0.640 | 1.045 | 0.828 | 0.691 | 0.922 | 0.748 | |
| SVR | 0.580 | 1.131 | 0.961 | 0.688 | 0.926 | 0.726 | |
| RF | 0.647 | 1.036 | 0.827 | 0.644 | 0.990 | 0.816 | |
| XGBoost | 0.867 | 0.636 | 0.559 | 0.682 | 0.935 | 0.800 | |
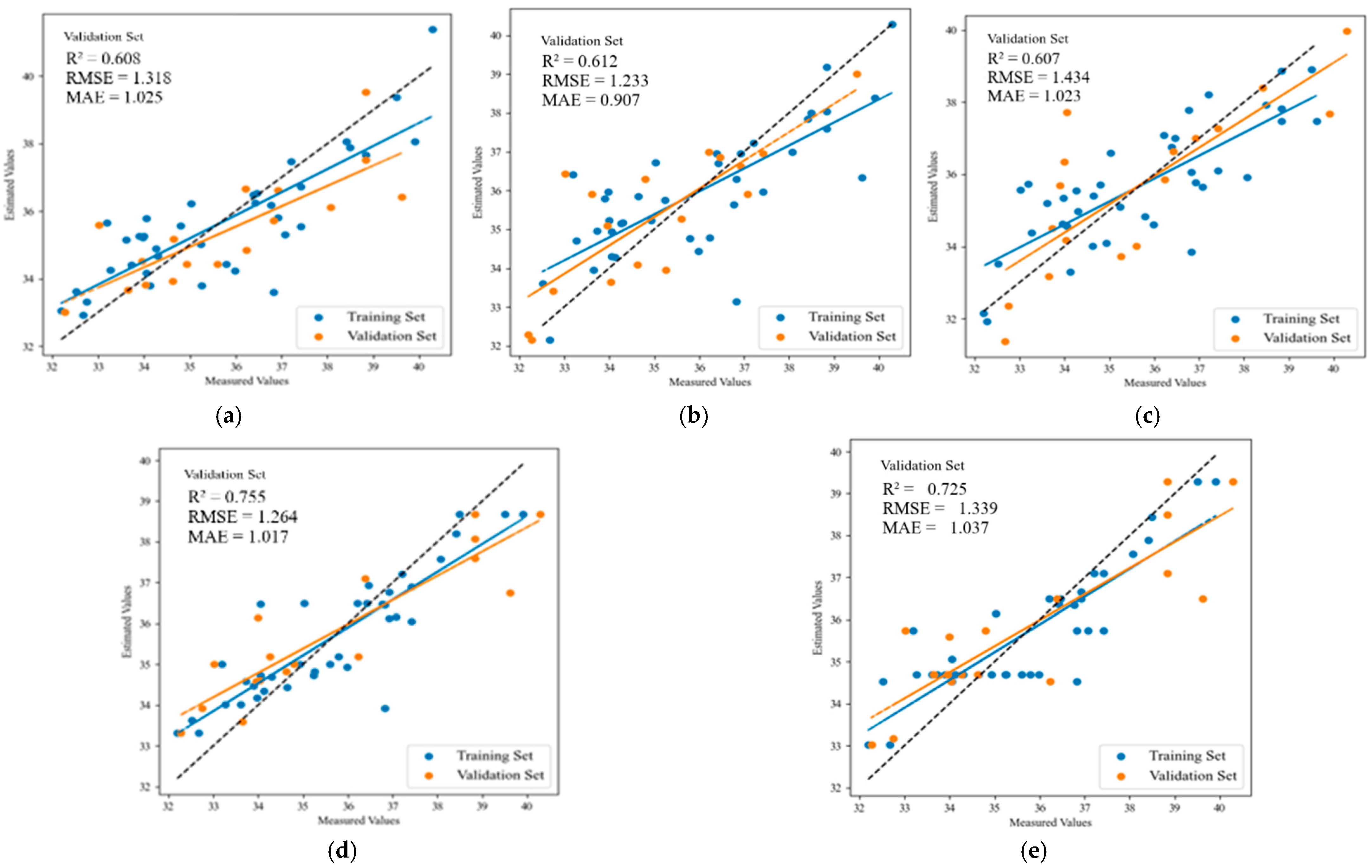
| Maturation stage | MLR | 0.685 | 1.212 | 0.983 | 0.608 | 1.318 | 1.025 |
| PLSR | 0.593 | 1.376 | 1.071 | 0.612 | 1.233 | 0.907 | |
| SVR | 0.625 | 1.272 | 1.078 | 0.607 | 1.434 | 1.023 | |
| RF | 0.759 | 0.947 | 0.716 | 0.755 | 1.264 | 1.017 | |
| XGBoost | 0.742 | 0.982 | 0.770 | 0.725 | 1.339 | 1.037 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Y.; Han, F.; Shi, Z.; Zhao, F.; Zhang, F.; Pan, W.; Zhang, Z.; Cui, Q. Estimation of Canopy Chlorophyll Content of Apple Trees Based on UAV Multispectral Remote Sensing Images. Agriculture 2025, 15, 1308. https://doi.org/10.3390/agriculture15121308
Wang J, Zhang Y, Han F, Shi Z, Zhao F, Zhang F, Pan W, Zhang Z, Cui Q. Estimation of Canopy Chlorophyll Content of Apple Trees Based on UAV Multispectral Remote Sensing Images. Agriculture. 2025; 15(12):1308. https://doi.org/10.3390/agriculture15121308
Chicago/Turabian StyleWang, Juxia, Yu Zhang, Fei Han, Zhenpeng Shi, Fu Zhao, Fengzi Zhang, Weizheng Pan, Zhiyong Zhang, and Qingliang Cui. 2025. "Estimation of Canopy Chlorophyll Content of Apple Trees Based on UAV Multispectral Remote Sensing Images" Agriculture 15, no. 12: 1308. https://doi.org/10.3390/agriculture15121308
APA StyleWang, J., Zhang, Y., Han, F., Shi, Z., Zhao, F., Zhang, F., Pan, W., Zhang, Z., & Cui, Q. (2025). Estimation of Canopy Chlorophyll Content of Apple Trees Based on UAV Multispectral Remote Sensing Images. Agriculture, 15(12), 1308. https://doi.org/10.3390/agriculture15121308





