Comparative Assessment of Injection and Compression Molding on Soy Protein Bioplastic Matrices for Controlled Iron Release in Horticulture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soy-Protein-Based Systems
2.3. Characterization of Soy-Protein-Based Systems
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Crosslinking Degree
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Mechanical Properties
2.3.5. Water Uptake Capacity (WUC) and Soluble Matter Loss (SML)
2.3.6. Iron Release in Water
2.3.7. Iron Release in Soil
2.3.8. Biodegradability
2.4. Statistical Analysis
3. Results and Discussion
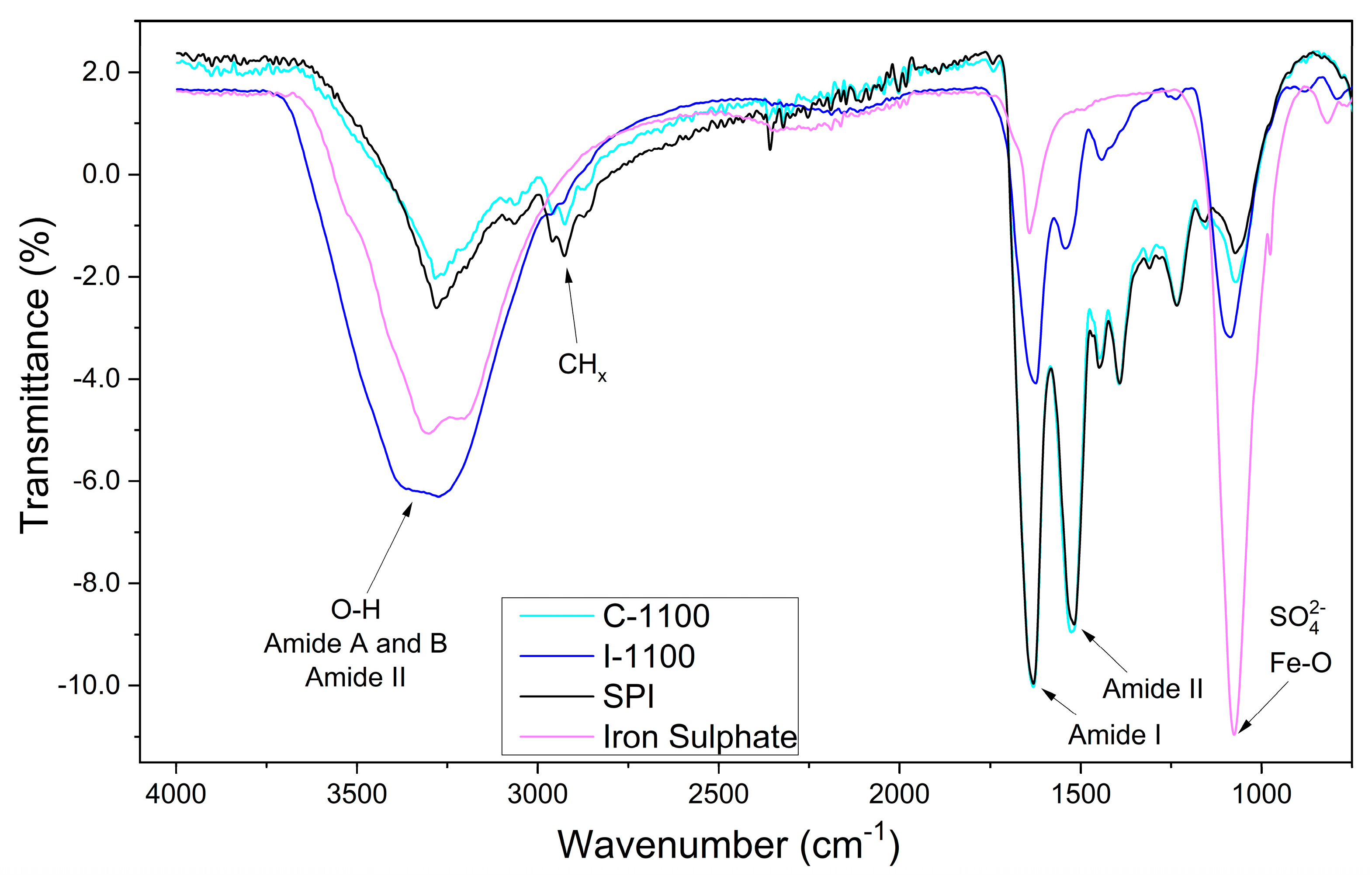
3.1. FTIR
3.2. Crosslinking Degree
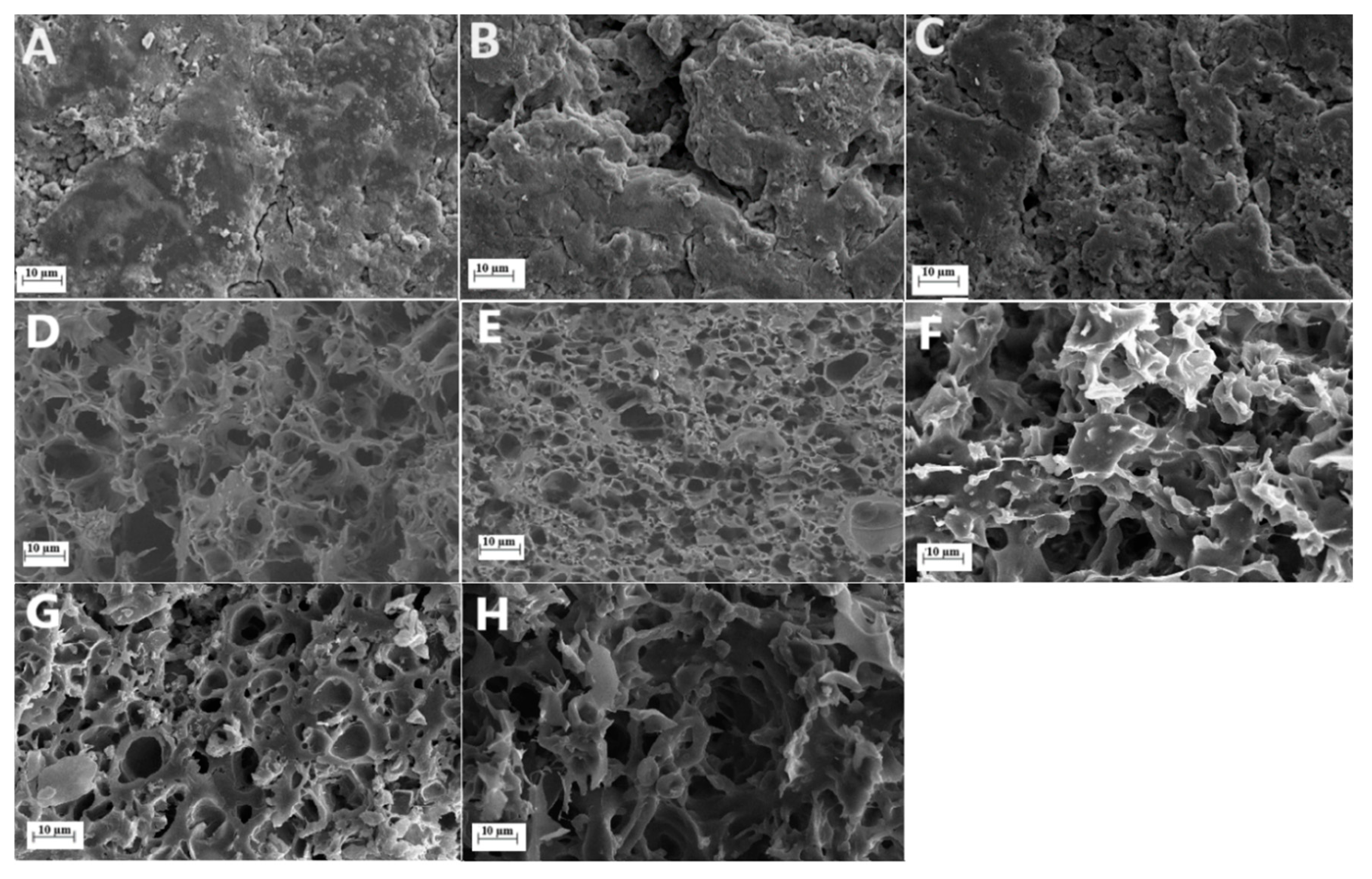
3.3. SEM
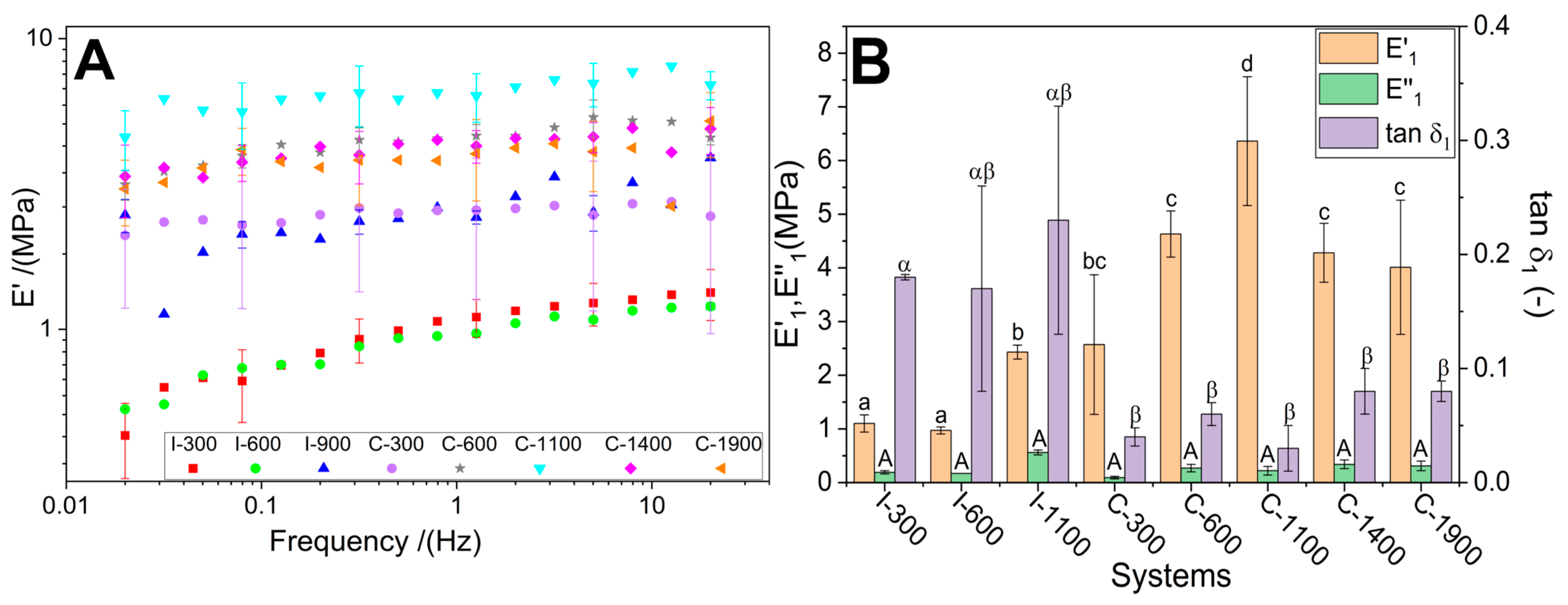
3.4. Mechanical Properties
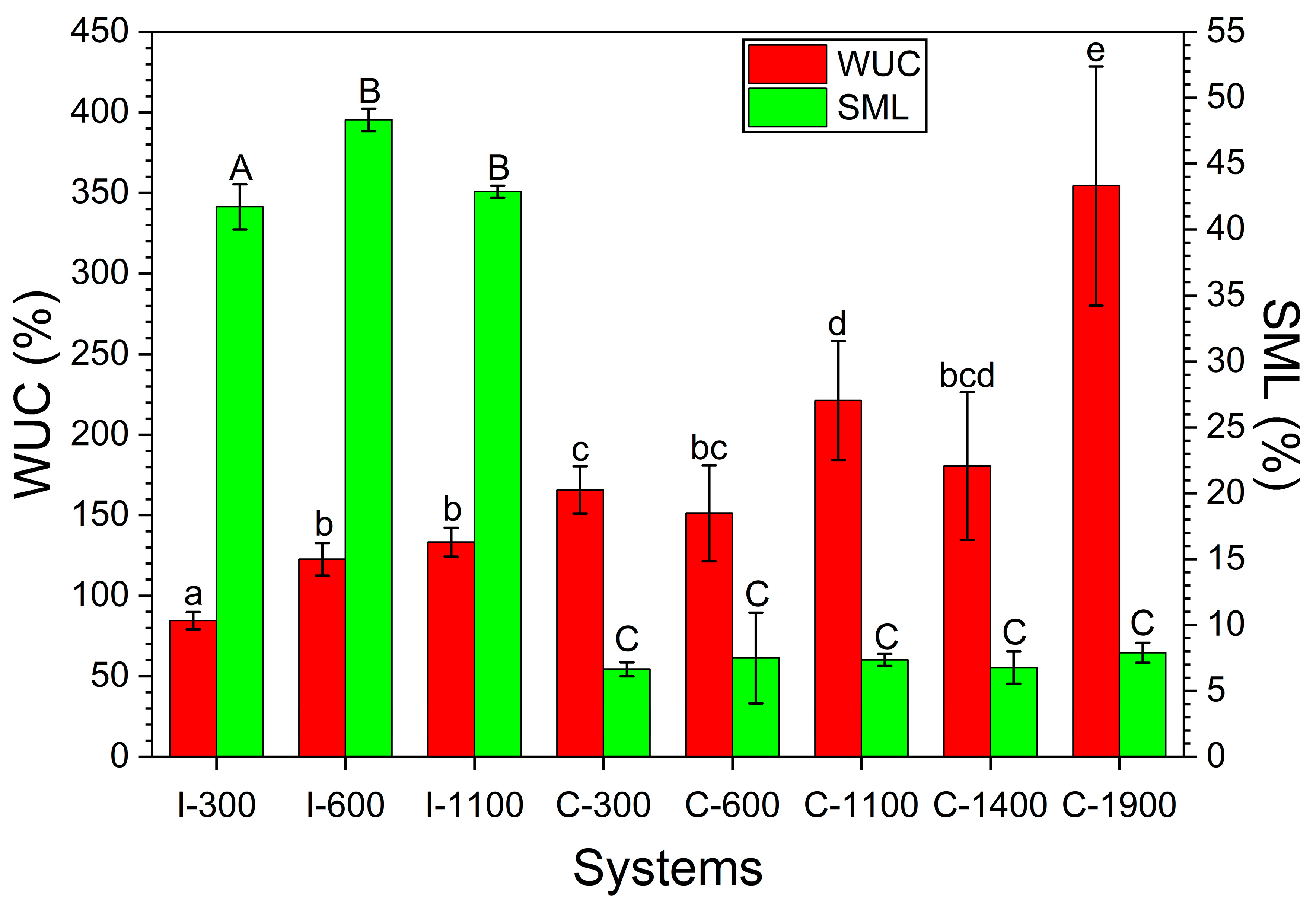
3.5. WUC and SML
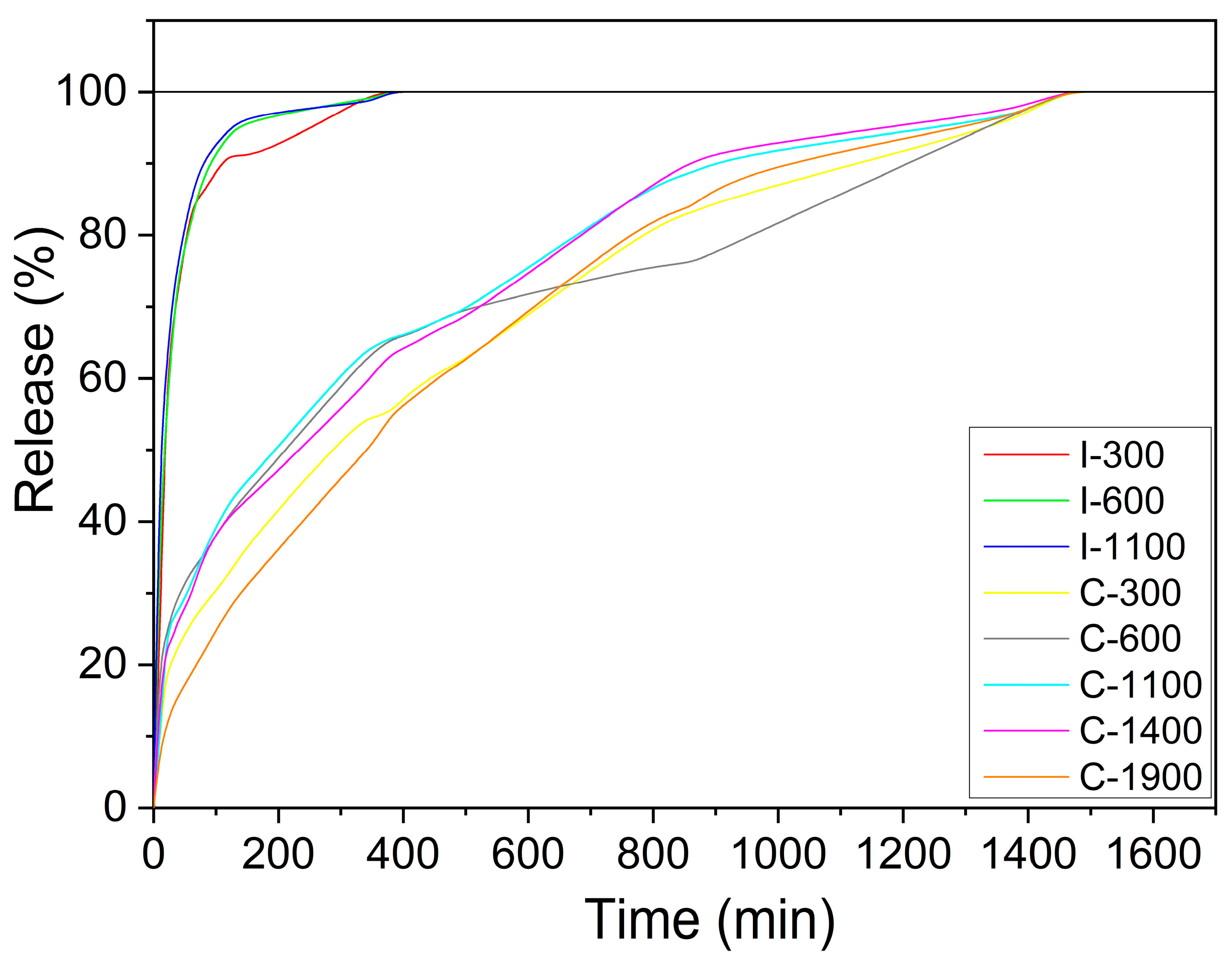
3.6. Iron Release in Water
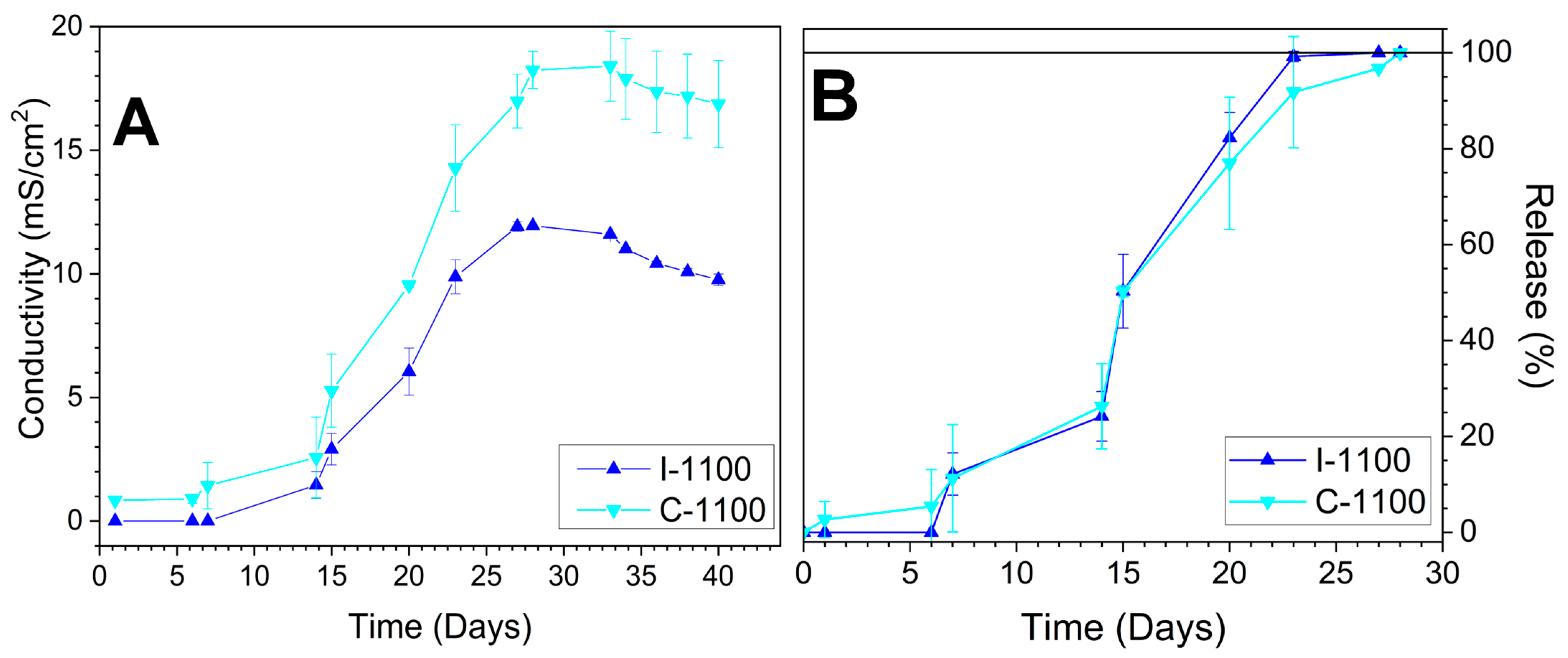
3.7. Iron Release in Soil
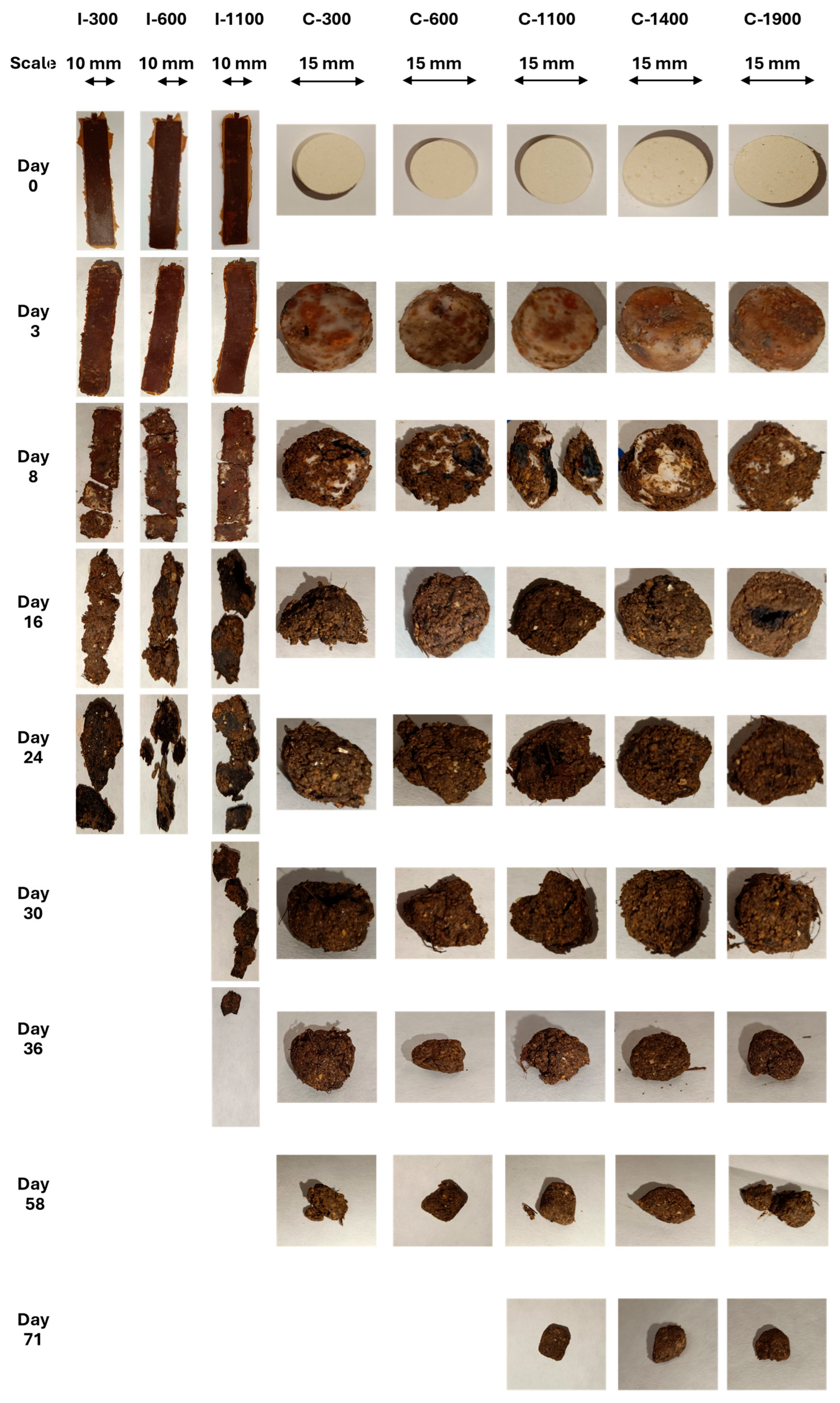
3.8. Biodegradability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economics and Social Affairs. Population Division Population Division. World Population Prospects. Available online: https://population.un.org/wpp/assets/Files/WPP2024_Summary-of-Results.pdf (accessed on 25 January 2024).
- Penuelas, J.; Coello, F.; Sardans, J. A Better Use of Fertilizers Is Needed for Global Food Security and Environmental Sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Chang, J.; Havlík, P.; Leclère, D.; de Vries, W.; Valin, H.; Deppermann, A.; Hasegawa, T.; Obersteiner, M. Reconciling Regional Nitrogen Boundaries with Global Food Security. Nat. Food 2021, 2, 700–711. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Morgan, K.T.; Cushman, K.E.; Sato, S. Release Mechanisms for Slow- and Controlled-Release Fertilizers and Strategies for Their Use in Vegetable Production. Horttechnology 2009, 19, 10–12. [Google Scholar] [CrossRef]
- Rajan, M.; Shahena, S.; Chandran, V.; Mathew, L. Controlled Release of Fertilizers—Concept, Reality, and Mechanism. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–56. [Google Scholar]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled Release Fertilizer: A Review on Developments, Applications and Potential in Agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Maya, M.G.; Abraham, J.; George, S.C.; Thomas, S. Exploring the Filler–Polymer Interaction and Solvent Transport Behavior of Nanocomposites Derived from Reduced Graphene Oxide and Polychloroprene Rubber. J. Appl. Polym. Sci. 2019, 136, 48168. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Controlled Release of Zinc from Soy Protein-Based Matrices to Plants. Agronomy 2021, 11, 580. [Google Scholar] [CrossRef]
- Cota-Arriola, O.; Onofre Cortez-Rocha, M.; Burgos-Hernández, A.; Marina Ezquerra-Brauer, J.; Plascencia-Jatomea, M. Controlled Release Matrices and Micro/Nanoparticles of Chitosan with Antimicrobial Potential: Development of New Strategies for Microbial Control in Agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Alonso-González, M.; Rubio-Valle, J.F.; Perez-Puyana, V.; Romero, A. Biodegradable Soy Protein-based Matrices for the Controlled Release of Zinc in Horticulture. J. Appl. Polym. Sci. 2020, 137, 49187. [Google Scholar] [CrossRef]
- Chanda, M.; Roy, S.K. Plastics Technology Handbook; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429124020. [Google Scholar]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-Based Biostimulants to Enhance Plant Growth—State-of-the-Art and Future Direction with Sugar Beet as an Example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Chan, W.Y. Proteins in the Design of Sustainable Plastics Alternatives. MRS Commun. 2023, 13, 1009–1024. [Google Scholar] [CrossRef]
- Fowler, P.A.; Hughes, J.M.; Elias, R.M. Biocomposites: Technology, Environmental Credentials and Market Forces. J. Sci. Food Agric. 2006, 86, 1781–1789. [Google Scholar] [CrossRef]
- Otálora González, C.M.; Alvarez Castillo, E.; Flores, S.; Gerschenson, L.N.; Bengoechea, C. Effect of Plasticizer Composition on the Properties of Injection Molded Cassava Starch-Based Bioplastics. Food Packag. Shelf Life 2023, 40, 101218. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Rubio-Valle, J.F.; Guerrero, A.; Romero, A. Processing of Biodegradable and Multifunctional Protein-based Polymer Materials for the Potential Controlled Release of Zinc and Water in Horticulture. J. Appl. Polym. Sci. 2020, 137, 49419. [Google Scholar] [CrossRef]
- Vass, P.; Nagy, Z.K.; Kóczián, R.; Fehér, C.; Démuth, B.; Szabó, E.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous Drying of a Protein-Type Drug Using Scaled-up Fiber Formation with HP-β-CD Matrix Resulting in a Directly Compressible Powder for Tableting. Eur. J. Pharm. Sci. 2020, 141, 105089. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Di Foggia, M.; Rosignoli, S.; Guerrero, A.; Rombolà, A.D.; Romero, A. Effect of Zinc and Protein Content in Different Barley Cultivars: Use of Controlled Release Matrices. Renew. Agric. Food Syst. 2023, 38, e34. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Selected Biopolymers’ Processing and Their Applications: A Review. Polymers 2023, 15, 641. [Google Scholar] [CrossRef]
- Etxabide, A.; de la Caba, K.; Guerrero, P. A Novel Approach to Manufacture Porous Biocomposites Using Extrusion and Injection Moulding. Eur. Polym. J. 2016, 82, 324–333. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Protein/Glycerol Blends and Injection-Molded Bioplastic Matrices: Soybean versus Egg Albumen. J. Appl. Polym. Sci. 2016, 133, 42980. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Castro-Criado, D.; Rubio-Valle, J.F.; Perez-Puyana, V.M.; Romero, A. Biodegradable Soy Protein-Based Tablets for the Controlled Release of Zinc. Ind. Crops Prod. 2024, 211, 118261. [Google Scholar] [CrossRef]
- Northover, G.H.R.; Mao, Y.; Ahmed, H.; Blasco, S.; Vilar, R.; Garcia-España, E.; Weiss, D.J. Effect of Salinity on the Zinc(II) Binding Efficiency of Siderophore Functional Groups and Implications for Salinity Tolerance Mechanisms in Barley. Sci. Rep. 2021, 11, 16704. [Google Scholar] [CrossRef]
- Nakib, D.; Slatni, T.; Di Foggia, M.; Rombolà, A.D.; Abdelly, C. Changes in Organic Compounds Secreted by Roots in Two Poaceae Species (Hordeum vulgare and Polypogon monspenliensis) Subjected to Iron Deficiency. J. Plant Res. 2021, 134, 151–163. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Romero, A.; Martínez, I.; Bengoechea, C.; Partal, P.; Guerrero, A. Effect of Aldehydes on Thermomechanical Properties of Gluten-Based Bioplastics. Food Bioprod. Process. 2014, 92, 20–29. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Bouroudian, E.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Evaluation of Different Strengthening Methods in the Mechanical and Functional Properties of Soy Protein-Based Bioplastics. J. Clean. Prod. 2020, 262, 121517. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Alonso-González, M.; Castro-Criado, D.; Felix, M.; Romero, A. Evaluation of Rice Bran Varieties and Heat Treatment for the Development of Protein/Starch-Based Bioplastics via Injection Molding. Int. J. Biol. Macromol. 2023, 253, 127503. [Google Scholar] [CrossRef]
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2005.
- Zhao, C.; Shen, Y.; Du, C.; Zhou, J.; Wang, H.; Chen, X. Evaluation of Waterborne Coating for Controlled-Release Fertilizer Using Wurster Fluidized Bed. Ind. Eng. Chem. Res. 2010, 49, 9644–9647. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and Their Potential in Controlled Release of Soil Nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of Biodegradable Polymers as Encapsulating Agents for the Development of a Urea Controlled-Release Fertilizer Using Biochar as Support Material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- ISO 20200:2004; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Simulated Composting Conditions in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2004.
- Türker-Kaya, S.; Huck, C. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Maigret, J.; Lourdin, D.; Guerrero, A.; Romero, A. Injection Molding versus Extrusion in the Manufacturing of Soy Protein-based Bioplastics with Zinc Incorporated. J. Appl. Polym. Sci. 2022, 139, 51630. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Gomez, M.A.; Capitani, G.; Sbroscia, M.; Sodo, A. High-Temperature Study of Basic Ferric Sulfate, FeOHSO4. Phys. Chem. Miner. 2020, 47, 43. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Maigret, J.-E.; Perez-Puyana, V.; Romero, A.; Lourdin, D. Revaluation of a Soy Protein by-Product in Eco-Friendly Bioplastics by Extrusion. J. Polym. Environ. 2022, 30, 1587–1599. [Google Scholar] [CrossRef]
- Ajomiwe, N.; Boland, M.; Phongthai, S.; Bagiyal, M.; Singh, J.; Kaur, L. Protein Nutrition: Understanding Structure, Digestibility, and Bioavailability for Optimal Health. Foods 2024, 13, 1771. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic Processing of Proteins for Film Formation—A Review. J. Food Sci. 2008, 73, R30–R39. [Google Scholar] [CrossRef]
- Capezza, A.J.; Robert, E.; Lundman, M.; Newson, W.R.; Johansson, E.; Hedenqvist, M.S.; Olsson, R.T. Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics. Polymers 2020, 12, 459. [Google Scholar] [CrossRef]
- Lay, S.; Guyon, A.; Chaix, J.-M.; Carry, C.; Pötschke, J. Grain Boundary Segregation in Sintered Materials: Effect on Densification and Grain Growth. In Proceedings of the European Congress and Exhibition on Powder Metallurgy, European PM Conference Proceedings, Hamburg, Germany, 9–13 October 2016; pp. 1–6. [Google Scholar]
- Alonso-González, M.; Felix, M.; Romero, A.; Sergi, C.; Bavasso, I.; Sarasini, F. Optimization of Processing Conditions for Rice Bran-Based Bioplastics Through Extrusion and Injection Molding. J. Polym. Environ. 2024, 33, 512–527. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Camacho-Hernández, I.L.; Rodríguez-González, F.J.; Martínez-Bustos, F.; Calderón-Castro, A.; Zazueta-Morales, J.d.J.; Aguilar-Palazuelos, E. Effect of Compounding and Plastic Processing Methods on the Development of Bioplastics Based on Acetylated Starch Reinforced with Sugarcane Bagasse Cellulose Fibers. Ind. Crops Prod. 2023, 192, 116084. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Thermomechanical Properties and Water Uptake Capacity of Soy Protein-Based Bioplastics Processed by Injection Molding. J. Appl. Polym. Sci. 2016, 133, 43524. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Effect of the Injection Moulding Processing Conditions on the Development of Pea Protein-based Bioplastics. J. Appl. Polym. Sci. 2016, 133, 43306. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Development of Eco-Friendly Biodegradable Superabsorbent Materials Obtained by Injection Moulding. J. Clean. Prod. 2018, 198, 312–319. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Sandía, J.A.; Cordobés, F.; Guerrero, A. Development of Novel Soy-Protein-Based Superabsorbent Matrixes through the Addition of Salts. J. Appl. Polym. Sci. 2019, 136, 47012. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Romero, A.; Bengoechea, C.; Guerrero, A. Natural Superabsorbent Plastic Materials Based on a Functionalized Soy Protein. Polym. Test. 2017, 58, 126–134. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and Characterization of a Novel Super-Absorbent Based on Wheat Straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Messa, L.; Froes, J.D.; Souza, C.; Faez, R. Híbridos de Quitosana-Argila Para Encapsulamento e Liberação Sustentada Do Fertilizante Nitrato de Potássio. Quim. Nova 2016, 39, 1215–1220. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Micronutrient-Controlled-Release Protein-Based Systems for Horticulture: Micro vs. Nanoparticles. Ind. Crops Prod. 2022, 185, 115128. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Function of Micronutrients in Plants. In Micronutrients in Agriculture; Mortvedt, J.J., Ed.; Soil Science Society of America: Madison, WI, USA, 1991; Volume 4, pp. 297–328. [Google Scholar]
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781003033615. [Google Scholar]
- Castro-Criado, D.; Abdullah, J.A.A.; Romero, A.; Jiménez-Rosado, M. Stabilization and Valorization of Beer Bagasse to Obtain Bioplastics. Polymers 2023, 15, 1877. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
| System | %DC | LSD |
|---|---|---|
| I-300 | 98.44 a | 1.73 |
| I-600 | 99.26 a | 1.73 |
| I-1100 | 98.12 a | 1.73 |
| C-300 | 89.84 bc | 1.73 |
| C-600 | 88.98 bc | 1.73 |
| C-1100 | 88.54 b | 1.73 |
| C-1400 | 91.51 c | 1.73 |
| C-1900 | 89.52 bc | 1.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Criado, D.; Jiménez-Rosado, M.; Pérez-Puyana, V.M.; Romero, A. Comparative Assessment of Injection and Compression Molding on Soy Protein Bioplastic Matrices for Controlled Iron Release in Horticulture. Agriculture 2025, 15, 1298. https://doi.org/10.3390/agriculture15121298
Castro-Criado D, Jiménez-Rosado M, Pérez-Puyana VM, Romero A. Comparative Assessment of Injection and Compression Molding on Soy Protein Bioplastic Matrices for Controlled Iron Release in Horticulture. Agriculture. 2025; 15(12):1298. https://doi.org/10.3390/agriculture15121298
Chicago/Turabian StyleCastro-Criado, Daniel, Mercedes Jiménez-Rosado, Víctor M. Pérez-Puyana, and Alberto Romero. 2025. "Comparative Assessment of Injection and Compression Molding on Soy Protein Bioplastic Matrices for Controlled Iron Release in Horticulture" Agriculture 15, no. 12: 1298. https://doi.org/10.3390/agriculture15121298
APA StyleCastro-Criado, D., Jiménez-Rosado, M., Pérez-Puyana, V. M., & Romero, A. (2025). Comparative Assessment of Injection and Compression Molding on Soy Protein Bioplastic Matrices for Controlled Iron Release in Horticulture. Agriculture, 15(12), 1298. https://doi.org/10.3390/agriculture15121298








