Sensory and Nutritional Characteristics of Organic Italian Hazelnuts from the Lazio Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Physical Features
2.3. Sensory Analysis
2.4. Antioxidant Activity
2.4.1. Extract Preparation for Polyphenol Compounds and Antioxidant Activity Determination
2.4.2. Total Phenolic Compounds (TPCs)
2.4.3. Total Antioxidant Capacity (TAC) Determination
2.5. HS-SPME/GC-MS Determination of Volatile Chemical Composition
2.6. Statistical Analyses
3. Results and Discussion
3.1. Physical Characteristics
3.2. Sensory Analysis
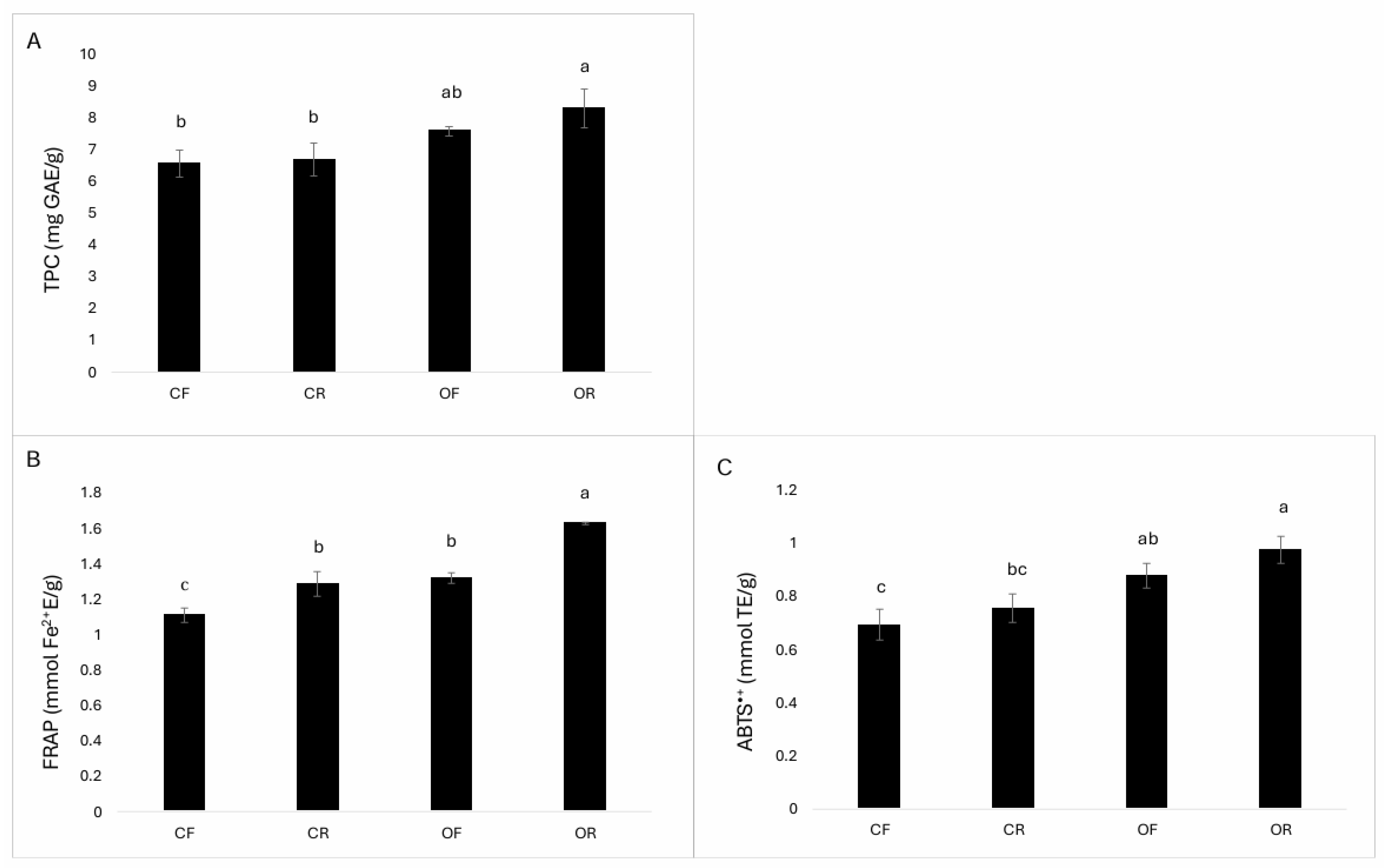
3.3. Total Phenolic Compounds (TPCs) and Total Antioxidant Capacity (TAC)
3.4. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT 2024. Food and Agriculture Organization of United Nations. Available online: http://faostat.fao.org/en/#data/QC (accessed on 24 March 2023).
- ISMEA. Institute of Services for the Agricultural and Food Market (Istituto di Servizi per il Mercato Agricolo Alimentare ISMEA). 2024. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/2284 (accessed on 31 January 2024).
- Marra, F.; Lavorgna, A.; Incarnato, L.; Malvano, F.; Albanese, D. Optimization of Hazelnut Spread Based on Total or Partial Substitution of Palm Oil. Foods 2023, 12, 3122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Lin, H.; Lin, Z. Hazelnut and its by-products: A comprehensive review of nutrition, phytochemical profile, extraction, bioactivities and applications. Food Chem. 2023, 413, 135576. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoğlu, H. Lipid characteristics, bioactive properties, and mineral content in hazelnut grown under different cultivation systems. J. Food Process. Preserv. 2022, 46, e16717. [Google Scholar] [CrossRef]
- Mohammed, D.; Freije, A.; Abdulhussain, H.; Khonji, A.; Hasan, M.; Ferraris, C.; Gasparri, C.; Aziz Aljar, M.A.; Ali Redha, A.; Giacosa, A.; et al. Analysis of the Antioxidant Activity, Lipid Profile, and Minerals of the Skin and Seed of Hazelnuts (Corylus avellana L.), Pistachios (Pistacia vera) and Almonds (Prunus dulcis) a Comparative Analysis. Appl. Chem. 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Pfeil, J.A.; Zhao, Y.; McGorrin, R.J. Chemical composition, phytochemical content, and antioxidant activity of hazelnut (Corylus avellana L.) skins from Oregon. LWT-Food Sci. Technol. 2024, 201, 116204. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
- Cui, N.; Zhao, T.; Han, Z.; Yang, Z.; Wang, G.; Ma, Q.; Liang, L. Characterisation of oil oxidation, fatty acid, carotenoid, squalene and tocopherol components of hazelnut oils obtained from three varieties undergoing oxidation. Int. J. Food Sci. Technol. 2022, 57, 3456–3466. [Google Scholar] [CrossRef]
- Costantini, L.; Frangipane, M.T.; Molinari, R.; Garzoli, S.; Massantini, R.; Merendino, N. Hazelnut Skin Waste as a Functional Ingredient to Nutritionally Improve a Classic Shortbread Cookie Recipe. Foods 2023, 12, 2774. [Google Scholar] [CrossRef]
- Daştan, E.; Çelik, O.F.; Baș, O.; Bulut, Z.; Lindemann, S.R.; Tugay, M.I.; Tunçil, E.Y. Sex-dependent colonic microbiota modulation by hazelnut (Corylus avellana L.) dietary fiber. Food Funct. 2023, 14, 2896–2907. [Google Scholar] [CrossRef]
- Meemken, E.M.; Qaim, M. Organic Agriculture, Food Security, and the Environment. Annu. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef]
- Karaosmanoglu, H.; Ustun, N.S. Fatty Acids, Tocopherol and Phenolic Contents of Organic and Conventional Grown Hazelnuts. J. Agric. Sci. Technol. 2021, 23, 167–177. [Google Scholar]
- Squara, S.; Stilo, F.; Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Genova, G.; Castello, G.; Bicchi, C.; Cordero, C. Corylus avellana L. Aroma Blueprint: Potent Odorants Signatures in the Volatilome of High Quality Hazelnuts. Front. Plant Sci. 2022, 13, 840028. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. Morphological Traits, Kernel Composition and Sensory Evaluation of Hazelnut (Corylus avellana L.) Cultivars Grown in Poland. Agronomy 2019, 9, 703. [Google Scholar] [CrossRef]
- International Nut; Dried Fruit Council (INC). Global Statistical Review, Hazelnut. 2024. Available online: https://inc.nutfruit.org/global-statistical-review-hazelnut/ (accessed on 31 January 2024).
- Commission Regulation (EC) No 667/2009 of 22 July 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R0667 (accessed on 31 January 2024).
- Milošević, T.; Milošević, N. Determination of size and shape features of hazelnuts using multivariate analysis. Acta Sci. Pol. Hortorum Cultus 2017, 16, 49–61. [Google Scholar] [CrossRef]
- Bioversity, FAO, CIHEAM. Descriptors for hazelnut (Corylus avellana L.); Bioversity International: Rome, Italy; Food and Agriculture Organization of the United Nations: Rome, Italy; International Centre for Advanced Mediterrenean: Zaragoza, Spain, 2008; pp. 1–64. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/e0e78206-b738-44c8-ac31-b0d5ee92ee50/content (accessed on 31 January 2024).
- Mohsenin, N.N. Physical Properties of Plant and Animal Material; Gordon and Breach Science Publishers Inc.: New York, NY, USA, 1980. [Google Scholar]
- ISO 8589; Sensory Analysis. General Guidance for the Design of Test Rooms. 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 31 January 2024).
- UNI EN ISO 13299; Sensory Analysis. Methodology. General Guidance for Establishing a Sensory Profile. 2016. Available online: https://www.iso.org/standard/58042.html (accessed on 31 January 2024).
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Di Prima, S.; Cavicchioli, M.; Cerutti, A.K.; Bounous, G. Setting a protocol for hazelnut roasting using sensory and colorimetric analysis: Influence of the roasting temperature on the quality Tonda Gentile delle Langhe cv. hazelnut. Czech J. Food Sci. 2013, 31, 390–400. [Google Scholar] [CrossRef]
- Demchik, M.C.; Fischbach, J.; Yates, M.D. Physical characteristics and sensory analysis of American hazelnuts in comparison to Tonda di Giffoni. Agroforest Syst. 2016, 90, 919–926. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Massantini, R.; Corona, P. Better selection of chestnut cultivars: Experimenting the sensory characterization of the Marrone chestnuts compared to the “Chataigne” chestnuts. Eur. Food Res. Technol. 2024, 250, 961–969. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Garzoli, S.; De Vita, D.; Massantini, R.; Corona, P. Identifying the sensory profile and fatty acid composition for quality valorization of Marrone chestnut cultivars. Eur. Food Res. Technol. 2024, 11, 2837–2847. [Google Scholar] [CrossRef]
- Warmund, M.R.; Elmore, J.R.; Adhikari, K.; McGraw, S. Descriptive sensory analysis and free sugar contents of chestnut cultivars grown in North America. J. Sci. Food Agric. 2011, 91, 1940–1945. [Google Scholar] [CrossRef]
- Mellano, M.G.; Rapalino, S.; Donno, D. Sensory profiles of Castanea sativa cultivars and eurojapanese hybrids. Castanea 2017, 2, 8–9. [Google Scholar]
- Corona, P.; Frangipane, M.T.; Moscetti, R.; Lo Feudo, G.; Castellotti, T.; Massantini, R. Chestnut Cultivar Identification through the Data Fusion of Sensory Quality and FT-NIR Spectral Data. Foods 2021, 10, 2575. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Luksic, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of organical fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Król, K.; Gantner, M. Morphological Traits and Chemical Composition of Hazelnut from Different Geographical Origins: A Review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Alasalvar, C.; Pelvan, E.; Bahar, B.; Korel, F.; Ölmez, H. Flavour of natural and roasted Turkish hazelnut varieties (Corylus avellana L.) by descriptive sensory analysis, electronic nose and chemometrics. Int. J. Food Sci. Technol. 2012, 47, 122–131. [Google Scholar] [CrossRef]
- Kiefl, J.; Schieberle, P. Evaluation of Process Parameters Governing the Aroma Generation in Three Hazelnut Cultivars (Corylus avellana L.) by Correlating Quantitative Key Odorant Profiling with Sensory Evaluation. J. Agric. Food Chem. 2013, 61, 5236–5244. [Google Scholar] [CrossRef]
- Contador, L.; Robles, B.; Shinya, P.; Medel, M.; Pinto, C.; Reginato, G.; Infante, R. Characterization of texture attributes of raw almond using a trained sensory panel. Fruits 2015, 70, 231–237. [Google Scholar] [CrossRef]
- Booth, M.; Fu, M.; Peterson, D.G. Consumer acceptance and sensory perception of roasted American-European hybrid hazelnuts. J. Food Sci. 2024, 89, 4440–4449. [Google Scholar] [CrossRef]
- Giacosa, S.; Belviso, S.; Bertolino, M.; Dal Bello, B.; Gerbi, V.; Ghirardello, D.; Giordano, M.; Zeppa, G.; Rolle, L. Hazelnut kernels (Corylus avellana L.) mechanical and acoustic properties determination: Comparison of test speed, compression or shear axis, roasting, and storage condition effect. J. Food Eng. 2006, 173, 59–68. [Google Scholar] [CrossRef]
- Kita, A.; Figiel, A.; Carbonell-Barrachina, A.; GwoŸdziowska, M. Texture profile analysis (TPA) of hazelnuts under different thermal treatment. Int. Agrophys. 2009, 23, 39–43. [Google Scholar]
- Lucchetti, S.; Ambra, R.; Pastore, G. Effects of peeling and/ or toasting on the presence of tocopherols and phenolic compounds in four Italian hazelnut cultivars. Eur. Food Res. Technol. 2018, 244, 1057–1064. [Google Scholar] [CrossRef]
- Carbonaro, M.; Mattera, M.; Nicoli, S.; Bergamo, P.; Cappelloni, M. Modulation of Antioxidant Compounds in Organic vs Conventional Fruit (Peach, Prunus persica L., and Pear, Pyrus communis L.). J. Agric. Food Chem. 2002, 50, 5458–5462. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is There Any Difference between the Phenolic Content of Organic and Conventional Tomato Juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Artik, N.; Akan, S.; Okay, Y.; Durmaz, N.; Köksal, A.I. Volatile aroma component of natural and roasted hazelnut varieties using solid-phase microextraction gas chromatography/mass spectrometry. Acta Sci. Pol. Hortorum Cultus 2021, 20, 85–96. [Google Scholar] [CrossRef]

| Descriptors | Sensory Attribute Definitions | Standards and Reference Materials |
|---|---|---|
| ease of peeling | ease of peeling the shell and pellicle away from the nut | different level of adherence of shell/pellicle to the nut (value 0 corresponds to hard, while value 10 corresponds to easy) |
| seed colour | external colour of the seed, after removing the pellicle | seed colour with a degree of darkness (value 0 corresponds to a light colour seed, while value 10 corresponds to dark) |
| crunchiness | amount of noise generated when the sample is chewed at a fast rate with the back teeth | value 0 corresponds to a dried apple piece, while 10 corresponds to a fresh celery piece |
| astringent | sensation of drying, drawing-up, or puckering of any of the mouth surfaces | diluted tannic acid solution (0.06–2 mg/mL) |
| sweetness | basic taste associated with sugar (sucrose) | diluted sucrose solution (0.5–6 g/L) |
| bitterness | basic taste associated with caffeine | diluted caffeine solution (0.03–0.2 g/L) |
| hazelnut aroma | intensity of aroma of hazelnut products | taste of hazelnut |
| oily aroma | oily taste | taste of hazelnut or vegetable oils |
| almond aroma | Sweet cherry pit-like nutty aromatic associated with almonds | taste of almond |
| butter aroma | aromatics commonly associated with natural, slightly salted butter | taste of butter |
| caramel aroma | aromatics associated with caramel | taste of caramel |
| walnut aroma | aromatics associated with walnuts | taste of walnut |
| woody | aromatics associated with woody | taste of wood |
| malty | aromatics associated with malty | taste of malt |
| popcorn-like | aromatics associated with popcorn-like | taste of popcorn |
| coffee-like | aromatics associated with coffee-like | taste of coffee |
| roasty | aromatics associated with roasty | taste of roasted |
| aromatic intensity | characteristic flavour of hazelnut at the seed break | aromatics commonly associated with hazelnut |
| Samples | Weight (g) | Length (mm) | Width (mm) | Thickness (mm) | Geometric Mean Diameter (mm) | Arithmetic Mean Diameter (mm) | Surface Area (mm2) | Sphericity (%) | Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|
| OF | 1.27 ± 0.17 a | 14.25 ± 1.98 a | 16.87 ± 1.72 a | 12.50 ± 1.21 a | 14.22 ± 0.89 a | 14.54 ± 0.92 a | 636.13 ± 76.27 a | 1.03 ± 0.09 a | 1523.89 ± 261.1 a |
| OR | 1.18 ± 0.17 a | 13.25 ± 1.98 a | 16.00 ± 1.78 a | 12.87 ± 1.21 a | 13.91 ± 0.89 ab | 14.04 ± 0.92 ab | 609.26 ± 76.27 ab | 1.05 ± 0.09 a | 1429.65 ± 261.1 a |
| CR | 1.26 ± 0.17 a | 12.12 ± 1.98 a | 15.00 ± 1.72 a | 11.75 ± 1.21 a | 12.83 ± 0.89 ab | 12.95 ± 0.92 b | 518.53 ± 76.27 ab | 1.05 ± 0.09 a | 1121.83 ± 261.1 a |
| CF | 1.20 ± 0.17 a | 12.37 ± 1.98 a | 14.50 ± 1.72 a | 11.75 ± 1.21 a | 1.78 ± 0.89 b | 12.87 ± 0.92 b | 513.98 ± 76.27 b | 1.03 ± 0.09 a | 1106.73 ± 261.1 a |
| Pr > F(Model) | 0.89 | 0.45 | 0.21 | 0.50 | 0.02 | 0.01 | 0.02 | 0.98 | 0.02 |
| Significant | No | No | No | No | Yes | Yes | Yes | No | Yes |
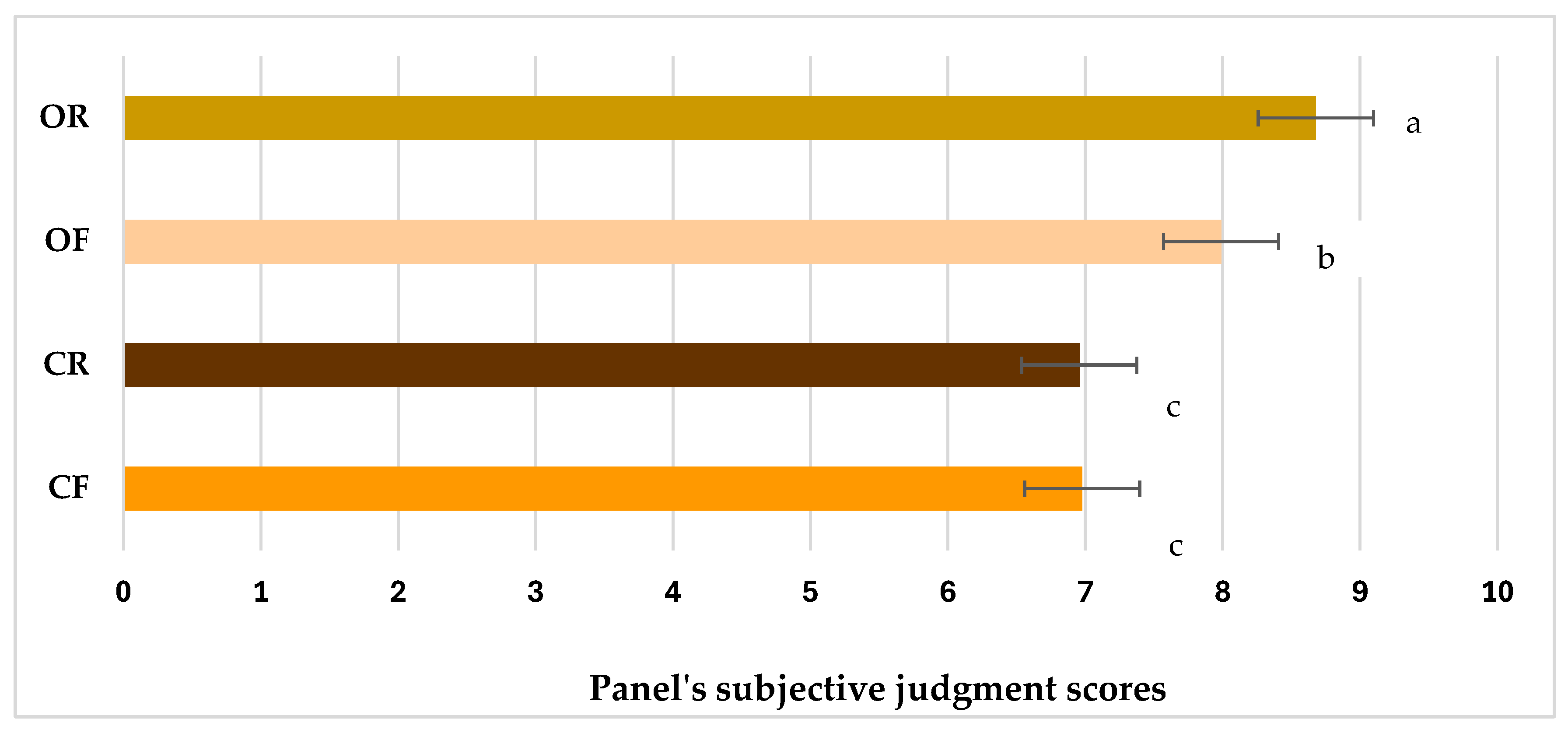
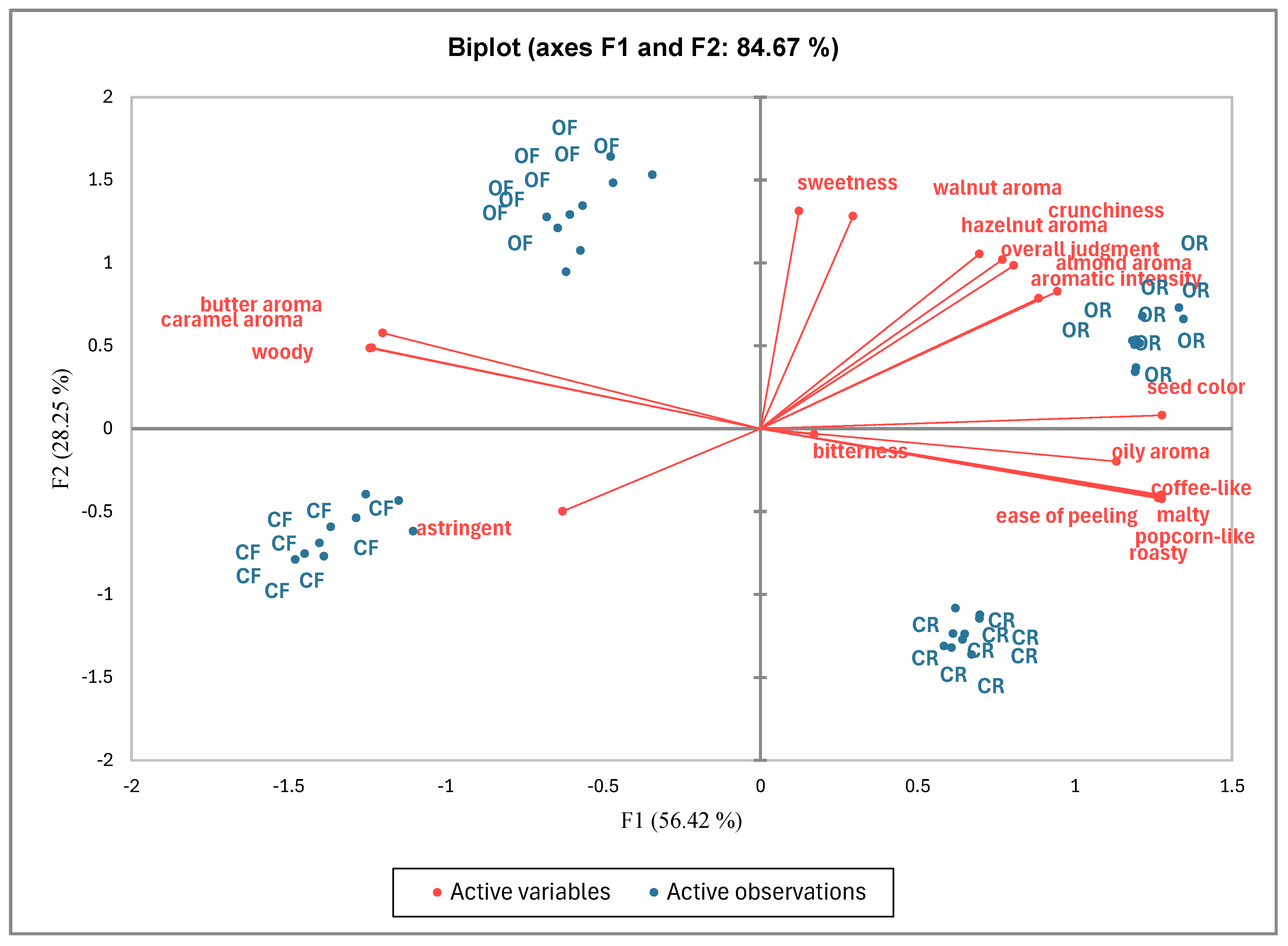
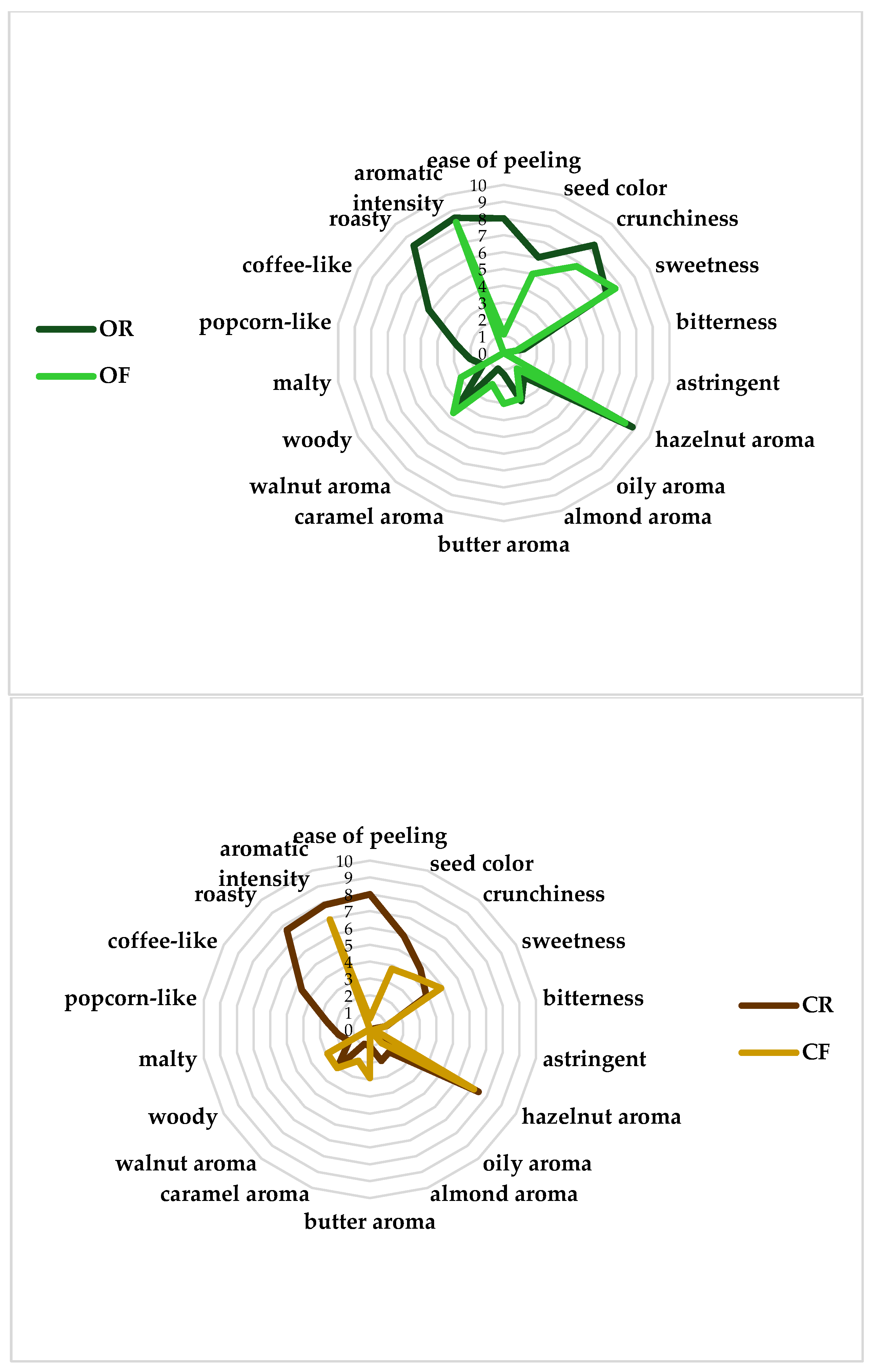
| Sample | Ease of Peeling | Seed Colour | Crunchiness | Sweetness | Bitterness | Astringent | Hazelnut Aroma | Oily Aroma | Almond Aroma | Butter Aroma | Caramel Aroma | Walnut Aroma | Woody | Malty | Popcorn-Like | Coffee-Like | Roasty | Aromatic Intensity | Overall Judgment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 8.0 a | 6.0 a | 8.3 a | 7.0 b | 1.2 a | 0.0 b | 8.8 a | 1.8 a | 3.0 a | 1.2 b | 1.0 b | 4.2 b | 1.4 b | 2.0 a | 2.8 a | 5.1 a | 8.3 a | 8.5 a | 8.6 a |
| OF | 1.1 b | 5.0 b | 6.7 b | 7.6 a | 0.7 a | 0.0 b | 8.3 b | 1.2 b | 2.9 a | 3.0 a | 1.9 a | 4.6 a | 2.9 a | 0.0 b | 0.0 c | 0.0 c | 0.0 c | 8.2 ab | 7.9 b |
| CR | 8.0 a | 5.8 a | 4.6 c | 3.8 d | 1.0 a | 0.0 b | 7.4 c | 1.7 a | 2.0 b | 1.2 c | 0.9 b | 3.0 c | 1.4 b | 1.8 a | 2.6 b | 4.6 b | 7.6 b | 7.8 b | 6.9 c |
| CF | 0.6 c | 3.8 c | 4.0 c | 4.8 c | 0.9 a | 0.2 a | 7.1 c | 1.0 b | 0.0 c | 2.9 a | 2.0 a | 3.0 c | 2.9 a | 0.0 b | 0.0 c | 0.0 c | 0.0 c | 6.9 c | 6.9 c |
| Pr > F(Model) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.927 | 0.002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Significant | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | CF (%) | CR (%) | OF (%) | OR (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2-pentanol | 660 | 664 | 8.4 ± 0.07 a | 6.5 ± 0.06 b | 7.2 ± 0.05 c | 9.1 ± 0.10 d |
| 2 | 2-hexanol | 800 | 801 | 10.2 ± 0.09 a | 9.4 ± 0.10 b | 12.2 ± 0.14 c | 10.5 ± 1.60 da |
| 3 | hexanal | 808 | 804 | 2.4 ± 0.02 a | 1.2 ± 0.02 b | 1.9 ± 0.03 cb | 0.9 ± 0.03 d |
| 4 | 4-heptanone | 872 | 869 | 2.6 ± 0.02 a | 3.1 ± 0.02 b | 4.5 ± 0.02 c | 2.0 ± 0.02 da |
| 5 | 2-furfurylthiol | 893 | 883 | 0.2 ± 0.02 a | 5.1 ± 0.04 b | Tr | 4.2 ± 0.05 c |
| 6 | 2(3H)-furanone | 904 | 914 | 1.5 ± 0.04 a | 9.1 ± 0.09 b | Tr | 5.2 ± 0.05 c |
| 7 | 3-methyl-4-heptanone | 927 | 932 | 6.3 ± 0.05 a | 4.2 ± 0.02 b | 2.5 ± 0.02 c | 3.2 ± 0.02 d |
| 8 | hexanoic acid | 970 | 978 | 18.5 ± 0.20 a | 15.5 ± 0.15 b | 20.7 ± 0.15 c | 22.7 ± 0.34 d |
| 9 | 1,8-cineole | 1028 | 1035 | Tr | 2.4 ± 0.02 a | 0.9 ± 0.05 b | 2.2 ± 0.03 ca |
| 10 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone | 1040 | 1055 | 0.9 ± 0.03 a | 3.6 ± 0.05 b | Tr | 2.1 ± 0.02 c |
| 11 | 2-nonanone | 1089 | 1091 | 4.1 ± 0.06 a | 2.1 ± 0.02 b | 3.6 ± 0.03 c | 4.0 ± 0.03 da |
| 12 | octanoic acid | 1169 | 1178 | 2.3 ± 0.05 a | 3.3 ± 0.03 b | 0.5 ± 0.02 c | 2.1 ± 0.07 da |
| 13 | 2-hexenal, (E)- | 1200 | 1205 | 2.2 ± 0.07 a | 1.8 ± 0.05 b | 2.5 ± 0.03 ca | 1.9 ± 0.12 db |
| 14 | decanal | 1207 | 1217 | 1.9 ± 0.03 a | 2.2 ± 0.04 b | 2.1 ± 0.04 cb | 3.3 ± 0.02 d |
| 15 | 1-pentanol | 1230 | 1233 | 6.2 ± 0.05 a | 5.5 ± 0.07 b | 9.3 ± 0.11 c | 4.1 ± 0.05 d |
| 16 | nonanoic acid | 1271 | 1276 | 3.4 ± 0.03 a | 4.2 ± 0.03 b | 2.1 ± 0.02 c | 5.5 ± 0.06 d |
| 17 | octanal | 1285 | 1278 | 6.5 ± 0.10 a | 4.3 ± 0.08 b | 5.5 ± 0.04 c | 3.2 ± 0.02 d |
| 18 | 2-heptanol | 1333 | 1325 | 14.5 ± 0.14 a | 12.3 ± 0.11 b | 20.1 ± 0.24 c | 10.9 ± 0.22 d |
| 19 | nonanal | 1387 | 1390 | 4.5 ± 0.03 a | 2.8 ± 0.07 b | 3.2 ± 0.05 c | 2.0 ± 0.03 d |
| 20 | acetic acid | 1420 | 1427 | 3.3 ± 0.03 a | 1.3 ± 0.02 b | 0.9 ± 0.06 c | 1.2 ± 0.02 db |
| SUM | 99.9 | 99.9 | 99.7 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frangipane, M.T.; Costantini, L.; Garzoli, S.; Merendino, N.; Massantini, R. Sensory and Nutritional Characteristics of Organic Italian Hazelnuts from the Lazio Region. Agriculture 2025, 15, 1279. https://doi.org/10.3390/agriculture15121279
Frangipane MT, Costantini L, Garzoli S, Merendino N, Massantini R. Sensory and Nutritional Characteristics of Organic Italian Hazelnuts from the Lazio Region. Agriculture. 2025; 15(12):1279. https://doi.org/10.3390/agriculture15121279
Chicago/Turabian StyleFrangipane, Maria Teresa, Lara Costantini, Stefania Garzoli, Nicolò Merendino, and Riccardo Massantini. 2025. "Sensory and Nutritional Characteristics of Organic Italian Hazelnuts from the Lazio Region" Agriculture 15, no. 12: 1279. https://doi.org/10.3390/agriculture15121279
APA StyleFrangipane, M. T., Costantini, L., Garzoli, S., Merendino, N., & Massantini, R. (2025). Sensory and Nutritional Characteristics of Organic Italian Hazelnuts from the Lazio Region. Agriculture, 15(12), 1279. https://doi.org/10.3390/agriculture15121279









