Abstract
Starch is a natural polymer. It is also an important food nutrient. Studies related to starch content testing can provide basic data for starch intake assessments and correlation studies. Meanwhile, data on the starch content in food are important for guiding the population to have a reasonable diet. Starch content directly affects the nutritional value, consumption quality, and processing quality of food. This paper summarized the common starch content detection techniques in food in the past five years, such as titration, spectrophotometry, near-infrared spectroscopy, and other methods. The principles, advantages, and disadvantages of these starch content detection techniques were described and discussed. Their problems in real sample detection (e.g., time-consuming, cumbersome operation, over-reliance on modeling algorithms, etc.) were analyzed. Challenges and future trends are also presented with the expectation of providing useful references for future research and practical applications. This paper provides a direction and research basis for the development of starch content detection techniques for food. It also provides value to related work in starch research.
1. Introduction
Carbohydrates in plants are often stored in the form of starch, which has great value and significance for plant growth and yield [1]. Plant starch content is an important reference for seed selection and breeding, quality evaluation, and exploitation [2]. Starch is a polysaccharide polymerized from glucose molecules. Starch has properties, such as thickening and moisturization. It is often used to improve the taste, appearance, and other properties [3]. Starch is one of the necessities for the food industry and people’s lives. The starch content directly affects the nutritional value, eating quality, and processing quality of some foods. The starch content of grains, potatoes, and other plants is generally high. According to nutritional statistics, starch accounts for approximately 50% of the carbohydrates consumed by humans [4]. This percentage is higher in countries or regions where cereals are staple foods. Some food standards designate the starch content as an important indicator of food conformity. For example, standards such as GB/T 13213-2017 and 36187-2018 [5,6] set clear requirements for the starch content in food [7]. Therefore, starch content testing technology is practically related to people’s lives and is an important basis for researching starch.
Currently, researchers often use the corresponding starch detection methods based on different types of food. Among them, the starch detection method using chemical methods has become highly standardized and accurate. It provides a common language for researchers, testing organizations, and consumers. This approach is preferred for scientific research. However, these methods often have drawbacks such as complicated sample pretreatment and reagent preparation [8]. However, these methods are time-consuming, inefficient, and unable to meet the needs of large-scale rapid testing. Therefore, novel methods for detecting starch content have received increasing attention. In the past ten years, researchers have been committed to exploring rapid and accurate solutions for food starch content detection. For example, spectroscopic detection technology can quickly obtain information related to starch content without compromising the integrity of food [9]. It has shown promising results in the determination of food starch content determination [10,11,12].
There are indirect and direct detection techniques for starch content. Indirect detection requires the hydrolysis of starch from experimental samples to reduce sugars. Subsequently, the detected value of reducing sugar is used to estimate the starch value. Indirect detection mainly involves titration methods, spectrophotometric methods, and chromatography. The direct assay involves the direct detection of starch content in the experimental samples. It does not hydrolyze starch in the experimental sample to reduce sugars [13]. Direct detection mainly includes thermogravimetric analysis (TGA), near-infrared spectroscopy (NIRS), hyperspectral imaging technology, and Raman spectroscopy. The relevant testing standards for starch content in foods at home and abroad are summarized in Table 1.

Table 1.
Food starch content detection standard.
2. Titration Methods
Titration is one of the most common methods used to determine the starch content of foods. It is based on the conversion of starch to reducing monosaccharides via hydrolysis. The reduced sugar content is then detected using titration. The actual starch content of the experimental samples is estimated by calculating the conversion coefficient [29]. During starch hydrolysis, other soluble sugars are converted into reducing sugars. This seriously interferes with the determination of starch content in the experimental samples. Therefore, it is often necessary for the tester to remove other soluble sugars by using a rinsing solvent (water or ethanol solution) first [30]. Titration has become a commonly used method for detecting reducing sugars because of its high accuracy and accessibility to conventional equipment [31].
In the process of using titration to detect the starch content in food, starch hydrolysis is an important step. Starch hydrolysis mainly involves enzymatic or acid hydrolysis. As starch granules have a microcrystalline structure, they are resistant to enzymes. Therefore, gelatinization (heating, high-pressure, chemical, etc.) is often required before enzymatic hydrolysis to destroy starch structure. This aids the action of amylase, thus improving the efficiency of enzymatic hydrolysis [32]. Enzymatic hydrolysis methods mainly include direct enzyme (amyloglucosidase) hydrolysis and dual enzyme (α-amylase and amyloglucosidase) hydrolysis, etc. In the last decade of research on amylase hydrolysis, Keshun, Liu et al. [33] investigated the use of the enzyme hydrolysis–titration method for the determination of starch content in cereals. They found that soaking the experimental samples in a 0.5 mol/L NaOH solution or high-pressure sterilization was the best pretreatment method before starch glucosidase hydrolysis dissolved starch. Souto, LRF et al. [34] studied the characterization and process of dual enzyme hydrolysis of cassava residues. Finally, they proved that dual enzyme hydrolysis was feasible for cassava residues. The disadvantages of the enzymatic hydrolysis titration method are cumbersome operation, long time consumption, and high cost; therefore, its popularity is low.
Acid hydrolysis is often used for the hydrolysis of starch using hydrochloric acid. However, this method is not as selective as enzymatic hydrolysis. This is due to the specificity of α-amylase for amyloglucosidase. Therefore, the treatment of experimental samples by enzymatic hydrolysis is not affected by non-starch polysaccharides, such as pectin [35]. Acid hydrolysis is a method of converting starch to reducing sugars using acid hydrolysis after the removal of other soluble sugars, fats, and other components. It is faster and simpler to perform than enzymatic hydrolysis. At the same time, it eliminates other influencing components. This improves the accuracy and detection efficiency. This method is suitable for experimental samples with relatively high starch contents and low contents of other polysaccharides [36]. Acid hydrolysis for the detection of starch content mainly adopts the acid hydrolysis–Farin reagent titration method. At present, some studies have improved the acid hydrolysis–Farin reagent titration method. In research on acid hydrolysis of starch in the last decade, e.g., Sun, LY et al. [37] investigated the effect of the starch hydrolysis method and other factors on the determination of sweet potato starch content. Nascimento, M.V.F. et al. [38] used ultrasound and reflux to study the feasible alternatives of starch hydrolysis. Zhang, Y et al. [39] assayed the starch content in rice using acid hydrolysis–Farin reagent titration. They also compared and analyzed techniques such as the 3,5-dinitrosalicylic acid colorimetric method (DNS method).
The acid hydrolysis–titration method uses a drenching solvent to eliminate soluble sugar components, but there is a lack of means to determine the total elimination of soluble sugar components. This often leads to residual sugar substances in the experimental samples, resulting in inaccurate test results. In the practical application of the acid hydrolysis–titration method, the staff often fails to determine the exact amount of drenching solvent [40]. Due to this problem, there are currently studies using the sugar discrimination (Molisch) reaction to detect whether the saccharides are completely removed. The sugar discrimination reaction involves the dehydration of sugar substances by inorganic acids to produce furfural and its derivatives. These products then react with α-naphthol to form a purplish-red substance [41]. This reaction can be used to identify saccharides such as monosaccharides and oligosaccharides. In the last decade of research on the Molisch reaction, Rautenstrauch, H et al. [42] discussed the feasibility of using thymol and carvacrol as substitutes for α-naphthol in the Molisch reaction. Zhao, CQ et al. [43] identified saccharides by the Molisch reaction to analyze the composition of bioflocculants.
Overall, the advantage of the acid hydrolysis–titration method is that its instrumentation is simple and easy to popularize. Certainly, these disadvantages are obvious. Many factors interfere with the results of this method, such as the intensity of the heat source and pH value, etc. However, the operation of this method is relatively complex and requires a high level of proficiency from staff. In addition, the detection results are calculated based on the total amount of hydrolyzed reducing sugars. Currently, there are few methods for determining the complete removal of soluble sugars.
3. Spectrophotometric Methods
The reducing sugars produced by the hydrolysis of starch can also be detected using spectrophotometry. The main spectrophotometric methods are anthrone colorimetry, the glucose oxidase–peroxidase method, the DNS method, and the hexokinase method. The anthrone colorimetric method begins with the elimination of other soluble sugars from the experimental sample using ethanol. Next, the starch in the residue is dissolved using perchloric acid, thus separating the starch from the other components. Then, anthrone and starch are reacted with concentrated sulfuric acid to produce a blue-green substance. Finally, absorbance at 640 nm was measured using a spectrophotometer [44]. Among the studies in the last decade using anthrone colorimetry to detect starch content, Wang, ZC et al. [45] determined carbon, N, P and non-structural carbohydrates in different parts of seedlings by the anthrone colorimetric method. Wu, Y et al. [46] determined the starch and other contents in the seed endosperm using the anthrone colorimetric method.
The glucose oxidase–peroxidase method involves the oxidation of glucose catalyzed by glucose oxidase to produce glucose and hydrogen peroxide. Then, an oxidase-catalyzed reaction of hydrogen peroxide and 4-aminoantipyrine with phenol produces red quinone imine. The starch content of the experimental samples was determined at a wavelength of 505 nm. The commonly used color developers are 4-amino antipyrine-phenol and o-anisidine [47]. In the last decade of research using the glucose oxidase–peroxidase method for the determination of starch content, Bastida, A et al. [48] demonstrated the possibility of this sustainable biocatalytic development of glucose testing by using biomass-derived materials as carriers for the immobilization of the enzyme (glucose oxidase–peroxidase method).
The DNS method involves the co-heating of reducing sugars with 3,5-dinitrosalicylic acid under alkaline conditions. 3,5-dinitrosalicylic acid is then reduced to brown-red 3-amino-5-nitrosalicylic acid. Finally, the starch content in the experimental samples is measured at a wavelength of 540 nm wavelength [49]. Among the studies in the last decade that used the DNS method to detect starch content, Xie, SD et al. [50] used the DNS method to detect the digestibility of yam starch. Liu, KS et al. [51] monitored in vitro antidiabetic activity using the DNS method. Currently, the hexokinase method is the most commonly used technique for detecting glucose concentration. It is also a recognized method of blood glucose detection [52].
Starch can also produce colored complexes by reacting with iodine reagents, without undergoing hydrolysis. This is then detected spectrophotometrically [53]. The colors of the complexes produced by the interaction of straight-chain starch and branched-chain starch with iodine are different. One complex with iodine is blue with an absorption wavelength of 500–800 nm, and the other complex is purplish-red with an absorption wavelength of 500–600 nm so that the absorption peaks of straight-chain starch and branched-chain starch partially overlap when using the single-wavelength method to detect starch content. They affect each other, significantly interfering with the accuracy of the determination [54]. To address this problem, dual-wavelength spectrophotometry has frequently been used in recent years to eliminate the mutual interference of the two absorption backgrounds. This effectively improves the sensitivity and accuracy of the method for determining the starch content. Dual-wavelength spectrophotometry for starch content is a means of calculating the total starch value by determining the straight-chain and branched-chain starch values and then summing them. Because this method can obtain the value of straight-chain starch and branched-chain starch, as well as the total starch value, it is widely used in the detection of starch value in food products. In the last decade of research on starch content using dual-wavelength spectrophotometry, Jiang, ZZ et al. [55] measured the content of straight-chain and branched-chain starch in transgenic maize plants using dual-wavelength spectrophotometry. Eventually, they found that overexpression of the ZmAPO1-9 gene increased the diameter of starch granules. Jie, He et al. [56] proposed a method based on dual-wavelength spectrophotometry for the detection of straight-chain starch and branched-chain starch in yam and taro. They also clarified the maximum absorption wavelength and reference wavelength. Zhang, XM et al. [57] investigated the effect of dual-wavelength spectrophotometry on rice starch content detection under different temperature and processing time conditions.
Overall, the advantages of spectrophotometric methods for the determination of starch content over titrimetric methods include a simpler operation and the possibility of testing a large number of experimental samples. However, it is susceptible to the influence of the colored components in the samples to be measured and has a weak anti-interference property.
4. Chromatography
Currently, ion chromatography and high-performance liquid chromatography (HPLC) are widely used for glucose determination. This method has excellent separability and can accurately separate glucose from reducing sugars such as fructose [58]. GB 5009.8-2016 [59] detects glucose in food using high-performance liquid chromatography. In a recent decade of research using ion chromatography and high-performance liquid chromatography to detect starch content, Li, OY et al. [60] detected the molecular mass distribution, monosaccharide composition, and content of extracts from Dictyophora echino-volvata Zane by high-performance gel permeation and ion chromatography. Eventually, they found that the main component of extracts from Dictyophora echino-volvata Zane was glucose, which was 75–90%. Xie, WQ et al. [61] proposed a new reaction headspace gas chromatographic (HS-GC) method for the efficient determination of total starch content in wheat flour. The relative error between this method and the metric method did not exceed 8.90%. Weng, CH et al. [62] proposed a method for the determination of starch content in meat floss products using high-performance liquid chromatography.
Currently, HPLC is often used to determine glucose content using an oscillometric and evaporative light scattering detector. This method is not as sensitive as ion chromatography–integral pulsed amperometry. However, it is now found that the sensitivity of the method can be further improved by derivatization or by combining other techniques. For example, Han, X et al. [63] quantified sugars in herbal medicines by the pre-column derivatization of 1-phenyl-3-methyl-5-pyrazolone coupled with high-performance liquid chromatography. Yeganeh-Zare, S et al. [64] used high-performance liquid chromatography coupled with a refractive index detector to determine the carbohydrate profile of experimental samples. Ultimately, they found that the glucose-to-fructose ratio and maltose content were the best indicators for detecting adulteration of apple juice concentrate. Depending on the adulterant, the limit for detecting adulteration of apple juice concentrate with cheaper sweeteners was 10%.
In general, chromatography provides more accurate detection of glucose content than titrimetric and spectrophotometric methods. At the same time, it can also eliminate the influence of other reducing sugars, making batch experimental sample detection easier. It has good precision. However, this method also has the disadvantages of a more complicated operation process and is time-consuming.
5. Polarimetric Methods
The principle of the polarimetric method for detecting starch content in food is that starch components and their hydrolysis products in food have polarimetric properties. Under certain conditions, the relationship between optical rotation and starch concentration is proportional [65]. In some domestic and international starch testing standards, a polarimetric method is used to detect the starch content of animal feed. In recent years, some scholars have attempted to apply the polarimetric method to the detection of starch content in food. For example, Zhou, XM et al. [66] investigated the uncertainty of the detection of cassava starch content by the polarimetric method. Ultimately, they found that the repeated detection of total optical rotation contributed the most to the uncertainty and that the weighing of experimental samples contributed the least to the total uncertainty. Na, BQ et al. [67] detected the starch content in rice by the polarimetric method. Ultimately, the experimental results proved that the technique was relatively precise and reproducible. However, the number of samples in this experiment was relatively small, and the range of the rice starch content was relatively narrow. The experimental procedure must be further improved.
In addition to hydrochloric acid hydrolysis, acidic calcium chloride solutions can also be used for starch extraction using the polarimetric method. The principle is that calcium ions are complex with the hydroxyl groups of the starch molecule, resulting in complexes with polarimetric properties. These complexes are uniformly distributed within the solution, and the starch value can be determined by detecting optical rotation [68]. In the last decade of studies using the acidic calcium chloride-polarimetric method for starch content, Valková, V et al. [69] applied the calcium chloride-polarimetric method to determine the starch content of wheat. They found that the starch content of wheat kernels was influenced by the wheat variety. Li, X et al. [70] examined the starch content of potatoes using the calcium chloride-acetic acid polarimetric method. Zhao, YM [71] found that the calcium chloride-polarimetric method for the determination of starch content in green bean food showed high average recoveries and high reproducibility and stability compared to the national standard method. Kong, DC et al. [72] detected the starch content in corn by colorimetric and calcium chloride-polarimetric methods, among others. Ultimately, they experimentally found that the calcium chloride-polarimetric method was more effective than other techniques for the detection of corn starch content. However, its accuracy and specificity were not as good as those of the other techniques.
In summary, the disadvantage of the polarimetric method is that it requires strict operating criteria such as pH and temperature of the acidic calcium chloride solution. Simultaneously, because it does not eliminate other sugar substances from the sample, it often causes its results to interfere with the optical rotation of other substances. Therefore, the results of this method for the detection of starch content in foodstuffs are generally high.
6. Thermogravimetric Analysis
Thermogravimetric analysis (TGA) is used to determine the relationship between the mass of the experimental sample and the change in temperature or time under controlled temperature conditions. The micro-quotient thermogravimetric curve can be calculated based on the first-order derivative of the thermogravimetric curve [73]. In the last decade of research using thermogravimetric analysis to detect starch content, Zhan, HJ et al. [74] proposed a technique based on differential thermogravimetric analysis for the detection of corn starch content. They used this method with infrared spectroscopy to clarify the existence of starch decomposition peaks at 250–370 °C. In addition, they optimized the interference factors. Subsequently, they [75] analyzed the characteristic thermal decomposition profile of soybeans based on thermogravimetric analysis. Their experimental conclusions proved that it was feasible to obtain indicators, such as soybean starch content, through linear equations and peak areas of thermal analysis profiles. Cui, LW et al. [76] constructed a model of infrared and thermogravimetric analysis to detect starch content in rice at 250–330 °C, respectively. By comparing the results with those of the national standard method, they found that the detection model was suitable for the detection of starch content in indica, glutinous, and japonica rice. This method has the advantages of low influence of the sample substrate, convenient pretreatment, and rapid determination.
Overall, thermogravimetric analysis is capable of simultaneous quantitative detection of multiple components, in contrast to the titration method, which is capable of detecting only a single component. At the same time, it has advantages in terms of speed and accuracy. However, thermogravimetric analysis is highly affected by experimental conditions and is not suitable for detecting samples with low starch content.
7. Near-Infrared Spectroscopy
In recent years, NIRS has been widely used to detect starch content in food [77,78]. GB/T 25219-2010 [16] determined the starch content of corn using NIRS. The determination of starch content by NIRS involves no complicated pretreatment, which saves time and labor. In addition, it does not use chemical reagents and is not destructive to experimental samples. This is a green and non-destructive determination technique. The principle of NIRS detection of starch content is to predict the starch content through the specific absorption peaks of C-H, O-H, etc. of starch, combined with data degradation and stoichiometry to establish a detection model [79]. The flow of the spectral data processing is illustrated in Figure 1.
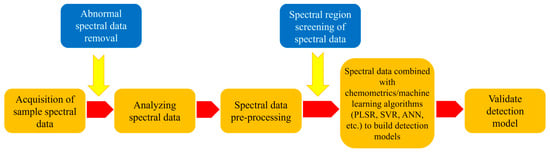
Figure 1.
Flow diagram of spectral data processing (Note: ANN = artificial neural network).
NIRS technology has been widely used in research on starch content detection in yams. Among them, there were more relevant studies on cassava, potato, sweet potato, and yam, such as Bantadjan, Y et al. [80] who used a portable visible and near-infrared spectrometer for fast and non-destructive estimation of starch content in fresh cassava roots. The starch prediction model obtained from the full wavelength region was able to predict the starch content better (Rp = 0.825 and the standard error of prediction = 2.502%). Mbanjo, EGN et al. [81] used NIRS to predict the starch content in fresh cassava roots. While PLSR and support vector regression (SVR) showed relatively good predictive abilities, the Random Forest (RF) model had the lowest performance. Maraphum, K et al. [82] used a portable near-infrared spectrometer with wavelengths of 570–1031 nm to assess starch content in fresh cassava tubers. They combined PLSR with different variable selection methods and spectral preprocessing. The results of this experiment showed that this calibrated model could be applied in the field to monitor the internal quality of cassava tubers. Posom, J et al. [83] selected effective wavelengths for the detection of cassava starch content using NIRS and machine learning. Finally, the coefficient of determination of prediction (R2) of their model was 0.88, and the root mean square error of prediction (RMSEP) was 1.38%. Chaiareekitwat, S et al. [84] applied NIRS to predict the quality of cassava leaves and roots. The prediction model that they developed better predicted the starch content of the root system. Ding, JG et al. [85] used visible/near-infrared diffuse transmission spectroscopy to determine potato blackheart and starch values. Finally, the starch quantitative model correlation coefficient of prediction (Rp) was 0.893, and RMSEP was 0.713%. Wang, F et al. [86,87] established a potato visible/near-infrared localized transmission spectroscopy acquisition system and portable device by combining the shapes of potatoes. Based on these studies, they proposed a method for determining potato starch content. Ultimately, the Rp of the model was 0.9122 and the RMSEP was 0.3404%. The mean deviation was 0.2536% and the maximum coefficient of variation for repeated sampling was 0.0124. Tang, CC et al. [88] used NIRS for high-throughput analysis of sweet potato root total starch and linear amylose contents, among others. They applied a dual optimization strategy (optimal sample subset partitioning and variable selection) for NIRS modeling. Alamu, EO et al. [89] evaluated the effect of different sampling methods on the starch content of yams detected by NIRS.
In recent years, research on methods for starch content determination using NIRS with legumes and cereals as the object of study has gradually matured. For example, John, R et al. [90] developed a combined near-infrared spectral prediction model for rice bean and adzuki bean flour samples to estimate the total starch and protein contents, among others. Padhi, SR et al. [91] developed an NIRS prediction model for the content of starch and other components in cowpeas. The prediction model was used for high-throughput screening of cowpea quality in a non-destructive manner. Zhang, J et al. [92] constructed a quantitative model for buckwheat starch and others based on near-infrared diffuse reflectance spectroscopy and verified the accuracy of the detection model. Joe, AAF et al. [93] evaluated the accuracy of NIRS for predicting content values such as starch and ash in wheat grains. They used 12 preprocessing prediction models combined with regression techniques to predict the physicochemical properties of wheat. He, MH et al. [94] detected the starch content of wheat, glutinous rice, millet, and other cereals by using near-infrared reflectance spectroscopy. The best determination results for starch were obtained at 1923–1961 nm by comparing partial least squares regression (PLSR) models in different wavelength regions. Tomar, M et al. [95] developed a model based on NIRS and modified partial least square regression (MPLS) for the prediction modeling of pearl millet starch and other components. Zhang, HY et al. [96] constructed an NIRS model for the detection of starch and other contents in foxtail millet. They used partial least squares (PLS) to analyze and validate the model. John, R et al. [97] developed a best-fit model for rice components, such as total protein and starch, using different spectral preprocessing and regression algorithms based on NIRS. However, the prediction results for starch content were unsatisfactory.
Currently, near-infrared spectroscopy has been applied to the study of starch detection in meat products. For example, Vichasilp, C et al. [98] proposed a method based on NIRS to predict the starch content of various meatballs. They combined the spectral data of beef meatballs, pork meatballs, and chicken meatballs to create a generalized model.
Generally, from the above studies, it can be concluded that NIRS has great potential for the detection of starch content in food products. However, the accuracy of the NIRS-based determination of starch content in foods such as polished rice needs to be further improved. Meanwhile, there are still some other issues that need to be investigated in the determination of starch content in some foods using NIRS, such as improving the sensitivity of the determination and eliminating the influence of other components on starch content in the experimental samples.
8. Hyperspectral Imaging Technology
NIRS usually acquires one-dimensional spectral data. However, hyperspectral imaging technology can acquire two-dimensional spectral images of the experimental samples [99]. A typical laboratory-based hyperspectral imaging system is shown in Figure 2. This technique combines spectral and imaging techniques to acquire images in the visible-near-infrared wavelength range. Simultaneously, it can acquire spatial, spectral, and chemical information from experimental samples. This information is then subjected to spectral preprocessing, data dimensionality reduction, and machine learning to construct a detection model [100]. Therefore, this method has great potential for the determination of food composition.
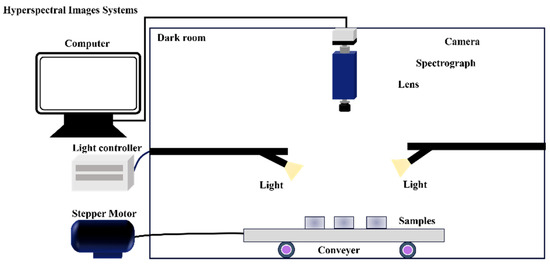
Figure 2.
The common laboratory-based hyperspectral imaging systems.
In contrast to NIRS, there are relatively few studies on the detection of starch content in yams using hyperspectral imaging technology. For example, Wang, FX et al. [101,102,103] investigated hyperspectral imaging technology and chemometrics methods for predicting potato starch content. Competitive adaptive reweighted sampling (CARS) and a successive projection algorithm (SPA) were used to extract the characteristic wavelengths. A PLSR model was constructed, and the final Rp was greater than 0.93. He, HJ et al. [104] used hyperspectral images with 900–1700 nm spectral information to detect the starch content in sweet potatoes. They applied multiple linear regression to process the spectra to obtain relatively good quantitative accuracy (Rp = 0.970, RMSEP = 0.874 g/100 g). Zhang, Y et al. [105] used hyperspectral imaging technology combined with chemometrics to develop a predictive model for Tiegun Yam starch content. They compared the effects of full-wavelength and characteristic-wavelength modeling.
Currently, research on starch content detection methods using hyperspectral imaging technology with legumes and grains as the research object is mostly based on spectral and image information combined with machine learning algorithms to construct detection models. For example, Hu, HQ et al. [106] predicted the starch content of Puerariae Thomsonii Radix using hyperspectral imaging and a deep learning algorithm. The R2 value of the one-dimensional convolutional neural network starch detection model was 0.9091. Bu, YH et al. [107] predicted the starch content in mixed sorghum using the hyperspectral technique. The detection model constructed by optimizing the backpropagation neural network through a genetic algorithm (GA-BPNN) obtained the best results. Zhang, J et al. [108] used near-infrared hyperspectral imaging technology to determine the starch content of wheat flour. The R2 and RMSEP of the optimal model for predicting starch were 0.9243 and 0.2068 g/100 g. Qiao, MM et al. [109] integrated the spectral and image features of visible-near-infrared hyperspectral imaging to determine the starch content of maize kernels. The best prediction model was established by multiplicative scattering correction (MSC), uninformative variable elimination (UVE), and extreme learning machine (ELM). Liu, C et al. [110] explored the possibility of using near-infrared hyperspectral imaging technology to determine the starch content of single corn seeds. The Rp of the prediction model based on the Levenberg–Marquardt algorithm (LMA) artificial neural network was 0.96. Liang, Y et al. [111] utilized hyperspectral imaging technology to predict starch content in fermented grains. The best prediction results were obtained from the detection model developed using spectral fusion and SVR. Zhang, ZH et al. [112] established a prediction model for the starch content of rice (with husks) from different rice varieties in China using hyperspectral imaging technology. They selected the optimal wavelength using regression coefficients as discriminants in the optimization process of the prediction model. The PLSR model had an R2 value of 0.8029. Lu, XZ [113] constructed a model for the determination of rice starch content at different storage times based on hyperspectral imaging technology. The R2 of the model was 0.991 and RMSEP was 0.669%. However, their experimental process only modeled and predicted the experimental rice starch content of one origin with different storage times. They did not consider the differences in the starch content of rice from multiple origins.
Overall, the current methods for determining starch content by hyperspectral imaging technology also face a number of urgent research issues. For example, the starch content detection model based on hyperspectral imaging technology has limited accuracy and is difficult to apply to the needs of starch content detection in experimental samples with complex compositions. However, the starch content detection model has poor generalization ability among different brands and models of equipment.
9. Mid-Infrared, Raman, and Terahertz Spectroscopy Technology
Compared with methods such as NIRS and hyperspectral imaging technology, which have been developed rapidly in recent years, studies on the detection of starch content in food based on mid-infrared, Raman, and THz spectroscopy are still very limited. In particular, the mechanism and methodology for detecting the starch content in food based on THz spectroscopy have rarely been reported. Mid-infrared, Raman, and THz spectroscopic techniques are often used to resolve digestibility [114], regeneration processes [115,116], pasting [117], crystallinity [118], and fingerprinting information [119,120], etc.
Mid-infrared spectroscopy (MIRS) can recognize the fundamental vibrational absorption of functional groups and molecular bonds in the mid-infrared region (4000–525 cm−1) [121]. Several studies have been conducted in the field of food starch content detection using MIRS technology. MIRS technology is often used in combination with other spectroscopic techniques. For example, Kandpal, LM et al. [122] developed a model for the detection of the chemical composition of tuber flours by the fusion of NIRS and MIRS. The best R2 value for the starch content detection model was 0.95. Pielorz, S et al. [123] used fluorescence spectroscopy and three vibrational spectroscopic methods (NIRS, MIRS, and Raman spectroscopy) to determine the content of starch and other components in colored-flesh potatoes. Ultimately, they applied PLSR modeling with an error of 3.45–4.55% for starch detection. Karunakaran, C et al. [124] investigated the stability of MIRS for rapid analysis of the nutrient content of pea seeds. They found that the correlation of the PLSR starch content detection model was greater than 0.70.
Raman spectroscopy is a scattering spectroscopy method. It has the advantage of being less susceptible to moisture interference and so on [125]. Currently, studies on the detection of starch content in food products using Raman spectroscopy are gradually beginning to be reported. For example, Wu, ML et al. [126] discovered a method for detecting moisture, protein, and starch content in mung beans using NIRS and Raman spectroscopy. However, the experimental results showed that the detection of starch content using these two techniques was not satisfactory. Nakajima, S et al. [127] investigated the selective detection of starch content in banana fruits using Raman spectroscopy. They found a correlation between Raman spectroscopy and starch content obtained by chemical analysis. Their linear regression (LR) model had an R2 of 0.88 and an RMSEP of 2.8%. Wei, X et al. [128] proposed a method for detecting starch content in rice using confocal Raman microspectroscopy (CRM). The Rp of the starch content detection model they developed was 0.8915 and the mean relative error was 1.08%.
THz spectroscopy lies between infrared and microwave [129]. It has low-energy transmission properties [130]. Therefore, it generally does not cause radiation damage in the experimental samples [131]. The weak interactions of molecules and low-frequency vibrational absorption are in the THz band [132]. Therefore, THz spectroscopy has unique detection advantages for the determination of specific food [133]. Currently, research on the detection of starch content in food based on THz spectroscopy is still in its infancy, and there are few related studies. For example, Nakajima, S et al. [134] found a correlation between the absorption peak at 9.0 THz in germinating mung bean seedlings and the change in starch. The intensity of this peak had an Rp of 0.98 with starch content.
Methods based on MIRS, Raman, and THz spectroscopic techniques for the determination of starch content have gradually begun to be reported in recent years. However, these methods are still in their nascent stage. Their mechanism analysis is not sufficiently thorough, and the detection algorithms are relatively simple. Meanwhile, the accuracy of their detection models is generally not high, and their stability is yet to be verified. To summarize this study, a summary of spectroscopic technique-based methods for the detection of starch content in food is shown in Table 2.

Table 2.
Summary of spectroscopy-based methods for the determination of starch content in foods.
10. Challenges and Future Trends
Based on the above literature, it was clear that researchers made a number of attempts in the field of food starch content detection based on advanced techniques. In short, these techniques overcome the shortcomings of traditional methods which were destructive and inconvenient. However, there were still many challenges in further improving the performance of methods for the determination of starch content in food. For example, the accuracy of reducing sugar detection in the indirect assay method during starch content determination directly affected the accuracy of its final results. The enzymatic hydrolysis–titration method had excellent specificity and accuracy. However, it had the disadvantages of cumbersome operation and high cost. Therefore, it is difficult to popularize this method. In addition, spectrophotometry requires additional chemical reagents and is not very efficient. At the same time, it is complicated and costly.
On the one hand, spectroscopic techniques such as NIRS relied heavily on data or image analysis algorithms. Accuracy, stability, and speed were directly determined by the chosen algorithm. Although complex algorithms could improve starch performance to some extent, they were very time-consuming. It was worthwhile for researchers to think about how to choose the right analysis algorithm quickly and efficiently. On the other hand, experimental equipment with high resolution and sensitivity was particularly expensive. This was a direct barrier to their application. In addition, some detection techniques had high requirements on the detection environment. External factors might interfere with the detection results. A comparison table of the advantages and disadvantages of starch content detection methods in food is shown in Table 3.

Table 3.
Comparison of advantages and disadvantages of methods for starch content detection in food.
Although there was a certain foundation for starch content detection technology in food, more research was needed. To put the detection technology into practical application, it should fulfill the requirements of high accuracy and stable results, fast detection speed, and affordable cost of detection equipment. For mature food starch detection methods (e.g., titration methods, spectrophotometric methods, chromatography, etc.), they generally had the disadvantages of time-consuming detection and cumbersome operation. However, these methods generally had standardized detection processes, which made it difficult to solve these problems. Because of the problems in the current research of spectroscopic detection techniques, the following aspects could be improved: (1) Although widely used algorithms for constructing models, such as PLSR, had achieved good detection results, the accuracy of these methods was low as the number of samples increases. Deep learning had demonstrated comparable or better performance than traditional chemometrics methods in handling large amounts of data. Due to the unique characteristics of deep learning (strong feature-learning ability, good migration learning, high efficiency of big data processing, etc.), its application in food starch content detection will surely become one of the hotspots for future research. (2) The cost of expensive testing equipment made it unsuitable for practical application. Therefore, researchers should focus on the development and improvement of affordable testing instruments from the perspective of cost reduction. (3) The research on the integration and fusion of various detection technologies should be strengthened. Combining two or more advanced detection techniques and making full use of their advantages played a crucial role in improving the results of food starch content detection.
11. Conclusions
With the continuous improvement of science and public awareness of health, environmental protection, and green requirements, the method of starch content determination in food is under continuous development and improvement. Indirect detection methods include titration methods, spectrophotometric methods, chromatography, and so on. Direct detection methods include polarimetric methods, thermogravimetric analysis, spectrometry, etc. Compared to indirect detection methods, direct detection methods are faster and easier to operate. At the same time, they have the advantage of the simultaneous detection of multiple components, and so on. Spectroscopic methods have unique advantages under the current policies related to environmental protection and energy savings. No additional waste or solvents are produced. They are more in line with the green detection requirements. However, these methods are susceptible to interference from nondetectable components, and their stability and accuracy are relatively weak. However, they are less versatile and difficult to adapt to the needs of starch content detection in various sample matrices. Moreover, the currently proposed spectral detection model for detecting starch content in food is still limited. In particular, the mechanism and methodology for detecting starch content in food based on spectroscopic techniques, such as MIDS, Raman, and THz, have rarely been reported. The advantages and disadvantages of indirect and direct detection methods are plotted against each other as shown in Table 3. In the future, with in-depth exploration of these issues, food starch content detection methods will be more intelligent, green, and batch. Simultaneously, they tend to be simpler, faster, and more efficient.
Author Contributions
Conception and design: X.W., F.L. and D.D.; Analysis and interpretation of the data: X.W. and Y.L. (Yinfeng Liu); The drafting of the paper: X.W. and S.L.; Revising it critically for intellectual content: X.W., F.L. and Y.L. (Yachao Liu); The final approval of the version to be published: X.W. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (32302212).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank Yong He, Fei Liu, Shiping Zhu, and Shengling Zhou for their valuable support.
Conflicts of Interest
The authors declare no conflicts of interest associated with this paper.
References
- Arefi, A.; Sturm, B.; Hoffmann, T. Explainability of deep convolutional neural networks when it comes to NIR spectral data: A case study of starch content estimation in potato tubers. Food Control 2025, 169, 110979. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, X.; Wang, H.; Liu, S.; Chen, S. A transfer learning method for near infrared models of potato starch content and traceability from different origins. J. Food Compos. Anal. 2025, 137, 106909. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, J.; Xue, S.; Jin, Q.; Zhu, J.; Lu, S.; Meng, Q.; Du, H.; Fu, M.; Zhong, Y. Development of comprehensive prediction models for pumpkin fruit sensory quality using physicochemical analysis, near-infrared spectroscopy, and machine learning. J. Food Compos. Anal. 2024, 134, 106530. [Google Scholar] [CrossRef]
- Gonzalez-Vidal, T.; Calvo-Malvar, M.; Fernandez-Merino, C.; Sanchez-Castro, J.; Lado-Baleato, O.; Diaz-Louzao, C.; Pazos-Couselo, M.; Alonso-Sampedro, M.; Matabuena, M.; Gude, F. Divergent hypoglycemic and hyperglycemic responses to the components of evening meals. A general adult population study in individuals without diabetes (aegis study). Clin. Nutr. 2024, 43, 379–390. [Google Scholar] [CrossRef] [PubMed]
- GB/T 13213-2017; Canned Pork Mince. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- GB/T 36187-2018; Frozen Surimi. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Zhao, G.; Xu, Z.; Tang, L.; Li, X.; Dai, Z.; Xie, Z.; Jiang, Y.; Wu, Y.; Zhang, P.; Wang, Q. Research on rapid determination methods for main compositions and sensory quality of pumpkins based on hyperspectral imaging technology. J. Food Compos. Anal. 2025, 138, 107028. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, G.; Chen, B.; Yan, B.; Ren, F.; Li, N.; Zhu, X.; He, S.; Mu, Z.; Liu, H. High-throughput near-infrared spectroscopy for detection of major components and quality grading of peas. Front. Nutr. 2024, 11, 1505407. [Google Scholar] [CrossRef]
- Namakula, B.F.; Nuwamanya, E.; Kanaabi, M.; Wembambazi, E.; Kawuki, R.S. Predicting starch content of cassava with near infrared spectroscopy in Ugandan cassava germplasm. J. Near Infrared Spectrosc. 2023, 31, 256–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G. Starch content and physicochemical properties of green wheat starch. Int. J. Food Prop. 2019, 22, 1463–1474. [Google Scholar] [CrossRef]
- Vitelli, M.; Mehrtash, H.; Assatory, A.; Tabtabaei, S.; Legge, R.L.; Rajabzadeh, A.R. Rapid and non-destructive determination of protein and starch content in agricultural powders using near-infrared and fluorescence spectroscopy, and data fusion. Powder Technol. 2021, 381, 620–631. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.Z.; Lee, T.C. Direct determination of the starch content in gravy by near-infrared spectroscopy. J. Agric. Food Chem. 1996, 44, 1460–1463. [Google Scholar] [CrossRef]
- Su, W.; Xue, H. Imaging spectroscopy and machine learning for intelligent determination of potato and sweet potato quality. Foods 2021, 10, 2146. [Google Scholar] [CrossRef]
- GB/T 20378-2006; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- ISO 10520-1997; Native Starch-Determination of Starch Content-Ewers Polarimetric Method. International Organization for Standardization: Geneva, Switzerland, 1997.
- GB/T 25219-2010; Inspection of Grain and Oils—Determination of Starch Content in Maize—Near-Infrared Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2010.
- 17. AOAC 940.30; Starch in Prepared Mustard. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1940.
- AOAC 925.50; Starch in Confectionery. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1925.
- 19. AOAC 920.10; Sulfur in Plants. Sodium Peroxide Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 920.44; Starch in Baking Powders. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 920.83; Starch in Cacao Products Direct Acid Hydrolysis Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1920.
- AOAC 979.10; Starch in Cereals. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1979.
- AOAC 996.11; Starch(Total)In Cereal Products Amyloglucosidase-a-Amylase Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1979.
- AOAC 958.06; Starch in Meat Titrimetric Method. Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1958.
- ISO 13965-1998; Meat and Meat Products-Determination of Starch and Glucose Contents-Enzymatic Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- NY/T 802-2004; Method for Determination of Starch in Raw Milk and Dairy Food Enzyme-Colorimetric method. The Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004.
- AACC 76-11; Starch—Glucoamylase Method with Subsequent Measurement of Glucose with Glucose Oxidase. American Association of Cereal Chemists: St. Paul, MN, USA, 1976.
- 28. GB 5009.9-2016; National Food Safety Standard Determination of Starch in Food. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- Tang, Y.; Zhang, J.; Xu, Y.; Li, C.; Li, Y.; Li, G.; Hu, Y.; Li, W. Combining with acid-base titration, HPLC, ATR-FTIR and chemometrics to study the effects of sulfur fumigation on medicinal and edible starchy samples. J. Food Compos. Anal. 2025, 137, 106967. [Google Scholar] [CrossRef]
- Letoffe, A.; Hosseinpourpia, R.; Silveira, V.; Adamopoulos, S. Effect of Fenton reaction parameters on the structure and properties of oxidized wheat starch. Carbohydr. Res. 2024, 542, 109190. [Google Scholar] [CrossRef]
- Yogurtcu, H.; Gurler, N. Evaluation of effect of boric acid on thermoplastic starch: Morphological, mechanical, barrier, and optical properties. Polym. Eng. Sci. 2024, 64, 2230–2240. [Google Scholar] [CrossRef]
- Huang, M.; Li, L.; Lei, G.; Qiu, R.; Wang, Y.; Wu, J.; Zong, X. Preparation of fern root resistant starch by pullulanase and glucoamylase combined with autoclaving-enzymatic method: Physicochemical properties and structural characterization. J. Sci. Food Agric. 2025, 105, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Q. Enzymatic determination of total starch and degree of starch gelatinization in various products. Food Hydrocoll. 2020, 103, 105639. [Google Scholar] [CrossRef]
- Souto, L.R.F.; Caliari, M.; Soares Junior, M.S.; Fiorda, F.A.; Garcia, M.C. Utilization of residue from cassava starch processing for production of fermentable sugar by enzymatic hydrolysis. Food Sci. Technol. 2017, 37, 19–24. [Google Scholar] [CrossRef]
- Lehoczki, G.; Kandra, L.; Gyemant, G. The use of starch azure for measurement of alpha-amylase activity. Carbohydr. Polym. 2018, 183, 263–266. [Google Scholar] [CrossRef]
- Halim, A.; Torley, P.J.; Farahnaky, A.; Majzoobi, M. Investigating the effects of acid hydrolysis on physicochemical properties of quinoa and faba bean starches as compared to cassava starch. Foods 2024, 13, 3885. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, X.; Cheng, J.; Lu, G.; Pang, L. Comparison and optimization of determination methods for starch in sweet potato. J. Chin. Cereals Oils Assoc. 2023, 38, 199–204. [Google Scholar] [CrossRef]
- Nascimento, M.V.F.; Vicente, J.; Silva, F.O.M.d.; La, O.R. Acid hydrolysis optimization of starch by lane-eynon method. Bol. Cent. Pesqui. Process. Aliment. 2016, 34, 37–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L. Study on determination of starch content in rice by acid hydrolysis—Fehlings reagent titration. Sci. Technol. Food Ind. 2017, 38, 256–259+265. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, R.; Fu, G.; Wen, J.; Xing, L.; Wu, S. Determination of starch in food by high temperature and high pressure acid hydrolysis liquid phase method. Cereal Feed. Ind. 2023, 2, 62–65. [Google Scholar]
- Gaspari, P.F.; Bernardes, C.F. Experimental characterization of carbohydrates. Rev. Ensino Bioquim. 2020, 18, 49–55. [Google Scholar]
- Rautenstrauch, H.; Rebenstorff, A.; Gudenschwager, S.; Ruppersberg, K. The new molisch sample for teaching a safe carbohydrate detection. Chem. Unserer Zeit 2023, 57, 172–179. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, X.; Gu, H.; Zhang, J.; Zou, W.; Liu, J.; Yang, Q. Qualitative analysis of components of bioflocculant prepared with Bacillus fusiformis for the treatment of tannery wastewater. Clean Technol. Environ. Policy 2016, 18, 973–978. [Google Scholar] [CrossRef]
- Fan, J.; Gu, D.; Xv, W.; Zhou, T.; Chen, A.; Lu, J.; Wang, Y.; Jin, H.; Wei, F.; Ma, S. Comparison of polysaccharide profiles of different seaweeds based on ion chromatography and ultrahigh-performance liquid chromatography. Sep. Sci. Plus 2024, 7, e202400060. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Arif, M.; Shamshad, J.; Wu, A.; Zhan, W.; Ahmad, B.; Tan, N.; Al-Anazi, K.M.; Farah, M.A.; et al. Analyzing the impact of phosphorous and nitrogen on Castanopsis sclerophylla early growth stages. J. King Saud Univ. Sci. 2024, 36, 103517. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, C.; Yu, X.; Shen, Y. Biochemical changes during fruit and seed development in Nanjing Linden (Tilia miqueiana M.). Forests 2023, 14, 969. [Google Scholar] [CrossRef]
- Kanchan, M.; Tambe, P.K.; Bharati, S.; Powar, O.S. Convolutional neural network for colorimetric glucose detection using a smartphone and novel multilayer polyvinyl film microfluidic device. Sci. Rep. 2024, 14, 28377. [Google Scholar] [CrossRef]
- Bastida, A.; Garrido, L.; Fernandez-Mayoralas, A. Use of supramolecular chemistry based on β-cyclodextrin-grafted chitosan beads to prepare green biocatalytic materials. Mater. Adv. 2025, 6, 311–318. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Blando, F.; Orfila, C.; Marshall, L.J.; Boesch, C. Chromogenic assay is more efficient in identifying α-amylase inhibitory properties of anthocyanin-rich samples when compared to the 3,5-dinitrosalicylic acid (DNS) assay. Molecules 2023, 28, 6399. [Google Scholar] [CrossRef]
- Xie, S.; Chen, H.; Jiang, X.; Zhou, B.; Guo, Z.; Zeng, H.; Zhang, Y. Structural and physicochemical properties of a Chinese yam starch-tea polyphenol complex prepared using autoclave-assisted pullulanase treatment. Foods 2023, 12, 3763. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Sumi, T.S.; Golder, M.; Biswas, B.; Maknoon Kerr, P.G. Antidiabetic profiling, cytotoxicity and acute toxicity evaluation of aerial parts of Phragmites karka (Retz.). J. Ethnopharmacol. 2021, 270, 113781. [Google Scholar] [CrossRef]
- Pakaew, K.; Chonpathompikunlert, P.; Wongmanee, N.; Rojanaverawong, W.; Sitdhipol, J.; Thaveethaptaikul, P.; Charoenphon, N.; Hanchang, W. Lactobacillus reuteri TISTR 2736 alleviates type 2 diabetes in rats via the hepatic IRS1/PI3K/AKT signaling pathway by mitigating oxidative stress and inflammatory mediators. Eur. J. Nutr. 2025, 64, 27. [Google Scholar] [CrossRef]
- Ichikawa, S.; Kodama, Y. Fluorescent staining and quantification of starch granules in chloroplasts of live plant cells using fluorescein. Bio-Protocol 2024, 14, e5103. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Kaur, C.; Devi, V.; Singh, A.; Das, A.K.; Rakshit, S.; Chaudhary, D.P. A rapid single kernel screening method for preliminary estimation of amylose in maize. Food Anal. Methods 2022, 15, 2163–2171. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, D.; Zhang, H.; Qu, J.; Liu, S.; Guan, S.; Ma, Y. Effects of overexpression of zmapo1-9 gene on maize yield. Plant Growth Regul. 2023, 99, 493–503. [Google Scholar] [CrossRef]
- He, J.; Yan, F.; Huang, F.; Xiao, Y.; Xie, L. Determination of amylose and amylopectin contents in yam and taros by dual-wavelength spectrophotometry. Sci. Technol. Food Ind. 2022, 43, 303–309. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Gao, F.; Zeng, Z.; Li, X.; Liang, Y.; Zhang, H.; Yang, S. The effects of different conditions (dispersion temperature and time) in dual wavelength violet spectrophotographic determination of rice starch content. J. Southwest Univ. (Nat. Sci. Ed.) 2020, 42, 49–55. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Wang, D.; Gao, H.; Ding, Y.; Cheng, J.; Han, Y. Fe(II)/Fe(III) cycle enhanced the Electro-Fenton degradation of methylene blue with Fe3O4@C as three-dimensional electrode. Appl. Surf. Sci. 2025, 683, 161764. [Google Scholar] [CrossRef]
- GB 5009.8-2016; Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Food According to National Food Safety Standards. National Health and Family Planning Commission of the People’s Republic of China, National Medical Products Administration: Beijing, China, 2016.
- Li, O.; Zhang, Q.; Shi, X.; Huang, Y.; Yin, J.; Zhang, S.; Nie, S. The quality control of extracts from Dictyophora echino-volvata zane by high performance gel permeation chromatography and ion chromatography. J. Chin. Inst. Food Sci. Technol. 2019, 19, 213–219. [Google Scholar] [CrossRef]
- Xie, W.; Gong, Y.; Yu, K. Quantitative analysis of total starch content in wheat flour by reaction headspace gas chromatography. Anal. Bioanal. Chem. 2017, 409, 5195–5200. [Google Scholar] [CrossRef] [PubMed]
- Weng, C. The Determination of Starch in Meat Floss and the Effect of Different Processing Techniques on Its Characteristics and Content Determination. Master’s Thesis, Zhejiang University of Technology, Zhejiang, China, 2018. [Google Scholar]
- Han, X.; Ando, H.; Kudo, Y.; Sasaki, Y. Development of highly sensitive method for sugar determination in herbal medicine; application of monosaccharides and oligosaccharides in Japanese Angelica root and Rehmannia root. Chem. Pharm. Bull. 2022, 70, 796–804. [Google Scholar] [CrossRef]
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid detection of apple juice concentrate adulteration with date concentrate, fructose and glucose syrup using HPLC-RID incorporated with chemometric tools. Food Chem. 2022, 370, 131015. [Google Scholar] [CrossRef]
- Puscas, A.; Tanislav, A.E.; Marc, R.A.; Muresan, V.; Muresan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity evaluation and antioxidant activity of a novel drink based on roasted avocado seed powder. Plants 2022, 11, 1083. [Google Scholar] [CrossRef]
- Zhou, X.; Lei, B.; Zhang, F.; Liu, Y.; Fan, X. Uncertainty evaluation for the determination of starch content in Manihot esculenta by polarimetric method. Food Sci. 2016, 37, 144–147. [Google Scholar]
- Na, B.; Ao, W.; Wang, X.; Bao, X. Determination of starch content in rice by the polarimetric method. J. North Pharm. 2020, 17, 6–8. [Google Scholar]
- Ma, J.; Jiang, Q.-T.; Zhang, X.-W.; Wei, L.; Chen, G.-Y.; Qi, P.-F.; Wei, Y.-M.; Lan, X.-J.; Lu, Z.-X.; Zheng, Y.-L. Effect of lipids on starch determination through various methods. Pak. J. Agric. Sci. 2014, 51, 751–757. [Google Scholar]
- Valkova, V.; Duranova, H.; Bilcikova, J.; Zofajova, A.; Havrlentova, M. The content and quality of starch in different wheat varieties growing in experimental conditions. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 462–466. [Google Scholar] [CrossRef]
- Li, X.; Xue, C.; Gao, L.; Ren, G.; Cao, Y.; Zhang, D. Potato starch content determination and comparision from different areas of Yulin city. Appl. Chem. Ind. 2015, 44, 1892–1896. [Google Scholar] [CrossRef]
- Zhao, Y. On determining starch content of green bean food with polarimetry. Jiangsu Condiment Subsid. Food 2014, 2, 37–39. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, R.; Wang, X.; Song, B. Comparison of starch contents of corn samples using different standard methods. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2016, 37, 23–27. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Correa-Pina, B.L.; Pineda-Gomez, P.; Barron-Garcia, O.Y.; Londono-Restrepo, S.M.; Rodriguez-Garcia, M.E. Structural, morphological, compositional, thermal, pasting, and functional properties of isolated achira (Canna indica L.) starch: Review. Int. J. Biol. Macromol. 2024, 282, 136710. [Google Scholar] [CrossRef]
- Zhan, H.; Cui, L.; Li, J.; Bai, J. Determination of starch content in corn by differential thermal analysis. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2012, 33, 31–36. [Google Scholar] [CrossRef]
- Zhan, H.; Zhang, J.; Xu, F.; Yang, D. Determination of main component content in soybean by hermogravimetric analysis. Cereal Feed Industry 2016, 11, 56–61. [Google Scholar]
- Cui, L.; Zhan, H.; Zhang, J.; Hu, Y. Determination of starch content in rice by thermogravimetric analysis. J. Chin. Cereals Oils Assoc. 2017, 32, 167–170. [Google Scholar]
- Lopez-Calabozo, R.; Liberal, A.; Fernandes, A.; Revilla, I.; Ferreira, I.C.F.R.; Barros, L.; Vivar-Quintana, A.M. Determination of carbohydrate composition in lentils using near-infrared spectroscopy. Sensors 2024, 24, 4232. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Tang, L.; Zhao, G.; Wu, Y.; Zhang, P.; Wang, Q. An effective moisture interference correction method for maize powder NIR spectra analysis. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2024, 312, 124033. [Google Scholar] [CrossRef]
- Hou, S.; Han, J.; Men, Y.; Yang, Y.; Long, L.; Liu, L.; Sun, Z. Analysis of genotype-by-environment effects on starch content in 281 Tartary buckwheat varieties and evaluation of the physicochemical properties of two elite varieties. LWT 2024, 197, 115866. [Google Scholar] [CrossRef]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Rapid starch evaluation in fresh cassava root using a developed portable visible and near-infrared spectrometer. ACS Omega 2020, 5, 11210–11216. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Hershberger, J.; Peteti, P.; Agbona, A.; Ikpan, A.; Ogunpaimo, K.; Kayondo, S.I.; Abioye, R.S.; Nafiu, K.; Alamu, E.O.; et al. Predicting starch content in cassava fresh roots using near-infrared spectroscopy. Front. Plant Sci. 2022, 13, 990250. [Google Scholar] [CrossRef]
- Maraphum, K.; Saengprachatanarug, K.; Wongpichet, S.; Phuphuphud, A.; Posom, J. Achieving robustness across different ages and cultivars for an NIRS-PLSR model of fresh cassava root starch and dry matter content. Comput. Electron. Agric. 2022, 196, 106872. [Google Scholar] [CrossRef]
- Posom, J.; Maraphum, K. Achieving prediction of starch in cassava (Manihot esculenta Crantz) by data fusion of Vis-NIR and Mid-NIR spectroscopy via machine learning. J. Food Compos. Anal. 2023, 122, 105415. [Google Scholar] [CrossRef]
- Chaiareekitwat, S.; Mahayothee, B.; Rungpichayapichet, P.; Khuwijitjaru, P.; Nagle, M.; Latif, S.; Mueller, J. The potential of near-infrared spectroscopy as a rapid method for quality evaluation of cassava leaves and roots. J. Food Compos. Anal. 2024, 126, 105913. [Google Scholar] [CrossRef]
- Ding, J.; Han, D.; Li, Y.; Peng, Y.; Wang, Q.; Han, X. Simultaneous non-destructive on-line detection of potato black-heart disease and starch content based on visible/near infrared diffuse transmission spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 1909–1915. [Google Scholar]
- Wang, F.; Li, Y.; Peng, Y.; Yang, B.; Li, L.; Liu, Y. Multi-parameter potato quality non-destructive rapid detection by visible/near-infrared spectra. Spectrosc. Spectr. Anal. 2018, 38, 3736–3742. [Google Scholar]
- Wang, F.; Li, Y.; Peng, Y.; Yang, B.; Li, L.; Yin, X. Hand-held device for non-destructive detection of potato quality parameters. Trans. Chin. Soc. Agric. Mach. 2018, 49, 348–354. [Google Scholar]
- Tang, C.; Jiang, B.; Ejaz, I.; Ameen, A.; Zhang, R.; Mo, X.; Wang, Z. High-throughput phenotyping of nutritional quality components in sweet potato roots by near-infrared spectroscopy and chemometrics methods. Food Chem. X 2023, 20, 100916. [Google Scholar] [CrossRef]
- Alamu, E.O.; Adesokan, M.; Asfaw, A.; Maziya-Dixon, B. Effect of sample preparation methods on the prediction performances of near infrared reflectance spectroscopy for quality traits of fresh yam (Dioscorea spp.). Appl. Sci. 2020, 10, 6035. [Google Scholar] [CrossRef]
- John, R.; Bartwal, A.; Jeyaseelan, C.; Sharma, P.; Ananthan, R.; Singh, A.K.; Singh, M.; Gayacharan Rana, J.C.; Bhardwaj, R. Rice bean-adzuki bean multitrait near infrared reflectance spectroscopy prediction model: A rapid mining tool for trait-specific germplasm. Front. Nutr. 2023, 10, 1224955. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.R.; John, R.; Bartwal, A.; Tripathi, K.; Gupta, K.; Wankhede, D.P.; Mishra, G.P.; Kumar, S.; Rana, J.C.; Riar, A.; et al. Development and optimization of NIRS prediction models for simultaneous multi-trait assessment in diverse cowpea germplasm. Front. Nutr. 2022, 9, 1001551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, J.; Zhang, M.; Zhang, X.; Jingjing, E. Establishment of rapid detection model of buckwheat nutritional components based on near infrared spectroscopy. J. Chin. Cereals Oils Assoc. 2020, 35, 151–158. [Google Scholar]
- Joe, A.A.F.; Gopal, A.; Pandian, R. Performance evaluation of chemometric prediction models-key components of wheat grain. J. Sci. Ind. Res. 2020, 79, 148–152. [Google Scholar]
- He, M.H.; Hu, J.Q.; Wu, Y.W.; Ouyang, J. Determination of starch and amylose contents in various cereals using common model of near-infrared reflectance spectroscopy. Int. Food Res. J. 2021, 28, 987–995. [Google Scholar] [CrossRef]
- Tomar, M.; Bhardwaj, R.; Kumar, M.; Singh, S.P.; Krishnan, V.; Kansal, R.; Verma, R.; Yadav, V.K.; Dahuja, A.; Ahlawat, S.P.; et al. Development of NIR spectroscopy based prediction models for nutritional profiling of pearl millet (Pennisetum glaucum (L.)) R.Br: A chemometrics approach. LWT 2021, 149, 111813. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, F.; Zhao, F.; Li, X.; Fan, G.; Zhao, Z.; Guo, P. Rapid prediction of apparent amylose, total starch, and crude protein by near-infrared reflectance spectroscopy for foxtail millet (Setaria italica). Cereal Chem. 2020, 97, 653–660. [Google Scholar] [CrossRef]
- John, R.; Bhardwaj, R.; Jeyaseelan, C.; Bollinedi, H.; Singh, N.; Harish, G.D.; Singh, R.; Nath, D.J.; Arya, M.; Sharma, D.; et al. Germplasm variability-assisted near infrared reflectance spectroscopy chemometrics to develop multi-trait robust prediction models in rice. Front. Nutr. 2022, 9, 946255. [Google Scholar] [CrossRef]
- Vichasilp, C.; Kawano, S. Prediction of starch content in meatballs using near infrared spectroscopy (NIRS). Int. Food Res. J. 2015, 22, 1501–1506. [Google Scholar]
- Lu, Y.; Saeys, W.; Kim, M.; Peng, Y.; Lu, R. Hyperspectral imaging technology for quality and safety evaluation of horticultural products: A review and celebration of the past 20-year progress. Postharvest Biol. Technol. 2020, 170, 111318. [Google Scholar] [CrossRef]
- Polder, G.; Dieleman, J.A.; Hageraats, S.; Meinen, E. Imaging spectroscopy for monitoring the crop status of tomato plants. Comput. Electron. Agric. 2024, 216, 108504. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C. Improved model for starch prediction in potato by the fusion of near-infrared spectral and textural data. Foods 2022, 11, 3133. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Song, S. A study of starch content detection and the visualization of fresh-cut potato based on hyperspectral imaging. RSC Adv. 2021, 11, 13636–13643. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Song, S.; Xie, S.; Kang, F. Study on starch content detection and visualization of potato based on hyperspectral imaging. Food Sci. Nutr. 2021, 9, 4420–4430. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Wang, Y.; Al-Maqtari, Q.A.; Liu, H.; Zhang, M.; Ou, X. Towards rapidly quantifying and visualizing starch content of sweet potato [Ipomoea batatas (L.) Lam] based on NIR spectral and image data fusion. Int. J. Biol. Macromol. 2023, 242, 124748. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhou, C.; Zhou, J.; Nan, T.; Yang, J.; Huang, L. Rapid and nondestructive identification of origin and index component contents of Tiegun yam based on hyperspectral imaging and chemometric method. J. Food Qual. 2023, 2023, 6104038. [Google Scholar] [CrossRef]
- Hu, H.; Wang, T.; Wei, Y.; Xu, Z.; Cao, S.; Fu, L.; Xu, H.; Mao, X.; Huang, L. Non-destructive prediction of isoflavone and starch by hyperspectral imaging and deep learning in Puerariae Thomsonii Radix. Front. Plant Sci. 2023, 14, 1271320. [Google Scholar] [CrossRef]
- Bu, Y.; Jiang, X.; Tian, J.; Hu, X.; Fei, X.; Huang, D.; Luo, H. Rapid and accurate detection of starch content in mixed sorghum by hyperspectral imaging combined with data fusion technology. J. Food Process Eng. 2022, 45, e14129. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Ren, Z.; Wang, S.; Yue, M.; Zhang, S.; Yin, X.; Gong, K.; Ma, C. Rapid determination of protein, starch and moisture content in wheat flour by near-infrared hyperspectral imaging. J. Food Compos. Anal. 2023, 117, 105134. [Google Scholar] [CrossRef]
- Qiao, M.; Cui, T.; Xia, G.; Xu, Y.; Li, Y.; Fan, C.; Han, S.; Dong, J. Integration of spectral and image features of hyperspectral imaging for quantitative determination of protein and starch contents in maize kernels. Comput. Electron. Agric. 2024, 218, 108718. [Google Scholar] [CrossRef]
- Liu, C.; Huang, W.; Yang, G.; Wang, Q.; Li, J.; Chen, L. Determination of starch content in single kernel using near-infrared hyperspectral images from two sides of corn seeds. Infrared Phys. Technol. 2020, 110, 103462. [Google Scholar] [CrossRef]
- Liang, Y.; Tian, J.; Hu, X.; Huang, Y.; He, K.; Xie, L.; Yang, H.; Huang, D.; Zhou, Y.; Xia, Y. Rapid determination of starch and alcohol contents in fermented grains by hyperspectral imaging combined with data fusion techniques. J. Food Sci. 2024, 89, 3540–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, X.; Ma, C. Development of simplified models for the nondestructive testing of rice with husk starch content using hyperspectral imaging technology. Anal. Methods 2019, 11, 5910–5918. [Google Scholar] [CrossRef]
- Lu, X. Research on Nondestructive Detection of Varieties, Moisture and Starch in Rice Based on Hyperspectral Imaging Technology. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2017. [Google Scholar]
- Ichinose, J.; Oba, K.; Arase, Y.; Kaneshiro, J.; Tate, S.-i.; Watanabe, T.M. Quantitative prediction of rice starch digestibility using Raman spectroscopy and multivariate calibration analysis. Food Chem. 2024, 435, 137505. [Google Scholar] [CrossRef]
- An, H.; Ma, Q.; Zhang, F.; Zhai, C.; Sun, J.; Tang, Y.; Wang, W. Insight into microstructure evolution during starch retrogradation by infrared and Raman spectroscopy combined with two-dimensional correlation spectroscopy analysis. Food Hydrocoll. 2024, 146, 109174. [Google Scholar] [CrossRef]
- An, H.; Zhai, C.; Ma, Q.-Y.; Zhang, F.; Wang, S.-Y.; Sun, J.-F.; Wang, W.-X. Quantitative characterization of wheat starch retrogradation by combining 2d-cos and spectral fusion. Spectrosc. Spectr. Anal. 2023, 43, 162–168. [Google Scholar]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Prediction of starch pasting properties in barley flour using ATR-MIR spectroscopy. Carbohydr. Polym. 2013, 95, 509–514. [Google Scholar] [CrossRef]
- Guo, H.; Shiraga, K.; Kondo, N.; Chen, S.; Yamashige, Y.; Ogawa, Y. Determining changes in crystallinity of rice starch after heat-moisture treatment using terahertz spectroscopy. Food Chem. 2023, 425, 136237. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Boschetto, F.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman molecular fingerprints of rice nutritional quality and the concept of Raman barcode. Front. Nutr. 2021, 8, 663569. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Hashimoto, Y.; Marin, E.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman fingerprints of rice nutritional quality: A comparison between Japanese Koshihikari and internationally renowned cultivars. Foods 2021, 10, 2936. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Babadi, A.A.; Ariffin, N.M.; Zou, X. Development of FTIR-ATR spectra and pls regression combination model for discrimination of pure and adulterated acacia honey. Food Control 2025, 169, 110996. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Mouazen, A.M.; Masithoh, R.E.; Mishra, P.; Lohumi, S.; Cho, B.-K.; Lee, H. Sequential data-fusion of near-infrared and mid-infrared spectroscopy data for improved prediction of quality traits in tuber flours. Infrared Phys. Technol. 2022, 127, 104371. [Google Scholar] [CrossRef]
- Pielorz, S.; Kita, A.; Rytel, E.; Szostak, R.; Mazurek, S. Application of vibrational and fluorescence spectroscopy to the compositional analysis of colored-flesh potatoes. J. Sci. Food Agric. 2024, 104, 1399–1407. [Google Scholar] [CrossRef]
- Karunakaran, C.; Vijayan, P.; Stobbs, J.; Bamrah, R.K.; Arganosa, G.; Warkentin, T.D. High throughput nutritional profiling of pea seeds using Fourier transform mid-infrared spectroscopy. Food Chem. 2020, 309, 125585. [Google Scholar] [CrossRef]
- Su, W.; Sun, D. Fourier transform infrared and Raman and hyperspectral imaging techniques for quality determinations of powdery foods: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Yuan, Y.; Li, S.; Song, X.; Yin, J. Comparison of NIR and Raman spectra combined with chemometrics for the classification and quantification of mung beans (Vigna radiata L.) of different origins. Food Control 2023, 145, 109498. [Google Scholar] [CrossRef]
- Nakajima, S.; Kuroki, S.; Ikehata, A. Selective detection of starch in banana fruit with Raman spectroscopy. Food Chem. 2023, 401, 134166. [Google Scholar] [CrossRef]
- Wei, X.; Li, F.; Perumal, A.B.; Sanaeifar, A.; Guindo, M.L.; Shi, Y.; He, Y.; Liu, F. Confocal Raman microspectroscopy combined with spectral screening algorithms for quantitative analysis of starch in rice. Food Hydrocoll. 2023, 141, 108737. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, W.; Zhu, S.; Zhou, S.; Wu, W.; Xie, Z. Application of terahertz spectrum and interval partial least squares method in the identification of genetically modified soybeans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118453. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, S.; Zhou, S.; Zheng, W.; Li, S. Identification of soybean origin by terahertz spectroscopy and chemometrics. IEEE Access 2020, 8, 184988–184996. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Zhu, S.; Zheng, W.; Zhou, S.; Wu, W.; Xie, Z. Quantitative analysis of soybean protein content by terahertz spectroscopy and chemometrics. Chemom. Intell. Lab. Syst. 2021, 208, 104199. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Zhu, S.; Zheng, W.; Xie, Y.; Zhou, S.; Hu, M.; Miao, Y.; Ma, L.; Wu, W.; et al. Terahertz spectroscopy combined with data dimensionality reduction algorithms for quantitative analysis of protein content in soybeans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119571. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Kong, D.; Zhu, S.; Li, S.; Zhou, S.; Wu, W. Rapid identification of soybean varieties by terahertz frequency-domain spectroscopy and grey wolf optimizer-support vector machine. Front. Plant Sci. 2022, 13, 823865. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Shiraga, K.; Suzuki, T.; Kondo, N.; Ogawa, Y. Quantification of starch content in germinating mung bean seedlings by terahertz spectroscopy. Food Chem. 2019, 294, 203–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).