Mechanisms Behind the Soil Organic Carbon Response to Temperature Elevations
Abstract
1. Introduction
2. Extrinsic Factors Affecting the SOC Response to Elevated Temperatures
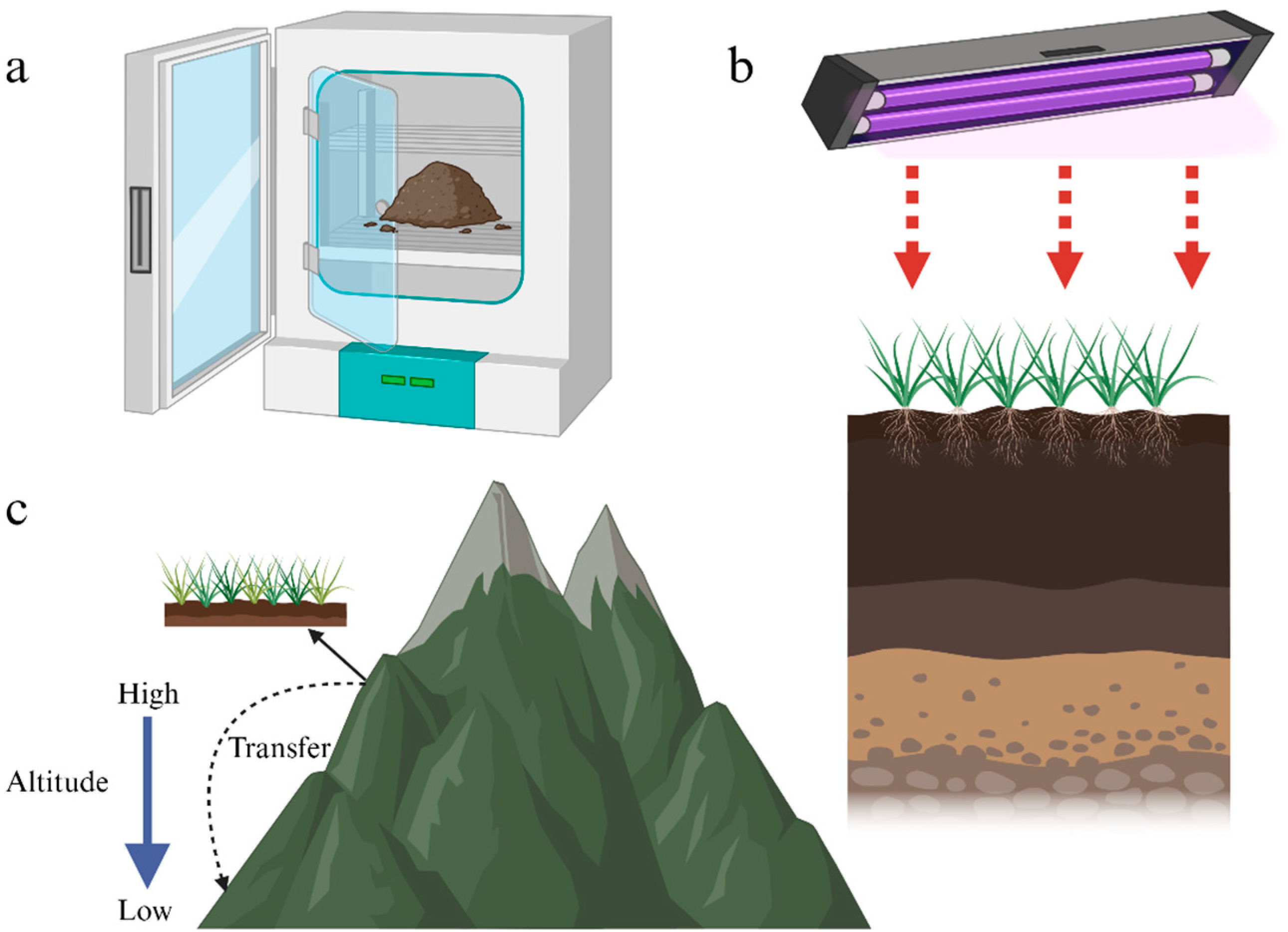
2.1. Variation in the SOC Response to Temperature Elevations in Different Temperature Elevation Methods
2.1.1. Indoor Temperature Elevations
2.1.2. Field Temperature Elevations
2.1.3. Soil Displacement
2.2. Variation in the SOC Response to Temperature Elevations in Different Land Use
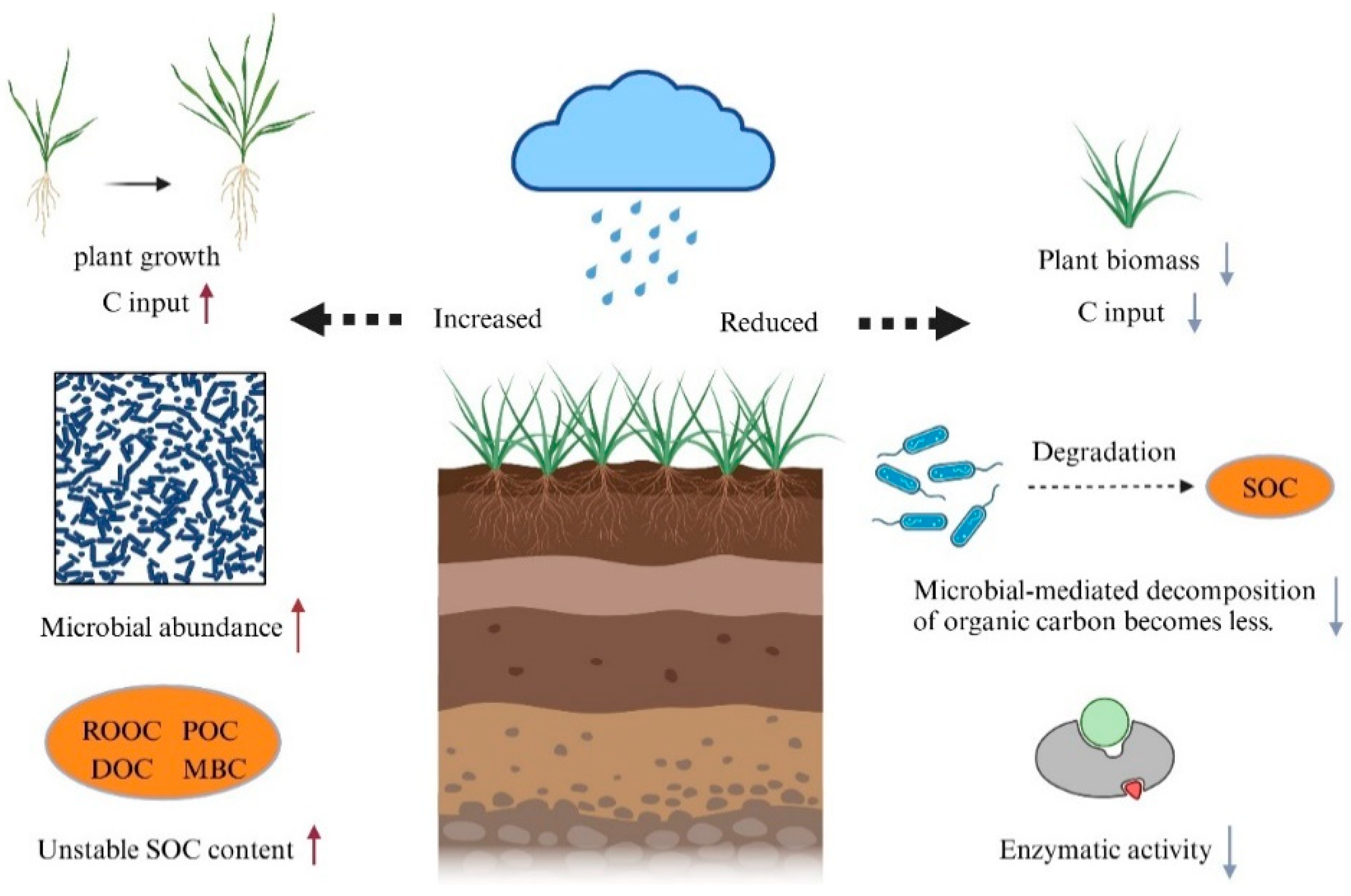
2.3. Variation in the SOC Response to Temperature Elevations in Different Rainfall Conditions
3. Intrinsic Factors Affecting the SOC Response to Elevated Temperatures
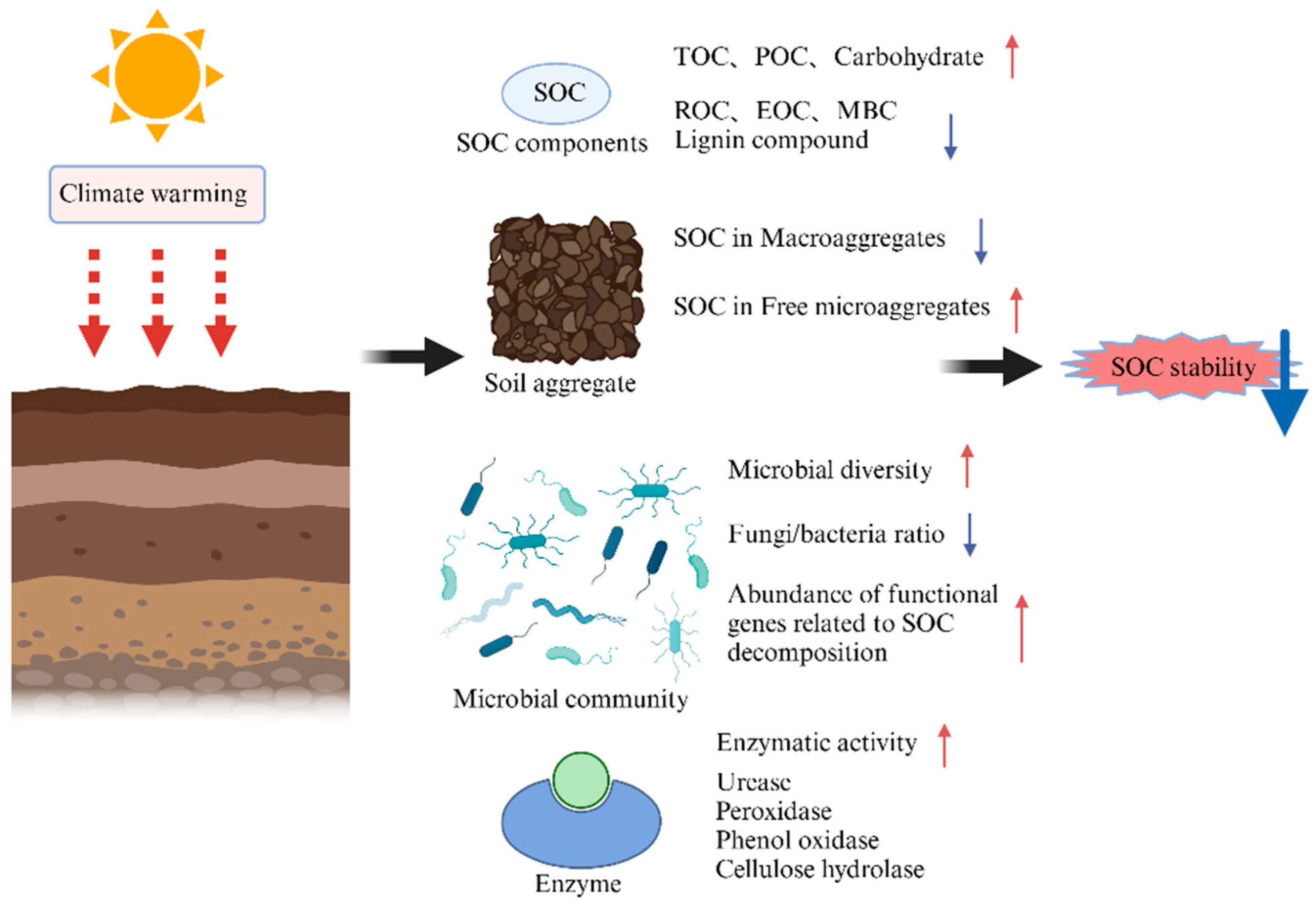
3.1. The Effect of Temperature Elevations on SOC Fractions
3.2. The Effect of Temperature Elevations on Soil Microorganisms and Enzymes
3.3. The Effect of Temperature Elevations on the Temperature Sensitivity of SOC Decomposition
3.4. The Effect of Temperature Elevations on SOC Turnover
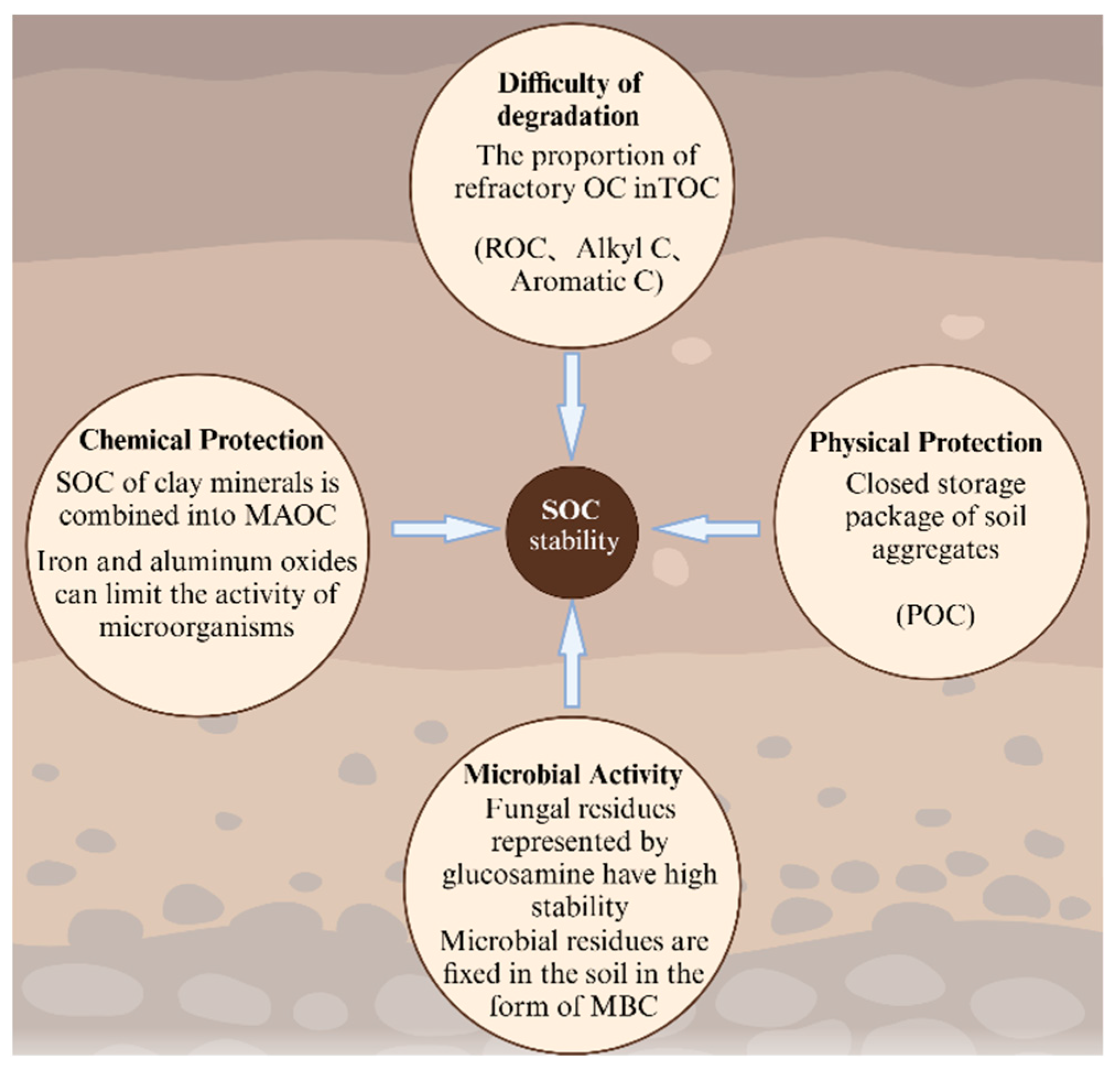
3.5. The Effect of Temperature Elevations on SOC Stability
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dlamini, P.; Chivenge, P.; Chaplot, V. Overgrazing Decreases Soil Organic Carbon Stocks the Most under Dry Climates and Low Soil pH: A Meta-Analysis Shows. Agric. Ecosyst. Environ. 2016, 221, 258–269. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S. Global Carbon Budget 2020. Earth Syst. Sci. Data Discuss. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Keenan, T.F.; Williams, C.A. The Terrestrial Carbon Sink. Annu. Rev. Environ. Resour. 2018, 43, 219–243. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, J. Impact of Land Use Change on Profile Distributions of Soil Organic Carbon Fractions in the Yanqi Basin. Catena 2014, 115, 79–84. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally Rising Soil Heterotrophic Respiration over Recent Decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.-A.; Wookey, P.A.; Ågren, G.I.; Sebastià, M.-T.; et al. Temperature Sensitivity of Soil Respiration Rates Enhanced by Microbial Community Response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Hall, S.J.; Wang, F.; Ye, C.; Li, Z.; Li, S.; Zhou, L.; Qiu, Y.; Guo, J.; et al. Interactive Global Change Factors Mitigate Soil Aggregation and Carbon Change in a Semi-arid Grassland. Glob. Change Biol. 2020, 26, 5320–5332. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing Uncertainty in Soil Carbon Feedbacks to Climate Change. Nat. Clim Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Q.; Liu, Y.; Liu, S.; Yu, D.; Shi, X.; Xing, S.; Chen, H.; Fan, X. Combined Effects of Temperature and Precipitation on Soil Organic Carbon Changes in the Uplands of Eastern China. Geoderma 2019, 337, 1105–1115. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, B.; Cheng, W. Decadally Cycling Soil Carbon Is More Sensitive to Warming than Faster-cycling Soil Carbon. Glob. Change Biol. 2015, 21, 4602–4612. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Meir, P.; Velasquez, E.; Turner, B.L. Soil Carbon Loss by Experimental Warming in a Tropical Forest. Nature 2020, 584, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, Y.; Qin, W.; Xu, T.; Mao, Y.; Wang, Q.; Chen, K.; Zhu, B. Plant and Microbial Regulations of Soil Carbon Dynamics under Warming in Two Alpine Swamp Meadow Ecosystems on the Tibetan Plateau. Sci. Total Environ. 2021, 790, 148072. [Google Scholar] [CrossRef]
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.J.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-Term Pattern and Magnitude of Soil Carbon Feedback to the Climate System in a Warming World. Science 2017, 358, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Boone, L.; Van Linden, V.; Roldán-Ruiz, I.; Sierra, C.A.; Vandecasteele, B.; Sleutel, S.; De Meester, S.; Muylle, H.; Dewulf, J. Introduction of a Natural Resource Balance Indicator to Assess Soil Organic Carbon Management: Agricultural Biomass Productivity Benefit. J. Environ. Manag. 2018, 224, 202–214. [Google Scholar] [CrossRef]
- Chen, Q.; Niu, B.; Hu, Y.; Luo, T.; Zhang, G. Warming and Increased Precipitation Indirectly Affect the Composition and Turnover of Labile-Fraction Soil Organic Matter by Directly Affecting Vegetation and Microorganisms. Sci. Total Environ. 2020, 714, 136787. [Google Scholar] [CrossRef]
- Chang, R.; Liu, S.; Chen, L.; Li, N.; Bing, H.; Wang, T.; Chen, X.; Li, Y.; Wang, G. Soil Organic Carbon Becomes Newer under Warming at a Permafrost Site on the Tibetan Plateau. Soil Biol. Biochem. 2021, 152, 108074. [Google Scholar] [CrossRef]
- Sistla, S.A.; Moore, J.C.; Simpson, R.T.; Gough, L.; Shaver, G.R.; Schimel, J.P. Long-Term Warming Restructures Arctic Tundra without Changing Net Soil Carbon Storage. Nature 2013, 497, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Berlin, W.; Reichel, V.; Hürkamp, A.; Dröder, K. Heat Control Simulation for Variothermal Injection Moulding Moulds Using Infrared Radiation. Int. J. Adv. Manuf. Technol. 2022, 119, 6073–6089. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.-Q. Experimental Warming, Not Grazing, Decreases Rangeland Quality on the Tibetan Plateau. Ecol. Appl. 2007, 17, 541–557. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-Dependent Warming in Mountain Regions of the World. Nat. Clim. Change 2015, 5, 424–430. [Google Scholar]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above-and Belowground Linkages Shape Responses of Mountain Vegetation to Climate Change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, C.; Zhou, Z. Globally Altitudinal Trends in Soil Carbon and Nitrogen Storages. Catena 2022, 210, 105870. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Qu, C.; Huang, W.; Zhang, D.; Li, Y.; Yi, Z.; Liu, J. Translocating Subtropical Forest Soils to a Warmer Region Alters Microbial Communities and Increases the Decomposition of Mineral-Associated Organic Carbon. Soil Biol. Biochem. 2020, 142, 107707. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Kumar, M.; Todaria, N.P.; Pandey, R. Biomass and Soil Carbon along Altitudinal Gradients in Temperate Cedrus Deodara Forests in Central Himalaya, India: Implications for Climate Change Mitigation. Ecol. Indic. 2020, 111, 106025. [Google Scholar] [CrossRef]
- Wei, D.; Tao, J.; Wang, Z.; Zhao, H.; Zhao, W.; Wang, X. Elevation-Dependent Pattern of Net CO2 Uptake across China. Nat. Commun. 2024, 15, 2489. [Google Scholar] [CrossRef] [PubMed]
- Leifeld, J.; Zimmermann, M.; Fuhrer, J.; Conen, F. Storage and Turnover of Carbon in Grassland Soils along an Elevation Gradient in the Swiss Alps. Glob. Change Biol. 2009, 15, 668–679. [Google Scholar] [CrossRef]
- Ma, H.; Yang, X.; Guo, Q.; Zhang, X.; Zhou, C. Soil Organic Carbon Pool along Different Altitudinal Level in the Sygera Mountains, Tibetan Plateau. J. Mt. Sci. 2016, 13, 476–483. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Z.; Ryan, M.G.; You, Y.; Sun, O.J. Changes in Soil Organic Carbon Contents and Fractionations of Forests along a Climatic Gradient in China. For. Ecosyst. 2019, 6, 1. [Google Scholar] [CrossRef]
- Du, B.; Kang, H.; Pumpanen, J.; Zhu, P.; Yin, S.; Zou, Q.; Wang, Z.; Kong, F.; Liu, C. Soil Organic Carbon Stock and Chemical Composition along an Altitude Gradient in the Lushan Mountain, Subtropical China. Ecol. Res. 2014, 29, 433–439. [Google Scholar] [CrossRef]
- Wu, M.; Pang, D.; Chen, L.; Li, X.; Liu, L.; Liu, B.; Li, J.; Wang, J.; Ma, L. Chemical Composition of Soil Organic Carbon and Aggregate Stability along an Elevation Gradient in Helan Mountains, Northwest China. Ecol. Indic. 2021, 131, 108228. [Google Scholar] [CrossRef]
- Zeeshan, M.; Zhou, W.; Wu, C.; Lin, Y.; Azeez, P.A.; Song, Q.; Liu, Y.; Zhang, Y.; Lu, Z.; Sha, L. Soil Heterotrophic Respiration in Response to Rising Temperature and Moisture along an Altitudinal Gradient in a Subtropical Forest Ecosystem, Southwest China. Sci. Total Environ. 2022, 816, 151643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, Y.; Blagodatskaya, E.; Berauer, B.J.; Schuchardt, M.; Holz, M.; Shi, L.; Dannenmann, M.; Kiese, R.; Jentsch, A.; et al. Response of Microbial Growth and Enzyme Activity to Climate Change in European Mountain Grasslands: A Translocation Study. Catena 2024, 239, 107956. [Google Scholar] [CrossRef]
- Li, S.; Delgado-Baquerizo, M.; Ding, J.; Hu, H.; Huang, W.; Sun, Y.; Ni, H.; Kuang, Y.; Yuan, M.M.; Zhou, J.; et al. Intrinsic Microbial Temperature Sensitivity and Soil Organic Carbon Decomposition in Response to Climate Change. Glob. Change Biol. 2024, 30, e17395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xiao, Y.; Zhang, X.; Sui, Y.; Xiao, L.; Lin, J.; Cruse, R.M.; Ding, G.; Liu, X. Warming-Dominated Climate Change Impacts on Soil Organic Carbon Fractions and Aggregate Stability in Mollisols. Geoderma 2023, 438, 116618. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Chen, J.; Zhao, F.; Wang, F.; Sun, Y.; Zhang, G.; Qiu, L. Responses of Soil Organic Carbon to Climate Change in the Qilian Mountains and Its Future Projection. J. Hydrol. 2021, 596, 126110. [Google Scholar] [CrossRef]
- Koven, C.D.; Hugelius, G.; Lawrence, D.M.; Wieder, W.R. Higher Climatological Temperature Sensitivity of Soil Carbon in Cold than Warm Climates. Nat. Clim. Change 2017, 7, 817–822. [Google Scholar] [CrossRef]
- Lei, J.; Guo, X.; Zeng, Y.; Zhou, J.; Gao, Q.; Yang, Y. Temporal Changes in Global Soil Respiration since 1987. Nat. Commun. 2021, 12, 403. [Google Scholar] [CrossRef]
- Chen, Y.; Han, M.; Yuan, X.; Hou, Y.; Qin, W.; Zhou, H.; Zhao, X.; Klein, J.A.; Zhu, B. Warming Has a Minor Effect on Surface Soil Organic Carbon in Alpine Meadow Ecosystems on the Qinghai–Tibetan Plateau. Glob. Change Biol. 2022, 28, 1618–1629. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.-X.; Sun, W.; Zhong, Z.-M.; Zhang, X.-Z.; Zhou, Y.-T. A Meta-Analysis of the Effects of Experimental Warming on Plant Physiology and Growth on the Tibetan Plateau. J. Plant Growth Regul. 2015, 34, 57–65. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, G.; Zhao, B.; Cong, N.; Zheng, Z.; Zhu, J.; Duan, X.; Zhang, Y. Climate Warming Alters the Relative Importance of Plant Root and Microbial Community in Regulating the Accumulation of Soil Microbial Necromass Carbon in a Tibetan Alpine Meadow. Glob. Change Biol. 2023, 29, 3193–3204. [Google Scholar] [CrossRef]
- Ding, X.; Chen, S.; Zhang, B.; Liang, C.; He, H.; Horwath, W.R. Warming Increases Microbial Residue Contribution to Soil Organic Carbon in an Alpine Meadow. Soil Biol. Biochem. 2019, 135, 13–19. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Li, C.; Deng, Q.; Fang, H.; Dai, T.; Chen, C.; Wang, J.; Fan, Z.; Shi, W.; et al. Divergent Responses of Cropland Soil Organic Carbon to Warming across the Sichuan Basin of China. Sci. Total Environ. 2022, 851, 158323. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Huang, T.; Ju, X.; Gu, B.; Huang, W.; Xu, L.; Rees, R.M.; Powlson, D.S.; Smith, P.; Cui, S. Chinese Cropping Systems Are a Net Source of Greenhouse Gases despite Soil Carbon Sequestration. Glob. Change Biol. 2018, 24, 5590–5606. [Google Scholar] [CrossRef]
- Liu, X.; Lie, Z.; Reich, P.B.; Zhou, G.; Yan, J.; Huang, W.; Wang, Y.; Peñuelas, J.; Tissue, D.T.; Zhao, M.; et al. Long-Term Warming Increased Carbon Sequestration Capacity in a Humid Subtropical Forest. Glob. Change Biol. 2024, 30, e17072. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Gloor, E.; Bååth, E.; Meir, P. Soil Carbon and Microbes in the Warming Tropics. Funct. Ecol. 2022, 36, 1338–1354. [Google Scholar] [CrossRef]
- Zeng, X.; Feng, J.; Yu, D.; Wen, S.; Zhang, Q.; Huang, Q.; Delgado-Baquerizo, M.; Liu, Y. Local Temperature Increases Reduce Soil Microbial Residues and Carbon Stocks. Glob. Change Biol. 2022, 28, 6433–6445. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Y.; Wang, C.; Zhou, Z. Mineral Protection Explains the Elevational Variation of Temperature Sensitivity of Soil Carbon Decomposition in the Eastern Himalaya. Appl. Soil Ecol. 2024, 197, 105346. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Wiesmeier, M.; Buness, V.; Berauer, B.J.; Schuchardt, M.A.; Jentsch, A.; Schlingmann, M.; Andrade-Linares, D.; Wolf, B.; Kiese, R.; et al. Rapid Loss of Organic Carbon and Soil Structure in Mountainous Grassland Topsoils Induced by Simulated Climate Change. Geoderma 2024, 442, 116807. [Google Scholar] [CrossRef]
- Ofiti, N.O.E.; Schmidt, M.W.I.; Abiven, S.; Hanson, P.J.; Iversen, C.M.; Wilson, R.M.; Kostka, J.E.; Wiesenberg, G.L.B.; Malhotra, A. Climate Warming and Elevated CO2 Alter Peatland Soil Carbon Sources and Stability. Nat. Commun. 2023, 14, 7533. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, X.; Song, Y.; Gao, S.; Ren, J.; Zhang, H.; Wang, X. Warming-Induced Labile Carbon Change Soil Organic Carbon Mineralization and Microbial Abundance in a Northern Peatland. Microorganisms 2022, 10, 1329. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Fang, C.; Li, B.; Nie, M. Drought May Exacerbate Dryland Soil Inorganic Carbon Loss under Warming Climate Conditions. Nat. Commun. 2024, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, P.; Maestre, F.T.; Moreno-Jiménez, E.; Delgado-Baquerizo, M.; Eldridge, D.J.; Saiz, H.; Gross, N.; Le Bagousse-Pinguet, Y.; Gozalo, B.; Ochoa, V.; et al. Vulnerability of Mineral-Associated Soil Organic Carbon to Climate across Global Drylands. Nat. Clim. Change 2024, 14, 976–982. [Google Scholar] [CrossRef]
- Hu, H.; Chen, J.; Zhou, F.; Nie, M.; Hou, D.; Liu, H.; Delgado-Baquerizo, M.; Ni, H.; Huang, W.; Zhou, J.; et al. Relative Increases in CH4 and CO2 Emissions from Wetlands under Global Warming Dependent on Soil Carbon Substrates. Nat. Geosci. 2024, 17, 26–31. [Google Scholar] [CrossRef]
- Eze, S.; Palmer, S.M.; Chapman, P.J. Negative Effects of Climate Change on Upland Grassland Productivity and Carbon Fluxes Are Not Attenuated by Nitrogen Status. Sci. Total Environ. 2018, 637, 398–407. [Google Scholar] [CrossRef]
- Wei, X.; Van Meerbeek, K.; Yue, K.; Ni, X.; Desie, E.; Heděnec, P.; Yang, J.; Wu, F. Responses of Soil C Pools to Combined Warming and Altered Precipitation Regimes: A Meta-analysis. Glob. Ecol. Biogeogr. 2023, 32, 1660–1675. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, D.; Zhang, Y.; McLaughlin, N.; Zhang, Y.; Wang, Y.; Chen, X.; Zhang, S.; Lu, Y.; Liang, A. Precipitation Increment Reinforced Warming-Induced Increases in Soil Mineral-Associated and Particulate Organic Matter under Agricultural Ecosystem. Appl. Soil Ecol. 2024, 196, 105301. [Google Scholar] [CrossRef]
- Feng, W.; Liang, J.; Hale, L.E.; Jung, C.G.; Chen, J.; Zhou, J.; Xu, M.; Yuan, M.; Wu, L.; Bracho, R.; et al. Enhanced Decomposition of Stable Soil Organic Carbon and Microbial Catabolic Potentials by Long-term Field Warming. Glob. Change Biol. 2017, 23, 4765–4776. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Castanha, C.; Porras, R.C.; Torn, M.S. The Whole-Soil Carbon Flux in Response to Warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Heimann, M.; Reichstein, M. Terrestrial Ecosystem Carbon Dynamics and Climate Feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The Temperature Response of Soil Microbial Efficiency and Its Feedback to Climate. Nat. Clim. Change 2013, 3, 395–398. [Google Scholar] [CrossRef]
- Ofiti, N.O.E.; Zosso, C.U.; Soong, J.L.; Solly, E.F.; Torn, M.S.; Wiesenberg, G.L.B.; Schmidt, M.W.I. Warming Promotes Loss of Subsoil Carbon through Accelerated Degradation of Plant-Derived Organic Matter. Soil Biol. Biochem. 2021, 156, 108185. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, X.; Vinci, G.; Sun, B.; Drosos, M.; Li, L.; Piccolo, A.; Pan, G. Aggregate Fractions Shaped Molecular Composition Change of Soil Organic Matter in a Rice Paddy under Elevated CO2 and Air Warming. Soil Biol. Biochem. 2021, 159, 108289. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Li, C.; Zhang, J.; Wang, L. Effects of Temperature on Soil Organic Carbon Fractions Contents, Aggregate Stability and Structural Characteristics of Humic Substances in a Mollisol. J Soils Sediments 2016, 16, 1849–1857. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Liu, J.; Wang, Z.; Zhang, Z.; Li, X.; Zhang, Q.; Liu, W.; Liu, H.; Zeng, J.; et al. Linking the Soil Carbon Pool Management Index to Ecoenzymatic Stoichiometry and Organic Carbon Functional Groups in Abandoned Land under Climate Change. Catena 2024, 235, 107676. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Heinzle, J.; Salas, E.; Kwatcho-Kengdo, S.; Borken, W.; Schindlbacher, A.; Wanek, W. Long-term Soil Warming Decreases Soil Microbial Necromass Carbon by Adversely Affecting Its Production and Decomposition. Glob. Change Biol. 2024, 30, e17379. [Google Scholar] [CrossRef]
- Guan, S.; An, N.; Zong, N.; He, Y.; Shi, P.; Zhang, J.; He, N. Climate Warming Impacts on Soil Organic Carbon Fractions and Aggregate Stability in a Tibetan Alpine Meadow. Soil Biol. Biochem. 2018, 116, 224–236. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Wang, Y.; Smaill, S.; Clinton, P. Warming Rather Than Increased Precipitation Increases Soil Recalcitrant Organic Carbon in a Semiarid Grassland after 6 Years of Treatments. PLoS ONE 2013, 8, e53761. [Google Scholar] [CrossRef]
- Zhao, G.; Liang, C.; Feng, X.; Liu, L.; Zhu, J.; Chen, N.; Chen, Y.; Wang, L.; Zhang, Y. Elevated CO2 Decreases Soil Carbon Stability in Tibetan Plateau. Environ. Res. Lett. 2020, 15, 114002. [Google Scholar] [CrossRef]
- Sáez-Sandino, T.; García-Palacios, P.; Maestre, F.T.; Plaza, C.; Guirado, E.; Singh, B.K.; Wang, J.; Cano-Díaz, C.; Eisenhauer, N.; Gallardo, A.; et al. The Soil Microbiome Governs the Response of Microbial Respiration to Warming across the Globe. Nat. Clim. Change 2023, 13, 1382–1387. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-Carbon Response to Warming Dependent on Microbial Physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Zhang, Q.; Lyu, M.; Xiong, X.; Liu, X.; Lin, T.; Yang, Y. Substrate Availability and Soil Microbes Drive Temperature Sensitivity of Soil Organic Carbon Mineralization to Warming along an Elevation Gradient in Subtropical Asia. Geoderma 2020, 364, 114198. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, B.; Xie, B.; Feng, K.; Zhang, Z.; Zhang, Z.; Li, S.; Du, X.; Zhang, Q.; Gu, S. Warming Reshaped the Microbial Hierarchical Interactions. Glob. Change Biol. 2021, 27, 6331–6347. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Chen, H.; Liu, H.; Nie, M. Enzymic Moderations of Bacterial and Fungal Communities on Short-and Long-Term Warming Impacts on Soil Organic Carbon. Sci. Total Environ. 2022, 804, 150197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; Van Nostrand, J.D.; Wu, L. Warming Enhances Old Organic Carbon Decomposition through Altering Functional Microbial Communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Chen, H.Y.; Niu, S. Global Meta-Analysis on the Responses of Soil Extracellular Enzyme Activities to Warming. Sci. Total Environ. 2020, 705, 135992. [Google Scholar] [CrossRef]
- Zi, H.B.; Hu, L.; Wang, C.T.; Wang, G.X.; Wu, P.F.; Lerdau, M.; Ade, L.J. Responses of Soil Bacterial Community and Enzyme Activity to Experimental Warming of an Alpine Meadow. Eur. J. Soil Sci. 2018, 69, 429–438. [Google Scholar] [CrossRef]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S. Soil Enzymes in Response to Climate Warming: Mechanisms and Feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Chen, Y.; Han, M.; Yuan, X.; Zhou, H.; Zhao, X.; Schimel, J.P.; Zhu, B. Long-Term Warming Reduces Surface Soil Organic Carbon by Reducing Mineral-Associated Carbon Rather than “Free” Particulate Carbon. Soil Biol. Biochem. 2023, 177, 108905. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Y.; Yuan, M.M.; Gao, Q.; Wu, L.; Qin, Z.; Shi, Z.J.; Schuur, E.A.; Cole, J.R.; Tiedje, J.M. Winter Warming Rapidly Increases Carbon Degradation Capacities of Fungal Communities in Tundra Soil: Potential Consequences on Carbon Stability. Mol. Ecol. 2021, 30, 926–937. [Google Scholar] [CrossRef]
- Chen, J.I.; Elsgaard, L.; van Groenigen, K.J.; Olesen, J.E.; Liang, Z.; Jiang, Y.U.; Lærke, P.E.; Zhang, Y.; Luo, Y.; Hungate, B.A. Soil Carbon Loss with Warming: New Evidence from Carbon-degrading Enzymes. Glob. Change Biol. 2020, 26, 1944–1952. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhao, L.; Zhou, G.; Huang, W.; Liu, J. Increased Litter Input Increases Litter Decomposition and Soil Respiration but Has Minor Effects on Soil Organic Carbon in Subtropical Forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Chen, X.; Lin, Y.; Niu, S.; Jiang, L.; Luo, R.; Luo, Y. Unchanged Carbon Balance Driven by Equivalent Responses of Production and Respiration to Climate Change in a Mixed-grass Prairie. Glob. Change Biol. 2016, 22, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhou, H.; Zhao, X.-Q.; Han, F.; Shi, L.-N.; Duan, J.-C.; Zhao, J.-Z. Influence of Simulated Warming Using OTC on Physiological–Biochemical Characteristics of Elymus Nutans in Alpine Meadow on Qinghai-Tibetan Plateau. Acta Ecol. Sin. 2010, 30, 166–171. [Google Scholar] [CrossRef]
- Wang, J.; Defrenne, C.; McCormack, M.L.; Yang, L.; Tian, D.; Luo, Y.; Hou, E.; Yan, T.; Li, Z.; Bu, W.; et al. Fine-root Functional Trait Responses to Experimental Warming: A Global Meta-analysis. New Phytol. 2021, 230, 1856–1867. [Google Scholar] [CrossRef]
- Tang, L.; Zhong, L.; Xue, K.; Wang, S.; Xu, Z.; Lin, Q.; Luo, C.; Rui, Y.; Li, X.; Li, M.; et al. Warming Counteracts Grazing Effects on the Functional Structure of the Soil Microbial Community in a Tibetan Grassland. Soil Biol. Biochem. 2019, 134, 113–121. [Google Scholar] [CrossRef]
- Johnston, E.R.; Hatt, J.K.; He, Z.; Wu, L.; Guo, X.; Luo, Y.; Schuur, E.A.G.; Tiedje, J.M.; Zhou, J.; Konstantinidis, K.T. Responses of Tundra Soil Microbial Communities to Half a Decade of Experimental Warming at Two Critical Depths. Proc. Natl. Acad. Sci. USA 2019, 116, 15096–15105. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Balser, T.C.; Firestone, M.K. Linking Microbial Community Composition and Soil Processes in a California Annual Grassland and Mixed-Conifer Forest. Biogeochemistry 2005, 73, 395–415. [Google Scholar] [CrossRef]
- Six, J.; Callewaert, P.; Lenders, S.; De Gryze, S.; Morris, S.J.; Gregorich, E.G.; Paul, E.A.; Paustian, K. Measuring and Understanding Carbon Storage in Afforested Soils by Physical Fractionation. Soil Sci. Soc. Am. J. 2002, 66, 1981–1987. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, J.; Yu, W.; Guo, X.; Xu, K.; Zhao, R.; Xiao, L.; Wang, M.; Jiang, Y.; Zhang, S.; et al. Climate-Induced Shifts in Composition and Protection Regulate Temperature Sensitivity of Carbon Decomposition through Soil Profile. Soil Biol. Biochem. 2022, 172, 108743. [Google Scholar] [CrossRef]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K. Widespread Coupling between the Rate and Temperature Sensitivity of Organic Matter Decay. Nat. Geosci. 2010, 3, 854–857. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Xiao, L.; Guo, X.; Zheng, J.; Zhu, B.; Luo, Z. Reconciling Carbon Quality with Availability Predicts Temperature Sensitivity of Global Soil Carbon Mineralization. Proc. Natl. Acad. Sci. USA 2024, 121, e2313842121. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, Y.; Zhou, J. Carbon Quality and the Temperature Sensitivity of Soil Organic Carbon Decomposition in a Tallgrass Prairie. Soil Biol. Biochem. 2012, 50, 142–148. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Pendall, E.; Reich, P.B.; Noh, N.J.; Li, B.; Fang, C.; Nie, M. Rising Temperature May Trigger Deep Soil Carbon Loss across Forest Ecosystems. Adv. Sci. 2020, 7, 2001242. [Google Scholar] [CrossRef]
- Poeplau, C.; Kätterer, T.; Leblans, N.I.W.; Sigurdsson, B.D. Sensitivity of Soil Carbon Fractions and Their Specific Stabilization Mechanisms to Extreme Soil Warming in a Subarctic Grassland. Glob. Change Biol. 2017, 23, 1316–1327. [Google Scholar] [CrossRef]
- Qin, S.; Chen, L.; Fang, K.; Zhang, Q.; Wang, J.; Liu, F.; Yu, J.; Yang, Y. Temperature Sensitivity of SOM Decomposition Governed by Aggregate Protection and Microbial Communities. Sci. Adv. 2019, 5, 1218. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Chen, L.; Yang, Q.; Chen, S.; Zhang, W. Global Synthesis of Temperature Sensitivity of Soil Organic Carbon Decomposition: Latitudinal Patterns and Mechanisms. Funct. Ecol. 2019, 33, 514–523. [Google Scholar] [CrossRef]
- Lefèvre, R.; Barré, P.; Moyano, F.E.; Christensen, B.T.; Bardoux, G.; Eglin, T.; Girardin, C.; Houot, S.; Kätterer, T.; Van Oort, F. Higher Temperature Sensitivity for Stable than for Labile Soil Organic Carbon–Evidence from Incubations of Long-term Bare Fallow Soils. Glob. Change Biol. 2014, 20, 633–640. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Amelung, W. The Temperature Sensitivity (Q10) of Soil Respiration: Controlling Factors and Spatial Prediction at Regional Scale Based on Environmental Soil Classes. Glob. Biogeochem. Cycles 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Varney, R.M.; Chadburn, S.E.; Friedlingstein, P.; Burke, E.J.; Koven, C.D.; Hugelius, G.; Cox, P.M. A Spatial Emergent Constraint on the Sensitivity of Soil Carbon Turnover to Global Warming. Nat. Commun. 2020, 11, 5544. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Don, A.; Schneider, F. Roots Are Key to Increasing the Mean Residence Time of Organic Carbon Entering Temperate Agricultural Soils. Glob. Change Biol. 2021, 27, 4921–4934. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, X.; Yu, P.; Xu, L.; Chen, G.; Cao, L.; Song, C.; Cai, C.; Li, J. Subsoil Organic Carbon Turnover Is Dominantly Controlled by Soil Properties in Grasslands across China. Catena 2021, 207, 105654. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-Term Sensitivity of Soil Carbon Turnover to Warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef]
- Fang, C. Decreased Temperature Sensitivity of Soil Respiration Induced by Warming Slowed Topsoil Carbon Turnover in a Semi-Arid Grassland. Appl. Soil Ecol. 2022, 180, 104620. [Google Scholar] [CrossRef]
- Mao, X.; Sun, T.; Liu, X.; Zhou, J.; Ma, Q.; Wu, L.; Zhang, M. Disentangle the Drivers of Soil Organic Carbon Mineralization and Their Temperature Sensitivity in Both Topsoil and Subsoil: Implication of Thermal Stability and Chemical Composition. Ecol. Indic. 2024, 158, 111399. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil Organic Matter Turnover Is Governed by Accessibility Not Recalcitrance. Glob. Change Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Wagai, R.; Kishimoto-Mo, A.W.; Yonemura, S.; Shirato, Y.; Hiradate, S.; Yagasaki, Y. Linking Temperature Sensitivity of Soil Organic Matter Decomposition to Its Molecular Structure, Accessibility, and Microbial Physiology. Glob. Change Biol. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-associated Forms to Address Global Change in the 21st Century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different Climate Sensitivity of Particulate and Mineral-Associated Soil Organic Matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- García-Palacios, P.; Bradford, M.A.; Benavente-Ferraces, I.; de Celis, M.; Delgado-Baquerizo, M.; García-Gil, J.C.; Gaitán, J.J.; Goñi-Urtiaga, A.; Mueller, C.W.; Panettieri, M. Dominance of Particulate Organic Carbon in Top Mineral Soils in Cold Regions. Nat. Geosci. 2024, 17, 145–150. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]

| Land Use | Characterization of SOC Response to Temperature Elevations | References |
|---|---|---|
| Grasslands soil | Warmer temperatures enhance soil respiration while increasing plant biomass and carbon content of microbial sources. It made the change in SOC insignificant. | [38,39,40,41] |
| Cropland soil | Changes in soil organic carbon in agricultural soils caused by temperature elevations are the result of a combination of factors such as soil texture, precipitation, and land management. | [42,43] |
| Alpine swamp soil | Temperature elevations increased the soil extractable organic carbon (EOC), MBC, and SOC content. Root inputs were greater than carbon degradation and release. | [12] |
| Temperate forest | Elevated temperatures caused a forest soil carbon loss of 710 gCm−2, which was a 31% reduction in carbon stocks. | [13] |
| Subtropical forest soil | Short-term temperature elevations increase soil heterotrophic respiration, which reduces soil carbon stocks. However, long-term temperature increases increase forest carbon sequestration capacity. | [31,44] |
| Tropical forest soil | Elevated temperatures accelerate the decomposition of soil organic matter, resulting in a 55% increase in CO2 emissions from soil heterotrophic respiration. | [11,45] |
| Hilly area soil | Elevated temperatures reduced carbon sequestration by increasing the decomposition of microbial residual charcoal, exacerbating temperature increases and CO2 emissions. Soil carbon decomposition temperature sensitivity decreases with elevation. | [46,47,48] |
| Peatland soil | Elevated temperature increases lignin phenols and induces enhanced degradation of more unstable SOC molecules. When the temperature was increased by 9 °C, soluble compounds from plant and microbial sources decreased by 30%, corresponding to a loss of −0.79 ± 0.2 mg g−1 per 1 °C increase in temperature. Temperature elevations by 5 °C significantly stimulated soil organic carbon mineralization, increased nitrogenous compounds, and decreased the activities of invertase and urease. | [49,50] |
| Drylands soil | The temperature sensitivity of dryland soil organic carbon is mainly regulated by soil physicochemical properties (pH and salinity ions). Due to the continuous temperature elevations, the protective effect of minerals cannot stop carbon loss in the arid regions of the world. | [51,52] |
| Wetlands soil | For low C/N (<12) soils, soil CH4 emissions are more sensitive to temperature, while for high C/N (>21) soils, soil CO2 emissions are more sensitive to temperature. | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, H.; Liang, X.; Jiang, M.; He, S.; He, Y. Mechanisms Behind the Soil Organic Carbon Response to Temperature Elevations. Agriculture 2025, 15, 1118. https://doi.org/10.3390/agriculture15111118
Wu Y, Li H, Liang X, Jiang M, He S, He Y. Mechanisms Behind the Soil Organic Carbon Response to Temperature Elevations. Agriculture. 2025; 15(11):1118. https://doi.org/10.3390/agriculture15111118
Chicago/Turabian StyleWu, Yonglin, Haitao Li, Xinran Liang, Ming Jiang, Siteng He, and Yongmei He. 2025. "Mechanisms Behind the Soil Organic Carbon Response to Temperature Elevations" Agriculture 15, no. 11: 1118. https://doi.org/10.3390/agriculture15111118
APA StyleWu, Y., Li, H., Liang, X., Jiang, M., He, S., & He, Y. (2025). Mechanisms Behind the Soil Organic Carbon Response to Temperature Elevations. Agriculture, 15(11), 1118. https://doi.org/10.3390/agriculture15111118






