Abstract
Alkaline fertilizers demonstrate significant potential in mitigating rice cadmium (Cd) accumulation, yet the combined effects of calcium–magnesium phosphate (CMP) with potassium (K) fertilizer types and split application strategies remain unclear. Through multi-site field trials in Cd-contaminated paddy soils, we evaluated split applications of K2CO3, K2SO4, and K2SiO3 at tillering and booting stages following basal CMP amendment. Optimized K regimes reduced brown rice Cd concentrations (up to 89% reduction) compared to conventional fertilization. Notably, at the CF site, split K2SiO3 application (TB-K2SiO3) and single tillering-stage K2SO4 (T-K2SO4) achieved brown rice Cd levels of 0.13 mg/kg, complying with China’s food safety standard (≤0.20 mg/kg), thereby eliminating non-carcinogenic risks. Mechanistically, TB-K2SiO3 enhanced soil pH by 0.21 units and increased available K (AK) by 50.26% and available Si (ASi) by 21.35% while reducing Cd bioavailability by 43.55% compared to non-split K2SiO3. In contrast, T-K2SO4 elevated sulfate-driven Cd immobilization. Structural equation modeling prioritized soil available Cd, root Cd, and antagonistic effects of AK and ASi as dominant factors governing Cd accumulation. The integration of CMP with split K2SiO3 application at the tillering and booting stages or single K2SO4 application at the tillering stage ensures safe rice production in Cd-contaminated soils, offering scalable remediation strategies for paddy ecosystems.
1. Introduction
Cadmium (Cd) contamination in paddy soils poses a critical threat to rice (Oryza sativa L.) safety, a concern exacerbated in southern China by acidic soil conditions that enhance Cd mobility [1]. This contamination originates from both anthropogenic activities (e.g., fertilizer application, industrial effluents) and geogenic processes (e.g., mining) [2,3]. The environmental risk is further amplified by Cd’s relatively high solubility in paddy ecosystems and rice’s pronounced capacity for Cd bioaccumulation compared to other cereal crops [4,5]. Notably, significant Cd contamination plagues China’s major rice-producing regions; for instance, in Hunan Province’s Yangtze River basin, Cd concentrations in some brown rice samples have reached 0.71–1.21 mg/kg [6], with 76–88% exceeding national safety standards in certain key production areas [7,8]. These dual contamination challenges urgently require strategies balancing agricultural productivity with food safety.
While immobilization technologies employing organic (e.g., biochar, compost) and inorganic amendments (e.g., lime, sepiolite) can effectively reduce Cd bioavailability through mechanisms like pH elevation, adsorption, and precipitation [9,10,11], their practical application faces challenges. Organic amendments may offer uncertain long-term efficacy due to decomposition [12,13], and inorganic agents often necessitate high application rates for substantial Cd reduction, potentially leading to adverse ecological impacts and increased costs [14,15,16]. For example, while combined lime–phosphate applications have shown significant Cd reduction, such intensive practices can compromise soil microbial diversity and economic viability [17,18]. These limitations highlight the need for more cost-effective and sustainable alternatives. In this context, strategic fertilizer management is emerging as a critical approach capable of regulating rice physiology and soil Cd bioavailability to concurrently enhance yield and reduce grain Cd concentrations [19,20,21,22]. Optimized fertilization, therefore, represents a promising avenue for sustainable Cd risk mitigation in rice production.
Potassium (K) fertilization is integral to agriculture for enhancing crop yields and stress resistance [23]. However, the choice of K fertilizer is critical in Cd-contaminated soils, as different K sources exert contrasting effects on Cd migration due to their anion components [24,25]. For instance, chloride-based fertilizers (e.g., KCl) can exacerbate Cd uptake through complexation, whereas sulfate-, carbonate-, and silicate-based K fertilizers (e.g., K2SO4, K2CO3, K2SiO3) generally reduce or suppress Cd accumulation via mechanisms like soil Cd immobilization and enhanced root sequestration [20,26,27,28]. Beyond K source selection, application strategies, including split applications of silicon (Si) and adjusted nitrogen timing, also significantly modulate efficacy [29,30,31]. Despite these advances, key knowledge gaps persist. Most studies focus on single K sources under controlled environments, neglecting field-scale interactions between fertilizer blends and heterogeneous soil properties. Our previous research has demonstrated that calcium–magnesium phosphate (CMP) fertilizers, serving as a phosphorus nutrient source, effectively reduce cadmium accumulation in rice [20,32], whereas synergistic scheduling of K with complementary nutrients remains systematically unvalidated for Cd minimization. Resolving these limitations necessitates field trials to establish anion-specific Cd inhibition thresholds and develop stage-specific fertilization protocols that simultaneously ensure yield stability and Cd safety—an imperative for sustainable rice production in contaminated agroecosystems.
To address these knowledge gaps, cross-site field trials were conducted in typical acidic Cd-contaminated paddy fields in southern China to evaluate the combined efficacy of CMP basal amendment with anion-specific K fertilizers (K2CO3, K2SO4, K2SiO3) and split application strategies. The specific objectives were to (1) quantify the effects of these optimized fertilization regimes on Cd translocation and accumulation in rice; (2) assess treatment-dependent variations in soil Cd bioavailability and related soil properties; and (3) elucidate the key pathways governing Cd accumulation in rice grains using structural equation modeling. This systematic approach advances field-scale Cd mitigation strategies while maintaining productivity, offering a practically implementable framework for targeted remediation in mild to moderate Cd-contaminated paddies.
2. Materials and Methods
2.1. Experimental Sites and Materials
Field trials were conducted at two Cd-contaminated paddy sites in Hunan Province, China: Changfeng (CF, 113.48° E, 28.21° N) and Huanggu Village (HG, 113.22° E, 27.57° N) (Figure 1). These sites were selected based on their distinct Cd contamination levels and agricultural significance. Soil physicochemical properties at both sites are detailed in Table S1. Total Cd contents were quantified as 0.90 mg/kg (CF) and 1.00 mg/kg (HG), both exceeding the national safety threshold for agricultural soils (0.30 mg/kg, GB 15618-2018) [33].
Figure 1.
Field trial locations.
The locally adapted rice cultivar Tianyouhuazhan was selected as the experimental material due to its regional cultivation prevalence. The fertilizers utilized in this study included compound fertilizer (NPK, total nutrients ≥ 45%, N-P2O5-K2O = 15-15-15), urea (total N ≥ 46%), CMP (P2O5 ≥ 16%, CaO ≥ 12%, MgO ≥ 2%), KCl (K2O ≥ 60%), K2CO3 (K2CO3 ≥ 99%), K2SiO3 (K2O ≥ 34%, SiO2 ≥ 52%), and K2SO4 (K2O ≥ 52%, S ≥ 17%). To ensure field applicability, all fertilizers were procured from local agricultural suppliers.
2.2. Field Plot Experimental Design
A randomized complete block design with three replicates per treatment was implemented across seven fertilization regimes. The experiment comprised the following. (1) CK: basal NPK + KCl applied at the tillering stage; (2) T-K2CO3: basal CMP + K2CO3 applied at the tillering stage; (3) TB-K2CO3: basal CMP + K2CO3 split between the tillering and booting stages; (4) T-K2SO4: basal CMP + K2SO4 applied at the tillering stage; (5) TB-K2SO4: basal CMP + K2SO4 split between the tillering and booting stages; (6) T-K2SiO3: basal CMP + K2SiO3 applied at the tillering stage; and (7) TB-K2SiO3: basal CMP + K2SiO3 split between the tillering and booting stages. Detailed fertilizer rates and application timings are provided in Table S2. Plot dimensions (4 × 5 m) were isolated by 50 cm bunds lined with impermeable membranes to prevent cross-contamination. Rice seedlings were transplanted at 25 × 20 cm spacing in early June and harvested in early October, with the cultivation period lasting approximately 125 days. Irrigation and pest management during rice growth followed local practices.
2.3. Sample Collection and Preparation
2.3.1. Plant Sampling and Preparation
At rice maturity, 15 rice plants per plot were randomly sampled from the central rows of each plot using the five-point method. The rice plants were thoroughly rinsed with deionized water to remove surface contaminants and then heated at 105 °C for 30 min and oven-dried at 60 °C until a constant mass was achieved. The samples were then separated into roots, straw, and grains. The grains were mechanically dehulled using a laboratory-scale rice mill to obtain brown rice.
2.3.2. Soil Sampling and Preparation
Concurrent with plant sampling, composite soil samples (0–20 cm depth) were collected from each plot. Five subsamples were taken randomly within each plot and combined to form one composite sample per plot. Soil samples were air-dried, sieved, and stored for further analysis.
2.4. Sample Digestion and Determination of Soil Characteristics
Rice samples were digested with a HNO3-HClO4 (4:1, v/v) mixture, whereas soil samples were subjected to tri-acid digestion (HCl-HNO3-HClO4). Cd concentrations in the digests were quantified using inductively coupled plasma mass spectrometry (ICP-MS; NexION 350X, PerkinElmer, Waltham, MA, USA). Quality assurance included procedural blanks and certified reference materials (CRMs: GBW07428-GSS-14 for soil and GBW07603-GSV-2 for plant tissues) during digestion and analysis. Soil pH was determined potentiometrically in a 1:2.5 (w/v) soil/water suspension using a calibrated pH meter (S400-K, Mettler Toledo, Zurich, Switzerland). Available Cd (ACd) was extracted with 0.01 mol/L of CaCl2 (1:10 w/v) and quantified [20]. Cation exchange capacity (CEC) was determined using the barium chloride–sulfate forced exchange protocol [19], whereas available sulfur (AS) was assessed through phosphate buffer extraction coupled with barium sulfate turbidimetric analysis [34]. Available K (AK) was extracted with 1.00 mol/L of ammonium acetate (pH 7.00) and quantified via flame photometry [35]. Available silicon (ASi) was extracted using a 0.5 mol/L acetate buffer (pH 4.00), with soluble silicic acid subsequently measured through molybdenum blue colorimetry at 660 nm [36].
2.5. Health Risk Assessment
To assess the potential health risks associated with Cd in brown rice, the exposed population was categorized into two demographic groups: children and adults. Health risks were evaluated using the hazard quotient (HQ) and carcinogenic risk (CR) indices, as per USEPA’s and Wei et al.’s methodologies [37,38]. The HQ and CR were calculated as follows:
where C (mg/kg) represents the Cd concentrations in brown rice; IR (g/day) represents daily rice intake rate; EF (day/year) is the exposure frequency; ED is the exposure duration (year); BW (kg) is the average body weight; AT (day) is the average exposure time; RfD is the oral reference dose (mg/kg-day); and SF is the slope factor for cancer. All parameter values employed in this assessment are comprehensively summarized in Table S3. An HQ value exceeding 1.0 indicates a significant non-carcinogenic risk, whereas values below 1.0 suggest no adverse health effects. CR values greater than 1.0 × 10−4 were classified as intolerable, while risks between 1.0 × 10−4 and 1.0 × 10−6 were deemed acceptable. CR values below 1.0 × 10−6 implied negligible carcinogenic risk [39,40].
ADI = C × IR × EF × ED/(BW × AT)
HQ = ADI/RfD
CR = ADI × SF
2.6. Statistical Analysis
The translocation factor (TF) of Cd, which indicates the capacity for Cd translocation among rice tissues, was calculated according to Deng et al. [20]:
where Cr, Cs, and Cb represent the Cd concentrations in rice roots, straw, and brown rice, respectively.
TFrs = Cs/Cr
TFsb = Cb/Cs
Statistical analyses were conducted using SPSS 27 to perform one-way analysis of variance (ANOVA) followed by Duncan’s test for post hoc comparisons, with statistical significance defined at p < 0.05. Data visualization was executed using Origin 2025. To elucidate relationships between soil physicochemical properties and Cd accumulation in rice, Mantel tests were implemented via the linkET package in R (v4.3.1). A structural equation model (SEM) was constructed using Amos 28 to quantify direct and indirect pathways regulating Cd accumulation in rice under optimized fertilization strategies.
3. Results
3.1. Cd Accumulation in Rice and Health Risk Assessment
At the CF site, T-K2SO4 reduced root and straw Cd concentrations by 41.7% and 59.0%, respectively, versus CK (p < 0.05), while T-K2CO3 decreased straw Cd by 23.2% (Figure 2A). In contrast, Cd accumulation in roots and straw was enhanced by the T-K2SiO3 treatment. Notably, split application strategies further modulated Cd distribution, as TB-K2SiO3 reduced root and straw Cd concentrations by 57.96% and 62.37%, respectively, compared to non-split application, whereas TB-K2SO4 exhibited an elevated effect. Brown rice Cd concentrations were reduced by 52.26%, 28.58%, and 88.89% in the T-K2CO3, T-K2SiO3, and T-K2SO4 treatments compared to CK, respectively. Further decreases in brown rice Cd concentrations (43.26% and 84.71%) were recorded in the TB-K2CO3 and TB-K2SiO3 treatments relative to their single-application equivalents, while TB-K2SO4 induced a 3.55-fold increase in brown rice’s Cd concentration compared to T-K2SO4. Notably, both TB-K2SiO3 and T-K2SO4 achieved compliance with China’s food safety standard limit (0.2 mg/kg; GB 2762-2022) [41], with brown rice Cd concentrations measured at 0.13 mg/kg. The health risk assessment demonstrated that optimized K fertilization strategies significantly mitigated risks associated with Cd intake via brown rice consumption (Figure 3). The HQ values from the intake of brown rice under the TB-K2SiO3 and T-K2SO4 treatments decreased to below 1.0 for both children and adults, representing reductions of 89.08% and 88.89% compared to CK, respectively.
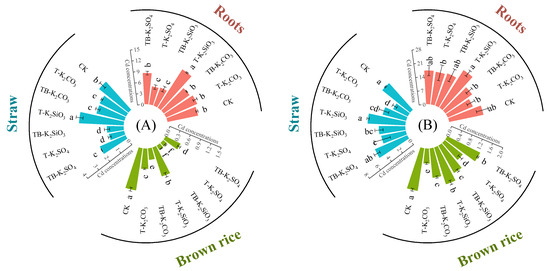
Figure 2.
Different fertilizer applications affect Cd accumulation in rice tissues at CF (A) and HG (B) sites. Statistically significant differences (p < 0.05) among fertilization treatments are denoted by distinct lowercase letters.
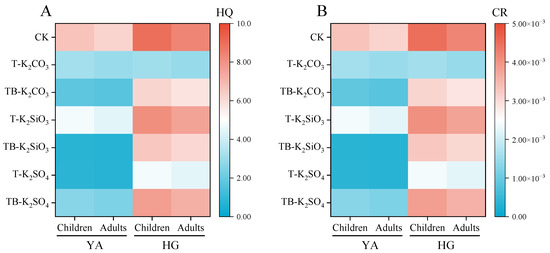
Figure 3.
The hazard quotient (A) and carcinogenic risk (B) of Cd from intake of brown rice across fertilization treatments. Risk magnitudes are represented through a chromatic gradient spanning blue (minimal risk) to red (elevated risk), with color intensity corresponding to health risk severity.
At the HG site, root Cd concentrations showed no significant differences under different K fertilization regimes (Figure 2B). Compared with the CK treatment, T-K2CO3 and T-K2SO4 significantly reduced straw Cd concentrations, whereas TB-K2CO3 and TB-K2SO4 enhanced Cd accumulation in straw relative to single-application treatments. In contrast, the straw Cd concentrations of TB-K2SiO3 decreased significantly by 29.81% compared to T-K2SiO3. Similarly, changes in brown rice Cd followed the same trend as straw Cd. Although brown rice Cd concentrations under different fertilization strategies were significantly reduced by 9.75–46.78% compared to CK, TB-K2CO3 and TB-K2SO4 significantly increased Cd concentrations by 96.77% and 60.93% compared to T-K2CO3 and T-K2SO4, respectively. Importantly, only TB-K2SiO3 further significantly inhibited Cd accumulation in brown rice. Despite substantial reductions in HQ and CR values from the intake of brown rice under optimized K fertilization (Figure 3), residual health risks persisted due to elevated soil Cd background levels and severe contamination severity.
3.2. Changes in Cd Translocation in Rice Tissues
At the CF site, TFrs was significantly reduced by 24.17–28.87% under all fertilization strategies compared to CK, except for the T-K2SiO3 treatment (Figure 4A). A similar trend was observed at the HG site, where TFrs decreased by 18.25–42.64% across treatments relative to CK, although significant differences were limited to the T-K2CO3 treatment (Figure 4B). Notably, TB-K2SiO3 reduced TFrs by 8.22% (CF) and 6.84% (HG) compared to its non-split application counterpart, whereas TB-K2CO3 and TB-K2SO4 treatments were associated with increases in TFrs (4.84–28.08%), albeit without statistical significance. Additionally, at the CF site, TFsb was significantly reduced by 39.31–75.39% under all K fertilization regimes compared to CK. Compared to single-application treatments, TFsb was further decreased by 41.96% and 58.79% under the TB-K2CO3 and TB-K2SiO3 treatments, respectively. In contrast, T-K2SO4 induced a 92.87% increase in TFsb. However, at the HG site, the efficacy of fertilization strategies in modulating TFsb was substantially attenuated, with no significant reductions observed compared to CK.
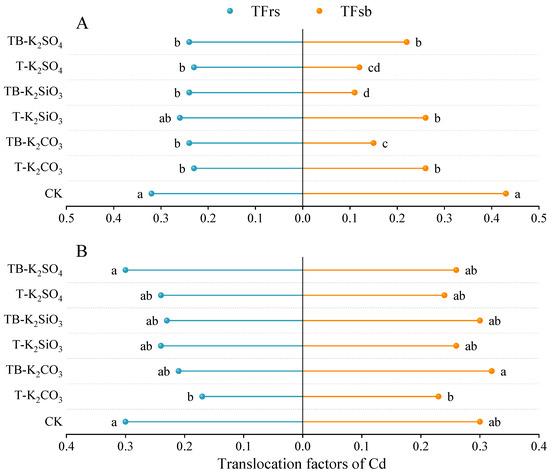
Figure 4.
Translocation factors of Cd in different rice tissues at CF (A) and HG (B) sties. TFrs: translocation factor of Cd from roots to straw; TFsb: translocation factor of Cd from straw to brown rice. Statistically significant differences (p < 0.05) among fertilization treatments are denoted by distinct lowercase letters.
3.3. Variation in Soil Cd Bioavailability and Soil Characteristics
All K fertilizer regimes significantly elevated the soil pH by 0.21–0.45 units relative to CK, with split applications further enhancing pH by 0.05–0.21 units compared to single applications (Figure 5A). The highest pH (5.40) was recorded under TB-K2SiO3, indicating its superior alkalizing capacity. The immobilization effect of soil Cd was significantly enhanced by split application K fertilization regimes (Figure 5B). In single-application treatments, Cd bioavailability followed a descending order: T-K2SiO3 (0.22 mg/kg) > T-K2CO3 (0.20 mg/kg) > T-K2SO4 (0.14 mg/kg). Compared to non-split treatments, ACd contents were further reduced by 22.03% and 43.55% under the TB-K2CO3 and TB-K2SiO3 treatments, respectively, whereas TB-K2SO4 significantly increased Cd bioavailability (p < 0.05). Analysis of AK contents showed that single-application treatments elevated AK levels relative to CK, with significant increments in T-K2CO3 (59.53%) and T-K2SO4 (79.17%) (Figure 5C). Importantly, the TB-K2SiO3 enhanced AK by 50.26% compared to T-K2SiO3, surpassing CK levels by 94.83%, while TB-K2CO3 and TB-K2SO4 showed no significant AK differences from their single-application counterparts. For sulfur (S) bioavailability, non-split K treatments increased AS contents by 84.25–113.21% relative to CK, with T-K2SO4 achieving peak AS mobilization (54.21 mg/kg) due to sulfate anion dominance (Figure 5D). Although split application treatments reduced AS contents by 2.16–18.23% compared to single applications, they remained 72.8–98.6% higher than CK. Critically, TB-K2SiO3 significantly improved soil Si bioavailability, yielding ASi contents 1.21-fold higher than T-K2SiO3 (p < 0.05). For CEC, only TB-K2SiO3 showed a significant increase relative to CK, with no significant differences among other treatments (Figure 5F).
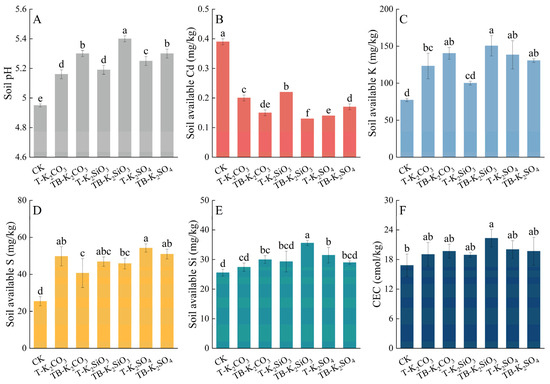
Figure 5.
Effects of different fertilizer applications on soil pH (A), available Cd (B), K (C), S (D), and Si (E) contents, and CEC (F). Statistically significant differences (p < 0.05) among fertilization treatments are denoted by distinct lowercase letters.
3.4. Relationship Between Soil Properties and Cd Accumulation in Rice
The Mantel test revealed that ASi contents exerted an extremely significant influence on root Cd concentrations (p < 0.01; Figure 6). Similarly, ASi and AK contents were identified as having extremely significant effects on straw Cd concentrations (p < 0.01), while CEC and ACd contents demonstrated significant impacts (p < 0.05). All measured factors significantly affected brown rice Cd accumulation, with soil pH, ACd, AK, and ASi contents displaying the highest significance (p < 0.01). Soil pH, AK, AS, and ASi contents exhibited highly significant negative correlations with ACd contents (p < 0.01), confirming their roles in Cd immobilization. Additionally, soil pH showed strong positive correlations with CEC, AK, AS, and ASi contents (p < 0.01), suggesting synergistic interactions in nutrient availability enhancement.
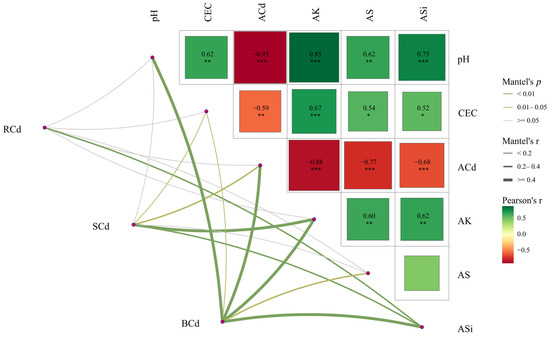
Figure 6.
Mantel test of soil physicochemical properties and rice Cd accumulation. Edge width corresponds to the Mantel’s r statistic for the corresponding distance correlations, and edge color indicates the statistical significance. Pairwise comparisons between soil indicators, with a color gradient representing Pearson’s correlation coefficient. Rcd, SCd, and BCd represent the Cd concentrations in roots, straw, and brown rice, respectively; ACd, AK, AS, ASi, and CEC represent soil available Cd, K, S, and Si contents and cation exchange capacity, respectively. * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001.
3.5. Integrated Pathway Analysis of Cd Accumulation in Rice
SEM was employed to assess the influence of soil environmental factors on Cd accumulation in brown rice under optimized K fertilizer regimes (Figure 7). The SEM analysis identified robust and statistically significant inverse relationships between soil pH, AK, and AS contents and ACd contents (p < 0.01). Additionally, ACd contents and root Cd concentrations were found to exert significant positive effects on Cd accumulation in brown rice and straw. Conversely, AK and ASi contents significantly inhibited root Cd accumulation. Notably, key drivers of brown rice Cd accumulation under optimized K fertilization were ranked: ACd (standardized effect: 0.85) > AK (−0.60) > RCd (0.33) > ASi (−0.22).
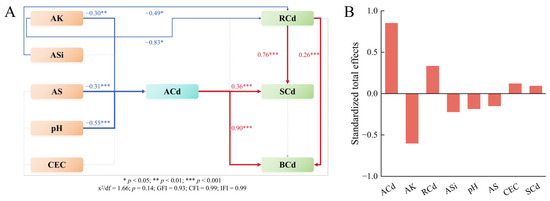
Figure 7.
Structural equation modeling (SEM) shows the direct and indirect effects of soil characteristics on the Cd accumulation in rice (A) and the standardized total effects of these factors on the Cd concentrations in brown rice (B). Red lines represent significant positive correlation; blue lines represent significant negative correlation. Rcd, SCd, and BCd represent the Cd concentrations in roots, straw, and brown rice, respectively; ACd, AK, AS, ASi, and CEC represent soil available Cd, K, S, and Si contents and cation exchange capacity, respectively.
4. Discussion
In situ passivation, primarily achieved by elevating soil pH and modifying Cd speciation to reduce bioavailability, is a widely recognized remediation strategy for the ecological restoration of Cd-contaminated farmland [4]. Nevertheless, a persistent challenge with many prevailing techniques is their reliance on high application rates of passivating agents, which can escalate costs and pose secondary ecological risks [17,42,43]. This necessitates a careful balance between remediation effectiveness, economic viability, and agricultural sustainability. Our multi-regional field trials have demonstrated that the synergistic application of CMP with optimized K fertilization regimes decreased Cd concentrations in brown rice by 28.58–89.08% at the CF site and 9.75–65.13% at the HG site. This highlights the potential to enhance remediation efficacy without resorting to excessively high amendment rates. The selection of the K fertilizer type is of paramount importance, attributable to its differential effects on plant Cd accumulation. For instance, pot experiments conducted by Wang et al. revealed that incremental KCl application rates elevated Cd concentrations in rice by 10.8–192.8%, whereas K2SO4 application led to a 32.5% reduction [26]. These findings are corroborated by Liu et al. [44] and Zahedifar et al. [45], who also reported that KCl application exacerbated Cd accumulation in crops. Furthermore, field trials by Deng et al. demonstrated that basal application of K2CO3 reduced Cd concentrations in brown rice by 23.57–41.51% compared to conventional fertilization practices [20]. Our investigation aligns with these findings under field conditions. Notably, at the CF site, TB-K2SiO3 and T-K2SO4 achieved brown rice Cd levels below China’s food safety standard (0.20 mg/kg; GB 2762-2022), eliminating non-carcinogenic risks for all age groups. Consequently, a split application of K2SiO3 (at the tillering and booting stages) or a single application of K2SO4 (at the tillering stage), when integrated with CMP, is recommended as an efficacious strategy for ensuring safe rice production in Cd-contaminated agroecosystems.
K, as one of the most abundant cations within plant cells, plays an indispensable role in the regulation of plant growth, developmental processes, and stress resistance mechanisms [46]. Beyond its established roles in fundamental cellular metabolic processes, K has been demonstrated to alleviate Cd toxicity via a concert of mechanisms. These include the diminished uptake and subsequent translocation of Cd within the plant, an augmented synthesis of stress-responsive metabolites (e.g., specific proteins), the amelioration of Cd-induced growth inhibition, and an attenuation of oxidative damage [47,48]. Additionally, it has been reported that elevated exogenous K concentrations supplied during the tillering stage significantly reduced Cd accumulation in rice tissues, primarily by impeding its translocation from roots to aerial plant parts [49]. This observation is congruent with our findings from SEM, which revealed strong negative correlations between AK and ACd/root Cd (Figure 7).
The observed variations in Cd accumulation with different K fertilizer types are primarily attributable to distinct anion-mediated mechanisms. As a common K fertilizer, KCl increases cadmium solubility through ligand displacement, forming soluble CdCln(2−n) complexes [50,51]. Chloride-induced soil acidification promotes cadmium desorption from soil colloids, thereby increasing bioavailability [52]. Furthermore, chloride upregulates cadmium transporter genes (OsNRAMP5, OsHMA2) in rice xylem, accelerating root-to-shoot translocation [53]. In contrast, K2CO3 tends to neutralize soil acidity, converting free Cd ions into less bioavailable carbonate-bound forms, thereby reducing Cd bioavailability [20]. K2SO4 offers another pathway for Cd immobilization, primarily involving sulfur-mediated processes. S can enhance the formation of root iron plaques, which effectively adsorb and immobilize Cd, restricting its entry into rice roots [54]. Under flooded conditions, SO42− can be microbially reduced to sulfide, leading to the precipitation of Cd as highly insoluble CdS [55]. Concurrently, Fe2+ derived from Fe3+ reduction synergistically forms iron sulfide–cadmium co-precipitates, significantly reducing Cd bioavailability [56]. Additionally, the hydroxyl ions generated during SO42− reduction can elevate soil pH, further promoting Cd adsorption to soil colloids and its transformation into stable hydroxides [34]. Congruent with these mechanisms, SEM analysis revealed significant negative correlations between soil pH and AS contents and ACd levels (Figure 7). Both T-K2SO4 and TB-K2SO4 treatments significantly increased soil pH and AS contents, reduced Cd bioavailability, and, consequently, suppressed Cd accumulation in brown rice. Si has been demonstrated to inhibit Cd accumulation in rice through multiple pathways. (1) Si forms polysilicic acid gel complexes or silicate–Cd complexes via adsorption and co-precipitation, effectively immobilizing Cd and reducing its bioavailability [27]. (2) Si enhances Cd retention in plant cell walls, promotes the synthesis of thiol compounds (e.g., glutathione, polysaccharides, and non-protein sulfhydryl groups), and strengthens Cd chelation, thereby limiting Cd translocation [57,58]. (3) Si has been shown to downregulate the expression of key Cd transporter genes, such as OsLCT1 and OsNRAMP5, directly suppressing Cd uptake by rice roots [59]. Field trials confirmed that soil ASi contents were significantly enhanced by K2SiO3 treatments, especially through split application (TB-K2SiO3) (Figure 5E). Furthermore, significant negative correlations were observed between soil ASi contents and both ACd levels and root Cd concentrations (Figure 6 and Figure 7), indicating that K2SiO3 application effectively reduced brown rice Cd by immobilizing soil Cd and inhibiting root Cd uptake.
Fertilization timing critically governs Cd accumulation dynamics in rice [30]. The booting and grain-filling stages represent critical growth phases for Cd accumulation in brown rice, during which targeted soil amendments can reduce root Cd uptake and inhibit translocation to grains, thereby minimizing contamination [60,61,62]. Previous studies indicate that phased application of Cd passivators outperforms single-dose treatments applied at transplanting [12]. Similarly, a nitrogen application ratio of 6:0:2:2 (pre-transplanting:tillering:booting:grain-filling) has been shown to reduce rice Cd content by 52.72–74.13% [63]. Equal Si allocation across the transplanting, tillering, and booting stages reduced grain Cd concentrations by 87.4% [30]. Split application, especially of fertilizers like K2SiO3, ensures a more continuous supply of beneficial elements (like K and Si) and can help maintain a more stable and favorable rhizosphere environment throughout critical growth stages [27]. This sustained effect, compared to a single basal application, could lead to more prolonged or strategically timed modulation of Cd transporter gene expression. For instance, consistent availability of Si from split K2SiO3 application might lead to more sustained downregulation of Cd uptake transporters like OsNRAMP5 and OsNRAMP1, as Si is known to affect their expression [28]. In this study, split K2SiO3 application at the tillering and booting stages reduced brown rice Cd concentrations by 84.71% (CF) and 19.22% (HG) compared to non-split treatments. This enhanced efficacy is likely due to the sustained release of Si, which alleviated potential ASi deficiency during critical growth stages, thereby promoting continuous Cd immobilization and reducing ACd [64]. Furthermore, K2SiO3, as an alkaline amendment, exhibited enhanced hydrolysis activity relative to H+, which activated alkaline nutrients (e.g., alkali-hydrolyzable N, available P, and AK) and improved soil fertility [28]. Phased Si fertilization has been previously linked to nutrient enrichment, enhanced Si cycling, and improved rhizosphere bioavailability [27]. Congruently, our experiments demonstrated that the TB-K2SiO3 treatment significantly increased soil pH, AK, and ASi contents compared to T-K2SiO3, while Cd bioavailability followed an inverse trend (Figure 5). SEM revealed significant negative correlations between soil pH, AK contents, and ACd contents, as well as between ASi contents and root Cd levels (Figure 7). These findings indicate that TB-K2SiO3 enhanced Cd immobilization and nutrient availability through sustained Si release, effectively inhibiting Cd uptake. Interestingly, a contrasting observation was made for K2SO4, where a single application at tillering (T-K2SO4) outperformed its split application counterpart in reducing brown rice Cd. This discrepancy may be attributed to the potentially diminished soil available sulfur levels under the split K2SO4 regimen (Figure 5D), which could have weakened the sulfate-mediated Cd immobilization mechanisms.
5. Conclusions
This multi-site field validation across diverse acidic Cd-contaminated paddy soils demonstrates that coupling CMP with optimized K fertilization regimes—specifically, a split K2SiO3 application at the tillering and booting stages or a single K2SO4 application at tillering—significantly reduces cadmium accumulation in rice grains. At the CF site, for instance, these strategies achieved brown rice Cd concentrations of 0.13 mg/kg, fully complying with China’s strict food safety standard (GB 2762-2022), while simultaneously reducing HQ to safe thresholds for both children and adults. Compared to conventional fertilization, these CMP-synergized strategies reduced brown rice Cd by 28.58% to 89.08% at CF and 9.75% to 65.13% at HG, with split K2SiO3 outperforming non-split application by reducing Cd bioavailability by an additional 43.55%. Mechanistic insights from SEM identified soil available Cd and root Cd as dominant drivers of grains’ Cd accumulation, which were counteracted by the antagonistic effects of increased available K and Si under optimized fertilization. Furthermore, the split K2SiO3 regime not only enhanced soil pH and AK and ASi contents but also promoted Cd sequestration, whereas single K2SO4 primarily leveraged sulfate-driven Cd immobilization. Collectively, these findings establish CMP-synergized K management as a scalable, low-input strategy for safer rice production in moderately Cd-contaminated paddies, prioritizing field applicability without compromising yield and offering a practical pathway to align agricultural productivity with public health goals.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15101052/s1, Table S1: Soil characteristics in field trials; Table S2: Different fertilizer application rates and methods; Table S3: Parameter values for the human health risk assessment.
Author Contributions
Conceptualization, X.D.; methodology, X.D., Y.Y. and Q.Z. (Qingru Zeng); visualization, Q.Z. (Qiying Zhang), W.W. and Y.Z.; formal analysis, Q.Z. (Qiying Zhang) and W.W.; investigation, Y.Z. and X.T.; data curation, W.W. and Y.Z.; writing—original draft preparation, Q.Z. (Qiying Zhang) and W.W.; writing—review and editing, X.D., Y.Y. and Q.Z. (Qingru Zeng); funding acquisition, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32201390), the Natural Science Foundation of Hunan Province (2025JJ50104), and the National Key Research and Development Program of China (2022YFD1700102).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jing, H.; Yang, W.; Chen, Y.; Yang, L.; Zhou, H.; Yang, Y.; Zhao, Z.; Wu, P.; Zia-ur-Rehman, M. Exploring the mechanism of Cd uptake and translocation in rice: Future perspectives of rice safety. Sci. Total Environ. 2023, 897, 165369. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, S.; Zhou, H.; Zeng, M.; Zhang, J.; Huang, F.; Shan, S.; Guo, Z.; Yi, H.; Sun, Z.; et al. Combined amendment reduces soil Cd availability and rice Cd accumulation in three consecutive rice planting seasons. J. Environ. Sci. 2022, 111, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, P.; Yang, W. Study on safe usage of agricultural land in typical Karst areas based on Cd in soil and maize: A case study of Northwestern Guizhou, China. Agriculture 2022, 12, 1156. [Google Scholar] [CrossRef]
- NaziaTahir; Ullah, A.; Tahir, A.; Rashid, H.U.; Rehman, T.U.; Danish, S.; Hussain, B.; Akca, H. Strategies for reducing Cd concentration in paddy soil for rice safety. J. Clean. Prod. 2021, 316, 128116. [Google Scholar] [CrossRef]
- Li, X.; Peng, P.; Long, J.; Dong, X.; Jiang, K.; Hou, H. Plant-induced insoluble Cd mobilization and Cd redistribution among different rice cultivars. J. Clean. Prod. 2020, 256, 120494. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F.-J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Yang, L.; Jing, H.; Mao, W.; Liu, J.; Zou, Y.; Wu, Y.; Zhou, H.; Yang, W.; et al. A Critical Review of Biochar Application for the remediation of greenhouse gas emissions and nutrient loss in rice paddies: Characteristics, mechanisms, and future recommendations. Agronomy 2023, 13, 893. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, W.; Zhou, H.; Zia-ur-Rehman, M.; Salam, M.; Ouyang, L.; Chen, Y.; Yang, L.; Wu, P. Exploring the mechanisms of organic fertilizers on Cd bioavailability in rice fields: Environmental behavior and effect factors. Ecotoxicol. Environ. Saf. 2024, 285, 117094. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lu, S. Soil inorganic amendments produce safe rice by reducing the transfer of Cd and increasing key amino acids in brown rice. J. Environ. Sci. 2024, 136, 121–132. [Google Scholar] [CrossRef]
- Mabagala, F.S.; Zhang, T.; Zeng, X.; He, C.; Shan, H.; Qiu, C.; Gao, X.; Zhang, N.; Su, S. A review of amendments for simultaneously reducing Cd and As availability in paddy soils and rice grain based on meta-analysis. J. Environ. Manag. 2024, 366, 121661. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, J.; Zhou, H.; Wang, Y.; Ni, L.; Liao, Y.; Gu, J.; Liao, B.; Li, Q. Long-term effects of compound passivator coupled with silicon fertilizer on the reduction of cadmium and arsenic accumulation in rice and health risk evaluation. Sci. Total Environ. 2024, 922, 171245. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, Z.; Li, X.; Chen, W.; Deng, Y.; Han, C. Lime and phosphate amendment can significantly reduce uptake of Cd and Pb by field-grown rice. Sustainability 2017, 9, 430. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Qiang, R.; Lu, E.; Li, C.; Zhang, J.; Gao, Q. Response of soil microbial community structure to phosphate fertilizer reduction and combinations of microbial fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, P.; Zhou, W.; Liu, H.; Cheng, K.; Xiao, X.; Tang, H.; Yi, Z. Response characteristics of soil Cd availability to microbes in paddy soil with long-term fertilization and its impact on Cd uptake in rice. Sci. Total Environ. 2024, 957, 177680. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Yang, Y.; Lu, L.; Yuan, X.; Zeng, H.; Zeng, Q. Cadmium accumulation in rice (Oryza sativa L.) alleviated by basal alkaline fertilizers followed by topdressing of manganese fertilizer. Environ. Pollut. 2020, 262, 114289. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Zhao, T.; Dahlgren, R.A.; Xu, J. Fertilizer application alters cadmium and selenium bioavailability in soil-rice system with high geological background levels. Environ. Pollut. 2024, 350, 124033. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, Q.-T.; Lee, C.C.C.; Jiang, C.A.; Wei, Z. Evaluation of manganese application after soil stabilization to effectively reduce cadmium in rice. J. Hazard. Mater. 2022, 424, 127296. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Gao, Z.; Lu, J.; Zhang, Y.; Li, X. Straw return combined with potassium fertilization improves potassium stocks in large-macroaggregates by increasing complex iron oxide under rice–oilseed rape rotation system. Soil Tillage Res. 2025, 248, 106404. [Google Scholar] [CrossRef]
- Bellarby, J.; Surridge, B.W.J.; Haygarth, P.M.; Liu, K.; Siciliano, G.; Smith, L.; Rahn, C.; Meng, F. The stocks and flows of nitrogen, phosphorus and potassium across a 30-year time series for agriculture in Huantai county, China. Sci. Total Environ. 2018, 619–620, 606–620. [Google Scholar] [CrossRef]
- Grant, C.A.; Sheppard, S.C. Fertilizer impacts on cadmium availability in agricultural soils and crops. Hum. Ecol. Risk Assess. 2008, 14, 210–228. [Google Scholar] [CrossRef]
- Wang, K.; Fu, G.; Yu, Y.; Wan, Y.; Liu, Z.; Wang, Q.; Zhang, J.; Li, H. Effects of different potassium fertilizers on cadmium uptake by three crops. Environ. Sci. Pollut. Res. 2019, 26, 27014–27022. [Google Scholar] [CrossRef]
- Pan, B.; Wang, W.; Liu, B.; Cai, K.; Tian, J.; Cai, Y. Significant difference in the efficacies of silicon application regimes on cadmium species and environmental risks in rice rhizosphere. Environ. Pollut. 2023, 327, 121521. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, B.; Liu, B.; Cai, K.; Tian, J.; Wang, W. The Cd sequestration effects of rice roots affected by different Si management in Cd-contaminated paddy soil. Sci. Total Environ. 2022, 849, 157718. [Google Scholar] [CrossRef]
- Pan, B.; Cai, Y.; Liu, B.; Cai, K.; Lv, W.; Tian, J.; Wang, W. Abatement of Cd in rice grain and toxic risks to human health by the split application of silicon at transplanting and jointing period. J. Environ. Manag. 2022, 302, 114039. [Google Scholar] [CrossRef]
- Rehman, M.Z.U.; Rizwan, M.; Rauf, A.; Ayub, M.A.; Ali, S.; Qayyum, M.F.; Waris, A.A.; Naeem, A.; Sanaullah, M. Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 2019, 226, 454–462. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, H.; Xu, C.; Zheng, S.; Wu, M.; Zhang, Q.; Liao, Y.; Zhu, H.; Zhu, Q.; Huang, D. Nitrogen application practices to reduce cadmium concentration in rice (Oryza sativa L.) grains. Environ. Sci. Pollut. Res. 2022, 29, 50530–50539. [Google Scholar] [CrossRef]
- Chen, B.; Deng, X.; Ma, Q.; Zhao, Y.; Wang, A.; Zhang, X.; Zeng, Q. Cadmium accumulation in brown rice (Oryza sativa L.) depends on environmental factors and nutrient transport: A three-year field study. Sci. Total Environ. 2023, 903, 166942. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality–Risk Control Standard for Soil Contamination of Agricultural Land. China Environment Publishing Group: Beijing, China, 2018.
- Wang, J.; Yu, L.; Qin, L.; Sun, X.; Zhou, W.; Wang, M.; Chen, S. Low pe+pH inhibits Cd transfer from paddy soil to rice tissues driven by S addition. Chemosphere 2023, 335, 139126. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, L.; Wei, X.; Li, T.; Tang, Y.; Zeng, X.; Lei, Z.; Wen, J.; Su, S. Promoted decomposition in straw return to double-cropped rice fields controls soil acidity, increases soil fertility and improves rice yield. Chem. Eng. J. 2025, 509, 161309. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, B.; Wang, X.; Chen, L.; Akhtar, K.; Tang, S.; Lu, H.; He, J.; Wen, R.; He, B. Differences in the response mechanism of cadmium uptake, transfer, and accumulation of different rice varieties after foliar silicon spraying under cadmium-stressed soil. Front. Plant Sci. 2023, 13, 1064359. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook 2011 Edition; US Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Wei, R.; Chen, C.; Kou, M.; Liu, Z.; Wang, Z.; Cai, J.; Tan, W. Heavy metal concentrations in rice that meet safety standards can still pose a risk to human health. Commun. Earth Environ. 2023, 4, 84. [Google Scholar] [CrossRef]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. CATENA 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Kang, M.; Wang, X.; Chen, J.; Fang, Q.; Liu, J.; Tang, L.; Liu, L.; Cao, W.; Zhu, Y.; Liu, B. Extreme low-temperature events can alleviate micronutrient deficiencies while increasing potential health risks from heavy metals in rice. Environ. Pollut. 2023, 334, 122165. [Google Scholar] [CrossRef]
- GB 2762-2022; National Standard for Food Safety: Limit of Contaminants in Food. NHFPCPRC and CFDA: Beijing, China, 2022.
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Huang, Q.; Tang, S.; Wang, J.; Hu, P.; Shao, G. Can liming reduce cadmium (Cd) accumulation in rice (Oryza sativa) in slightly acidic soils? A contradictory dynamic equilibrium between Cd uptake capacity of roots and Cd immobilisation in soils. Chemosphere 2018, 193, 547–556. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, P.; Li, Z.; Zou, B.; Wang, G.; Li, N.; Qiu, J. Effects of fertiliser and intercropping on cadmium uptake by maize. Chem. Ecol. 2013, 29, 489–500. [Google Scholar] [CrossRef]
- Zahedifar, M.; Akbar, M.A.; Mahshid, S.; Zahra, Z.; Karimian, F. Cadmium accumulation and partitioning in Ocimum basilicum as influenced by the application of various potassium fertilizers. Arch. Agron. Soil Sci. 2016, 62, 663–673. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture-status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Zaheer, M.M.; Ahmad, Y.N.; Rashid, A.S.; Ullah, K.W.; Aqeel, A.; Aamir, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2018, 41, 461–476. [Google Scholar] [CrossRef]
- Zhang, S. Effects of Anion Attendant, pH Value, and Concentrations of Ca and K in Rhizosphere on Cd Uptake Andtanslolcation in Rice Root; Zhejiang University: Hangzhou, China, 2020. (In Chinese) [Google Scholar]
- Guo, J.; Chen, M.; Huang, Y.; Xie, S.; Zhang, X.; Zuo, T.; Hu, C.; Wang, G. Chloride application weakens cadmium immobilization by lime in paddy rice soil. Ecotoxicol. Environ. Saf. 2022, 241, 113761. [Google Scholar] [CrossRef]
- Dahlin, A.S.; Eriksson, J.; Campbell, C.D.; Öborn, I. Soil amendment affects Cd uptake by wheat—Are we underestimating the risks from chloride inputs? Sci. Total Environ. 2016, 554–555, 349–357. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Khaliq, M.A.; Xie, T.; Chen, Y.; Wang, G. Chlorine weaken the immobilization of Cd in soil-rice systems by biochar. Chemosphere 2019, 235, 1172–1179. [Google Scholar] [CrossRef]
- Guo, J.; Ge, C.; Abulimiti, M.; Zhou, D.; Hu, C.; Wang, G. Detrimental effect of chloride on suppressing cadmium accumulation in rice grains: A field-based investigations. Environ. Technol. Innov. 2024, 36, 103883. [Google Scholar] [CrossRef]
- Miao, F.; Zhang, X.; Fu, Q.; Hu, H.; Islam, M.S.; Fang, L.; Zhu, J. Sulfur enhances iron plaque formation and stress resistance to reduce the transfer of Cd and As in the soil-rice system. Sci. Total Environ. 2024, 927, 171689. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Q.; Li, G.; Guo, C.; Li, L.; Wang, J. Evaluating water management efficiency in regulating cadmium and arsenic accumulation in rice in typical Japonica paddy soils at varied pH levels. Agriculture 2024, 14, 407. [Google Scholar] [CrossRef]
- Huang, H.; Ji, X.-B.; Cheng, L.-Y.; Zhao, F.-J.; Wang, P. Free radicals produced from the oxidation of ferrous sulfides promote the remobilization of cadmium in paddy soils during drainage. Environ. Sci. Technol. 2021, 55, 9845–9853. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia ur Rehman, M.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Peera Sheikh Kulsum, P.G.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Tang, J.; Yang, J.; Guo, Z.; Xiao, Y.; Ge, Y.; Liu, T.; Hu, Q.; Ao, H.; et al. Cadmium absorption and translocation in rice plants are influenced by lower air temperatures during grain filling stage. Sci. Total Environ. 2024, 954, 176742. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Zhao, F.-J.; Wang, P. The relative contributions of root uptake and remobilization to the loading of Cd and As into rice grains: Implications in simultaneously controlling grain Cd and As accumulation using a segmented water management strategy. Environ. Pollut. 2022, 293, 118497. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, M.; Dai, H.; Tian, T.; Pan, W.; Xu, J.; Lin, D. Safe production of rice in Cd-polluted paddy fields by rhizosphere application of zero-valent iron nanoplates at specific growth stages. Nano Today 2024, 56, 102289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Xiao, H.; Ao, H.; Tian, W.; Xiao, F.; Xiang, Y.; Zhang, X. Effect of nitrogen fertilizer on iron plaque formation on the root surface of double cropping rice and cadmium accumulation in double-season rice. J. Agro-Environ. Sci. 2021, 40, 260–268. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Li, G.; Zhao, Q.; Wang, W.; Jiang, J.; Wang, Y.; Yuan, L. Stabilization of arsenic, antimony, and lead in contaminated soil with montmorillonite modified by ferrihydrite: Efficiency and mechanism. Chem. Eng. J. 2023, 457, 141182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).