Abstract
Abiotic stresses significantly disrupt plant physiology at the molecular, biochemical, and morphological levels, often causing irreversible damage. To ensure sustainable coffee production, it is essential to understand how environmental stresses—such as drought, heat, excess light, and salinity—affect plant growth, and to develop strategies to mitigate their impact. Despite the limited number of studies on this topic, compiling existing knowledge can provide valuable insights into how coffee plants respond to such stresses. Specifically, understanding whether coffee plants can endure damage caused by these stresses and the mechanisms they employ to do so is critical. This review aims to (i) summarize key findings on the effects of drought, heat, excess light, and salinity on coffee plants and their coping mechanisms; and (ii) explore plant breeding and nutrition as potential strategies to mitigate these abiotic stresses and enhance coffee production.
1. Introduction
Coffee is one of the most essential commodities in the international agricultural trade and represents a significant source of income to several countries, including Brazil, the world’s largest coffee producer and exporter [1]. For this reason, there has been a growing concern about coffee production since this is considered a high-risk crop, bearing in mind upcoming climate changes predicted to continue throughout the XXI century [2]. The forecasted weather variations include disturbances in rainfall patterns, more frequent drought periods, high temperatures, and a shift in the geographical regions suitable for coffee-growing [3].
Approximately 99% of global coffee production is obtained from cultivations of two species: Arabica, Coffea arabica L., 1753, and Robusta, Coffea canephora Pierre ex A. Froehner, 1897, both belonging to the order Gentianales, family Rubiaceae [4]. Arabica grows better in temperatures between 18 and 22 °C and Robusta between 22 and 28 °C. Damage to bean quality and yield of both species can occur out of these temperature ranges [5], suggesting significant sensitivity to shifts in climatic conditions. In addition, recurrent periods of drought accompanied by excess light and/or episodes of frost have caused instability in the annual coffee supply [6,7] by affecting plant development, flowering, fruit set, and the production of both Arabica and Robusta [4,8].
Drought, excess light, high temperatures, inadequate soil nutrient availability, and salinity are agriculture’s most important abiotic stresses [9,10,11,12,13]. Abiotic stresses affect the physiological conditions of plants, with considerable impacts at the molecular, biochemical, and morphological levels, and may cause irreversible damage to the plants, representing the leading causes of agricultural yield losses [11]. This topic is highly relevant to coffee production but remains underexplored compared to research on other crops (Figure 1A). Significant efforts to understand how coffee plants respond to various abiotic stresses and to develop mitigation strategies have increased since 2006 (Figure 1B).
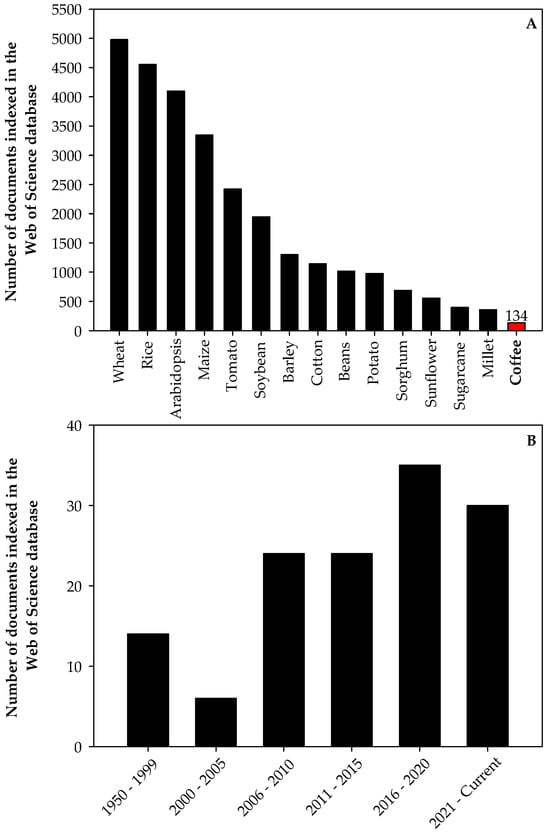
Figure 1.
Number of documents indexed in the Web of Science database about stress in the most important economic crops (except the Arabidopsis genus, which was used as a reference, as it is a model plant) worldwide (A) and number of documents indexed in the Web of Science database about stress in coffee (B). The numbers presented in these figures were obtained from the search of studies published containing the keywords “stress” and “crop name” in the title on 17 July 2024. For example, for wheat, we inserted the keywords “stress” and “wheat” in different rows and selected the option “title”, in the field search. These numbers are subjected to changes according to the keywords used in the search.
Based on the above data, our aims in this brief review were (i) to summarize the main results concerning the impact of drought, heat, excess light, and salinity on coffee production and the main mechanisms employed by the plants to cope with the environmental stresses; and (ii) to address plant breeding and plant nutrition as strategies to mitigate abiotic stress in coffee cultivation.
2. Abiotic Stress Factors and Their Relationship with Coffee Plant Growth
In this section, we describe in a general way the main effects of drought, heat, excess light, and salinity on plant growth, and present the main mechanisms employed by coffee plants to deal with environmental stresses (Figure 2).

Figure 2.
Summary of the stress symptoms induced by drought, heat and excess light, and salinity in coffee plants, as well as tolerance mechanisms and strategies to improve plant tolerance to these stresses. Figure created by the authors.
2.1. Drought Stress in Coffee
Most studies on abiotic stress in coffee focus on drought stress and have been published in the past 15 years (Figure 3). Drought is considered the most stressful environmental factor affecting plant growth and yield, and its negative impacts are expected to become even more significant due to climate change [2,14]. In this scenario, maintaining growth and crop yield under one of the most stressful environmental conditions, such as water deficit, represents one of the main challenges of modern agriculture [15].
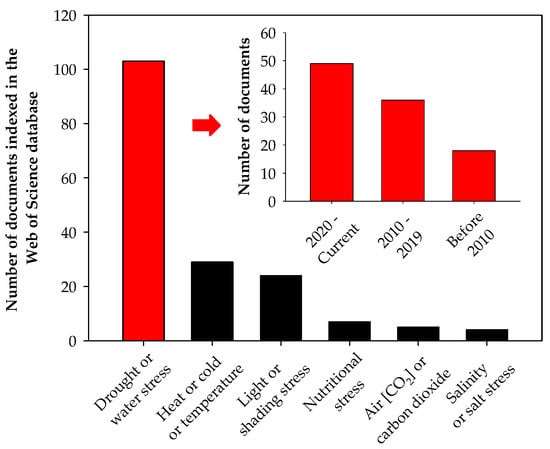
Figure 3.
Number of documents indexed in the Web of Science database concerning drought or water stress; heat, cold, or temperature stress; light or shading stress; nutritional stress; air [CO2] or carbon dioxide stress; and salinity or salt stress in coffee. The number of documents regarding drought or water stress in coffee indexed by the Web of Science database over time is also presented. The numbers presented in the figure were obtained from the search with the keywords described in the x-axis of this figure on 17 July 2024. These numbers are subjected to changes according to keywords used for search.
Water deficit in plants is characterized by an imbalance between water uptake by roots and water loss by leaf transpiration; i.e., the water deficit occurs when the transpiration from the leaf surface is higher than the water uptake by the roots [16]. Many factors can cause a plant water deficit, including environmental factors like inadequate precipitation, high evaporation, decreased groundwater level, soil compaction, and water retention by soil particles [17], as well as plant-related ones, like the root system. This period of abnormally dry weather, resulting in soil water deficit and, subsequently, plant water deficit, is defined as drought. The main symptoms of drought syndrome in plants vary depending on the species, stress intensity, developmental stage, growth conditions, and interaction with other environmental factors. The main morphological symptoms generally include loss of leaf turgor, inhibition of cell extension, drooping, wilting, etiolation, yellowing, and premature leaf downfall [18,19,20]. Considering coffee plants, the main symptoms of drought stress are depicted in Table 1 and Figure 4.

Table 1.
Changes induced by drought in coffee plants and main tolerance mechanisms for stress adaptation.

Figure 4.
Symptoms of drought stress in coffee plants include loss of leaf turgor (a,c,d), wilting (a,c,d), drooping (b,e), yellowing (d,e), necrosis (b–e), and premature leaf downfall (b,e).
In a very simplified reaction chain, the water deficit stress causes stomatal closure in response to the reduction in xylem water potential, preventing excess water vapor loss from the leaves to the atmosphere. However, transpiration reduction increases leaves’ temperature and reduces CO2 influx through diffusion into leaves’ mesophyll. With continuous interception and absorption of the photosynthetically active radiation (PAR) by these lower-transpiration leaves, the biochemical power (NADPH and ATP) from the photochemical phase of the photosynthesis would be less used by the biochemical phase due to the lower CO2 availability to ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), causing an over-reduction in the electron transport chain in the chloroplast thylakoids. Due to the biophysical nature of PAR absorption, the oxygen evolution complex, and the excitation of the reaction center in photosystem II (PSII), the free electrons from water photolysis react with free oxygen in the chloroplast lumen and generate an accumulation of reactive oxygen species (ROS), which would be counter-balanced by an antioxidant system (AOS). If the AOS cannot scavenge ROS, oxidative stress can destroy cells, damaging the tissues permanently [30,31].
Coffee plants exposed to drought stress use the following strategies to mitigate eventual damage: (i) decreased leaf area to reduce transpiration; (ii) sustained movement of sugars from sources to sinks; (iii) altered leaf morphology and root development; (iv) shoot-to-root signaling of water status; (v) maximizing photosynthesis versus transpiration; and (vi) replacement of ROS-damaged proteins and phospholipids [25]. It has been demonstrated that, in tolerant clones of C. canephora, transcriptional memory may contribute to drought tolerance during cycles of drought exposure [32]. More specifically, the RNAseq approach identified differentially expressed genes in tolerant (826) and sensitive (135) clones and their enriched categories. Such analysis indicated that sensitive clones may trigger an oxidative stress response, possibly leading to programmed cell death, when exposed to multiple drought episodes [32]. However, the acclimation of tolerant plants apparently involves antioxidant secondary metabolism and the abscisic acid (ABA) response. Additionally, 49 memory genes were identified in the tolerant clones, mainly linked to the ABA pathway, protein folding, and biotic stress [32].
Most research on plant responses to drought treats it as a single, isolated event, unlike natural conditions where recurring drought episodes often coincide with other environmental challenges. Drought-sensitive and drought-tolerant clones of C. canephora exposed to multiple drought cycles developed a differential acclimation that potentiated their defense mechanisms [14], with more significant acclimation in drought-tolerant clones than in its drought-sensitive counterpart. Plants subjected to multiple drought events showed higher photosynthetic rates and high activities of RuBisCO and other enzymes associated with carbon and antioxidant metabolism compared to those subjected to drought for the first time. In addition, acclimation to multiple drought events involved the expression of trainable genes related to drought tolerance and was also associated with a deep metabolite reprogramming with concordant alterations in central metabolic processes such as respiration and photorespiration, allowing them to be kept in an “alert state” to cope with further drought events [14] successfully. Both respiration and photorespiration can be affected by air [CO2], so it is crucial to understand how elevated air [CO2] induced by climate change can affect coffee production.
The elevated air [CO2] is likely to mitigate drought stress. Elevated [CO2] often diminishes leaf stomatal conductance and density [33,34]. In addition, arabica coffee grown for 4 years under high [CO2] showed a reduction in canopy leaf area associated with improved net photosynthesis [35]. This fact has been associated with increased RuBisCO activity and decreased oxygenase activity, which reduces photorespiration and ROS production [34,36]. In coffee subjected to drought stress, elevated [CO2] improved carbon assimilation rates with unchanged stomatal conductance, increased water use efficiency, respiration rates, and biomass accumulation, and decreased photorespiration rates and oxidative stress, regardless of watering. The elevated [CO2] also promoted key allometric adjustments linked to drought tolerance in coffee, such as more biomass partitioning towards roots with greater root length [10].
2.2. Heat and Excess Light Stress in Coffee
As sessile organisms, plants are largely affected by heat stress, which limits their growth, metabolism, and yields worldwide [30]. The interest in knowing the plant’s responses to heat stress is increasing since there is a concern about how ecosystems will be affected by climate change. Global annual temperatures are predicted to rise by 0.3–4.8 °C by 2100 [2], and many areas are likely to warm above the global average with increased numbers of heat waves and impacts worsened by simultaneous drought events.
The primary plant responses to heat stress include modifications in its morphology, such as an increase in the root system and a reduction in the number and conductance of stomata. Furthermore, the leaves curl, fold, and decrease their areas to avoid water loss by evapotranspiration [37,38]. According to Lima et al. [9], the leaf cell wall of coffee plants subjected to heat stress differs from that of non-stressed plants in terms of monosaccharide composition and molar mass profiles of polysaccharides. Heat stress increased the leaf hemicellulose content, whereas the pectin content decreased by almost 50% compared to plants in control conditions. Still, the final insoluble residue yields were not affected at the end of heat treatment. Also, alterations in palisade cells and ultrastructural damage in chloroplasts were observed. These data show that the chemical profile of coffee cell wall polymers and structural cell anatomy change under heat stress [9].
High temperatures are frequently accompanied by excess light in nature [39], which can restrict coffee growth since coffee plants are traditionally known for needing to be shade-grown. However, coffee can be grown in full sun exposure without losing yield. Martins et al. [40] demonstrated that some coffee plants can adjust their metabolic machinery to excess light through marked increases in their antioxidant capacity associated with enhanced consumption of reducing equivalents. Photorespiration and alternative pathways are the main ones responsible for reductant consumption under excess light. In such conditions, it was found that primary and secondary metabolisms were reprogrammed to down-regulate intermediates of the tricarboxylic acid (TCA) cycle and upregulate the amino acids, sugars and sugar alcohols, polyamines, and flavonoids [40]. These data suggest that metabolic alterations are primarily associated with oxidative stress avoidance rather than representing adjustments to prevent the plants from utilizing the additional light to improve their photosynthetic performance.
Although some coffee plants can adjust their metabolic machinery to excess light conditions [40], other coffee plants or genotypes are sensitive to heat and excess light. Some of the primary visual symptoms caused by excess light and heat include scalding, sunburn, and sunscald (Figure 5). Sunburn occurs when the coffee plant absorbs a large amount of energy from the sun and cannot dissipate it, resulting in oxidative damage. This type of damage is commonly observed following the pruning and planting of young trees, as the intense sunlight (and heat) causes the breakdown of chlorophyll in the leaf [41]. Initial damage appears as pale, bleached, or faded areas that become brown and necrotic until they finally drop. Unless extremely severe, plants typically recover from sunburn damage but may delay vegetative and/or reproductive growth, affecting yield. Unfortunately, there are only a few studies evaluating the response of coffee plants to heat and excess light stresses (Table 2), which makes it challenging to apply efficient strategies to mitigate these stressful conditions.

Figure 5.
The initial symptoms of sunburn in coffee leaves and fruits appear as pale, bleached, or faded areas that become brown (a,c) and necrotic (b,d,e). The images used in this figure were taken from the following websites: (a) “https://revistacampoenegocios.com.br/escaldadura-em-folhas-e-frutos-do-cafeeiro/” (accessed on 20 December 2024), (b) “https://www.cafepoint.com.br/noticias/tecnicas-de-producao/escaldadura-em-cafeeiros-ocorre-em-larga-escala-neste-ano-212432/” (accessed on 20 December 2024), (c) “https://www.cafepoint.com.br/noticias/tecnicas-de-producao/escaldadura-em-folhas-e-frutos-do-cafeeiro-93382/” (accessed on 20 December 2024), (d) “https://www.revistaprocampo.com.br/2019/02/06/escaldadura-em-cafeeiros-ocorre-em-larga-escala-neste-ano/” (accessed on 20 December 2024), and (e) “http://www.redepeabirus.com.br/redes/form/post?topico_id=103141” (accessed on 20 December 2024).

Table 2.
Changes induced by heat and excess light stress in coffee plants and main tolerance mechanisms for stress adaptation.
2.3. Salinity Stress in Coffee
Salinity is one of the principal abiotic stresses in agriculture that causes growth decreases, physiological abnormalities, and lower yields of crops throughout the world [49]. Salt stress remains a significant growth limitation factor, mostly in arid and semiarid zones [50]. Soil salinity is a measure of the concentration of all the soluble salts in the soil solution and is usually expressed as electrical conductivity (EC) [51]. A soil is considered saline when its pH is lower than 8.5, EC is higher than 4.0 dS m−1 (equal to 40 mM NaCl), and the exchangeable sodium percentage (ESP) is higher than 15% [52].
Soil salinization may occur naturally or through human action. Natural geological, hydrological, and pedological processes constitute some natural causes, but climate change and water management may accelerate salinization. In both arid and semiarid lands, evapotranspiration plays a vital role in the pedogenesis of saline and sodic soils. Soil salinity caused by anthropogenic action is mainly due to improper irrigation methods. Poor-quality water is often used for irrigation, so eventually, salt builds up in the soil unless the management of the irrigation system is such that salts are leached from the soil profile. Other anthropogenic causes are deforestation, accumulation of air-borne or water-borne salts in the soils, contamination with chemicals, and overgrazing [53]. More than 100 countries are estimated to face saline conditions in the soil or groundwater [54].
The roots are usually the first organ to sense the salt signal after plants are exposed to saline conditions [38]. The root architecture modifications in response to salinity are mediated by the suppression of cell division, initiation, elongation, or growth redirection away from salt. The earliest visual plant response to salinity is a reduction in leaf expansion rate, followed by a cessation of expansion as the stress intensifies. Also, exposure to salinity leads to stomatal closure, which reduces the photosynthesis ratio due to a decrease in stomatal conductance, which restricts the access of CO2 for the Calvin–Benson cycle [54,55].
In C. arabica, salinity induced structural damage in the mesophyll cells and changes in the organization of pectins, hemicelluloses, and lignin composition in the cell wall [56]. Beyond the observed changes in composition, the size of the hemicellulose fractions from coffee leaves increased under salinity, suggesting increased cross-linking between polysaccharides. Establishing stronger cross-linking between polysaccharides and lignification of cell walls could contribute to the cell’s stiffening, resulting in diffusion restriction, thus acting as a barrier for salt entrance. The increase in the negatively charged cell wall polysaccharides has been pointed out to play a role in coping with salt by facilitating ion transport at high salt concentrations. However, it is also possible that the negatively charged polysaccharides delay the entrance of sodium (Na+) [56].
The number of studies assessing the effect of salinity in coffee plants is very low (Figure 3), but climate change may accelerate salinization by changing water availability [49]. Therefore, new studies must be conducted to better understand how coffee plants cope with salinity to find early solutions to this problem.
3. Strategies to Improve Coffee Plant Tolerance to Abiotic Stress
The frequency of the stress factors mentioned above will progressively increase in the future due to a rise in climate change events. Thus, it is crucial to develop or better understand strategies to mitigate the adverse effects of these challenges on agriculture and improve crop resilience and yield. Several methods have been tested in recent years, such as the development of genotypes more tolerant to climate change through the application of conventional and modern breeding techniques and the utilization of plant growth regulators, osmoprotectants, nutrient and water management, planting time, seed priming, microbial seed treatment, and arbuscular mycorrhizae. The application of biochar, kaolin, chitosan, superabsorbent, yeast extract, and seaweed extract has also been assessed as tools to mitigate plant stress [57].
Fresh coffee husk for biochar (BC) has been tested as a promising alternative because it can be incorporated into the soil to improve the physical, chemical, and microbiological characteristics while increasing crop yields [58,59]. Soil properties such as water retention [60], pH [61,62], hydraulic conductivity, and nutrient availability increase with the application of BC [63]. By evaluating four different biochar doses (0, 4, 8, and 16 t ha−1) combined with a progressive reduction in irrigation (25, 50, 75, and 90% of water lost via evapotranspiration), these authors showed that the application of 8 t ha−1 of BC alleviated the effects of moderate water deficit in coffee plants. Reduced irrigation negatively affected the maximum quantum yield of photosystem II (Fv/Fm), leaf gas exchange, biomass, and water status. On the other hand, the application of 8 t ha−1 of BC increased photosynthesis in well-irrigated plants (~6 µmol m−2 s−1) and less-irrigated plants (~3.5 µmol m−2 s−1) compared with plants grown without application of BC (well-irrigated plants: ~3.9 µmol m−2 s−1; and less-irrigated plants: ~1.8 µmol m−2 s−1). The authors concluded that using BC can be recommended for coffee production not only to capture carbon and reintroduce it to the soil but also to alleviate the effects of moderate water deficit [64]. Other products have been soil-applied or used via foliar sprays to minimize drought-induced stress. It has been demonstrated that melatonin (phytomelatonin) plays an interesting role in abiotic stress tolerance [65,66]. Melatonin enhanced antioxidant enzyme activities and reduced free radicals, hydrogen peroxide (H2O2), and malondialdehyde generation in apples, grapes, maize, sunflower, tomato, and wheat [67]. The treatment with melatonin increases the tolerance to water deficit in plants [68,69] by maintaining the turgor pressure in the leaves at satisfactory levels [70]. Regarding the coffee responses, Campos et al. [71] found that 300 µM of melatonin applied to the soil 4 weeks after transplantation promoted increased drought tolerance by increasing root growth and reducing leaf water potential. The maintenance of leaf water potential by melatonin may have favored gas exchanges and, thus, biochemical reactions, since among plants exposed to water deficit, those treated with melatonin showed higher stomatal conductance and higher photosynthetic and transpiratory rates, allowing a greater supply of assimilates for growing tissues. These findings show that melatonin may enhance coffee plants’ drought tolerance. Other strategies, such as pre-exposing coffee seedlings to deficit irrigation [72] or grafting sensitive genotypes onto tolerant rootstocks [73], have also been tested and have shown promising results in enhancing plant tolerance to abiotic stress, especially to water deficit. Nevertheless, in this section, we highlight plant breeding and plant nutrition as potential strategies to help coffee plants grow under stressful conditions.
3.1. Plant Breeding Towards Abiotic Stress Tolerance
Coffee genetic breeding is a critical practice that aims to develop coffee genotypes with desirable agronomic traits, such as greater yield, resistance to diseases and pests, better bean quality, and adaptation to different climatic conditions. Coffee genotypes vary significantly in their responses to environmental conditions [27,28]. This variation is due to genetic differences affecting how each genotype interacts with precipitation, temperature, and soil fertility. There is evidence for significant inter-genotypic variation in response to high temperatures [74], which could be helpful to consider in tandem with findings regarding water stress tolerance since the average global temperature is increasing continuously [2]. However, the improvement of coffee through traditional breeding is slow due to the perennial nature of the plant. On the other hand, in the last 30 years, significant progress has been made in coffee biotechnology, creating many possibilities in this research area [75]. Nowadays, coffee breeding programs are primarily looking to identify environmental stress tolerance genes and transcription factors (TFs) to enable the development of new genotypes. Pyrosequencing of RNA extracted from the shoot apices of drought-tolerant and -susceptible C. arabica genotypes subjected to drought stress revealed that the expression of the gene CaSTK1 (protein kinase), which encodes a putative oxidative stress response (serine/threonine protein kinase) was highly induced by drought in the tolerant genotype [76]. Similar profiles were observed for CaSAMT1 (SAM-dependent methyltransferase), a gene encoding a putative S-adenosyl-L-methionine-dependent methyltransferase, CaSLP1 (plant development), CaMAS1 (ABA biosynthesis), and nsLTP (lipid transfer protein) genes [76]. These authors suggested that nsLTP may be related to the thicker cuticle observed on the abaxial leaf surface of tolerant genotypes compared to susceptible ones. The gene GolS is one of the main genes involved in the biosynthesis of raffinose family oligosaccharides (RFOs), which include raffinose, stachyose, and verbascose. In coffee, three isoforms of GolS were identified and characterized (CaGolS1, 2, and 3) under abiotic stress conditions. Moreover, it has been indicated that transcripts CaM6PR, CaPMI, and CaMTD involved in mannitol biosynthesis are modulated in distinct ways in response to abiotic stress like drought, heat, and salinity [77]. The RFO-regulating genes in coffee are essential for maintaining cellular osmoprotection under drought and salinity, making them promising genes for genetic breeding programs [77].
Transcription factors, such as the Dehydration Responsive Element Binding (DREB) proteins, are very important for plants dealing with environmental stresses since they activate the expression of many genes involved in cell protection, detoxification, and repair, among other things [78]. The regulation of coffee DREB-like genes was studied in coffee plants subjected to cold, heat, low relative humidity, exogenous ABA supply, and high light stress [79]. In C. arabica, the gene CaERF017 was upregulated in response to cold, and the genes CaERF053 and CaERF014 were upregulated in response to low humidity and high temperature, respectively. The genes CcDREB1B, CcRAP2.4, CcERF027, CcDREB1D, and CcTINY were upregulated under drought stress in C. canephora [79]. Studying these genes in coffee can contribute significantly to developing stress-tolerant coffee genotypes, ensuring the sustainability of coffee production in the face of climate change.
Although genetic transformation in coffee is feasible and has been explored extensively in research, the development and commercialization of transgenic coffee genotypes engineered for higher tolerance to drought, heat, and salinity are still limited. Abiotic stress tolerance is a complex trait often controlled by multiple genes. This makes it challenging to identify and manipulate the specific genetic factors contributing to improved tolerance [80]. Also, the coffee plant has a complex genome, and achieving stable and efficient transformation can be technically challenging. This includes issues related to gene insertion, expression, and inheritance in subsequent generations. Finally, much of the research on coffee genetic transformation has focused on traits such as disease resistance (e.g., coffee leaf rust) and coffee quality (e.g., caffeine content). As a result, there has been relatively less emphasis on developing genotypes specifically for abiotic stress tolerance [81]. However, there is ongoing research to address these challenges.
Advances in genomic and biotechnological tools, such as CRISPR/Cas9, may offer new opportunities to develop coffee genotypes with enhanced abiotic stress tolerance in the near future. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a natural defense mechanism found in bacteria. It consists of repetitive DNA sequences interspersed with “spacer” sequences derived from viruses that have previously attacked the bacteria. These spacers serve as a genetic memory of past infections. The Cas9 protein is an enzyme that acts as molecular scissors, capable of cutting DNA at specific locations. In such a technique, a synthetic RNA molecule that matches the particular DNA sequence target is made, and then the gRNA is combined with the Cas9 enzyme to form a complex that can find and cut the target DNA. The cell’s natural repair mechanisms kick in, and during this repair process, it is possible to introduce specific changes such as inserting, deleting, or replacing DNA sequences [82]. However, no genomic editing has been reported for agronomic traits of interest in coffee. Two studies conducted with C. canephora plants used the PDS gene (phytoene desaturase) as a target gene. The editing efficiencies were 30.4% [83] and 76.9% [84], with 7.6% and 54% of homozygous mutations, respectively, which are encouraging to the breeding programs but still a challenge considering the allopolyploid nature of coffee.
3.2. Plant Nutrition as a Tool to Improve Coffee Tolerance to Abiotic Stress
Developing solutions to deal with temperature changes, water availability, and light intensity induced by climate change is a considerable challenge for agricultural researchers. Abiotic stresses have an impact on the uptake, translocation, and utilization of nutrients, which results in the disruption of ion homeostasis in plant cells [12]. For instance, decreasing water availability may limit the nutrient availability in soil, decrease the nutrient uptake by roots, and finally reduce their tissue concentrations in plants [85]. Drought stress shows adverse effects on nitrogen (N) and phosphorus (P) concentrations [86] but has no definitive impact on the concentrations of other nutrients in the plant tissues [87]. In coffee plants, the negative effect of drought on N concentration is associated with a decreased Vmax (maximum velocity of absorption), Km (Michaelis–Menten constant), and Cmin (external concentration at which the net uptake of ions is zero) related to nitrate (NO3−) uptake [88]. Nutrient imbalances significantly impact the growth pattern, antioxidant defense mechanisms, and tolerance to biotic and abiotic stresses [12]. Therefore, the proper nutrient supply is essential to mitigate plant stress and enhance food production [12,89].
Many plant nutrients are reported to alleviate plant stress by participating in events that range from stress perception to tolerance acquisition [89]. For example, N is involved in (i) signaling processes through nitric oxide, (ii) osmoregulation processes and activation of components of the antioxidant system, and (iii) increasing root plasticity for water and nutrient absorption and in the construction of carbohydrates [12]. In turn, P is associated with processes like (i) maintaining cell turgidity and membrane stability, (ii) carbohydrate partitioning and adjusting root architecture, and (iii) the action of both antioxidant and photosynthetic systems [12,89]. Nutrients and beneficial elements play essential roles in plant protection from biotic and abiotic stresses (see [12]), but some elements have been studied more than others. Table 3 summarizes the main results of applying nutrients and beneficial elements to mitigate plant stress in coffee production. The number of studies assessing the contribution of nutrients, beneficial elements (Table 3), and elements not considered beneficial for plants, such as iodine (I), on the mitigation of abiotic stress in coffee production is low. However, most studies have shown promising results regarding increased plant tolerance and enhanced bean quality. To exemplify, Andrade et al. [90] reported that the application of I (2.5 mg dm3 of KIO3) attenuated the water deficit stress in C. arabica cv. Catuaí 99 by increasing photosynthetic efficiency, relative water content and water deficit tolerance index, content of photosynthetic pigments, and compatible osmolytes. The authors also observed that applying I stimulated the antioxidant enzymatic system, allowing higher cell membrane stability. These findings are fascinating since damage to cell membranes under water deficit conditions is an essential factor leading to the disruption of ion homeostasis in plants [12], which decreases the plant’s ability to deal with stress. Plant nutrition could serve as a powerful tool for mitigating stress [89]. Further studies are needed to assess the role of mineral elements (including those not traditionally considered beneficial or essential for plants) in reducing various types of stress.

Table 3.
Role of plant nutrients and beneficial elements on stress mitigation in coffee plants.
4. Concluding Remarks and Future Perspectives
The number of studies assessing damage induced by drought, heat, excess light, and salinity on coffee production is very low compared to other crops, which limits the understanding of plant responses to these environmental stresses and, consequently, the ability to use strategies more efficiently to mitigate plant stress. Many institutions like the World Meteorological Organization (WMO) frequently point out that the climate has been changing year by year, which has profound implications for coffee production. Therefore, many more studies must be conducted urgently to understand coffee’s responses to drought, heat, excess light, and salinity to support the application of strategies such as plant breeding and/or plant nutrition for stress mitigation.
Some studies have pointed out that plant memory is crucial for coffee plants to deal with drought, and based on these studies, the pre-exposure of coffee seedlings to deficient irrigation was proposed with promising results concerning plant stress mitigation. Such results are related to a faster plant metabolism adjustment induced by recurrent episodes of the same stress. This plant adjustment can be obtained from plant breeding programs and better plant nutrition. Due to the coffee plants’ perennial nature, plant breeding is frequently slow. However, plant breeding programs could be accelerated with new tools like CRISPR and a better comprehension of coffee responses to environmental stresses. Moreover, plant breeding programs related to coffee must focus on obtaining plants that are more tolerant to environmental stress.
Plant nutrition is another alternative that can be used to support plant metabolism adjustment, but it is necessary to manage the mineral nutrients more holistically, following the Sustainable Development Goals (https://sdgs.un.org/goals (accessed on 20 December 2024)). Thus, an alternative to mineral nutrient use is the employment of biostimulants [89]. However, few studies have evaluated the effect of biostimulants on coffee protection against drought, heat, excess light, and/or salinity. In addition, plant nutrition as a tool for plant stress mitigation must follow the core concept of 4R Nutrient Stewardship (applying the right source of plant nutrients at the right rate, at the right time, and in the right place).
Author Contributions
L.B.: methodology, formal analysis, and investigation, and writing—original draft preparation. F.H.S.R.: methodology and writing—original draft preparation. P.E.R.M.: writing—review and editing. L.R.G.G.: conceptualization, writing—review and editing, and supervision. L.G.-G.: writing—review and editing. M.L.V.d.R.: conceptualization, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank The Brazilian National Council for Scientific and Technological Development (CNPq) from Brazil for the scholarship to LB (grant number 370333/2024-1), research fellowship to PERM (grant number 312663/2021-8), and financial support for the National Institute of Science and Technology (INCT) on Soil and Food Security (grant number 406577/2022-6), INCT for Coffee (grant number 465580/2014-9), and INCT Plant Stress Physiology (grant number 406455/2022-8). The authors also thank The Minas Gerais State Research Support Foundation, FAPEMIG (grant number RED-00144-23), and The São Paulo Research Foundation, FAPESP (grant number 2021/06968-3), for financial support.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- USDA—United States Department of Agriculture. Brazil: Coffee Annual. 2023. Available online: https://fas.usda.gov/data/brazil-coffee-annual-8 (accessed on 16 July 2024).
- WMO—World Meteorological Organization. State of the Global Climate 2023. WMO-No. 1347. 2024. Available online: https://library.wmo.int/idurl/4/68835 (accessed on 21 October 2024).
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Rigal, C.; Vaast, P.; Ramalho, J.C.; et al. Shaded-Coffee: A Nature-Based Strategy for Coffee Production under Climate Change? A Review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef] [PubMed]
- Magrach, A.; Ghazoul, J. Climate and pest-driven geographic shifts in global coffee production: Implications for forest cover, biodiversity and carbon storage. PLoS ONE 2015, 10, e0133071. [Google Scholar] [CrossRef] [PubMed]
- Rigal, C.; Xu, J.; Vaast, P. Young shade trees improve soil quality in intensively managed coffee systems recently converted to agroforestry in Yunnan Province, China. Plant Soil 2020, 453, 119–137. [Google Scholar] [CrossRef]
- Braga, G.B.; Imbuzeiro, H.M.A.; Pires, G.F.; Oliveira, L.R.D.; Barbosa, R.A.; Vilela, K.D.F. Frost risk and rural insurance in Brazil. Rev. Bras. Meteorol. 2021, 36, 703–711. [Google Scholar] [CrossRef]
- Jayakumar, M.; Rajavel, M.; Surendran, U.; Gopinath, G.; Ramamoorthy, K. Impact of climate variability on coffee yield in India—With a micro-level case study using long-term coffee yield data of humid tropical Kerala. Clim. Change 2017, 145, 335–349. [Google Scholar] [CrossRef]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; Petkowicz, C.L.O. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef]
- Avila, R.T.; Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; Souza, R.P.B.; Martino, P.B.; Juárez, M.A.T.; Marçal, D.M.S.; Martins, S.C.V.; et al. Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ. Exp. Bot. 2020, 177, 104137. [Google Scholar] [CrossRef]
- dos Santos, T.B.; da Silva Ferreira, M.F.; Marques, I.; Oliveira, S.C.; Zaidan, I.R.; Oliveira, M.G.; Rodrigues, W.P.; Ribas, A.F.; Guyot, R.; Ramalho, J.C.; et al. Current challenges and genomic advances towards the development resilient coffee genotypes to abiotic stresses. In Genomic Designing for Abiotic Stress Resistant Technical Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–69. [Google Scholar]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An Effective Way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.; Ávila, R.T.; Morais, L.E.; Martins, S.C.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Gimenez, C.; Gallardo, M.; Thompson, R.B. Plant water relations. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 231–238. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Maestri, M.; Barros, R.S.; Regazzi, A.J. Water relations of coffee leaves (Coffea arabica and C. canephora) in response to drought. J. Hortic. Sci.-India 1993, 68, 741–746. [Google Scholar] [CrossRef]
- Lima, A.A.; Santos, I.S.; Torres, M.E.L.; Cardon, C.H.; Caldeira, C.F.; Lima, R.R.; Davies, W.J.; Dodd, I.C.; Chalfun-Junior, A. Drought and re-watering modify ethylene production and sensitivity, and are associated with coffee anthesis. Environ. Exp. Bot. 2021, 181, 104289. [Google Scholar] [CrossRef]
- Cavatte, P.C.; Oliveira, A.A.G.; Morais, L.E.; Martins, S.C.V.; Sanglard, L.M.V.P.; DaMatta, F.M. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol. Plant. 2012, 144, 111–122. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Maestri, M.; Barros, R.S. Phothosynthetic performance of two coffee species under drought. Photosynthetica 1997, 34, 257–264. [Google Scholar] [CrossRef]
- de Andrade, L.I.F.; Linhares, P.C.A.; da Fonseca, T.M.; Silva, A.A.; Santos, J.P.; Pereira, M.P.; Silva, V.A.; Marchiori, P.E.R. Photosynthetic efficiency and root plasticity promote drought tolerance in coffee genotypes. Acta Physiol. Plant. 2022, 44, 109. [Google Scholar] [CrossRef]
- Chekol, H.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dąbrowska, G.B.; Degu, A. Drought Stress Responses in Arabica Coffee Genotypes: Physiological and Metabolic Insights. Plants 2024, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Chekol, H.; Bezuayehu, Y.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dąbrowska, G.B.; Degu, A. Unraveling Drought Tolerance and Sensitivity in Coffee Genotypes: Insights fromSeed Traits, Germination, and Growth-Physiological Responses. Agriculture 2023, 13, 1754. [Google Scholar] [CrossRef]
- Aman, M.; Worku, M.; Shimbir, T.; Astatkie, T. Root traits and biomass production of drought-resistant and drought-sensitive arabica coffee varieties growing under contrasting watering regimes. Agrosyst. Geosci. Environ. 2024, 7, e20488. [Google Scholar] [CrossRef]
- Praxedes, S.C.; DaMatta, F.M.; Loureiro, M.E.; Ferrão, M.A.G.; Cordeiro, A.T. Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ. Exp. Bot. 2006, 56, 263–273. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Carvalho, M.H.C. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- de Guedes, F.A.F.; Nobres, P.; Ferreira, D.C.R.; Menezes-Silva, P.E.; Ribeiro-Alves, M.; Correa, R.L.; DaMatta, F.M.; Alves-Ferreira, M. Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environ. Exp. Bot. 2018, 147, 220–233. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions: Photosynthesis and stomatal conductance responses to rising [CO2]. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Rakocevic, M.; Ribeiro, R.V.; Marchiori, P.E.R.; Filizola, H.F.; Batista, E.R. Structural and functional changes in coffee trees after 4 years under free air CO2 enrichment. Ann. Bot. 2018, 121, 1065–1078. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Sicher, R.C.; Timlin, D.; Bailey, B. Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J. Plant Physiol. 2012, 169, 686–695. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 2318. [Google Scholar] [CrossRef]
- Gerganova, M.; Popova, A.V.; Stanoeva, D.; Velitchkova, M. Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol. Biochem. 2016, 104, 234–241. [Google Scholar] [CrossRef]
- Martins, S.C.V.; Araújo, W.L.; Tohge, T.; Fernie, A.R.; DaMatta, F.M. In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS ONE 2014, 9, e94862. [Google Scholar] [CrossRef]
- Roda, N.d.M.; Branchi, B.A.; Longo, R.M.; Pontin, J.; Abreu, D.P.d.; Santos, P.R.d.; Campostrini, E. The Advantages of Using Kaolin-Based Particle Films to Improve Coffee Production in the Minas Gerais Cerrado Biome. Sustainability 2022, 14, 4485. [Google Scholar] [CrossRef]
- de Oliveira, R.R.; Ribeiro, T.H.C.; Cardon, C.H.; Fedenia, L.; Maia, V.A.; Barbosa, B.C.F.; Caldeira, C.F.; Klein, P.E.; Chalfun-Junior, A. Elevated Temperatures Impose Transcriptional Constraints and Elicit Intraspecific Differences Between Coffee Genotypes. Front. Plant Sci. 2020, 11, 1113. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Silva, J.R.; Ferreira, L.S.; Machado Filho, J.A.; Figueiredo, F.A.M.M.A.; Ferraz, T.M.; Bernado, W.P.; Bezerra, L.B.S.; Abreu, D.P.; Cespom, L.; et al. Stomatal and photochemical limitations of photosynthesis in coffee (Coffea spp.) plants subjected to elevated temperatures. Crop Pasture Sci. 2018, 69, 317–325. [Google Scholar] [CrossRef]
- Vilas-Boas, T.; Duarte, A.A.; Della Torre, F.; Lovato, M.B.; Lemos-Filho, J.P. Does acclimation in distinct light conditions determine differences in the photosynthetic heat tolerance of coffee plants? Plant Biol. 2023, 25, 1101–1108. [Google Scholar] [CrossRef]
- Thioune, E.-H.; McCarthy, J.; Gallagher, T.; Osborne, B. A humidity shock leads to rapid, temperature dependent changes in coffee leaf physiology and gene expression. Tree Physiol. 2017, 37, 367–379. [Google Scholar]
- Liu, X.G.; Wan, M.D.; Wu, H.; Yang, Q.L. Photosynthetic response and use of water and light of Arabica coffee leaf under different irrigation and light levels. Oxid. Commun. 2016, 39, 873–883. [Google Scholar]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, N.F.; Cavatte, P.C.; Silva, P.E.M.; Martins, S.C.V.; Morais, L.E.; Medina, E.F.; DaMatta, F.M. Physiological and biochemical abilities of robusta coffee leaves for acclimation to cope with temporal changes in light availability. Physiol. Plant. 2013, 149, 4555. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Soil Letters Salt-Affected Soils Are a Global Issue. Intergovernmental Technical Panel on Soils. 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/8b5c687f-5a9b-4034-a4e2-0457066c1ae3/content (accessed on 16 July 2024).
- Majeed, A.; Muhammad, Z. Salinity: A major agricultural problem—Causes, impacts on crop productivity and management strategies. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 83–99. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chhabra, R. Classification of salt-affected soils. Arid Land Res. Manag. 2004, 19, 61–79. [Google Scholar] [CrossRef]
- Yadav, S.; Irfan, M.; Ahmad, A.; Hayat, S. Causes of salinity and plant manifestations to salt stress: A review. J. Environ. Biol. 2011, 32, 667–685. [Google Scholar]
- Hnilickova, H.; Hnilicka, F.; Martinkova, J.; Kraus, K. Effects of Salt Stress on Water Status; Photosynthesis and Chlorophyll Fluorescence of Rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O.; Donatti, L.; Petkowicz, C.L.O. Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydr. Polym. 2014, 112, 686–694. [Google Scholar] [CrossRef]
- Oyebamiji, Y.O.; Adigun, B.A.; Shamsudin, N.A.A.; Ikmal, A.M.; Salisu, M.A.; Malike, F.A.; Lateef, A.A. Recent Advancements in Mitigating Abiotic Stresses in Crops. Horticulturae 2024, 10, 156. [Google Scholar] [CrossRef]
- Lehmann, J.; Amonette, J.E.; Roberts, K. Role of biochar in mitigation of climate change. In Handbook of Climate Change and Agroecosystems; Hillel, D., Rosenzweig, C., Eds.; Imperial College Press: London, UK, 2010; Volume 1, pp. 343–363. [Google Scholar]
- Jeffery, S.; Verheijen, F.G.; Kammann, C.; Abalos, D. Biochar effects on methane emissions from soils: A meta-analysis. Soil Biol. Biochem. 2016, 101, 251–258. [Google Scholar] [CrossRef]
- Amoakwah, E.; Frimpong, K.A.; Okae-Anti, D.; Arthur, E. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geoderma 2017, 307, 189–197. [Google Scholar] [CrossRef]
- Sorrenti, G.; Masiello, C.A.; Dugan, B.; Toselli, M. Biochar physico-chemical properties as affected by environmental exposure. Sci. Total Environ. 2016, 563–564, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Wei, X.; Shaukat, N.; Chen, J.; Raza, A.; Younis, A.; Nafees, M.; Abideen, Z.; Zaid, A.; Latif, N.; et al. Effects of biochar and biochar-compost mix on growth, performance and physiological responses of potted Alpinia zerumbet. Sustainability 2021, 13, 11226. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Reyes-Herrera, D.F.; Sánchez-Reinoso, A.D.; Lombardini, L.; Restrepo-Díaz, H. Physiological Responses of Coffee (Coffea arabica L.) Plants to Biochar Application under Water Deficit Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 12873. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, P.; Wang, R.; Sun, L.; Ju, Q.; Xu, J. Comparative physiological responses and transcriptome analysis reveal the roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol. 2018, 18, 362. [Google Scholar] [CrossRef]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Ayyaz, A.; Shahzadi, A.K.; Fatima, S.; Yasin, G.; Zafar, Z.U.; Athar, H.U.R.; Farooq, M.A. Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol. 2022, 34, 335–346. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, H.; Hu, W.; Chen, L.; He, C.; Shi, H. Comparative transcriptional profiling of melatonin synthesis and catabolic genes indicates the possible role of melatonin in developmental and stress responses in rice. Front. Plant Sci. 2016, 7, 676. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar]
- Campos, C.N.; Ávila, R.G.; Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Sseremba, G.; Tongoona, P.B.; Musoli, P.; Eleblu, J.S.Y.; Melomey, L.D.; Bitalo, D.N.; Atwijukire, E.; Mulindwa, J.; Aryatwijuka, N.; Muhumuza, E.; et al. Viability of Deficit Irrigation Pre-Exposure in Adapting Robusta Coffee to Drought Stress. Agronomy 2023, 13, 674. [Google Scholar] [CrossRef]
- Silva, V.A.; Antunes, W.C.; Guimarães, B.L.S.; Paiva, R.M.C.; Silva, V.F.; Ferrão, M.A.G.; DaMatta, F.M.; Loureiro, M.E. Physiological response of Conilon coffee clone sensitive to drought grafted onto tolerant rootstock. Pesqui. Agropecu. Bras. 2010, 45, 457–464. [Google Scholar]
- Teixeira, A.L.; Souza, F.D.F.; Pereira, A.A.; Oliveira, A.C.B.D.; Rocha, R.B. Selection of arabica coffee progenies tolerant to heat stress. Cienc. Rural 2015, 45, 1228–1234. [Google Scholar] [CrossRef]
- Naik, B.J.; Kim, S.C.; Seenaiah, R.; Basha, P.A.; Song, E.Y. Coffee cultivation techniques, impact of climate change on coffee production, role of nanoparticles and molecular markers in coffee crop improvement, and challenges. J. Plant Biotechnol. 2021, 48, 207–222. [Google Scholar] [CrossRef]
- Mofatto, L.S.; Carneiro, F.D.A.; Vieira, N.G.; Duarte, K.E.; Vidal, R.O.; Alekcevetch, J.C.; Cotta, M.G.; Verdeil, J.-L.; Fabienne, L.-M.; Lartaud, M.; et al. Identification of candidate genes for drought tolerance in coffee by high-throughput sequencing in the shoot apex of different Coffea arabica cultivars. BMC Plant Biol. 2016, 16, 94. [Google Scholar] [CrossRef]
- De Carvalho, K.; Petkowicz, C.L.O.; Nagashima, G.T.; Bespalhok Filho, J.C.; Vieira, L.G.E.; Pereira, L.F.P.; Domingues, D.S. Homeologous genes involved in mannitol synthesis reveal unequal contributions in response to abiotic stress in Coffea arabica. Mol. Genet. Genom. 2014, 289, 951–963. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef]
- Torres, L.F.; Reichel, T.; Déchamp, E.; de Aquino, S.O.; Duarte, K.E.; Alves, G.S.C.; Silva, A.T.; Cotta, M.G.; Costa, T.S.; Diniz, L.E.C.; et al. Expression of DREB-like genes in Coffea canephora and C. arabica subjected to various types of abiotic stress. Trop. Plant Biol. 2019, 12, 98–116. [Google Scholar] [CrossRef]
- Duque, A.S.; Almeida, A.M.; Bernardes da Silva, A.; Marques da Silva, J.; Farinha, A.P.; Santos, D.; Fevereiro, P.; Araújo, S.S. Abiotic stress responses in plants: Unraveling the complexity of genes and networks to survive. In Abiotic Stress: Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; INTECH Open: Rijeka, Croatia; London, UK, 2013; pp. 49–102. [Google Scholar]
- Mishra, M.K.; Slater, A. Recent Advances in the Genetic Transformation of Coffee. Biotechnol. Res. Int. 2012, 2012, 580857. [Google Scholar] [CrossRef]
- Vats, S.; Kumawat, S.; Kumar, V.; Patil, G.B.; Joshi, T.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Genome editing in plants: Exploration of technological advancements and challenges. Cell 2019, 8, 1386. [Google Scholar] [CrossRef] [PubMed]
- Breitler, J.-C.; Dechamp, E.; Campa, C.; Rodrigues, L.A.Z.; Guyot, R.; Marraccini, P.; Etienne, H. CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora. Plant Cell Tissue Organ Cult. 2018, 134, 383–394. [Google Scholar] [CrossRef]
- Casarin, T.; Freitas, N.C.; Pinto, R.T.; Breitler, J.-C.; Rodrigues, L.A.Z.; Marraccini, P.; Etienne, H.; Diniz, L.E.C.; Andrade, A.C.; Paiva, L.V. Multiplex CRISPR/Cas9-mediated knockout of the phytoene desaturase gene in Coffea canephora. Sci. Rep. 2022, 12, 17270. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. B Soil. Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Silva, E.C.; Nogueira, R.J.M.C.; Silva, M.A.; Albuquerque, M.B. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Martinez, H.E.P.; Souza, B.P.; Caixeta, E.T.; Carvalho, F.P.; Clemente, J.M. Water deficit changes nitrate uptake and expression of some nitrogen related genes in coffee-plants (Coffea arabica L.). Sci. Hortic. 2020, 267, 109254. [Google Scholar] [CrossRef]
- Rabêlo, F.H.S. Role of Plant Nutrition in Mitigating Biotic and Abiotic Stresses in Plants. Inform. Agron. 2024, 2, 5–22. (In Portuguese) [Google Scholar]
- Andrade, O.V.S.; Lima, J.S.; Neves, T.T.; Benevenute, P.A.N.; Santos, L.C.; Nascimento, V.L.; Guilherme, L.R.G.; Marchiori, P.E.R. The role of potassium iodate in mitigating the damages of water deficit in coffee plants. J. Soil Sci. Plant Nutr. 2024, 24, 5772–5788. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Foliar nitrogen supply enhances the recovery of photosynthetic performance of cold-stressed coffee (Coffea arabica L.) seedlings. Photosynthetica 2020, 58, 951–960. [Google Scholar] [CrossRef]
- Nunes, M.A.; Ramalho, J.D.C.; Dias, M.A. Effect of Nitrogen Supply on the Photosynthetic Performance of Leaves from Coffee Plants Exposed to Bright Light. J. Exp. Bot. 1993, 44, 893–899. [Google Scholar] [CrossRef]
- Salamanca-Jimenez, A.; Doane, T.A.; Horwath, W.R. Performance of Coffee Seedlings as Affected by Soil Moisture and Nitrogen Application. Adv. Agron. 2016, 136, 221–244. [Google Scholar]
- Vinecky, F.; Davrieux, F.; Mera, A.C.; Alves, G.S.C.; Lavagnini, G.; Leroy, T.; Bonnot, F.; Rocha, O.C.; Bartholo, G.F.; Guerra, A.F.; et al. Controlled irrigation and nitrogen, phosphorous and potassium fertilization affect the biochemical composition and quality of Arabica coffee beans. J. Agric. Sci. 2017, 155, 902–918. [Google Scholar] [CrossRef]
- Ramirez-Builes, V.H.; Kusters, J.; Thiele, E.; Lopez-Ruiz, J.C. Physiological and Agronomical Response of Coffee to Different Nitrogen Forms with and without Water Stress. Plants 2024, 13, 1387. [Google Scholar] [CrossRef]
- Rocha, B.C.P.; Martinez, H.E.P.; Ribeiro, C.; Brito, D.S. Nitrogen Metabolism in Coffee Plants Subjected to Water Deficit and Nitrate Doses. Braz. Arch. Biol. Technol. 2023, 66, e23210060. [Google Scholar]
- Rakocevic, M.; Marchiori, P.E.R.; Zambrosi, F.C.B.; Machado, E.C.; Maia, A.H.N.; Ribeiro, R.V. High phosphorus supply enhances leaf gas exchange and growth of young Arabica coffee plants under water deficit. Exp. Agric. 2022, 58, e30. [Google Scholar] [CrossRef]
- Ramírez-Builes, V.H.; Küsters, J.; de Souza, T.R.; Simmes, C. Calcium Nutrition in Coffee and Its Influence on Growth, Stress Tolerance, Cations Uptake, and Productivity. Front. Agron. 2020, 2, 590892. [Google Scholar] [CrossRef]
- Silva, D.M.; Souza, K.R.D.; Boas, L.V.V.; Alves, Y.S.; Alvez, J.D. The effect of magnesium nutrition on the antioxidant response of coffee seedlings under heat stress. Sci. Hortic. 2017, 224, 115–125. [Google Scholar] [CrossRef]
- Dias, K.G.L.; Guimarães, P.T.G.; Furtini Neto, A.E.; Silveira, H.R.O.; Lacerda, J.J.J. Effect of Magnesium on Gas Exchange and Photosynthetic Efficiency of Coffee Plants Grown under Different Light Levels. Agriculture 2017, 7, 85. [Google Scholar] [CrossRef]
- Ramirez-Builes, V.H.; Küsters, J.; Thiele, E.; Leal-Varon, L.A. Boron Nutrition in Coffee Improves Drought Stress Resistance and, Together with Calcium, Improves Long-Term Productivity and Seed Composition. Agronomy 2024, 14, 474. [Google Scholar] [CrossRef]
- de Sousa, G.F.; Silva, M.A.; de Morais, E.G.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; de Oliveira, R.R.; Amaral, D.; Brown, P.; Chalfun-Junior, A.; Guilherme, L.R.G. Selenium enhances chilling stress tolerance in coffee species by modulating nutrient, carbohydrates, and amino acids content. Front. Plant Sci. 2022, 13, 1000430. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.F.d.; Silva, M.A.; Carvalho, M.R.d.; Morais, E.G.d.; Benevenute, P.A.N.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; Guilherme, L.R.G. Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect? Plants 2023, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).