Abstract
Maintaining biodiversity in agricultural landscapes is a major challenge for environmental protection in Europe. Vineyards rely heavily on agrotechnical interventions such as herbicide use and tillage for weed control, which affect biodiversity and can lead to soil erosion and resistant weed populations. The fragmentation of agricultural landscapes affects biodiversity by altering community composition and often reducing plant population sizes and genetic diversity. However, it can also increase the abundance of certain species and enhance population resilience to environmental change. Vineyards can support high levels of biodiversity and provide ecosystem services due to their semi-natural habitat structure. This research evaluates vegetation biodiversity using phytosociological relevés in different vineyards. Our results show that species richness and biodiversity are significantly influenced by vineyard age and management type. This study highlights differences in the representation of plant functional groups, with perennial taxa in grassy inter-row contributing to anti-erosion functions and serving as food sources for pollinators. The root zone around vine trunks shows an increase in invasive species with vineyard age, posing a risk to the agroecosystem. Vineyards predominantly follow a ruderal ecological strategy, using nutrients and light efficiently, while tolerating management disturbances. Understanding these dynamics is critical for developing sustainable vineyard management practices that support biodiversity and ecological resilience, counteract the homogenization of agricultural landscapes, and promote the coexistence of viticulture and species-rich ecosystems.
1. Introduction
Preserving biodiversity in agricultural landscapes remains one of the main challenges for environmental protection in Europe [1,2]. Traditional small-scale agriculture co-created the current shape of the entire landscape and its biodiversity character [3,4]. The extensive management of traditional agriculture co-formed a high biodiversity [5,6]. However, current agriculture trends either intensify cultivation practices or abandon land use altogether, both of which threaten existing biodiversity [7].
Vineyards are often intensively managed and highly dependent on agrotechnical interventions in Europe [8,9,10]. The relationship between agriculture, biodiversity, and weed control in vineyards has become very important [11]. Different land management and weed control techniques significantly affect species richness and weed distribution in vineyards [12,13]. Certain weed species directly compete with the grapevine for water and nutrients, and, apart from this, they limit the vegetation biodiversity in the vineyard, which results in the reduced stability of vineyard ecosystems and a higher occurrence of pathogens and vine pests [14,15,16,17].
Weed control in vineyards is primarily achieved through herbicides and tillage. However, these methods promote erosion [18] and the emergence of resistant weed populations [19]. Soil erosion, exacerbated by a low soil organic matter content and a sloping terrain, is a major disadvantage of tillage [20,21]. While herbicides are cost-effective and efficient for weed control, their toxicity and negative environmental effects affect their use [22,23]. An alternative for vegetation management in vineyards is the establishment of cover crops in the inter-rows [24]. The choice of inter-row management strategy depends on winemaking traditions, pedo-climatic conditions, vineyard slope, and available machinery [25]. Inter-row cover crops are becoming increasingly popular. However, they compete with vines and can reduce vegetative growth and grape yield [21,26]. Often, a combination of vegetation management techniques, such as the “sandwich” rotation, is employed to mitigate these negative effects. This technique, developed in Switzerland, alternates strips of cultivated rows with grassy rows and is particularly recommended in areas with low rainfall [27,28,29,30].
In agricultural landscapes, the phenomenon of fragmentation is associated with a range of changes in biodiversity structure, community composition, abiotic conditions, and biotic interactions [31]. Fragmentation affects specific functional groups of organisms to different degrees [32] and alters community composition and competitive relationships between populations. Fragmentation and, especially, the edge effects of habitats can affect the distribution of living resources and, thus, evoke changes in the abiotic characteristics of habitats [33,34]. Changes in abiotic and biotic environmental characteristics induced by fragmentation can represent a selection pressure on populations of organisms. Fragmentation is often associated with reduced plant population size and availability of pollinators [35]. In plant populations, this induces a reduction in genetic diversity and an increase in inbreeding depression in the plant population [36]. Habitat fragmentation alters the landscape seed dispersal of wind-dispersed species [37]. However, most reactions of organisms to habitat fragmentation are positive and enhance the resistance of populations to a wide range of environmental conditions [38]. Fragmentation has the ability to produce persistent and often unpredictable outcomes, including surprising increases in the abundance of certain species. Long-time monitoring scales are essential to fully understand the consequences of fragmentation and be allowed to duly assess the effects of landscape fragmentation [31,39]. Increasing the diversity of vegetation in vineyards can maintain a higher landscape biodiversity and provide refuge and food source for various vertebrates and arthropods, including those which limit pests [40,41]. Although vineyards are intensively managed agroecosystems, they can host a great biodiversity of organisms [42,43] and provide a range of ecosystem services. Thanks to their special habitat structure, they have a semi-natural-to-natural character [21,44,45]. Vineyard vegetation can directly and indirectly provide several ecosystem services for grapevine production [21,46] and increase biological activity [47], infiltration water [48], and organic matter availability [49].
Thanks to the presence of vegetation in vineyards, the stability of the soil structure improves, organic matter stabilizes soil aggregates, and root systems protect the soil from erosion [49]. Vineyard vegetation also supports beneficial organisms important to grapevines [50] and boosts biodiversity [51,52]. Inter-rows can promote sustainability in viticulture by enabling a management system that fuels permanent or temporary vegetation cover with non-crop plant species, either as a mixture of sown cover crops or as spontaneous vegetation. The positive effect of cover crops on different levels of biodiversity and the related ecosystem services has been demonstrated by many studies [4,43,53,54].
The cultivation of grapevines creates conditions for a unique ecosystem consisting of vine plants, non-target vegetation, and other organisms. However, the study of vineyard vegetation has often overlooked the important feature of succession. Long-term and fragmented vineyard management, together with specific soil-climate conditions, influence vegetation succession in vineyards. Our study hypothesized that long-term vineyard conditions alter the course of succession and influence the representation of functional plant groups. The current study aimed to (i) determine trends in vineyard vegetation succession, (ii) identify changes in plant functional group representation during vineyard ageing, and (iii) clarify the application of plant ecological strategies during vineyard ecosystem succession. The central question of this research was the following: how do long-term vineyard conditions in the Dyjskosvratecký Valley, Moravia (Czech Republic), influence vegetation succession, changes in plant functional groups over time, and the application of plant ecological strategies within vineyard ecosystems?
2. Materials and Methods
2.1. Study Area and Charactersistics of the Vineyards
The selected vineyards are located on the edge of the Dyjskosvratecký Valley in South Moravia (Czech Republic). The altitude ranges between 240 and 320 m. The average annual temperature is 8.5 °C, and the annual rainfall is 470 mm. These data were taken from the nearest meteorological station of the Czech Hydrometeorological Institute in Kuchařovice [55,56,57].
This area belongs to the Moravian wine region, specifically the Znojmo wine subregion. A total of 44 vineyards of various ages were selected. The selected vineyards are located in the cadastral territories of 4 wine-growing municipalities. The Stará hora vineyard in the wine-growing village of Horní Dunajovice was established in 1995, 2000, 2020, and 2021. The Volné pole vineyard in the wine-growing village of Hostěradice was established in the years 1972, 2003, 2014, 2015, 2016, 2017, 2018, 2020, and 2021. The vine lines U Vinohradu in the wine-growing village of Miroslav were established in 2003, 2004, 2007, 2014, and 2019. The Weinperky vine line in the wine-growing village of Miroslav were established in the years 1996, 1998, 1999, 2001, 2002, 2003, 2004, 2008, 2009, 2011, 2014, 2015, and 2017. The Stará hora vineyards in the wine-growing village of Miroslavské Knínice were established in 2001. The Zolos vineyards in the wine-growing village of Miroslavské Knínice were established in 2014. The name of the vineyards, their year of establishment and GPS coordinates, the predominant soil type, and the area are shown in Table 1.

Table 1.
General characteristics of the selected vineyards. The soil types follow the IUSS Working Group WRB [58].
A conventional management system was applied in the evaluated vineyards. A similar type of management was employed in selected vineyards (Figure 1). There were 3 different habitats in the vineyards—a grassy inter-row (M1), a cultivated inter-row (M2), and a strip under the vine (PP) area around the trunks.
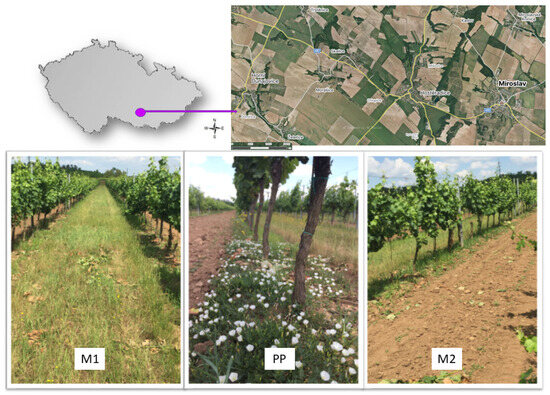
Figure 1.
Map of the area of interest, vineyards, and three methods of vegetation management: (M1) grassy inter-row; (PP) strip under the vine; and (M2) cultivated inter-row.
A mixture of grasses (Lolium multiflorum, Lolium perenne, Festuca arundinacea, and Festuca pratensis) and leguminous plants (Onobrychis viciifolia and Trifolium pratense) were sown in the grassy inter-row (M1) during the establishment of the vineyards. The vegetation of the grassy inter-row was maintained by mowing and mulching the biomass.
The cultivated inter-row (M2) in vineyards at most 3 years old was sown with a mixture of annual crops (Phacelia tanacetifolia, Pisum sativum, Raphanus sativus, Sinapis alba, Trifolium alexandrinum, Trifolium incarnatum, and Vicia pannonica) in the spring. During the summer, the vegetation was cut and ploughed into the soil.
In the strip area around the trunks where mechanization was not used, the vegetation was regulated by the application of herbicides and, alternatively, mechanically removed.
2.2. Method of Vegetation Assessment
The vegetation was evaluated using the standard method of phytosociological relevés [59]. The size of each plot was 6 m2, a rectangle measuring 1 by 6 m. The cover of all plant species was estimated and recorded as a percentage. The observation took place each year during the period between 2020 and 2023, in three vegetation optima (April, June, and October). The taxonomic nomenclature of the plants follows Kaplan et al. [60]. The full names of the plants and their groupings are given in Appendix A. Specimens of some of the plant species found were preserved and deposited in the herbarium of the Department of Plant Biology, Faculty of AgroSciences, Mendel University.
In each vineyard, three permanent plots (grassy inter-row (M1), cultivated inter-row (M2) and strip under the vine (PP)) were recorded in 4 repetitions. Permanent areas for recording the phytosociological relevés were chosen based on following parameters: the areas were adjacent to each other and located in three different habitats. During the four-year monitoring period, a total of 132 permanent relevés were recorded three times a year. The total number of phytosociological relevé records was 1584.
The identified plant species were divided into functional groups according to their biological properties. The first criterion was the division according to life span and taxonomy: (i) annual dicotyledons, (ii) perennial dicotyledons, (iii) annual monocotyledons, and (iv) perennial monocotyledons. The information was drawn from the Pladias database [61,62].
The second criterion was the division of the plant species into functional groups according to their importance in the vineyard ecosystem: (i) crops, (ii) legumes (Fabaceae family), (iii) annual weeds, (iv) deep-rooting species, (v) species with an anti-erosion effect (perennial grasses), and (vi) entomophilous species [61,62,63].
The third criterion divided the plant species into groups—native or alien—according to their origin with respect to the territory of the Czech Republic. Native plant species had to be present on the territory of the Czech Republic before the Neolithic time period. Alien taxa were divided into archaeophytes and neophytes according to the time at which they had been introduced by humans into the territory of the Czech Republic. The sorting of the species was carried out according to Pyšek et al. [64]. The alien species (archaeophytes and neophytes) were divided according to their invasion status. Casually introduced plant species were taxa whose survival in the area depended on repeated diaspore supply induced by human activity. Naturalized plant species had regularly multiplied in the area for a long time, independent of human activity. Invasive plant species were alien taxa that quickly spread over considerable distances over land. Following [64], the species were divided into the following categories: (i) native, (ii) casual archaeophytes, (iii) naturalized archaeophytes, (iv) invasive archaeophytes, (v) casual neophytes, (vi) naturalized neophytes, and (vii) invasive neophytes.
The fourth criterion divided the species according to ecological strategy. Grime [65,66] distinguished three basic ecological strategies of plants: (i) the competitive strategy (C), convenient in stable habitats where the resources are abundant, the conditions are not extreme, and disturbance is limited; (ii) the stress tolerance strategy (S), convenient in habitats with scarce resources and extreme and highly variable conditions, but with limited disturbance; and (iii) the ruderal strategy (R), convenient in habitats where the resources are abundant, the conditions are not extreme, but disturbance is frequent. Scores expressing a degree of “C”, “S”, or “R” were applied to the identified species. The rate was expressed as a percentage, and the sum of all three scores for each individual taxon was 100% [67].
The fifth criterion divided the species based on the categories of threatened species according to the national Red List of Vascular Plants of the Czech Republic [68]. The main category “A” includes taxa that are extinct or missing, while the main category “C” includes taxa which are threatened, including rarer taxa requiring attention and unclear cases.
The sixth criterion divided the species according to the successional age optimum, which is expressed as the median time in years from the moment of disturbance to the time when the taxon occurs during the succession. The optimum was determined to be in the range of 1 to 50 years. For the taxa whose optimum was a time longer than 50 years since the last disturbance and could not be calculated precisely due to a low number of successional stages, the value was set to 75 years [61].
2.3. Statistical Data Evaluation
The Kruskal–Wallis test was conducted separately for the strip under the vine (PP) and the two differently managed inter-rows (M1, M2) across seasons (spring, summer, and autumn). Analogously, the Kruskal–Wallis test was conducted separately for the seasons (spring, summer, autumn) and separately across the rows with grapevine (PP) and the two differently managed inter-rows (M1, M2). The Shapiro–Wilk test was utilized to assess the normality of the data distributions within each group, while the Levene test was employed to examine the homogeneity of the variances across groups. Both tests indicated significant departures from normality (all results with p < 0.05), justifying the use of the Kruskal–Wallis test for data analysis. Subsequently, post hoc pairwise comparisons were performed using the Dunn test, following significant results from the Kruskal–Wallis test. To account for multiple comparisons, the Bonferroni correction was applied. Linear regression analysis using the lm function in R was used to investigate the relationship between the number of species and the age of the vineyard. The lm model function was utilized to fit the linear regression models separately for each combination of seasons (spring, summer, and autumn) and variants (M1, M2, PP), resulting in a total of nine distinct models. Data analyses were conducted using the R statistical software [69].
3. Results
During the four-year monitoring period, 172 plant species were identified in the selected vineyards. The following taxa belonging to annual dicotyledonous species were identified: Amaranthus powellii, Amaranthus retroflexus, Anagallis arvensis, Anagallis foemina, Anthemis arvensis, Atriplex patula, Atriplex sagittata, Brassicca napus subsp. napus, Camelina microcarpa, Camelina sativa, Capsella bursa-pastoris, Cerastium holosteoides, Consolida regalis, Conyza canadensis, Datura stramonium, Descurainia sophia, Erigeron annuus, Erodium cicutarium, Euphorbia helioscopia, Fagopyrum esculentum, Fallopia convolvulus, Fumaria officinalis, Galeopsis tetrahit, Galinsoga parviflora, Galium aparine, Geranium pusillum, Geranium robertianum, Holosteum umbellatum, Chelidonium majus, Chenopodium album, Chenopodium hybridum, Chenopodium polyspermum, Lactuca serriola, Lamium amplexicaule, Lamium purpureum, Lathyrus sativus, Linum usitatissimum, Malva neglecta, Matricaria discoidea, Mercurialis annua, Microthlaspi perfoliatum, Myosotis arvensis, Papaver rhoeas, Phacelia tanacetifolia, Pisum sativum, Polygonum aviculare, Portulaca oleracea, Raphanus sativus, Raphanus raphanistrum, Scleranthus annuus, Senecio jacobaea, Senecio vulgaris, Silene noctiflora, Sinapis alba, Solanum nigrum, Sonchus oleraceus, Stellaria media, Trifolium alexandrinum, Trifolium incarnatum, Tripleurospermum inodorum, Urtica urens, Veronica hederifolia, Vicia pannonica, and Viola arvensis.
The following taxa belonging to perennial dicotyledonous species were identified: Agrimonia eupatoria, Achillea millefolium, Ailanthus altissima, Alcea biennis, Anthyllis vulneraria, Arctium lappa, Arctium tomentosum, Artemisia absinthium, Artemisia vulgaris, Berteroa incana, Carduus acanthoides, Carlina vulgaris, Centaurea jacea, Cichorium intybus, Cirsium arvense, Convolvulus arvensis, Crataegus laevigata, Crepis biennis, Crepis capillaris, Cynoglossum officinale, Daucus carota, Echinops sphaerocephalus, Echium vulgare, Eryngium campestre, Euphorbia esula, Falcaria vulgaris, Fragaria vesca, Galium album, Galium verum, Geranium pyrenaicum, Geum urbanum, Humulus lupulus, Hypericum perforatum, Inula salicina, Juglans regia, Lamium album, Lamium maculatum, Lathyrus tuberosus, Lepidium draba, Ligustrum vulgare, Linaria vulgaris, Lotus corniculatus, Medicago lupulina, Medicago sativa, Melilotus albus, Melilotus officinalis, Nonea pulla, Onobrychis viciifolia, Onopordum acanthium, Parthenocissus inserta, Petrorhagia prolifera, Physalis alkekengi, Pilosella aurantiaca, Pilosella officinarum, Plantago lanceolata, Plantago major, Plantago media, Potentilla argentea, Potentilla reptans, Quercus petraea, Reseda lutea, Ribes aureum, Robinia pseudoacacia, Rosa canina, Rubus sect. Rubus, Rumex crispus, Rumex obtusifolius, Salvia pratensis, Sambucus nigra, Scabiosa ochroleuca, Securigera varia, Silene latifolia, Silene vulgaris, Symphytum officinale, Tanacetum vulgare, Taraxacum sect. Taraxacum, Tragopogon dubius, Tragopogon orientalis, Trifolium campestre, Trifolium pratense, Trifolium repens, Urtica dioica, and Vicia cracca.
The following taxa belonging to annual monocotyledonous species were identified: Apera spica-venti, Avena fatua, Bromus hordeaceus, Digitaria sanguinalis, Echinochloa crus-galli, Hordeum murinum, Panicum miliaceum, Poa annua, Secale cereale, Setaria pumila, Setaria viridis, Setaria verticillata, and Triticum aestivum.
The following taxa belonging to perennial monocotyledonous species were identified: Arrhenatherum elatius, Calamagrostis epigejos, Dactylis glomerata, Elymus repens, Festuca arundinacea, Festuca pratensis, Festuca rubra, Lolium multiflorum, Lolium perenne, Luzula campestris, Poa pratensis, and Stipa pennata.
The mean number of species per plot (6 m2) differed, ranging from 1 up to 32. In general, higher species numbers were observed on the plots situated in the inter-rows (M1, M2), contrary to the rows below the grape trunks (PP). Increasing species numbers over the same season were found for all the three differently managed variants (Figure 2).
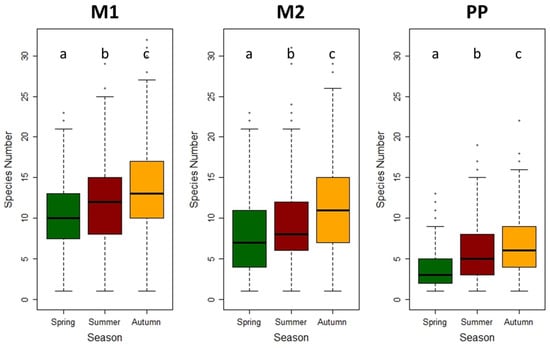
Figure 2.
The box and whisker plots present the number of species in the phytosociological relevés. Individual seasons of the year are presented separately, and the differences are tested using the Kruskal–Wallis test (the letters above the boxes show the statistically significant results). Variants: M1 = grassy inter-row; M2 = cultivated inter-row; and PP = stripe below the grape trunks.
The number of plant species increased statistically significantly in the grassy inter-row (M1) with the age of vineyards. The number of plant species also increased statistically significantly in the strip area around the grapevine trunks with the age of the vineyard, but only during the summer season. In spring and autumn, the differences were not statistically significant. The changes in the number of plant species in the cultivated inter-rows were not statistically significant (Figure 3).
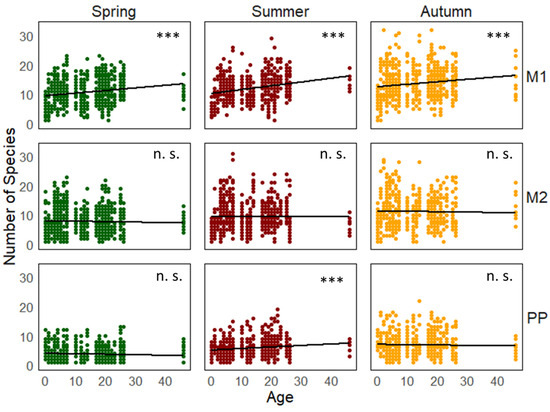
Figure 3.
The relationship between the number of plant species and the age of the vineyards (years after establishment). The variants include M1 for grassy inter-rows, M2 for cultivated inter-rows, and PP for stripes below the grape trunks. The asterisks (***) denote significant changes in species numbers when comparing vineyards with different establishment times (n. s.: M2 spring p = 0.18; M2 summer p = 0.13; M2 autumn p = 0.15; PP spring p = 0.11; and PP autumn p = 0.12).
The coverage development of the plant groups according to the length of the growing season and botanical division is shown in Figure 4. The representation of the plant groups changed with the age of the vineyard, the season, and the type of inter-row.
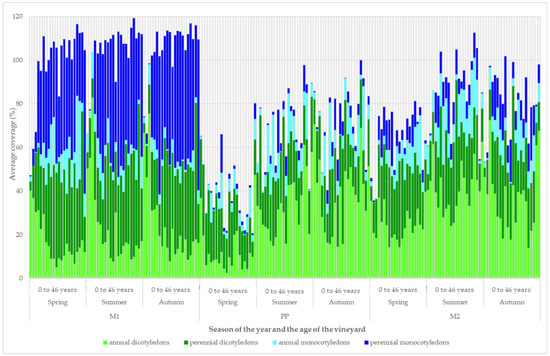
Figure 4.
Development of the structure of vineyard vegetation cover according to biological properties.
In the grassy inter-row (M1), there was an increase in coverage in the vineyards that were up to 4 years old, in both the perennial dicotyledon and perennial monocotyledon groups. The increase was no longer evident in older vineyards. The coverage of the group of annual dicotyledonous plants gradually decreased with the age of the vineyards, up to 6 years, and then the share of coverage stagnated. The coverage of the group of annual monocots was higher in vineyards older than 6 years, but only in the spring evaluation period; in the other evaluation periods, the coverage stagnated.
There was a significant difference in coverage between seasons in the strip area around the grapevine trunks. In the spring, the coverage was significantly lower than in the summer or autumn. In the spring, the cover was dominated by perennial dicotyledonous species. In vineyards up to 7 years old, there was a decrease in coverage in the group of annual dicotyledons in summer and autumn. In older vineyards, the coverage of the group of annual dicotyledons increased.
In the cultivated inter-row (M2), the group of annual dicotyledons had a high coverage, especially in young and old vineyards. In vineyards between 5 and 14 years old, there was a decrease in coverage. In old vineyards, the coverage was higher in the group of annual monocotyledons.
The development of the coverage of the plant groups according to the functional groups is shown in Figure 5. In vineyards up to 4 years of age, there was an increase in the coverage of representatives of the Fabaceae family, entomophilous plants, and species with an anti-erosion effect in the grassy inter-row (M1). There was a significant difference in the coverage of plant groups between the seasons. During the spring, the coverage of the group of deep-rooting species was dominant. During the summer, the coverage of annual weeds decreased. In the older vineyards, the coverage of annual weeds increased in the cultivated inter-row (M2). There was a high coverage of the group of crops and representatives of the leguminous family.
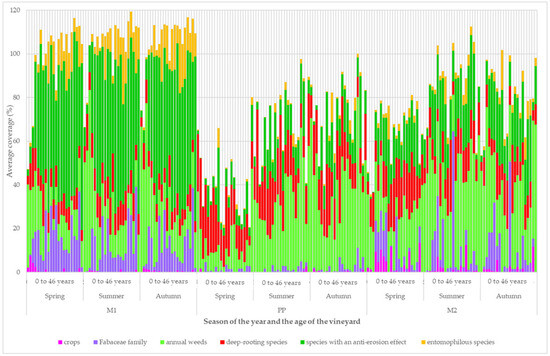
Figure 5.
Development of the structure of vineyard vegetation cover according to functional groups.
In vineyards up to 7 years old, during the summer and autumn, the coverage of the group of annual weeds decreased. In older vineyards, the coverage of the annual weed group increased. In the cultivated inter-row (M2) of young vineyards, there was a high coverage of plant groups and representatives of the leguminous family. In vineyards aged 5 to 14 years, there was an increase in the coverage of deep-rooting species.
The development of plant group coverage according to origin and invasion status is demonstrated in Figure 6. The high coverage of species from the invasive archaeophyte group and invasive neophyte group was mainly in the strip area around the grapevine trunks and in the cultivated inter-row (M2). Indigenous plant species had the least coverage in these habitats.
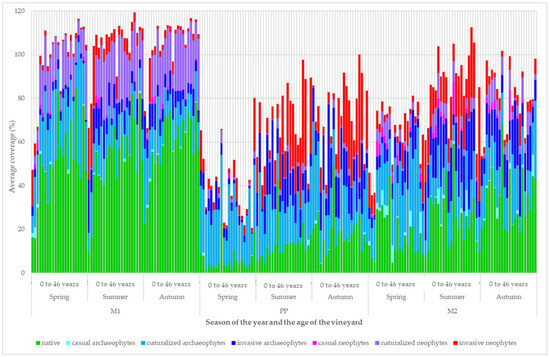
Figure 6.
Development of the structure of vineyard vegetation cover according to functional groups.
The development of the coverage of the plant groups according to their ecological strategy is shown in Figure 7. In the grassy inter-row (M1) in vineyards aged up to 4 years, there was a stable ratio between the three basic strategies, with a slight predominance of the ruderal strategy. There was a markedly lower proportion of species with a stress-tolerant strategy in the strip area around the grapevine trunks. In the cultivated inter-row (M2), there was also a lower share of species with a stress-tolerant strategy and a stronger predominance of species with a ruderal strategy.
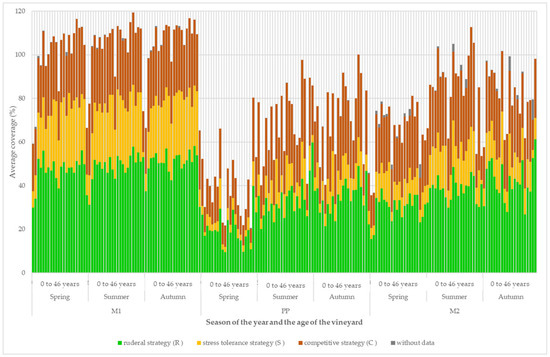
Figure 7.
Development of the structure of vineyard vegetation cover according to the ecological strategy.
The development of the coverage of groups of species according to the extinction risk is depicted in Figure 8. The occurrence of threatened plant species was not high. They were found at all three sites, especially in vineyards older than 3 years. These were the taxa Inula salicina, Nonea pulla, Petrorhagia prolifera, Pilosella aurantiaca, Silene noctiflora, Stipa pennata, and Urtica urens.
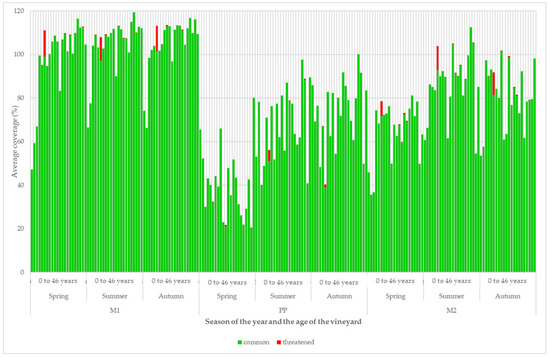
Figure 8.
Development of the structure of vineyard vegetation cover according to the extinction risk.
The development of the coverage of the group of species according to the optimal age of succession is shown in Figure 9. Figure 9 illustrates the progressive development of vegetation cover within a vineyard, focusing on changes over time, as the vegetation matures and reaches its optimal successional age. The figure provides a detailed visual representation of how the structure of the vineyard’s vegetation evolves, highlighting key stages and characteristics at various points in the succession process.
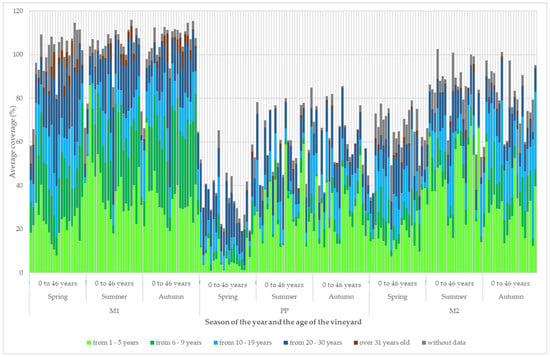
Figure 9.
Development of the structure of vineyard vegetation cover according to the optimal successional age.
In the grassy inter-row (M1) in vineyards aged 12 to 16 years, there was a decrease in the coverage of the group with an optimal age of succession of 1 to 5 years, and, on the contrary, there was an increase in the coverage of the other groups. In vineyards older than 16 years, the proportion of groups according to the optimal age of succession did not change extensively. Species from the group with an optimum successional age of 1 to 5 years maintained their dominant coverage in the strip area around the grapevine trunk and in the cultivated inter-row (M2). In the cultivated inter-row (M2), the proportion of species with an optimum successional age of 10 to 29 years increased with growing vineyard age.
4. Discussion
Vineyards in agricultural landscapes support higher vegetation species diversity. The results show that different vegetation management practices create habitat heterogeneity within vineyard areas. This heterogeneity leads to the formation of habitats with varying levels of plant species representation, contributing to overall biodiversity.
The grassy inter-rows created more favourable conditions for the occurrence of a higher number of plant species. On the contrary, the lowest number of plant species appeared in the strip area around the grapevine trunks. It is evident that the method of regulation markedly affects the number of plant species. The applied herbicides have an important role in weed control in vineyards [70,71] but are also the main reason for the decline in biodiversity in agroecosystems [72,73,74]. The types of herbicides used are critical. As pointed out by Winkler et al. [75], a reduction in the use of herbicides leads to a change in the spectrum of vegetation species. Soil cultivation in vineyards is used primarily to control annual weeds [76,77,78]; however, certain plant taxa are able to adapt to this type of weed control.
The grassy inter-rows in vineyards are areas where certain plant groups thrive. Perennial taxa, known for their anti-erosion benefits, and indigenous taxa have substantial coverage in these areas. Although we did not measure erosion directly, the existing literature supports the role of perennial plants in reducing erosion. The species composition indicates that grassy inter-rows are important habitats with anti-erosion functions. Additionally, the high proportion of indigenous taxa limits the spread of non-indigenous species. The long-term, stable management of grass communities fosters the development of species-rich vegetation. Major changes in grassland management lead to shifts in species composition [79], and the grassy inter-rows of vineyards are well-suited for creating diverse plant communities. The vegetation of the strip area around the grapevine trunks has a markedly different composition. Annual dicotyledonous taxa, deep-rooting taxa, and taxa with an invasive status have substantial coverage there. The strip area around grapevine trunks is a habitat within vineyards where the vegetation is most regulated. Nevertheless, there is an important share of taxa that directly compete with the grapevine or pose a danger to the entire ecosystem. A high level of vegetation regulation apparently suppresses indigenous taxa and species with beneficial functions in relation to grapevines.
A cultivated inter-row favours to occurrence of annual taxa, deep-rooting taxa, indigenous archaeophytes, and domesticated archaeophytes. The repeated cultivation of the soil creates similar conditions to those on arable land; therefore, there is a higher coverage of annual taxa. The typical annual taxa in field crop stands are weeds. According to Kazakou et al. [80], tillage and herbicide use favour the profile of ruderal taxa, plants with a lower competitiveness and high seed production. According to Storkey et al. [81], regulatory interventions lead to the selection of plant taxa that have a rapid life cycle as well as taxa that competitively limit optimal grapevine development. This can be observed, from our results, mainly in habitats with intensive regulatory interventions (M2, PP). Despite their adverse properties, weeds also provide other functions: e.g., they serve as a source of food and shelter for a whole range of animals, which gives them an important place in the vineyard ecosystem [82,83,84]. Biomass regulation is also of great importance for reducing the risk of fires [85]. Moreover, the intensification of agriculture has led to the loss of biodiversity, the simplification of the plant community, and the deterioration of ecosystem stability [86,87,88]; yet, threatened species can also be part of the vineyard vegetation. However, their representation is not considerable.
The vegetation of vineyards is influenced by their age. This relationship was particularly evident in the grassy inter-rows, where there was a gradual increase in the number of plant species. However, when considering the changes in cover, these changes occurred mainly in young vineyards, up to 8 years of age (Figure 4, Figure 5 and Figure 6). After this amount of time, the changes stabilized and became less pronounced. It could be assumed that the further course of vegetation succession was blocked due to vegetation regulation. This is also shown in Figure 8, where the share of species in the vegetation cover does not vary much in vineyards older than 5 years. The vegetation of the cultivated inter-rows changed only to a limited extent with the age of the vineyards, due to the cultivation of the soil, which returned the succession process to the initial stage of phytocenosis.
The conditions that the strip area around the grapevine trunks creates clearly increased the number of plant species with the growing age of the vineyards, but this was only noticeable in the summer season. The increase in the number of species was caused mainly by taxa with an invasive status. Plant taxa in vineyards most frequently apply a ruderal ecological strategy. This indicates sufficient nutrients and light in the vineyards and substantial disturbances. At sites in the cultivated inter-rows and strip areas around the grapevine trunks, the share of the R-strategy was more significant, while the share of the S-strategy decreased. The application of the S-strategy had a higher share in the grassy inter-rows. A similar proportion of S-strategy was reported by Winkler et al. [85] in the case of urban lawns. It can be presumed that the conditions of vineyards are of a stressful nature. Above all, the lack of water and the high insolation evoke stress in some taxa and can be part of the winemaking conditions—terroir. Plant taxa that apply a stress tolerance strategy allow these conditions to prevail. Regularly recurring disturbances and the application of herbicides generally occur in the cultivated inter-row and the strip area around the grapevine trunks. These factors give evidence for the application of an anthropogenic life strategy (A). According to Winkler et al. [89], the A-strategy is an adaptation to the conditions created by human civilization. It is therefore probable that the application of the A-strategy enables certain plant taxa to survive in vineyard conditions.
The primary ecosystem service of vineyards for farmers is the yield and quality of the grapes. These factors are influenced by grape variety, cultivation techniques, and the unique “terroir” conditions [90,91]. It is important to note that “terroir” conditions also play a crucial role in shaping vineyard vegetation. Griesser et al. [92] suggest that vineyard management strategies can adapt to climate change, maintain ecosystem functions, and enhance biodiversity. Similarly, Boinot et al. [93] emphasize that non-crop vegetation is essential for agroecosystems, particularly in mitigating climate change impacts and biodiversity loss crises.
The promotion of biodiversity is increasingly recognized among wine producers seeking to enhance the cultural value of agricultural landscapes [8,9]. Enhancing vegetation diversity in vineyards not only supports biodiversity but also enhances their esthetic appeal, fostering the coexistence of viticulture and diverse ecosystems.
5. Conclusions
The vegetation in vineyards creates a habitat for other organisms. It undergoes a succession with a very specific course. The succession of vegetation in vineyards differs according to the type of management used. In grassy inter-rows (M1), the number of plant species increases especially in young vineyards, until the ages of 5 to 8 years. After this time, the number of plant species stabilizes, which can be considered a blocked succession. The vegetation of the cultivated inter-row (M2) and the root zone changes little with age and remains in an initial phase of phytocenosis.
The representation of plant functional groups differs between habitats with different management types. During the ageing of vineyards, mainly perennial dicotyledonous and perennial monocotyledonous taxa predominate in the grassy inter-rows. Thanks to these taxa, the vegetation of a vineyard can serve primarily as a protection against erosion and a source of food for pollinators. In the strip area around the vine trunks, the number of plant species clearly increases with the age of the vineyard, mainly due to taxa with an invasive status. This habitat represents a risk zone from the point of view of the possible spread of non-native plant taxa in the agroecosystem.
The plant taxa growing in vineyards mainly follow a ruderal ecological strategy. This allows them to use nutrients and light more efficiently and survive the considerable disturbances associated with the regulation of vegetation in vineyards. Vineyards are an ecological niche that helps to create the conditions for the A-strategy as an adaptation to the conditions of human civilisation [89]. According to Mahaut et al. [94], understanding the impact of human activities on ecological and evolutionary dynamics requires a re-evaluation of ecological theories that were originally developed for natural ecosystems. They can hardly explain the reciprocal interactions between human activities and ecological and evolutionary processes.
Vineyards are an ecosystem with fragmented habitats that allow heterogeneous vegetation to flourish. As vineyards age, the vegetation changes, and the dynamics of the vineyard ecosystems become apparent. It is important to perceive vineyard vegetation not as an unchanging and stable community but as changing with changing dynamics. In young vineyards, the dynamics of change are faster, and, as the vineyard ages, the changes become slower. The vegetation of vineyards is a means of preventing the homogenisation of the agricultural landscape and allows the permanent coexistence of viticulture and species-rich ecosystems.
Author Contributions
Conceptualization, J.W., E.H., M.J. and L.H.; methodology, J.W., M.J. and E.H.; validation, J.W., E.H. and P.M.B.; formal analysis, J.W., M.J., E.H. and Y.R.L.; investigation, M.J., E.H., I.D. and Y.R.L.; resources, J.W.; data curation, J.W., E.H. and M.J.; writing—original draft preparation, J.W., E.H. and A.M.; writing—review and editing, J.W., I.D., P.M.B. and E.H.; visualization, J.W. and E.H.; project administration, E.H., L.H. and I.D.; and funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was created within the following project: IGA-ZF/2021-ST2001 Evaluation of ecosystem services of vegetation in permanent crops.
Data Availability Statement
All datasets generated and analysed during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Names of the plant species present in the vineyards under study and classification of the plant species in groups.
Table A1.
Names of the plant species present in the vineyards under study and classification of the plant species in groups.
| Name | Authorship | Family | Biological Properties | Functional Groups | Groups on Native and Invasion Status | Ecological Strategy | Treatened Species | Successional Age Optimum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (C) | (S) | (R) | ||||||||
| Agrimonia eupatoria | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 47.8 | 36.7 | 15.5 | common | 32 |
| Achillea millefolium | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 22 | 58.8 | 19.2 | common | 23 |
| Ailanthus altissima | (Mill.) Swingle | Simaroubaceae | perennial dicotyledons | deep-rooting species | invasive neophytes | 74.9 | 21.3 | 3.8 | common | 27 |
| Alcea biennis | Winterl | Malvaceae | perennial dicotyledons | entomophilous species | native | 0 | 0 | 0 | C2b | without data |
| Amaranthus powellii | S. Watson | Amaranthaceae | annual dicotyledons | annual weeds | invasive neophytes | 57.4 | 20.4 | 22.2 | common | 1.5 |
| Amaranthus retroflexus | L. | Amaranthaceae | annual dicotyledons | annual weeds | invasive neophytes | 52.1 | 23.7 | 24.2 | common | 1.5 |
| Anagallis arvensis | L. | Primulaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 9.7 | 0 | 90.3 | common | 5 |
| Anagallis foemina | Mill. | Primulaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 16.7 | 9.9 | 73.6 | C3 | 4 |
| Anthemis arvensis | L. | Asteraceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 21.3 | 0 | 78.4 | common | 10 |
| Anthyllis vulneraria | L. | Fabaceae | perennial dicotyledons | legumes | native | 51.6 | 4.6 | 43.8 | common | 18 |
| Apera spicaventi | (L.) P. Beauv. | Poaceae | annual monocotyledons | species with an anti-erosion effect | naturalized archaeophytes | 24.7 | 42.6 | 32.7 | common | 3 |
| Agrimonia eupatoria | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 47.8 | 36.7 | 15.5 | common | 32 |
| Arctium lappa | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 79.1 | 7.9 | 13 | common | 8.5 |
| Arctium tomentosum | Mill. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 79.1 | 7.9 | 13 | common | 8 |
| Arrhenatherum elatius | (L.) J. Presl et C. Presl | Poaceae | perennial monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 23.1 | 40.3 | 36.5 | common | 25 |
| Artemisia absinthium | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 36.9 | 21.9 | 41.2 | common | 8 |
| Artemisia vulgaris | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 41.6 | 29.6 | 28.8 | common | 10 |
| Atriplex patula | L. | Chenopodiaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 53.9 | 0 | 46.1 | common | 4.5 |
| Atriplex sagittata | Borkh. | Chenopodiaceae | annual dicotyledons | annual weeds | invasive archaeophytes | 38.3 | 29 | 32.8 | common | 5 |
| Avena fatua | L. | Poaceae | annual monocotyledons | species with an anti-erosion effect | naturalized archaeophytes | 48.8 | 25.5 | 25.8 | common | 4 |
| Berteroa incana | (L.) DC. | Brassicaceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 9.9 | 52.1 | 38 | common | 16.5 |
| Brassicca napus subsp. napus | L. | Brassicaceae | annual dicotyledons | crops | casual archaeophytes | 58 | 0.3 | 41.8 | common | without data |
| Bromus hordeaceus | L. | Poaceae | annual monocotyledons | species with an anti-erosion effect | naturalized archaeophytes | 11.6 | 47.2 | 41.2 | common | 12 |
| Calamagrostis epigejos | (L.) Roth | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 40.8 | 51.2 | 8 | common | 16 |
| Camelina microcarpa | DC. | Brassicaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 33.6 | 0 | 66.4 | common | 6.5 |
| Camelina sativa | (L.) Crantz | Brassicaceae | annual dicotyledons | crops | casual archaeophytes | 33.6 | 0 | 66.4 | common | without data |
| Capsella bursa-pastoris | (L.) Medik. | Brassicaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 43.4 | 0 | 56.6 | common | 2 |
| Carduus acanthoides | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 71.5 | 0 | 28.5 | common | 8 |
| Carlina vulgaris | L. | Asteraceae | annual dicotyledons | entomophilous species | native | 27.8 | 0 | 72.3 | common | 4.5 |
| Centaurea jacea | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 39.2 | 24.4 | 36.5 | common | 25 |
| Cerastium holosteoides | (Spenn.) Möschl | Caryophyllaceae | annual dicotyledons | entomophilous species | native | 0 | 9.7 | 90.3 | common | 15 |
| Cichorium intybus | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 74.4 | 0 | 25.6 | common | 11 |
| Cirsium arvense | (L.) Scop. | Asteraceae | perennial dicotyledons | deep-rooting species | invasive archaeophytes | 80.8 | 0 | 19.2 | common | 10 |
| Consolida regalis | S. F. Gray | Ranunculaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 20.4 | 36.9 | 42.7 | common | 4 |
| Convolvulus arvensis | L. | Convolvulaceae | perennial dicotyledons | deep-rooting species | naturalized archaeophytes | 36 | 4.7 | 59.3 | common | 22 |
| Conyza canadensis | (L.) Cronquist | Asteraceae | annual dicotyledons | annual weeds | invasive neophytes | 35.4 | 9.6 | 55.1 | common | 5 |
| Crataegus laevigata | (Poir.) DC. | Rosaceae | perennial dicotyledons | deep-rooting species | native | 18 | 56.9 | 25.1 | common | 5 |
| Crepis biennis | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 58.8 | 0 | 41.2 | common | 12.5 |
| Crepis capillaris | (L.) Wallr. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 42.8 | 0 | 57.2 | common | 9.5 |
| Cynoglossum officinale | L. | Boraginaceae | perennial dicotyledons | entomophilous species | native | 58.3 | 0 | 41.7 | common | 20 |
| Dactylis glomerata | L. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 29 | 37.3 | 33.7 | common | 32 |
| Datura stramonium | L. | Solanaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 71.3 | 0 | 28.7 | common | without data |
| Daucus carota | L. | Apiaceae | perennial dicotyledons | entomophilous species | native | 29.2 | 41.3 | 29.5 | common | 15 |
| Descurainia sophia | (L.) Prantl | Brassicaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 30.2 | 10.7 | 59.1 | common | 4.5 |
| Digitaria sanguinalis | (L.) Scop. | Poaceae | annual monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 17 | 3.5 | 79.5 | common | 2 |
| Echinochloa crus-galli | (L.) P. Beauv. | Poaceae | annual monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 41.8 | 12.4 | 45.8 | common | 1 |
| Echinops sphaerocephalus | L. | Asteraceae | perennial dicotyledons | entomophilous species | invasive neophytes | 0 | 0 | 0 | common | 16 |
| Echium vulgare | L. | Boraginaceae | perennial dicotyledons | entomophilous species | native | 78.1 | 0 | 21.9 | common | 14.5 |
| Elymus repens | (L.) Gould | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 33.8 | 35.9 | 30.3 | common | 8 |
| Erigeron annuus | (L.) Pers. | Asteraceae | annual dicotyledons | annual weeds | invasive neophytes | 41.3 | 13 | 45.8 | common | 30 |
| Erodium cicutarium | (L.) L‘Hér. | Geraniaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 37.7 | 18.6 | 43.7 | common | 6 |
| Eryngium campestre | L. | Apiaceae | perennial dicotyledons | entomophilous species | native | 84.1 | 15.9 | 0 | common | 40 |
| Euphorbia esula | L. | Euphorbiaceae | perennial dicotyledons | entomophilous species | native | 5.7 | 27.8 | 66.5 | common | 19 |
| Euphorbia helioscopia | L. | Euphorbiaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 18.9 | 18.3 | 62.3 | common | 12 |
| Fagopyrum esculentum | Moench | Polygonaceae | annual dicotyledons | crops | casual archaeophytes | 0 | 0 | 0 | common | without data |
| Falcaria vulgaris | Bernh. | Apiaceae | perennial dicotyledons | entomophilous species | native | 75.4 | 11.7 | 12.9 | common | 27.5 |
| Fallopia convolvulus | (L.) Á. Löve | Polygonaceae | annual dicotyledons | annual weeds | native | 25.6 | 22.8 | 51.6 | common | 7.5 |
| Festuca arundinacea | Schreb. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 49.9 | 27.4 | 22.7 | common | 1 |
| Festuca pratensis | Huds. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 17.4 | 42.2 | 40.3 | common | 32 |
| Festuca rubra | L. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 16.3 | 49.6 | 34.1 | common | 30 |
| Fragaria vesca | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 27.2 | 49.2 | 23.6 | common | 35 |
| Fumaria officinalis | L. | Fumariaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 19.3 | 0 | 80.7 | common | 19 |
| Galeopsis tetrahit | L. | Lamiaceae | annual dicotyledons | annual weeds | native | 33.1 | 0 | 66.9 | common | 9.5 |
| Galinsoga parviflora | Cav. | Asteraceae | annual dicotyledons | annual weeds | invasive neophytes | 15.5 | 32 | 52.5 | common | 2 |
| Galium album | Mill. | Rubiaceae | perennial dicotyledons | entomophilous species | native | 4.5 | 35.4 | 60.1 | common | 26 |
| Galium aparine | L. | Rubiaceae | annual dicotyledons | annual weeds | native | 12.2 | 0 | 87.8 | common | 28 |
| Galium verum | L. | Rubiaceae | perennial dicotyledons | entomophilous species | native | 1.3 | 71.8 | 26.9 | common | 34 |
| Geranium pusillum | Burm. f. | Geraniaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 38.9 | 18.4 | 42.7 | common | 6 |
| Geranium pyrenaicum | Burm. f. | Geraniaceae | perennial dicotyledons | entomophilous species | naturalized neophytes | 48.8 | 16.2 | 35 | common | 11 |
| Geranium robertianum | L. | Geraniaceae | annual dicotyledons | entomophilous species | native | 32 | 11.3 | 56.7 | common | 39 |
| Geum urbanum | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 31.7 | 32.8 | 35.5 | common | 40 |
| Holosteum umbellatum | L. | Caryophyllaceae | annual dicotyledons | annual weeds | native | 1.8 | 0 | 98.2 | common | 75 |
| Hordeum murinum | L. | Poaceae | annual monocotyledons | species with an anti-erosion effect | naturalized archaeophytes | 19.8 | 18 | 62.2 | common | without data |
| Humulus lupulus | L. | Cannabaceae | perennial dicotyledons | deep-rooting species | native | 47.3 | 30.1 | 22.7 | common | 35 |
| Hypericum perforatum | L. | Hypericaceae | perennial dicotyledons | entomophilous species | native | 6.7 | 52.6 | 40.7 | common | 23 |
| Inula salicina | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 21.7 | 33.5 | 44.8 | C4a | 23 |
| Chelidonium majus | L. | Papaveraceae | annual dicotyledons | annual weeds | native | 54.7 | 6.6 | 38.8 | common | 29 |
| Chenopodium album | L. | Chenopodiaceae | annual dicotyledons | annual weeds | native | 30.5 | 34.1 | 35.4 | common | 3 |
| Chenopodium hybridum | L. | Chenopodiaceae | annual dicotyledons | annual weeds | native | 43.1 | 0 | 56.9 | common | 3 |
| Chenopodium polyspermum | L. | Chenopodiaceae | annual dicotyledons | annual weeds | native | 33.8 | 1.1 | 65 | common | 2 |
| Juglans regia | L. | Juglandaceae | perennial dicotyledons | deep-rooting species | invasive archaeophytes | 52.4 | 31.3 | 16.3 | common | 35 |
| Lactuca serriola | L. | Asteraceae | annual dicotyledons | annual weeds | invasive archaeophytes | 66.3 | 12.1 | 21.6 | common | 5 |
| Lamium album | L. | Lamiaceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 39.8 | 2.4 | 57.8 | common | 21 |
| Lamium amplexicaule | L. | Lamiaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 20.3 | 0 | 79.7 | common | 10.5 |
| Lamium maculatum | L. | Lamiaceae | perennial dicotyledons | entomophilous species | native | 32.1 | 16.1 | 51.8 | common | 15 |
| Lamium purpureum | L. | Lamiaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 23.2 | 0 | 76.8 | common | 25 |
| Lathyrus sativus | L. | Fabaceae | annual dicotyledons | crops | casual archaeophytes | 0 | 0 | 0 | common | without data |
| Lathyrus tuberosus | L. | Fabaceae | perennial dicotyledons | deep-rooting species | naturalized archaeophytes | 21.9 | 19.9 | 58.3 | common | 18 |
| Lepidium draba | L. | Brassicaceae | perennial dicotyledons | deep-rooting species | naturalized archaeophytes | 47.6 | 0 | 52.4 | common | 11 |
| Ligustrum vulgare | L. | Oleaceae | perennial dicotyledons | deep-rooting species | native | 27.7 | 64 | 8.3 | common | 43 |
| Linaria vulgaris | Mill. | Scrophulariaceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 10.7 | 25 | 64.3 | common | 10.5 |
| Linum usitatissimum | L. | Linaceae | annual dicotyledons | crops | casual archaeophytes | 2.1 | 0 | 97.9 | common | without data |
| Lolium multiflorum | Lam. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | naturalized neophytes | 17.4 | 38.6 | 44 | common | 1 |
| Lolium perenne | L. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 18.5 | 29.3 | 52.3 | common | 8 |
| Lotus corniculatus | L. | Fabaceae | perennial dicotyledons | legumes | native | 11.5 | 19.1 | 69.4 | common | 15 |
| Luzula campestris | (L.) DC. | Juncaceae | perennial monocotyledons | species with an anti-erosion effect | native | 15.7 | 47.9 | 36.4 | common | 15 |
| Malva neglecta | Wallr. | Malvaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 11 | 33.6 | 55.5 | common | 1 |
| Matricaria discoidea | DC. | Asteraceae | annual dicotyledons | annual weeds | naturalized neophytes | 26.6 | 0 | 73.4 | common | 3 |
| Medicago lupulina | L. | Fabaceae | perennial dicotyledons | legumes | native | 22.4 | 19.9 | 57.7 | common | 13 |
| Medicago sativa | L. | Fabaceae | perennial dicotyledons | legumes | naturalized neophytes | 31 | 25.9 | 43.1 | common | 10.5 |
| Melilotus albus | Medik. | Fabaceae | perennial dicotyledons | legumes | naturalized archaeophytes | 36.5 | 0 | 63.5 | common | 18 |
| Melilotus officinalis | (L.) Pall. | Fabaceae | perennial dicotyledons | legumes | naturalized archaeophytes | 23.5 | 40.1 | 36.4 | common | 23 |
| Mercurialis annua | L. | Euphorbiaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 24.4 | 0 | 75.6 | common | without data |
| Microthlaspi perfoliatum | (L.) F. K. Mey. | Brassicaceae | annual dicotyledons | annual weeds | native | 0 | 0 | 0 | common | 45 |
| Myosotis arvensis | (L.) Hill | Boraginaceae | annual dicotyledons | annual weeds | native | 31.7 | 0 | 68.3 | common | 25 |
| Nonea pulla | (L.) DC. | Boraginaceae | perennial dicotyledons | entomophilous species | native | 48.4 | 0 | 51.9 | C4a | 30 |
| Onobrychis viciifolia | Scop. | Fabaceae | perennial dicotyledons | legumes | naturalized neophytes | 44.1 | 31.1 | 24.7 | common | 25 |
| Onopordum acanthium | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 61.5 | 22.7 | 15.9 | common | 75 |
| Panicum miliaceum | L. | Poaceae | annual monocotyledons | species with an anti-erosion effect | casual neophytes | 0 | 0 | 0 | common | without data |
| Papaver rhoeas | L. | Papaveraceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 55 | 0 | 45 | common | 3 |
| Parthenocissus inserta | (A. Kern.) Fritsch | Vitaceae | perennial dicotyledons | deep-rooting species | invasive neophytes | 65.9 | 15.6 | 18.5 | common | 47 |
| Petrorhagia prolifera | (L.) P. W. Ball et Heywood | Caryophyllaceae | perennial dicotyledons | entomophilous species | native | 4.4 | 51.7 | 43.9 | C4a | 7 |
| Phacelia tanacetifolia | Benth. | Hydrophyllaceae | annual dicotyledons | crops | casual neophytes | 51.6 | 0 | 48.4 | common | without data |
| Physalis alkekengi | L. | Solanaceae | perennial dicotyledons | crops | naturalized archaeophytes | 0 | 0 | 0 | common | without data |
| Pilosella aurantiaca | (L.) F. W. Schultz et Sch. Bip. | Asteraceae | perennial dicotyledons | entomophilous species | native | 42.4 | 0 | 57.6 | C3 | 20 |
| Pilosella officinarum | Vaill. | Asteraceae | perennial dicotyledons | entomophilous species | native | 9.6 | 26.3 | 64.1 | 27 | |
| Pisum sativum | L. | Fabaceae | annual dicotyledons | crops | casual archaeophytes | 0 | 0 | 0 | common | without data |
| Plantago lanceolata | L. | Plantaginaceae | perennial dicotyledons | entomophilous species | native | 61.1 | 0.9 | 38 | common | 16 |
| Plantago major | L. | Plantaginaceae | perennial dicotyledons | entomophilous species | native | 82.3 | 0 | 17.7 | common | 6 |
| Plantago media | L. | Plantaginaceae | perennial dicotyledons | entomophilous species | native | 68.2 | 0 | 31.8 | common | 30 |
| Poa annua | L. | Poaceae | annual monocotyledons | species with an anti-erosion effect | native | 6.8 | 35 | 58.2 | common | 4 |
| Poa pratensis | L. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 12.4 | 0 | 87.6 | common | 30 |
| Polygonum aviculare | L. | Polygonaceae | annual dicotyledons | annual weeds | native | 15.3 | 29.7 | 55 | common | 1 |
| Portulaca oleracea | L. | Portulacaceae | annual dicotyledons | annual weeds | invasive archaeophytes | 0.3 | 0 | 99.7 | common | without data |
| Potentilla argentea | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 21.3 | 50.4 | 28.3 | common | 19 |
| Potentilla reptans | L. | Rosaceae | perennial dicotyledons | entomophilous species | native | 35.3 | 29.3 | 35.4 | common | 22 |
| Quercus petraea | (Matt.) Liebl. | Fagaceae | perennial dicotyledons | deep-rooting species | native | 28.1 | 63.2 | 8.6 | common | 35 |
| Raphanus sativus | L. | Brassicaceae | annual dicotyledons | crops | casual archaeophytes | 62.1 | 0 | 37.9 | common | without data |
| Raphanus raphanistrum | L. | Brassicaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 63.2 | 0 | 36.8 | common | 4.5 |
| Reseda lutea | L. | Resedaceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 51 | 0 | 49.1 | common | 13 |
| Ribes aureum | Pursh | Grossulariaceae | perennial dicotyledons | deep-rooting species | casual neophytes | 31 | 58.3 | 10.8 | common | without data |
| Robinia pseudoacacia | L. | Fabaceae | perennial dicotyledons | deep-rooting species | invasive neophytes | 28.8 | 41.4 | 29.8 | common | 30 |
| Rosa canina | L. | Rosaceae | perennial dicotyledons | deep-rooting species | native | 16.6 | 50.2 | 33.2 | common | 35 |
| Rubus sect. Rubus | Kaplan et al. | Rosaceae | perennial dicotyledons | deep-rooting species | native | 46 | 39.1 | 14.9 | common | without data |
| Rumex crispus | L. | Polygonaceae | perennial dicotyledons | deep-rooting species | native | 65.1 | 0 | 35 | common | 7 |
| Rumex obtusifolius | L. | Polygonaceae | perennial dicotyledons | deep-rooting species | native | 80.6 | 0 | 19.5 | common | 7.5 |
| Salvia pratensis | L. | Lamiaceae | perennial dicotyledons | entomophilous species | native | 62.8 | 2.4 | 34.8 | common | 35 |
| Sambucus nigra | L. | Sambucaceae | perennial dicotyledons | deep-rooting species | native | 36.2 | 23.4 | 40.4 | common | 30 |
| Scabiosa ochroleuca | L. | Dipsacaceae | perennial dicotyledons | entomophilous species | native | 25.6 | 61.6 | 12.8 | common | 30 |
| Scleranthus annuus | L. | Caryophyllaceae | annual dicotyledons | annual weeds | native | 0 | 0.7 | 99.3 | common | 2 |
| Secale cereale | L. | Poaceae | annual monocotyledons | crops | casual archaeophytes | 0 | 0 | 0 | common | without data |
| Securigera varia | (L.) Lassen | Fabaceae | perennial dicotyledons | legumes | native | 39.6 | 1.6 | 58.9 | common | 25 |
| Senecio jacobaea | L. | Asteraceae | annual dicotyledons | entomophilous species | native | 65.6 | 0 | 34.4 | common | 35 |
| Senecio vulgaris | L. | Asteraceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 26.4 | 0 | 73.6 | common | 4 |
| Setaria pumila | (Poir.) Roem. et Schult. | Poaceae | annual monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 38.4 | 24.7 | 37 | common | 4 |
| Setaria viridis | (L.) P. Beauv. | Poaceae | annual monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 23.6 | 41.5 | 34.9 | common | 3 |
| Setaria verticillata | (L.) P. Beauv. | Poaceae | annual monocotyledons | species with an anti-erosion effect | invasive archaeophytes | 25.2 | 33.2 | 41.6 | common | 3 |
| Silene latifolia subsp. alba | (Mill.) Greuter et Burdet | Caryophyllaceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 51.2 | 0 | 48.8 | common | 10 |
| Silene noctiflora | L. | Caryophyllaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 49.3 | 4.8 | 45.9 | C4a | 3.5 |
| Silene vulgaris | (Moench) Garcke | Caryophyllaceae | perennial dicotyledons | entomophilous species | native | 48.1 | 0.2 | 51.7 | common | 26.5 |
| Sinapis alba | L. | Brassicaceae | annual dicotyledons | crops | casual archaeophytes | 56 | 0 | 44 | common | without data |
| Solanum nigrum | L. | Solanaceae | annual dicotyledons | annual weeds | native | 0 | 0 | 100 | common | 4 |
| Sonchus oleraceus | L. | Asteraceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 61.1 | 0 | 38.9 | common | 4 |
| Stellaria media | (L.) Vill. | Caryophyllaceae | annual dicotyledons | annual weeds | native | 5 | 0 | 95 | common | 11.5 |
| Stipa pennata | L. | Poaceae | perennial monocotyledons | species with an anti-erosion effect | native | 16.5 | 83.5 | 0 | C3 | 75 |
| Symphytum officinale | L. | Boraginaceae | perennial dicotyledons | entomophilous species | native | 68.1 | 0 | 31.9 | common | 32 |
| Tanacetum vulgare | L. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 59.1 | 18.3 | 22.6 | common | 18 |
| Taraxacum sect. Taraxacum | Kirschner et al. | Asteraceae | perennial dicotyledons | entomophilous species | native | 55.4 | 0 | 44.7 | common | without data |
| Tragopogon dubius | Scop. | Asteraceae | perennial dicotyledons | entomophilous species | naturalized archaeophytes | 17.4 | 22.4 | 60.2 | common | 13 |
| Tragopogon orientalis | L. | Asteraceae | perennial dicotyledons | entomophilous species | native | 42 | 0 | 58 | common | 16 |
| Trifolium alexandrinum | L. | Fabaceae | annual dicotyledons | legumes | casual neophytes | 0 | 0 | 0 | common | without data |
| Trifolium campestre | Schreb. | Fabaceae | perennial dicotyledons | legumes | native | 9.2 | 41.7 | 49.1 | common | 14.5 |
| Trifolium incarnatum | L. | Fabaceae | annual dicotyledons | legumes | casual neophytes | 40.4 | 11.8 | 47.8 | common | without data |
| Trifolium pratense | L. | Fabaceae | perennial dicotyledons | legumes | native | 24.7 | 31.5 | 43.8 | common | 13 |
| Trifolium repens | L. | Fabaceae | perennial dicotyledons | legumes | native | 25.4 | 12.7 | 61.9 | common | 7 |
| Tripleurospermum inodorum | (L.) Sch. Bip. | Asteraceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 23.5 | 0 | 76.5 | common | 5 |
| Triticum aestivum | L. | Poaceae | annual monocotyledons | crops | casual archaeophytes | 39.3 | 22.8 | 37.9 | common | without data |
| Urtica dioica | L. | Urticaceae | perennial dicotyledons | deep-rooting species | native | 44.8 | 18.2 | 37.1 | common | 25 |
| Urtica urens | L. | Urticaceae | annual dicotyledons | annual weeds | naturalized archaeophytes | 22.1 | 32.1 | 45.8 | C3 | 25 |
| Veronica hederifolia | L. | Scrophulariaceae | annual dicotyledons | annual weeds | native | 19.3 | 0 | 80.7 | common | 4.8 |
| Vicia cracca | L. | Fabaceae | perennial dicotyledons | legumes | native | 27.3 | 28.7 | 44 | common | 30 |
| Vicia pannonica | Crantz | Fabaceae | annual dicotyledons | legumes | naturalized archaeophytes | 0 | 0 | 0 | C2t | without data |
| Viola arvensis | Murray | Violaceae | annual dicotyledons | annual weeds | native | 30.8 | 1.6 | 67.6 | common | 6 |
References
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Pe’er, G.; Zinngrebe, Y.; Hauck, J.; Schindler, S.; Dittrich, A.; Zingg, S.; Tscharntke, T.; Oppermann, R.; Sutcliffe, L.M.; Sirami, C.; et al. Adding some green to the greening: Improving the EU’s Ecological Focus Areas for biodiversity and farmers. Conserv. Lett. 2017, 10, 517–530. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Paiola, A.; Assandri, G.; Brambilla, M.; Zottini, M.; Pedrini, P.; Nascimbene, J. Exploring the potential of vineyards for biodiversity conservation and delivery of biodiversity-mediated ecosystem services: A global-scale systematic review. Sci. Total Environ. 2020, 706, 135839. [Google Scholar] [CrossRef] [PubMed]
- Henle, K.; Alard, D.; Clitherow, J.; Cobb, P.; Firbank, L.; Kull, T.; McCracken, D.; Moritz, R.F.; Niemelä, J.; Rebane, M.; et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe—A review. Agric. Ecosyst. Environ. 2008, 124, 60–71. [Google Scholar] [CrossRef]
- Sutcliffe, L.M.; Batáry, P.; Kormann, U.; Báldi, A.; Dicks, L.V.; Herzon, I.; Kleijn, D.; Tryjanowski, P.; Apostolova, I.; Arlettaz, R.; et al. Harnessing the biodiversity value of Central and Eastern European farmland. Divers. Distrib. 2015, 21, 722–730. [Google Scholar] [CrossRef]
- Loos, J.; Von Wehrden, H. Beyond biodiversity conservation: Land sharing constitutes sustainable agriculture in European cultural landscapes. Sustainability 2018, 10, 1395. [Google Scholar] [CrossRef]
- Brugisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyards management on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Paoletti, M.G. Organic farming benefits local plant diversity in vineyard farms located in intensive agricultural landscapes. Environ. Manag. 2012, 49, 1054–1060. [Google Scholar] [CrossRef]
- Rusch, A.; Beaumelle, L.; Giffard, B.; Ugaglia, A.A. Chapter Seven—Harnessing biodiversity and ecosystem services to safeguard multifunctional vineyard landscapes in a global change context. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Vanbergen, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 65, pp. 305–335. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Hall, R.M.; Penke, N.; Kriechbaum, M.; Kratschmer, S.; Jung, V.; Chollet, S.; Guernion, M.; Nicolai, A.; Burel, F.; Fertil, A.; et al. Vegetation management intensity and landscape diversity alter plant species richness, functional traits and community composition across European vineyards. Agric. Syst. 2020, 177, 102706. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Valencia-Gredilla, F.; Royo-Esnal, A.; Recasens, J. Organic mulches as an alternative to conventional under-vine weed management in Mediterranean irrigated vineyards. Plants 2022, 11, 2785. [Google Scholar] [CrossRef] [PubMed]
- Maixner, M.; Ahrens, U.; Seemüller, E. Detection of the German grapevine yellows (Vergilbungskrankheit) MLO in grapevine, alternative hosts and a vector by a specific PCR procedure. Eur. J. Plant Pathol. 1995, 101, 241–250. [Google Scholar] [CrossRef]
- Castillo, P.; Rapoport, H.F.; Rius, J.P.; Díaz, R.J. Suitability of weed species prevailing in Spanish vineyards as hosts for root-knot nematodes. Eur. J. Plant Pathol. 2008, 120, 43–51. [Google Scholar] [CrossRef]
- Gualandri, V.; Asquini, E.; Bianchedi, P.; Covelli, L.; Brilli, M.; Malossini, U.; Bragagna, P.; Saldarelli, P.; Si-Ammour, A. Identification of herbaceous hosts of the Grapevine Pinot gris virus (GPGV). Eur. J. Plant Pathol. 2017, 147, 21–25. [Google Scholar] [CrossRef]
- Demian, E.; Jaksa-Czotter, N.; Varallyay, E. Grapevine Pinot gris virus is present in different non-Vitis hosts. Plants 2022, 11, 1830. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, M.; Cerdà, A.; Tarolli, P. Soil water erosion on Mediterranean vineyards: A review. Catena 2016, 141, 1–21. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Biddoccu, M.; Ferraris, S.; Opsi, F.; Cavallo, E. Long-term monitoring of soil management effects on runoff and soil erosion in sloping vineyards in Alto Monferrato (North-West Italy). Soil Res. 2016, 155, 176–189. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdes-Gomez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Donnini, S.; Tessarin, P.; Ribera-Fonseca, A.; Di Foggia, M.; Parpinello, G.P.; Rombola, A.D. Glyphosate impacts on polyphenolic composition in grapevine (Vitis vinifera L.) berries and wine. Food Chem. 2016, 213, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zaller, J.G.; Cantelmo, C.; Dos Santos, G.; Muther, S.; Gruber, E.; Pallua, P.; Mandl, K.; Friedrich, B.; Hofstetter, I.; Schmuckenschlager, B.; et al. Herbicides in vineyards reduce grapevine root mycorrhization and alter soil microorganisms and the nutrient composition in grapevine roots, leaves, xylem sap and grape juice. Environ. Sci. Pollut. R. 2018, 25, 23215–23226. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Steenwerth, K. Influence of Floor Management Technique on Grapevine Growth, Disease Pressure, and Juice and Wine Composition: A Review. Am. J. Enol. Viticult. 2012, 63, 149–164. [Google Scholar] [CrossRef]
- Steenwerth, K.; Belina, K.M. Cover crops and cultivation: Impacts on soil N dynamics and microbiological function in a Mediterranean vineyard agroecosystem. Appl. Soil Ecol. 2008, 40, 370–380. [Google Scholar] [CrossRef]
- Coniberti, A.; Ferrari, V.; Disegna, E.; Petillo, M.G.; Lakso, A.N. Under-trellis cover crop and planting density to achieve vine balance in a humid climate. Sci. Hortic. 2018, 227, 65–74. [Google Scholar] [CrossRef]
- Ragasová, L.; Kopta, T.; Winkler, J.; Pokluda, R. The Current Stage of Greening Vegetation in Selected Wine-Regions of South Moravian Region (Czech Republic). Agronomy 2019, 9, 541. [Google Scholar] [CrossRef]
- Schmid, A.; Weibel, F. Das sandwich system—ein Verfahren zur herbizidfreien Baumstreifenbewirtschaftung? [The sandwich system, a procedure for herbicide free in-row weed control?]. Obstbau 2000, 25, 214–217. [Google Scholar]
- Mia, M.J.; Furmanczyk, E.M.; Golian, J.; Kwiatkowska, J.; Malusá, E.; Neri, D. Living Mulch with Selected Herbs for Soil Management in Organic Apple Orchards. Horticulturae 2021, 7, 59. [Google Scholar] [CrossRef]
- Mia, M.J.; Massetani, F.; Murri, G.; Neri, D. Sustainable alternatives to chemicals for weed control in the orchard—A review. Hortic. Sci. 2020, 47, 1–12. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Körner, K.; Jeltsch, F. Detecting general plant functional type responses in fragmented landscapes using spatially-explicit simulations. Ecol. Model. 2008, 210, 87–300. [Google Scholar] [CrossRef]
- Murphy, H.T.; Lovett-Doust, J. Context and connectivity in plant metapopulations and landscape mosaics: Does the matrix matter? Oikos 2004, 105, 3–14. [Google Scholar] [CrossRef]
- Ries, L.; Sisk, T.D. A Predictive model of edge effects. Ecology 2004, 85, 2917–2926. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Córdova Martínez, A.; Caballero García, A.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Cornez, L.; Samkari, W.; Mazzella, J.M.; Venisse, A.; Boccio, V.; Auribault, K.; Keren, B.; Benistan, K.; Germain, D.P.; et al. Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with pseudoxanthoma elasticum. Genet. Med. 2017, 19, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Dener, E.; Ovadia, O.; Shemesh, H.; Altman, A.; Chen, S.C.; Giladi, I. Direct and indirect effects of fragmentation on seed dispersal traits in a fragmented agricultural landscape. Agric. Ecosyst. Environ. 2021, 309, 107273. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Ibanez, I.; Katz, D.S.W.; Peltier, D.; Wolf, S.M.; Barrie, B.T.C. Assessing the integrated effects of landscape fragmentation on plants and plant communities: The challenge of multiprocess-multiresponse dynamics. J. Ecol. 2014, 102, 882–895. [Google Scholar] [CrossRef]
- Sanguankeo, P.P.; León, R.G. Weed management practices determine plant and arthropod diversity and seed predation in vineyards. Weed Res. 2011, 51, 404–412. [Google Scholar] [CrossRef]
- Arlettaz, R.; Maurer, M.L.; Mosimann-Kampe, P.; Nusslé, S.; Abadi, F.; Braunisch, V.; Schaub, M. New vineyard cultivation practices create patchy ground vegetation, favouring Woodlarks. J. Ornithol. 2012, 153, 229–238. [Google Scholar] [CrossRef]
- Fernandez-Mena, H.; Frey, H.; Celette, F.; Garcia, L.; Barkaoui, K.; Hossard, L.; Naulleau, A.; Metral, R.; Gary, C.; Metay, A. Spatial and temporal diversity of service plant management strategies across vineyards in the south of France. Analysis through the Coverage Index. Eur. J. Agron. 2021, 123, 126191. [Google Scholar] [CrossRef]
- Geldenhuys, M.; Gaigher, R.; Pryke, J.S.; Samways, M.J. Diverse herbaceous cover crops promote vineyard arthropod diversity across different management regimes. Agric. Ecosyst. Environ. 2021, 307, 107222. [Google Scholar] [CrossRef]
- Candiago, S.; Winkler, K.J.; Giombini, V.; Giupponi, C.; Egarter Vigl, L. An ecosystem service approach to the study of vineyard landscapes in the context of climate change: A review. Sustain. Sci. 2023, 18, 997–1013. [Google Scholar] [CrossRef]
- Winkler, K.J.; Viers, J.H.; Nicholas, K.A. Assessing ecosystem services and multifunctionality for vineyard systems. Front. Environ. Sci. 2017, 5, 15. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, L.A.; Silva, R.A.; Godoy, R.; Jansen, K.; Matos, M.B.; Tavares Pinheiro, K.A.; Pinheiro, R.T. The impact of maternal post-partum depression on the language development of children at 12 months. Child Care Health Dev. 2012, 38, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Colmenero, M.; Bienes, R.; Eldridge, D.J.; Marques, M.J. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena 2013, 104, 153–160. [Google Scholar] [CrossRef]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Campos-Herrera, R.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Ground cover management in a Mediterranean vineyard: Impact on insect abundance and diversity. Agric. Ecosyst. Environ. 2019, 283, 106571. [Google Scholar] [CrossRef]
- Altieri, M.A.; Ponti, L.; Nicholls, C.I. Manipulating vineyard biodiversity for improved insect pest management: Case studies from northern California. Int. J. Biodivers. Sci. Manag. 2005, 1, 191–203. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 20 December 2023).
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Campos-Herrera, R.; Marco-Mancebón, V.S.; Pérez-Moreno, I. Effects of Ground Cover Management on Insect Predators and Pests in a Mediterranean Vineyard. Insects 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Zanettin, G.; Bullo, A.; Pozzebon, A.; Burgio, G.; Duso, C. Influence of Vineyard inter-row groundcover vegetation management on arthropod assemblages in the Vineyards of North-Eastern Italy. Insects 2021, 12, 349. [Google Scholar] [CrossRef]
- CGS. Geological Map of the Czech Republic, 1:50,000; Czech Geological Society: Prague, Czech Republic, 2018; Available online: https://mapy.geology.cz/geocr50/ (accessed on 8 November 2022).
- CGS. Map of Soil Types of the Czech Republic, 1:50,000; Czech Geological Society: Prague, Czech Republic, 2017; Available online: https://mapy.geology.cz/pudy/ (accessed on 8 November 2022).
- Culek, M. (Ed.) Biogeographical Division of the Czech Republic (Biogeografické Členění České Republiky), 1st ed.; Enigma: Prague, Czech Republic, 1996; p. 347. (In Czech) [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 236p, Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 20 December 2023).
- Biondi, E. Phytosociology today: Methodological and conceptual evolution. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2011, 145, 19–29. [Google Scholar] [CrossRef]
- Kaplan, Z.; Danihelka, J.; Chrtek, J.; Kirschner, J.; Kubát, K.; Štech, M.; Štěpánek, J. (Eds.) Key to the Flora of the Czech Republic [Klíč ke květeně České Republiky], 2nd ed.; Academia: Prague, Czech Republic, 2019; p. 1168. (In Czech) [Google Scholar]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Řezníčková, M.; Rohn, M.; Dřevojan, P.; Grulich, V.; et al. Pladias Database of the Czech Flora and Vegetation. Preslia 2021, 93, 1–87. [Google Scholar] [CrossRef]
- Dřevojan, P.; Čeplová, N.; Štěpánková, P.; Axmanová, I. Life Form. 2022. Available online: www.floraveg.eu (accessed on 20 December 2023).
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Pyšek, P.; Sádlo, J.; Chrtek, J., Jr.; Chytrý, M.; Kaplan, Z.; Pergl, J.; Pokorná, A.; Axmanová, I.; Čuda, J.; Doležal, J.; et al. Catalogue of alien plants of the Czech Republic (3rd edition): Species richness, status, distributions, habitats, regional invasion levels, introduction pathways and impacts. Preslia 2022, 94, 447–577. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; Wiley: Chichester, UK, 1979. [Google Scholar]
- Guo, W.Y.; Pierce, S. Life Strategy [Životní Strategie]. 2019. Available online: www.pladias.cz (accessed on 20 December 2023). (In Czech).
- Grulich, V. The Red List of vascular plants of the Czech Republic [Červený seznam cévnatých rostlin ČR]. Příroda 2017, 35, 75–132. (In Czech) [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 December 2023).
- El Titi, A. Implications of Soil tillage for Weed Communities. In Soil Tillage in Agroecosystems; El Titi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 147–185. [Google Scholar] [CrossRef]
- Kudsk, P.; Streibig, J.C. Herbicides—A two-edged sword. Weed Res. 2003, 43, 90–102. [Google Scholar] [CrossRef]
- Freemark, K.; Boutin, C. Impacts of agricultural herbicide use on terrestrial wildlife intemperate landscapes: A review withspecial reference to NorthAmerica. Agric. Ecosyst. Environ. 1995, 52, 67–91. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W.; Watson, R.N.; Nicholson, K.S. The detritus foodweb and the diversity of soil fauna as indicators of disturbance regimes in agroecosystems. Plant Soil 1995, 179, 35–43. [Google Scholar] [CrossRef]
- El Jaouhari, M.; Damour, G.; Tixier, P.; Coulis, M. Glyphosate reduces the biodiversity of soil macrofauna and benefits exotic over native species in a tropical agroecosystem. Basic Appl. Ecol. 2023, 73, 18–26. [Google Scholar] [CrossRef]
- Winkler, J.; Ricica, T.; Hubacíková, V.; Koda, E.; Vaverková, M.D.; Havel, L.; Zółtowski, M. Water Protection Zones—Impacts on Weed Vegetation of Arable Soil. Water 2023, 15, 3161. [Google Scholar] [CrossRef]
- Chan, K.Y.; Munro, K. Evaluating mustard extracts for earthworm sampling. Pedobiology 2001, 45, 272–278. [Google Scholar] [CrossRef]
- Emmerling, C. Response of earthworm communities to different types of soil tillage. Appl. Soil Ecol. 2001, 17, 91–96. [Google Scholar] [CrossRef]
- Pommeresche, R.; Loes, A.K. Relations between agronomic practice and earthworms in Norvegian arable soils. (Global Science Books). Dyn. Soil Dyn. Plant 2009, 3, 129–142. [Google Scholar]
- Winkler, J.; Mazur, Ł.; Smékalová, M.; Podlasek, A.; Hurajová, E.; Koda, E.; Jiroušek, M.; Jakimiuk, A.; Vaverková, M.D. Influence of land use on plant community composition in Vysocina Region grasslands, Czech Republic. Environ. Prot. Eng. 2022, 48, 21–33. [Google Scholar] [CrossRef]
- Kazakou, E.; Fried, G.; Richarte, J.; Gimenez, O.; Violle, C.; Metay, A. A plant trait-based response-and-effect framework to assess vineyard inter-row soil management. Bot. Lett. 2016, 163, 373–388. [Google Scholar] [CrossRef]
- Storkey, J.; Meyer, S.; Still, K.S.; Leuschner, C. The impact of agricultural intensification and land-use change on the European arable flora. Proc. R. Soc. B Biol. Sci. 2012, 279, 1421–1429. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Ragasová, L.; Kopta, T.; Winkler, J.; Šefrová, H.; Pokluda, R. The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region. Agronomy 2021, 11, 1073. [Google Scholar] [CrossRef]
- Hurajová, E.; Martínez Barroco, P.; Havel, L.; Děkanovský, I.; Winkler, J. Relationship Between Vegetation Succession and Earthworm Population in Vineyards. J. Ecol. Eng. 2024, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ježová, M.; Punčochář, R.; Hurajová, E.; Martínez Barroso, P.; Kopta, T.; Semerádová, D.; Vaverková, M.D. Fire Hazard: Undesirable Ecosystem Function of Orchard Vegetation. Fire 2023, 6, 25. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Peña, J.M.; Dorado, J. How weed management influence plant community composition, taxonomic diversity and crop yield: A long-term study in a Mediterranean vineyard. Agric. Ecosyst. Environ. 2022, 326, 107816. [Google Scholar] [CrossRef]
- Winkler, J.; Koda, E.; Červenková, J.; Děkanovský, I.; Nowysz, A.; Mazur, Ł.; Jakimiuk, A.; Vaverková, M.D. Green space in an extremely exposed part of the city center “Aorta of Warsaw”-Case study of the urban lawn. Urban Ecosyst. 2023, 26, 1225–1238. [Google Scholar] [CrossRef]
- Winkler, J.; Vaverková, M.D.; Havel, L. Anthropogenic life strategy of plants. Anthr. Rev. 2023, 10, 455–462. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.W.; Duchene, E.; Truong, T.T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple-choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Roby, J.P.; de Resseguier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Griesser, M.; Steiner, M.; Pingel, M.; Uzman, D.; Preda, C.; Giffard, B.; Tolle, P.; Memedemin, D.; Forneck, A.; Reineke, A.; et al. General trends of different inter-row vegetation management affecting vine vigor and grape quality across european 445 vineyards. Agric. Ecosyst. Environ. 2022, 338, 108073. [Google Scholar] [CrossRef]
- Boinot, S.; Fried, G.; Storkey, J.; Metcalfe, H.; Barkaoui, K.; Lauri, P.E.; Mézière, D. Alley cropping agroforestry systems: Reservoirs for weeds or refugia for plant diversity? Agric. Ecosyst. Environ. 2019, 284, 106584. [Google Scholar] [CrossRef]
- Mahaut, L.; Cheptou, P.O.; Fried, G.; Munoz, F.; Storkey, J.; Vasseur, F.; Violle, C.; Bretagnolle, F. Weeds: Against the Rules? Trends Plant Sci. 2020, 25, 1107–1116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).