Brewers’ Spent Grain as an Alternative Plant Protein Component of Honey Bee Feed
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Bee Feed
2.2. Proximate Analysis
2.3. Bees
2.4. Cage Test
2.5. Hemolymph Collecting and Analysis
2.6. Enzyme Activity Analysis
2.7. Non-Enzymatic Concentration of Selected Biochemical Markers in Bees’ Hemolymph
2.8. Data Evaluation
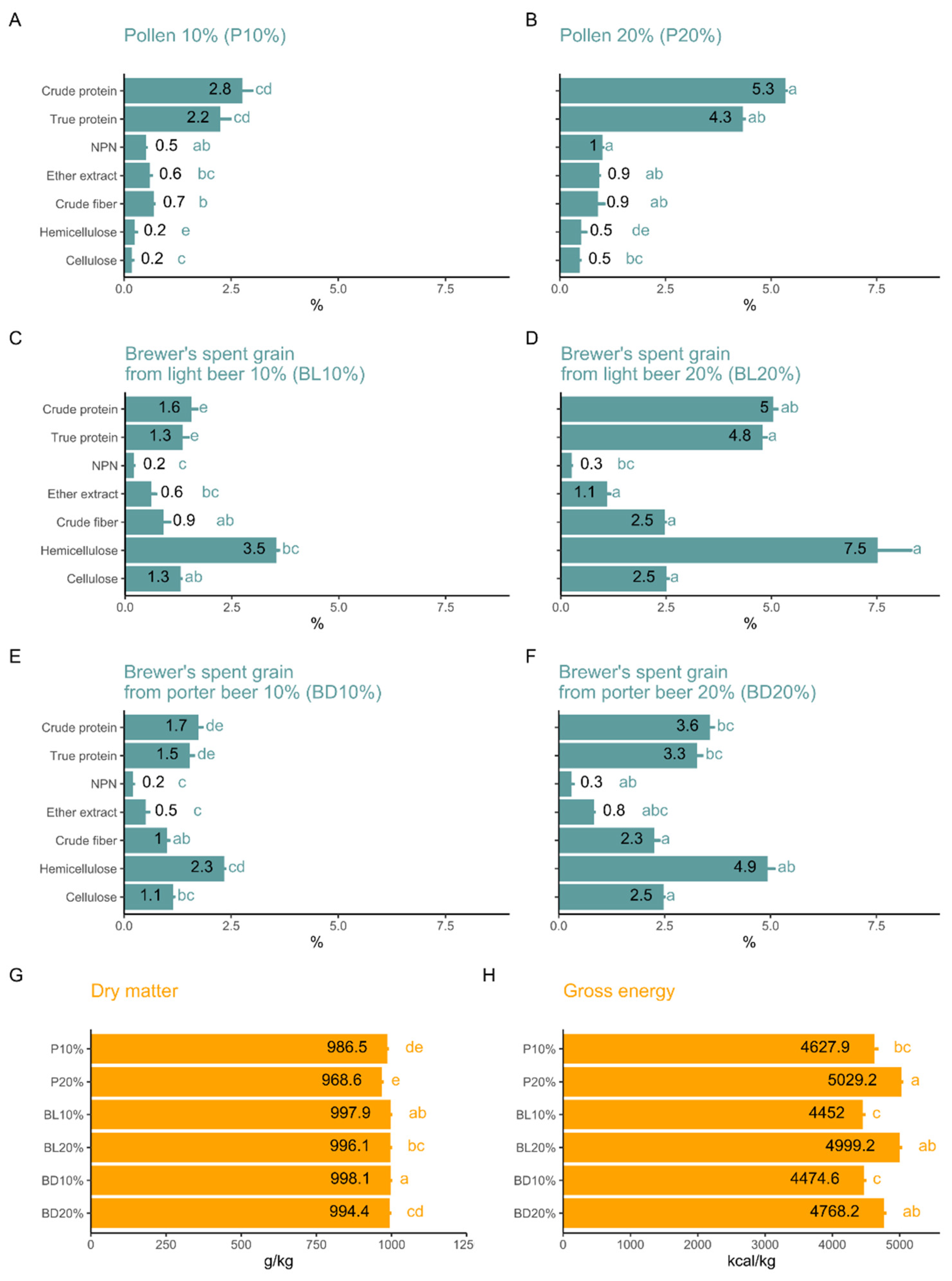
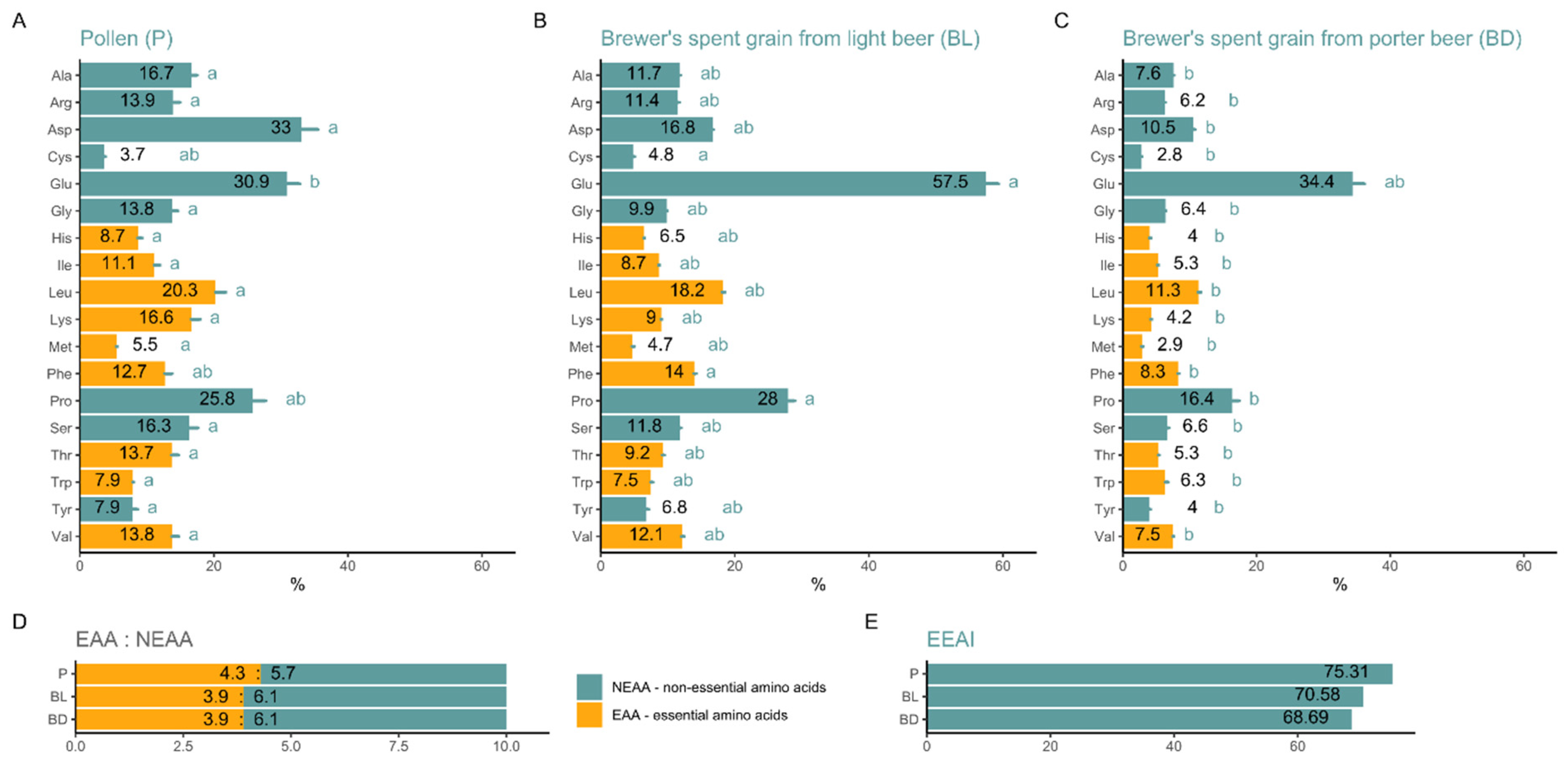
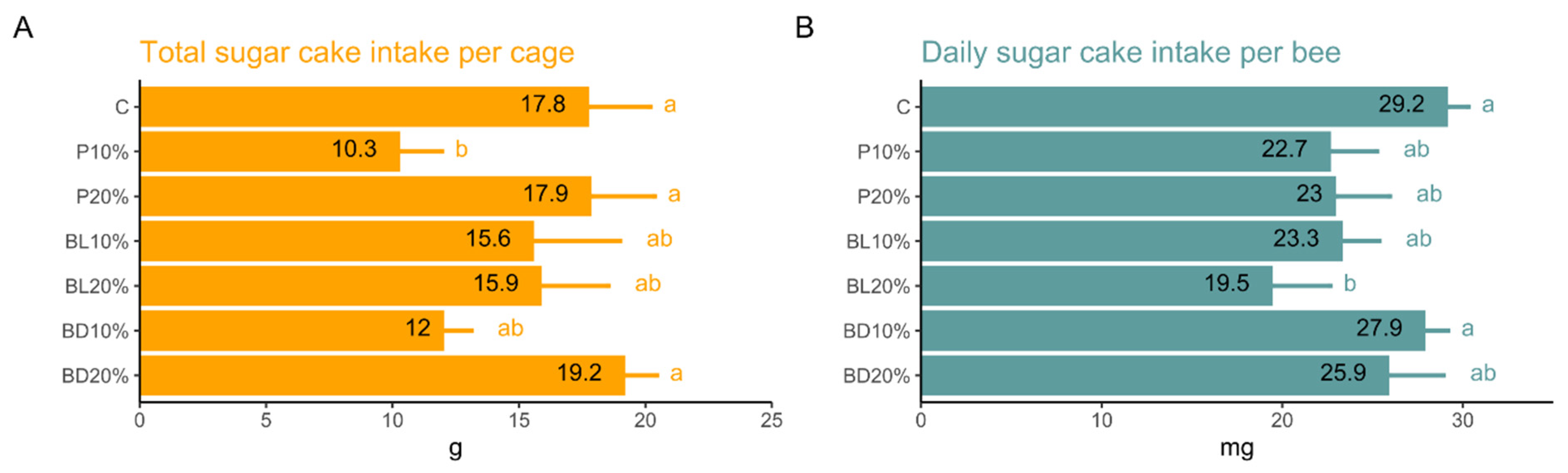
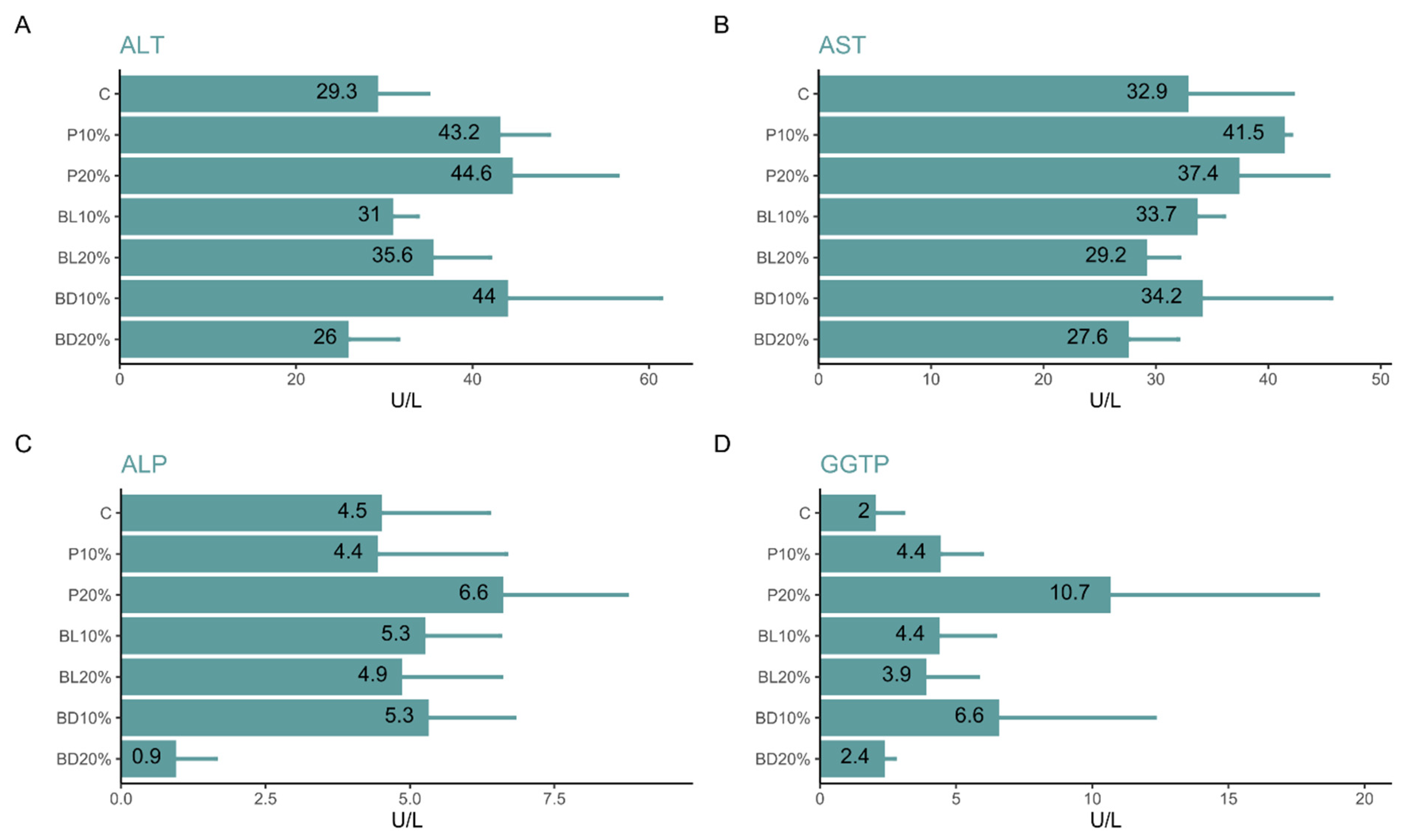
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey Bee Nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Apis mellifera L.). J. Econom. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.M.; Turcatto, A.P.; Francoy, T.M.; Gonçalves, L.S.; Cappelari, F.A.; De Jong, D. Evaluation of inexpensive pollen substitute diets through quantification of haemolymph proteins. J. Apic. Res. 2013, 52, 119–121. [Google Scholar] [CrossRef]
- De Jong, D.; da Silva, E.J.; Kevan, P.G.; Atkinson, J.L. Pollen substitutes increase honey bee haemolymph protein levels as much as or more than does pollen. J. Apic. Res. 2009, 48, 34–37. [Google Scholar] [CrossRef]
- Alqarni, A.S. Influence of some protein diets on the longevity and some physiological conditions of honeybee Apis mellifera L. workers. J. Biol. Sci. 2006, 6, 734–737. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. How honeybees (Apis mellifera L.) change their broodcare behaviour in response to non-foraging conditions and poor pollen conditions. Behav. Ecol. Sociobiol. 2002, 51, 415–425. [Google Scholar] [CrossRef]
- Walton, A.; Dolezal, A.G.; Bakken, M.A.; Toth, A.L. Hungry for the queen: Honeybee nutritional environment affects worker pheromone response in a life stage-dependent manner. Funct. Ecol. 2018, 32, 2699–2706. [Google Scholar] [CrossRef]
- Wright, G.A.; Lillvis, J.L.; Bray, H.J.; Mustard, J.A. Physiological State Influences the Social Interactions of Two Honeybee Nest Mates. PLoS ONE 2012, 7, e32677. [Google Scholar] [CrossRef]
- Ellis, A.M.; Hayes, G.W., Jr. An evaluation of fresh versus fermented diets for honey bees (Apis mellifera). J. Apic. Res. 2009, 48, 215–216. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. Cannibalism and early capping: Strategy of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol. A 2001, 187, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, T.; Crailsheim, K. Inner nest homeostasis in a changing environment with special emphasis on honey bee brood nursing and pollen supply. Apidologie 2004, 35, 249–263. [Google Scholar] [CrossRef]
- Prevéy, J.S. Climate change: Flowering time may be shifting in surprising ways. Curr. Biol. 2020, 30, R112–R114. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, A.; Olofsson, T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Abd El-Wahab, T.E.; Ghania, A.M.M.; Zidan, E.W. Assessment a new pollen supplement diet for honey bee colonies and their effects on some biological activities. Int. J. Agric. Technol. 2016, 12, 55–62. [Google Scholar]
- Dastouri, M.R.; Maheri-Sis, N.; Aghajanzadeh-Golshani, A.; Ebrahim-Nezhad, Y. The effect of replacement feeding of some protein sources with pollen on honey bee population and colony performance. J. Anim. Vet. Adv. 2007, 6, 1258–1261. [Google Scholar]
- Dodologlu, A.; Emsen, B. Effect of supplementary feeding on honey bee colony. J. Appl. Anim. Res. 2007, 32, 199–200. [Google Scholar] [CrossRef]
- Irandoust, H.; Ebadi, R. Nutritional effects of high protein feed on growth, development, Performance and overwintering of honey bee (Apis mellifera L.). Int. J. Adv. Biol. Biomed. Res. 2013, 1, 601–613. [Google Scholar]
- Kumari, I.; Kumar, R. Pollen Substitute Diet for Apis mellifera: Consumption and Effects on Colony Parameters in Sub-Tropical Himalaya. Indian J. Agric. Res. 2020, 54, 147–153. [Google Scholar] [CrossRef]
- Paray, B.A.; Kumari, I.; Hajam, Y.A.; Sharma, B.; Kumar, R.; Albeshr, M.F.; Farah, M.A.; Khan, J.M. Honeybee nutrition and pollen substitutes: A review. Saudi J. Biol. Sci. 2021, 28, 1167–1176. [Google Scholar] [CrossRef]
- Sihag, R.C.; Gupta, M. Development of an artificial pollen substitute/supplement diet to help tide the colonies of honeybee (Apis mellifera L.) over the dearth season. J. Apic. Sci. 2011, 55, 15–28. [Google Scholar]
- Blatt, J.; Roces, F. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): Dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J. Exp. Biol. 2001, 204 Pt 15, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K. Intestinal transport of sugars in the honeybee (Apis mellifera L.). J. Insect Physiol. 1988, 34, 839–845. [Google Scholar] [CrossRef]
- Guler, A.; Ekinci, D.; Biyik, S.; Garipoglu, A.V.; Onder, H.; Kocaokutgen, H. Effects of Feeding Honey Bees (Hymenoptera: Apidae) With Industrial Sugars Produced by Plants Using Different Photosynthetic Cycles (Carbon C3 and C4) on the Colony Wintering Ability, Lifespan, and Forage Behavior. J. Econ. Entomol. 2018, 111, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, B. Regulation of intermediary metabolism, with special reference to the control mechanisms in insect flight muscle. Adv. Insect Physiol. 1970, 7, 267–347. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Z.; Chen, X.; Hong, Z.; Lin, W.; Mu, X.; Hu, X.; Zheng, H. High-Fat Diets with Differential Fatty Acids Induce Obesity and Perturb Gut Microbiota in Honey Bee. Int. J. Mol. Sci. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed]
- Bajda, M.; Łoś, A.; Merska, M. Effect of amphotericin B on the biochemical markers in the haemolymph of honey bees. Med. Weter. 2014, 70, 766–769. [Google Scholar]
- Migdał, P.; Murawska, A.; Bieńkowski, P.; Strachecka, A.; Roman, A. Effect of E-field at frequency 50 Hz on protein, glucose, and triglycerides concentration in honeybee hemolymph. Eur. Zool. J. 2021, 88, 1170–1176. [Google Scholar] [CrossRef]
- Chang, H.; Ding, G.; Jia, G.; Feng, M.; Huang, J. Hemolymph Metabolism Analysis of Honey Bee (Apis mellifera L.) Response to Different Bee Pollens. Insects 2023, 14, 37. [Google Scholar] [CrossRef]
- Cremonz, T.M.; De Jong, D.; Bitondi, M.M.G. Quantification of Hemolymph Proteins as a Fast Method for Testing Protein Diets for Honey Bees (Hymenoptera: Apidae). J. Econom. Entomol. 1998, 91, 1284–1289. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC, 18th ed.; 4th Revision; AOAC: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Amino Acid Nutrition; Albanese, A.A., Ed.; Academic Press: New York, NY, USA, 1959; pp. 295–311. [Google Scholar]
- Migdał, P.; Murawska, A.; Roman, A. A Modified Standardized Method to Extract and Store Insect Hemolymph with Use of a Glass Capillary. J. Apic. Sci. 2020, 64, 165–168. [Google Scholar] [CrossRef]
- Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Honey bees and their brood: A potentially valuable resource of food, worthy of greater appreciation and scientific attention. J. Ecol. Environ. 2021, 45, 31. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Berbeć, E.; Plotnik, M.; Skorus, A.; Latarowski, K. Selected Biochemical Markers Change after Oral Administration of Pesticide Mixtures in Honey Bees. Toxics 2022, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Şapcaliu, A.; Pavel, C.; Savu, V.; Cãuia, E.; Matei, M.; Rãdoi, I. Biochemical and cytological investigations on haemolymph of Apis mellifera carpathica bee in stressful conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj. Napoca. 2010, 67, 313–320. [Google Scholar]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Cannabis extract has a positive–immunostimulating effect through proteolytic system and metabolic compounds of honey bee (Apis mellifera) workers. Animals 2021, 11, 2190. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Jouni, Z.E.; Zamora, J.; Wells, M.A. Absorption and tissue distribution of cholesterol in Manduca sexta. Arch. Insect Biochem. Physiol. 2002, 49, 167–175. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “blood” and body surface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Zirbes, L.; Nguyen, B.K.; de Graaf, D.C.; De Meulenaer, B.; Reybroeck, W.; Haubruge, E.; Saegerman, C. Hydroxymethylfurfural: A Possible Emergent Cause of Honey Bee Mortality? J. Agric. Food Chem. 2013, 61, 11865–11870. [Google Scholar] [CrossRef] [PubMed]
- Krainer, S.; Brodschneider, R.; Vollmann, J.; Crailsheim, K.; Riessberger-Gallé, U. Effect of hydroxymethylfurfural (HMF) on mortality of artificially reared honey bee larvae (Apis mellifera carnica). Ecotoxicology 2015, 25, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Henle, T. Maillard Reaction Products in Different Types of Brewing Malt. J. Agric. Food Chem. 2020, 68, 1427–14285. [Google Scholar] [CrossRef]
- Gregorc, A.; Jurišić, S.; Sampson, B. Hydroxymethylfurfural Affects Caged Honey Bees (Apis mellifera carnica). Diversity 2020, 12, 18. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migdał, P.; Wilk, M.; Berbeć, E.; Białecka, N. Brewers’ Spent Grain as an Alternative Plant Protein Component of Honey Bee Feed. Agriculture 2024, 14, 929. https://doi.org/10.3390/agriculture14060929
Migdał P, Wilk M, Berbeć E, Białecka N. Brewers’ Spent Grain as an Alternative Plant Protein Component of Honey Bee Feed. Agriculture. 2024; 14(6):929. https://doi.org/10.3390/agriculture14060929
Chicago/Turabian StyleMigdał, Paweł, Martyna Wilk, Ewelina Berbeć, and Natalia Białecka. 2024. "Brewers’ Spent Grain as an Alternative Plant Protein Component of Honey Bee Feed" Agriculture 14, no. 6: 929. https://doi.org/10.3390/agriculture14060929
APA StyleMigdał, P., Wilk, M., Berbeć, E., & Białecka, N. (2024). Brewers’ Spent Grain as an Alternative Plant Protein Component of Honey Bee Feed. Agriculture, 14(6), 929. https://doi.org/10.3390/agriculture14060929





