Genetic Characterization of Gardenia jasminoides Ellis Genotypes Derived from Seeds and Selection Based on Their Morphological Traits and Flower Aromatic Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Genetic Assessment
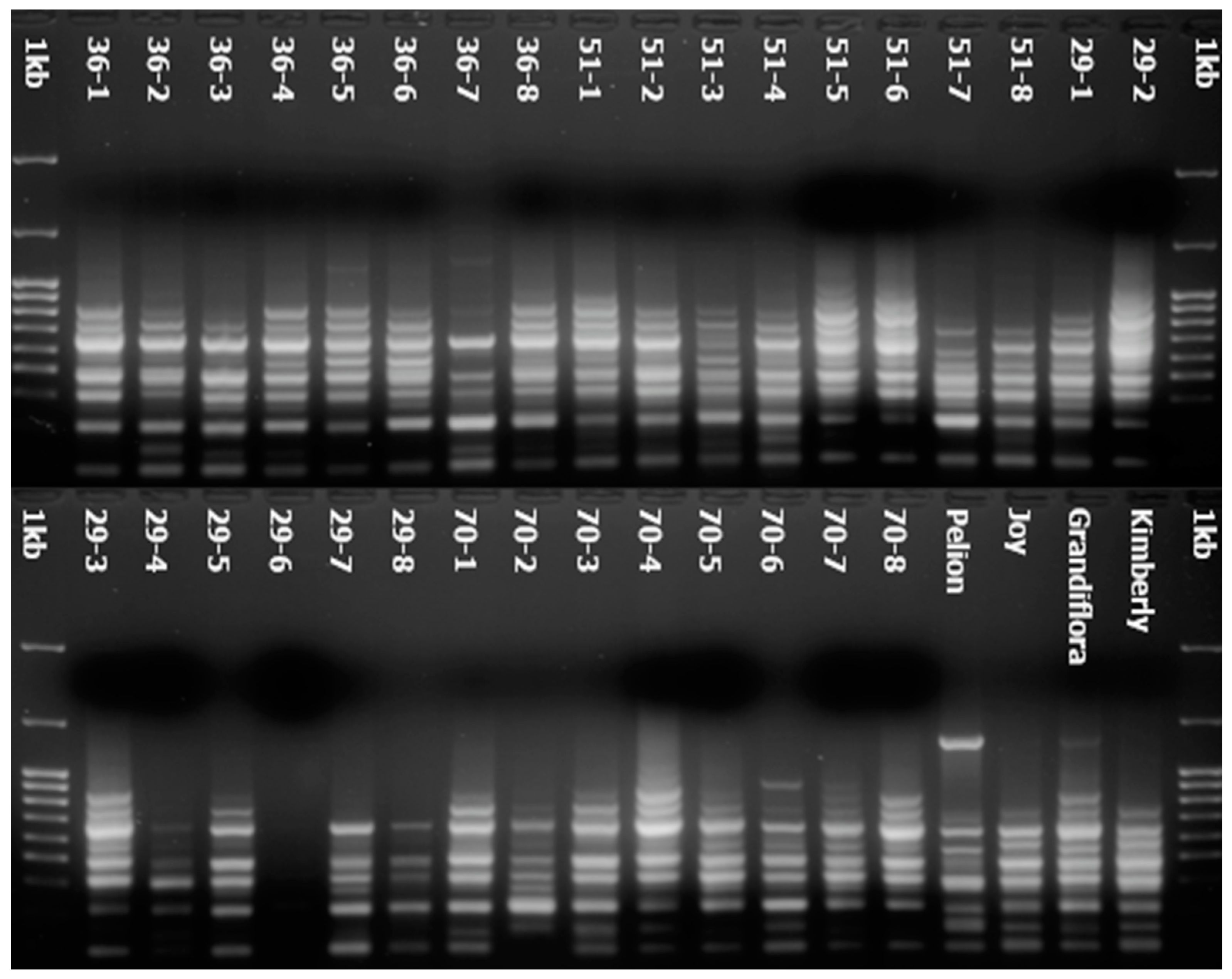
2.2.1. DNA Extraction
2.2.2. Molecular Markers and PCR Amplification
2.2.3. Data Analysis
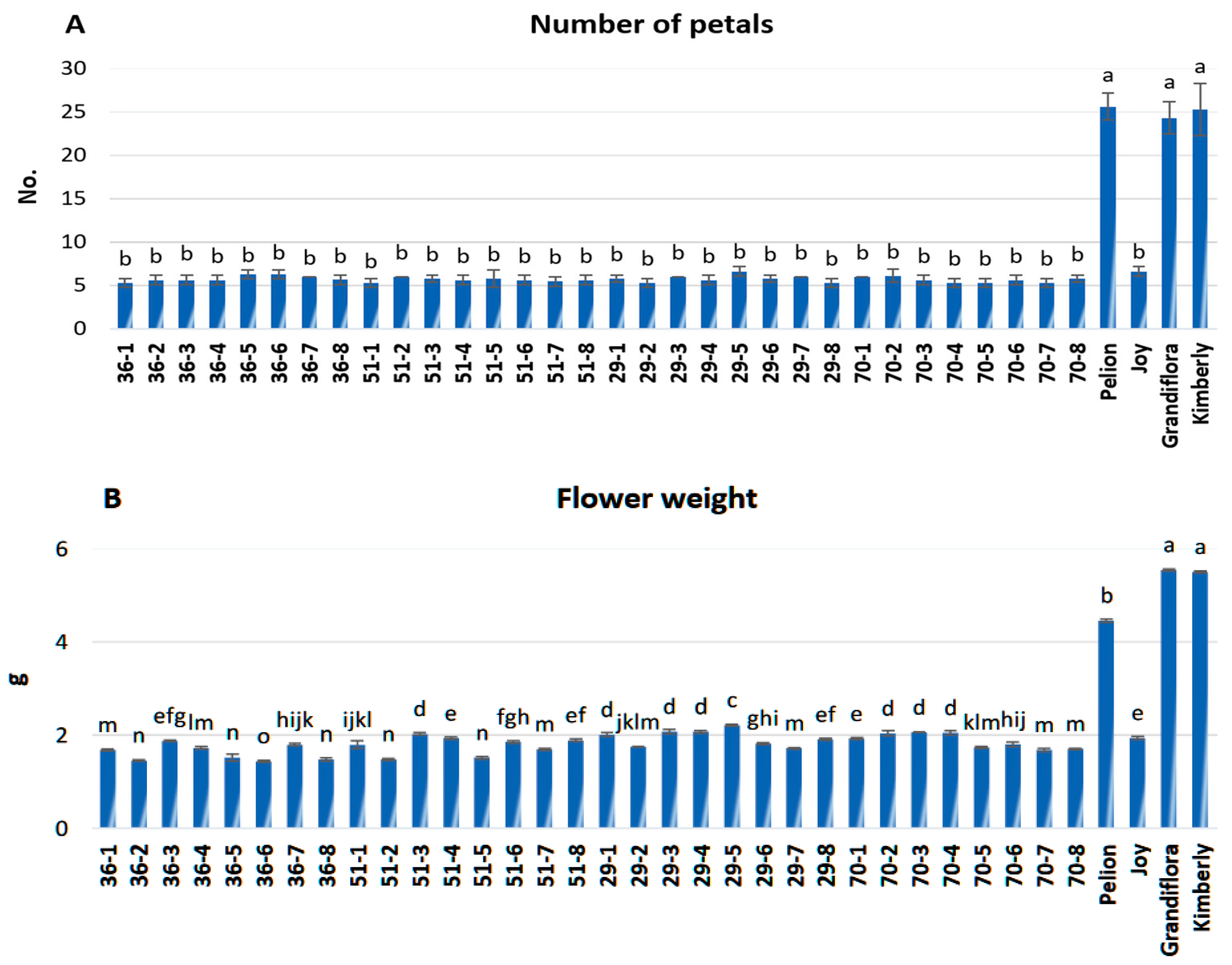
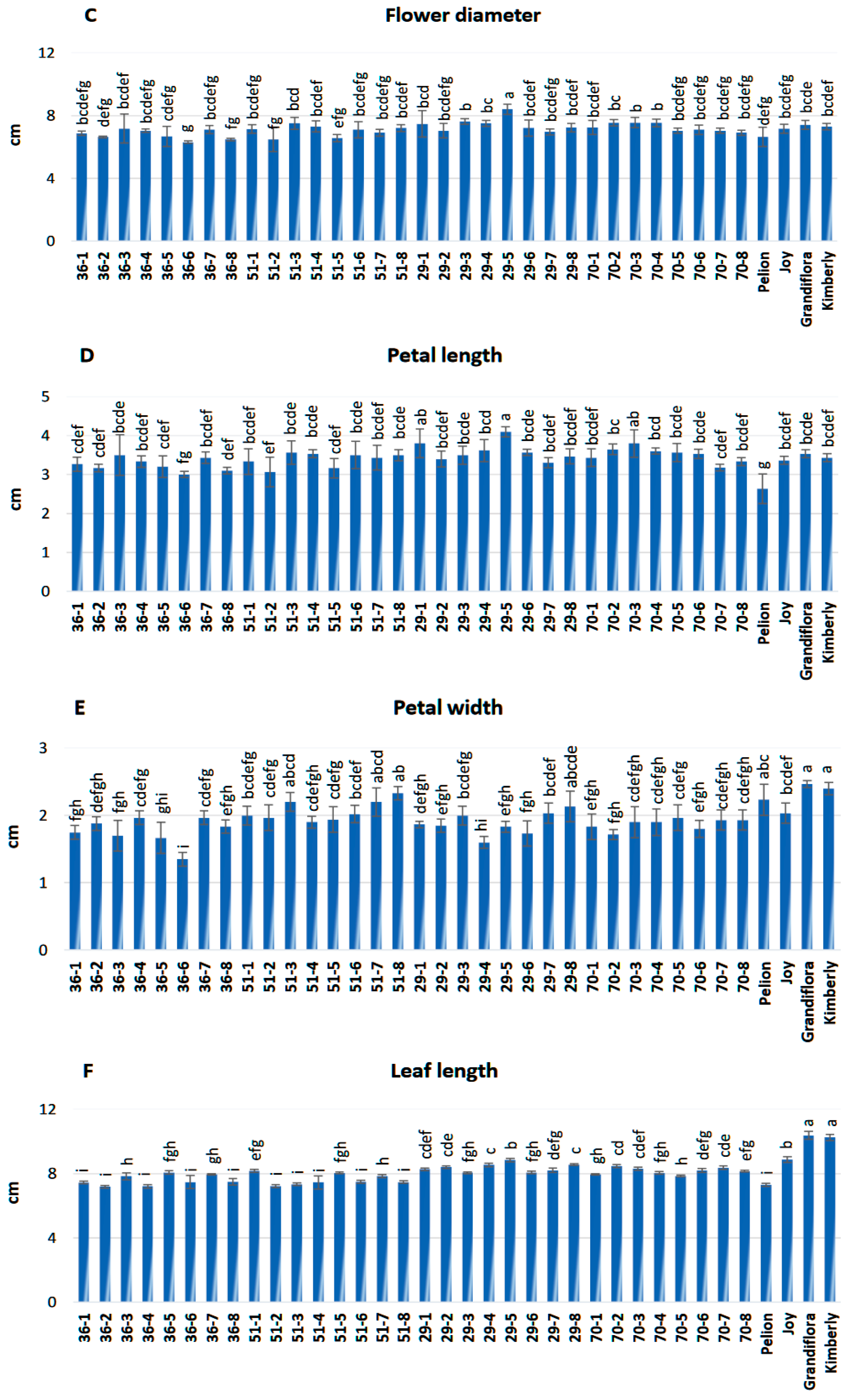
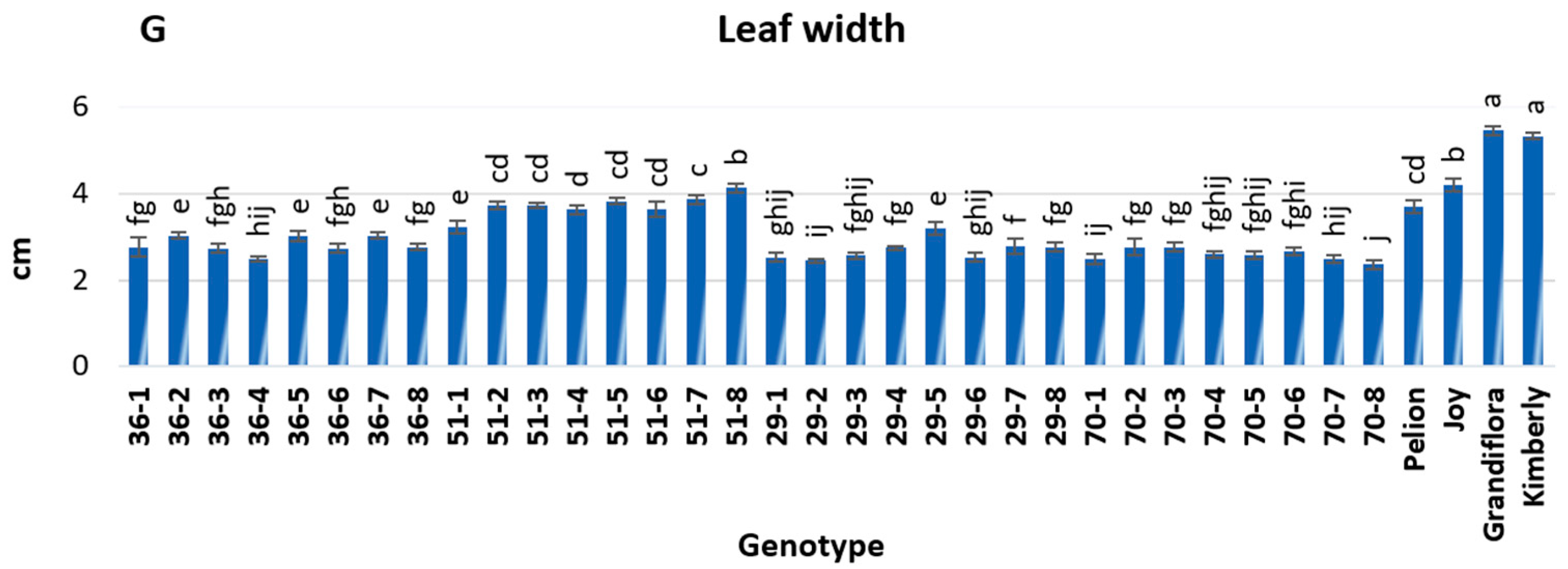
2.3. Morphological Traits
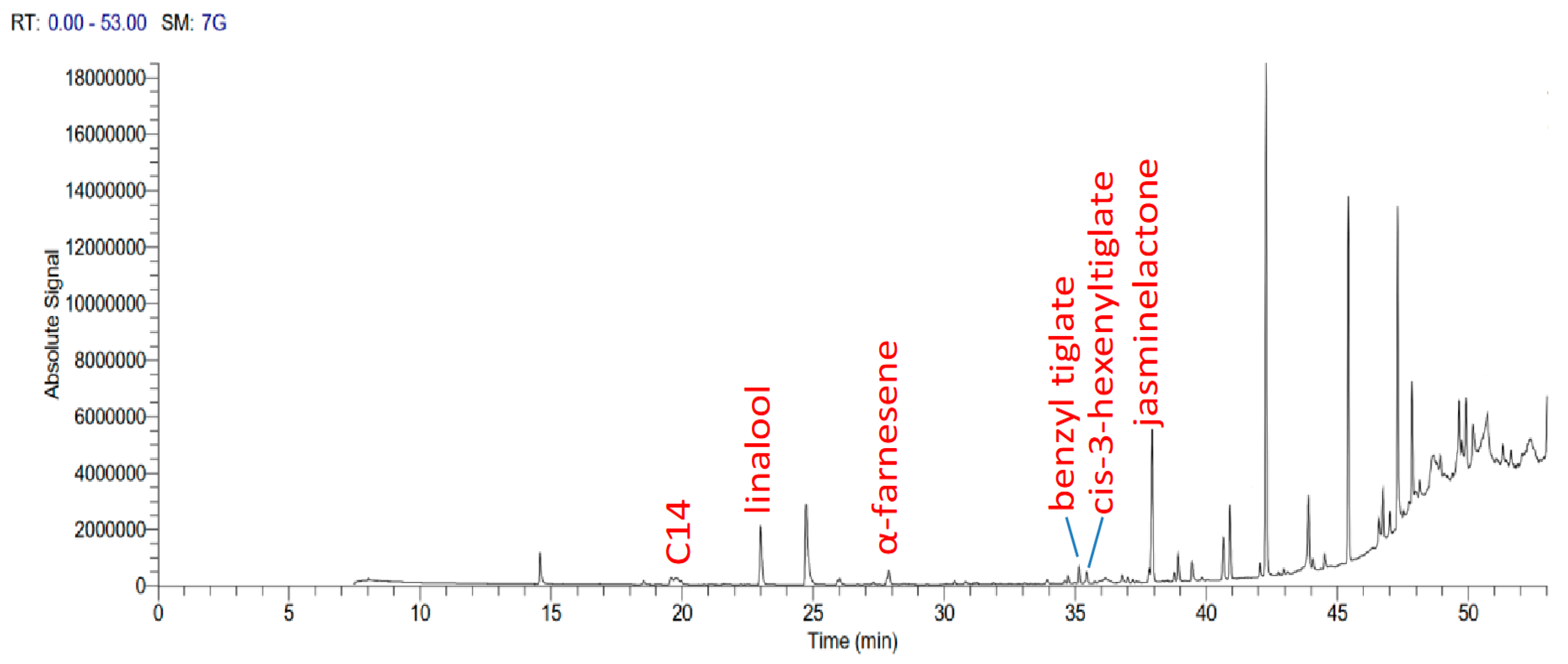
2.4. Extraction and GC-MS Analysis of Volatile Compounds from Flowers
2.5. Statistical Analysis
3. Results and Discussion
3.1. Genetic Analysis
3.1.1. ISSR and SCoT Polymorphism
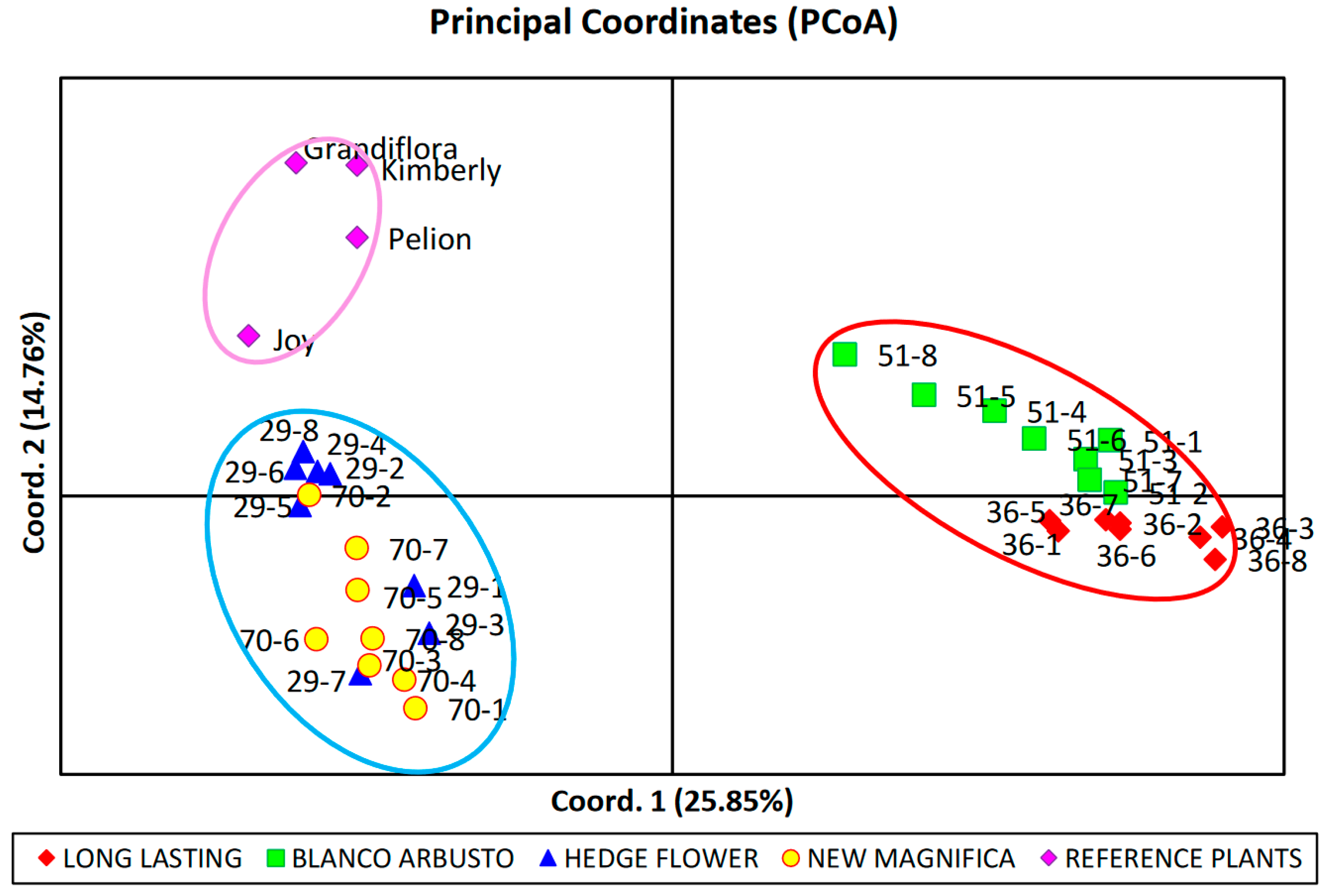
3.1.2. Principal Coordinate Analysis (PCoA)
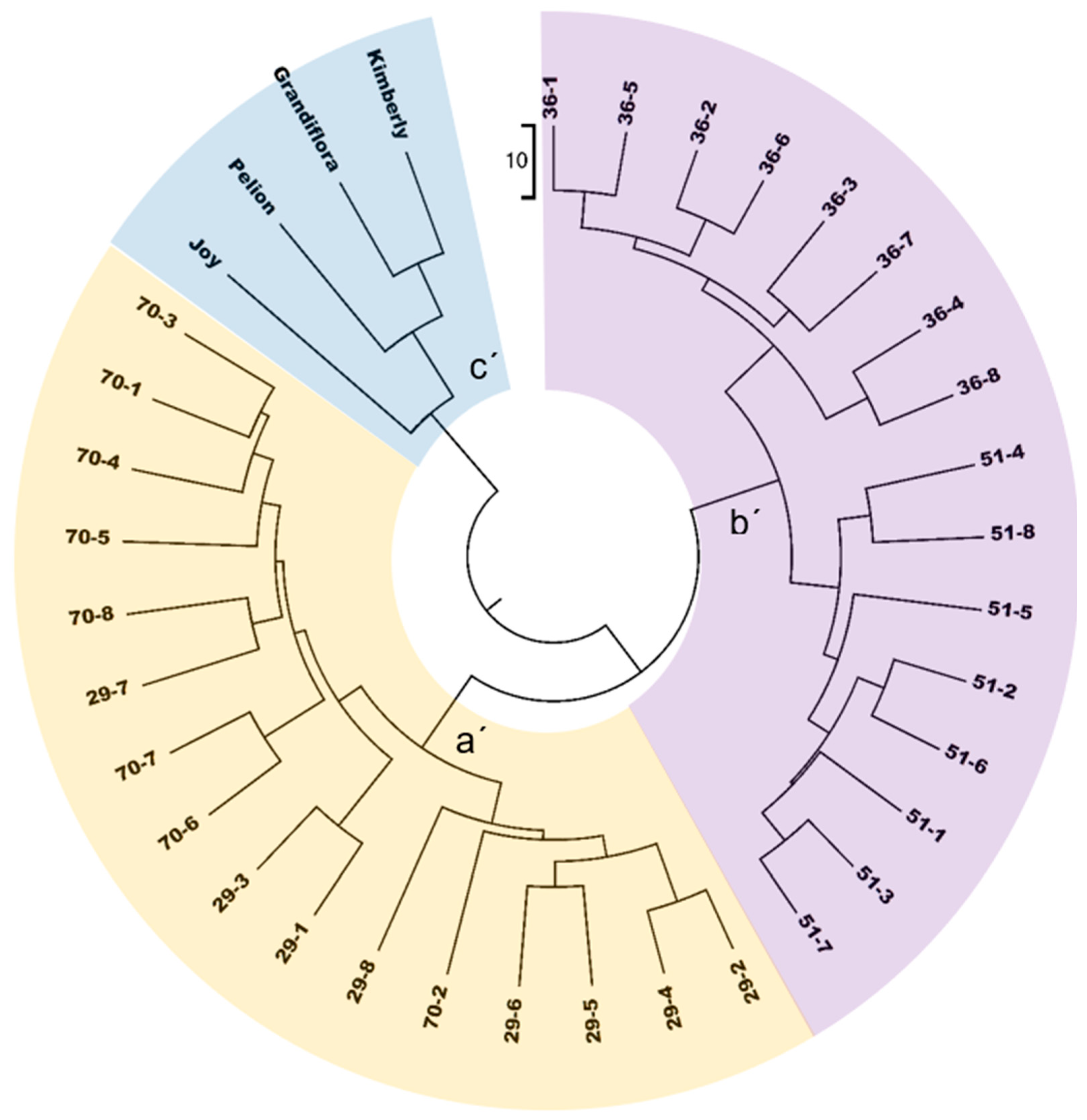
3.1.3. Unweighted Pair Group Method with Arithmetic Mean (UPGMA)
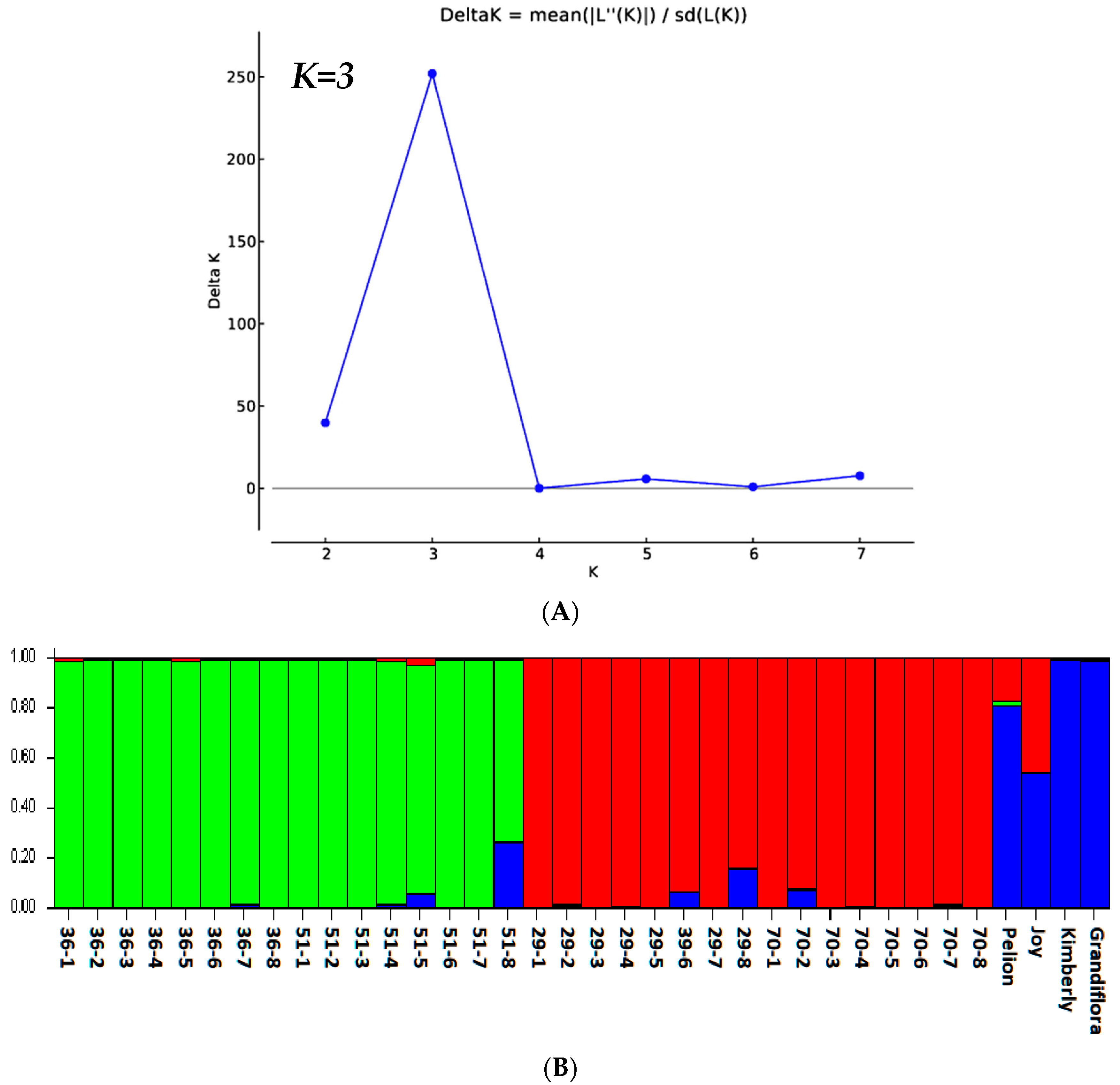
3.1.4. STRUCTURE Analysis
3.2. Evaluation of Morphological Traits
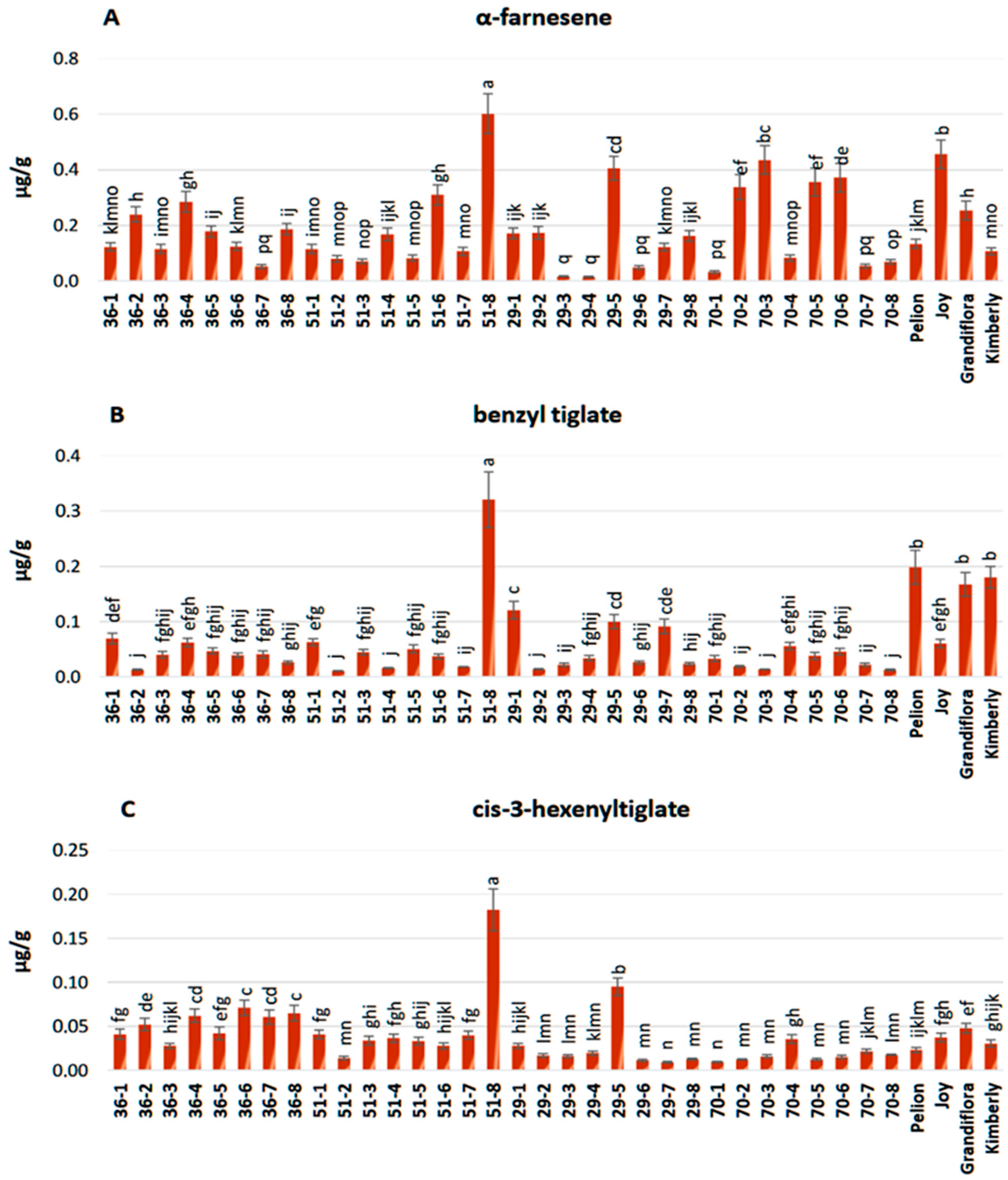
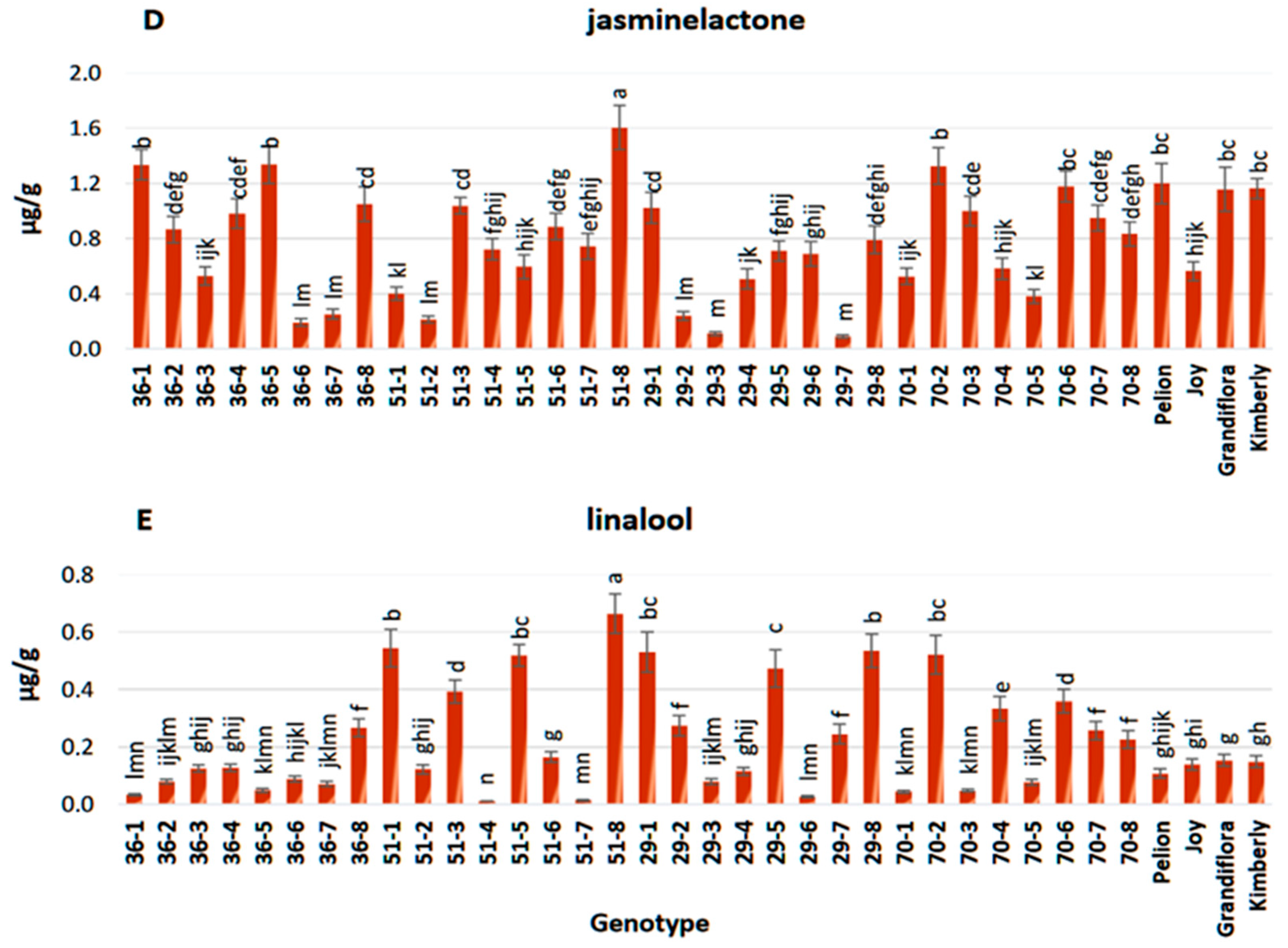
3.3. Chemical Composition of Flower Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarvis, C.E.; DuVal, A.; Crane, P.R. GARDENIA JASMINOIDES: A Traditional Chinese Dye Plant Becomes A Garden Ornamental in Europe. Curtis’s Bot. Mag. 2014, 31, 80–98. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Tsanakas, G.F.; Polidoros, A.; Economou, A. Genetic variation in gardenia grown as pot plant in Greece. Sci. Hortic. 2013, 162, 213–217. [Google Scholar] [CrossRef]
- Lim, T.K. Gardenia jasminoides. In Edible Medicinal and Non Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 8, pp. 705–729. [Google Scholar] [CrossRef]
- Çelikel, F.G.; Reid, M.S.; Jiang, C.-Z. Postharvest physiology of cut Gardenia jasminoides flowers. Sci. Hortic. 2020, 261, 108983. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, R.; Meng, J.; Li, Z.; Wu, Y.; Tao, J. Ameliorative effects of melatonin on dark-induced leaf senescence in gardenia (Gardenia jasminoides Ellis): Leaf morphology, anatomy, physiology and transcriptome. Sci. Rep. 2017, 7, 10423. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Xian, X.; Lin, X.; Pang, Z.; Liu, J.; He, S. Nano-silver pretreatment delays wilting of cut gardenia foliage by inhibiting bacterial xylem blockage. Sci. Hortic. 2019, 246, 791–796. [Google Scholar] [CrossRef]
- Huh, M.-K. Genetic Relationships between Gardenia jasminoides var. radicans and G. jasminoides for. grandiflora Using ISSR Markers. J. Life Sci. 2007, 17, 24–30. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Nendou, T.; Economou, A. Conservation, Regeneration and Genetic Stability of Regenerants from Alginate-Encapsulated Shoot Explants of Gardenia jasminoides Ellis. Polymers 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, R.; Zhang, B.; Jiang, T.; Li, N.; Qian, K.; Liu, B.; Zhang, J. Epigenetic instability in genetically stable micropropagated plants of Gardenia jasminoides Ellis. Plant Growth Regul. 2012, 66, 137–143. [Google Scholar] [CrossRef]
- Mei, Z.; Khan, M.A.; Yang, L.; Yang, M.; Fu, J. Genetic characterization and authentication of Gardenia jasminoides in different regions of China by using improved RAPD analysis. Ind. J. Exp. Biol. 2015, 53, 164–169. [Google Scholar]
- Mei, Z.; Zhou, B.; Wei, C.; Cheng, J.; Imani, S.; Chen, H.; Fu, J. Genetic Authentication of Gardenia jasminoides Ellis var. grandiflora Nakai by Improved RAPD-Derived DNA Markers. Molecules 2015, 20, 20219–20229. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Sharma, V.; Sureja, A.K.; Das Munshi, A.; Arya, L.; Saha, D.; Verma, M. Genetic diversity and population structure detection in sponge gourd (Luffa cylindrica) using ISSR, SCoT and morphological markers. Physiol. Mol. Biol. Plants 2020, 26, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.W.; Gao, M.R.; Huang, S.Y.; Zhang, S.W.; Zhang, X.J.; Li, T.; Yu, W.H.; Meng, P.; Shi, Q. Genetic Diversity and DNA Fingerprinting of Dendrobium Officinale Based On ISSR and Scot Markers. Appl. Ecol. Environ. Res. 2023, 21, 421–438. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Gorji, A.M.; Poczai, P.; Polgar, Z.; Taller, J. Efficiency of Arbitrarily Amplified Dominant Markers (SCOT, ISSR and RAPD) for Diagnostic Fingerprinting in Tetraploid Potato. Am. J. Potato Res. 2011, 88, 226–237. [Google Scholar] [CrossRef]
- Mulpuri, S.; Muddanuru, T.; Francis, G. Start codon targeted (SCoT) polymorphism in toxic and non-toxic accessions of Jatropha curcas L. and development of a codominant SCAR marker. Plant Sci. 2013, 207, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.K.; Sabana, A.A.; Rachana, K.E.; Rahman, S.; Jerard, B.A.; Karun, A. Genetic relationship and diversity among coconut (Cocos nucifera L.) accessions revealed through SCoT analysis. 3 Biotech 2015, 5, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, H.; Zarei, A.; Erfani-Moghadam, J. Phylogeny relationship among commercial and wild pear species based on morphological characteristics and SCoT molecular markers. Sci. Hortic. 2018, 235, 323–333. [Google Scholar] [CrossRef]
- Etminan, A.; Pour-Aboughadareh, A.; Noori, A.; Ahmadi-Rad, A.; Shooshtari, L.; Mahdavian, Z.; Yousefiazar-Khanian, M. Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol. Biotechnol. Equip. 2018, 32, 610–617. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Haq, S.U.; Jatav, P.K.; Kothari, S.; Kachhwaha, S. Assessment of genetic diversity in 29 rose germplasms using SCoT marker. J. King Saud Univ.-Sci. 2019, 31, 780–788. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.-A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable Exploitation of Greek Rosmarinus officinalis L. Populations for Ornamental Use through Propagation by Shoot Cuttings and In Vitro Cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Vasileiadis, A.; Magklaras, P.; Smyrnioudis, I.; Vasilakis, T.; Chazakis, M.; Anastasiadi, V.; Ziogou, F.-T.; et al. Propagation of Pistacia lentiscus var. Chia Genotypes and Determination of Their Ornamental Traits Combined with a Genetic Analysis Using ISSR Markers. Agronomy 2021, 11, 205. [Google Scholar] [CrossRef]
- Watanabe, N.; Nakajima, R.; Watanabe, S.; Moon, J.-H.; Inagaki, J.; Sakata, K.; Yagi, A.; Ina, K. Linalyl and bornyl disaccharide glycosides from Gardenia jasminoides flowers. Phytochemistry 1994, 37, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Pimenta, L.E.N.; Rideout, J.A. Iridoids from Gardenia jasminoides. Nat. Prod. Res. 2007, 21, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-L.; Wang, R.; Shi, Y.-P.; Qi, H.-Y. Iridoids from the flowers of Gardenia jasminoides Ellis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2014, 56, 267–270. [Google Scholar] [CrossRef]
- Song, J.-L.; Yang, Y.-J.; Qi, H.-Y.; Li, Q. Chemical constituents from flowers of Gardenia jasminoides. J. Chin. Med. Mat. 2013, 36, 752–755. [Google Scholar]
- Yu, R.; Li, Y.; Si, D.; Yan, S.; Liu, J.; Si, J.; Zhang, X. Identification, quantitative and bioactivity analyses of aroma and alcohol-soluble components in flowers of Gardenia jasminoides and its variety during different drying processes. Food Chem. 2023, 420, 135846. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.; Muraki, S.; Yoshida, T. Chemical Composition of the Absolute from Gardenia Flower. Agric. Biol. Chem. 1978, 42, 1351–1356. [Google Scholar] [CrossRef]
- Liu, B.Z.; Gao, Y. Analysis of headspace constituents of Gardenia flower by GC/MS with solid-phase microextraction and dynamic headspace sampling. Chin. J. Chromat. 2000, 18, 452–455. [Google Scholar]
- Ishikawa, M.; Honda, T.; Fujita, A.; Kurobayashi, Y.; Kitahara, T. “Aqua-space®”, a New Headspace Method for Isolation of Natural Floral Aromas Using Humidified Air as a Carrier Gas. Biosci. Biotechnol. Biochem. 2004, 68, 454–457. [Google Scholar] [CrossRef]
- Chaichana, J.; Niwatananun, W.; Vejabhikul, S.; Somna, S.; Chansakaow, S. Volatile constituents and biological activities of Gardenia jasminoides. J. Health Res. 2009, 23, 141–145. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N. Volatile profile and sensory property of Gardenia jasminoides aroma extracts. J. Cosmet. Sci. 2015, 66, 371–377. [Google Scholar]
- Zhang, N.; Luo, M.; He, L.; Yao, L. Chemical Composition of Essential Oil from Flower of ‘Shanzhizi’ (Gardenia jasminoides Ellis) and Involvement of Serotonergic System in Its Anxiolytic Effect. Molecules 2020, 25, 4702. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Tsaktsira, M.; Chavale, E.; Kostas, S.; Pipinis, E.; Tsoulpha, P.; Hatzilazarou, S.; Ziogou, F.-T.; Nianiou-Obeidat, I.; Iliev, I.; Economou, A.; et al. Vegetative Propagation and ISSR-Based Genetic Identification of Genotypes of Ilex aquifolium ‘Agrifoglio Commune’. Sustainability 2021, 13, 10345. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Wen, X.; Falush, D. Documentation for Structure Software: Version 2.3; University of Chicago: Chicago, IL, USA, 2010; p. 38. [Google Scholar]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Rusanov, K.E.; Kovacheva, N.M.; Atanassov, I.I. Comparative GC/MS Analysis of Rose Flower and Distilled Oil Volatiles of The Oil Bearing Rose Rosa Damascena. Biotechnol. Biotechnol. Equip. 2011, 25, 2210–2216. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Dehghan, E. Efficacy of SCoT and ISSR markers in assessment of genetic diversity in some Iranian pistachio (Pistacia vera L.) cultivars. Pist. Health J. 2018, 1, 37–43. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ciftci, V. Genetic relationships and diversity analysis in Turkish laurel (Laurus nobilis L.) germplasm using ISSR and SCoT markers. Mol. Biol. Rep. 2021, 48, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Mansoory, A.; Khademi, O.; Naji, A.M.; Rohollahi, I.; Sepahvand, E. Evaluation of Genetic Diversity in Three Diospyros Species, Collected from Different Regions in Iran, Using ISSR and SCoT Molecular Markers. Int. J. Fruit Sci. 2022, 22, 235–248. [Google Scholar] [CrossRef]
- Ziogou, F.-T.; Kotoula, A.-A.; Hatzilazarou, S.; Papadakis, E.-N.; Avramis, P.-G.; Economou, A.; Kostas, S. Genetic Assessment, Propagation and Chemical Analysis of Flowers of Rosa damascena Mill. Genotypes Cultivated in Greece. Horticulturae 2023, 9, 946. [Google Scholar] [CrossRef]
- González, J.M.; Galindo, Y.N.; Chávez, M.d.R.C.; Hernández, K.P.B.; Valencia, M.G.; Corredor, J.A.H. Effect of open field crop management on the floral development of gardenia (Gardenia jasminoides Ellis). Agro Prod. 2022, 15, 81–90. [Google Scholar] [CrossRef]
- Mousa, G.T.; Abdul-Hafeez, E.Y.; Ibrahim, O.H.M. Response of gardenia plants grown under various growth media and ferrous sulfate application. Pak. J. Agric. Sci. 2015, 52, 651–658. [Google Scholar]
- Shahin, S.M.; Khedr, H.M.; Ahmed, M.; Mohamed, R. Effect of some natural extracts on growth and flowering of Gardenia jasminoides J. Ellis plant. Egypt. J. Agric. Res. 2020, 98, 580–589. [Google Scholar] [CrossRef]


| Primer | Sequence (5′→3′) | Ta (°C) |
|---|---|---|
| UBC 808 | AGAGAGAGAGAGAGAGC | 58 |
| UBC 809 | AGAGAGAGAGAGAGAGG | 58 |
| UBC 810 | GAGAGAGAGAGAGAGAT | 52 |
| UBC 811 | GAGAGAGAGAGAGAGAC | 54 |
| UBC 815 | CTCTCTCTCTCTCTCTG | 50 |
| UBC 818 | CACACACACACACACAG | 56 |
| UBC 821 | GTGTGTGTGTGTGTGTT | 56 |
| UBC 834 | AGAGAGAGAGAGAGAGYT | 54 |
| UBC 841 | GAGAGAGAGAGAGAGAYC | 52 |
| UBC 888 | CACACACACACACABDB | 50 |
| SCoT 313 | ACGACATGGCGACCATCG | 50 |
| SCoT 333 | CCATGGCTACCACCGCAG | 50 |
| Morphological Trait | Description |
|---|---|
| 1. Flower form | Single or double |
| 2. Number of petals | Number of expanded petals per flower |
| 3. Flower weight | In g, using six replications per genotype |
| 4. Flower diameter | In cm, measured at the widest part of fully opened flowers |
| 5. Petal length | In cm, measured from the base of the claw to the tip of the blade |
| 6. Petal width | In cm, measured at the widest part of the blade |
| 7. Leaf length | In cm, measured from the end of the petiole to the top of the lamina |
| 8. Leaf width | In cm, measured at the widest part of the lamina |
| Primer | Size Range (bp) | NAB | NPB | PPB (%) |
|---|---|---|---|---|
| UBC 808 | 270–850 | 12 | 12 | 100 |
| UBC 809 | 300–1500 | 20 | 20 | 100 |
| UBC 810 | 300–1500 | 21 | 21 | 100 |
| UBC 811 | 200–1350 | 17 | 17 | 100 |
| UBC 815 | 250–1600 | 19 | 19 | 100 |
| UBC 818 | 200–1350 | 20 | 20 | 100 |
| UBC 821 | 380–1350 | 13 | 13 | 100 |
| UBC 834 | 200–1450 | 22 | 22 | 100 |
| UBC 841 | 150–1000 | 18 | 18 | 100 |
| UBC 888 | 200–1250 | 20 | 20 | 100 |
| SCoT 313 | 200–1500 | 19 | 19 | 100 |
| SCoT 333 | 450–1700 | 17 | 16 | 94.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadi, V.; Hatzilazarou, S.; Papadakis, E.-N.; Kotoula, A.-A.; Tsalouchos, N.; Labis, T.; Economou, A.; Kostas, S. Genetic Characterization of Gardenia jasminoides Ellis Genotypes Derived from Seeds and Selection Based on Their Morphological Traits and Flower Aromatic Substances. Agriculture 2024, 14, 650. https://doi.org/10.3390/agriculture14050650
Anastasiadi V, Hatzilazarou S, Papadakis E-N, Kotoula A-A, Tsalouchos N, Labis T, Economou A, Kostas S. Genetic Characterization of Gardenia jasminoides Ellis Genotypes Derived from Seeds and Selection Based on Their Morphological Traits and Flower Aromatic Substances. Agriculture. 2024; 14(5):650. https://doi.org/10.3390/agriculture14050650
Chicago/Turabian StyleAnastasiadi, Vasiliki, Stefanos Hatzilazarou, Emmanouil-Nikolaos Papadakis, Aikaterini-Angeliki Kotoula, Nikolaos Tsalouchos, Taxiarchis Labis, Athanasios Economou, and Stefanos Kostas. 2024. "Genetic Characterization of Gardenia jasminoides Ellis Genotypes Derived from Seeds and Selection Based on Their Morphological Traits and Flower Aromatic Substances" Agriculture 14, no. 5: 650. https://doi.org/10.3390/agriculture14050650
APA StyleAnastasiadi, V., Hatzilazarou, S., Papadakis, E.-N., Kotoula, A.-A., Tsalouchos, N., Labis, T., Economou, A., & Kostas, S. (2024). Genetic Characterization of Gardenia jasminoides Ellis Genotypes Derived from Seeds and Selection Based on Their Morphological Traits and Flower Aromatic Substances. Agriculture, 14(5), 650. https://doi.org/10.3390/agriculture14050650







