Next-Generation Biofertilizers: Nanoparticle-Coated Plant Growth-Promoting Bacteria Biofertilizers for Enhancing Nutrient Uptake and Wheat Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Traits of PGPB Isolates
2.2. Preparation of Nanoparticles
2.2.1. Mesoporous Silica Nanoparticles
2.2.2. Zinc Oxide Nanoparticles
2.2.3. Copper Oxide Nanoparticles
2.3. Nanoencapsulation of PGPB Isolates
2.3.1. Physical Adherence of Bacteria to the NPs
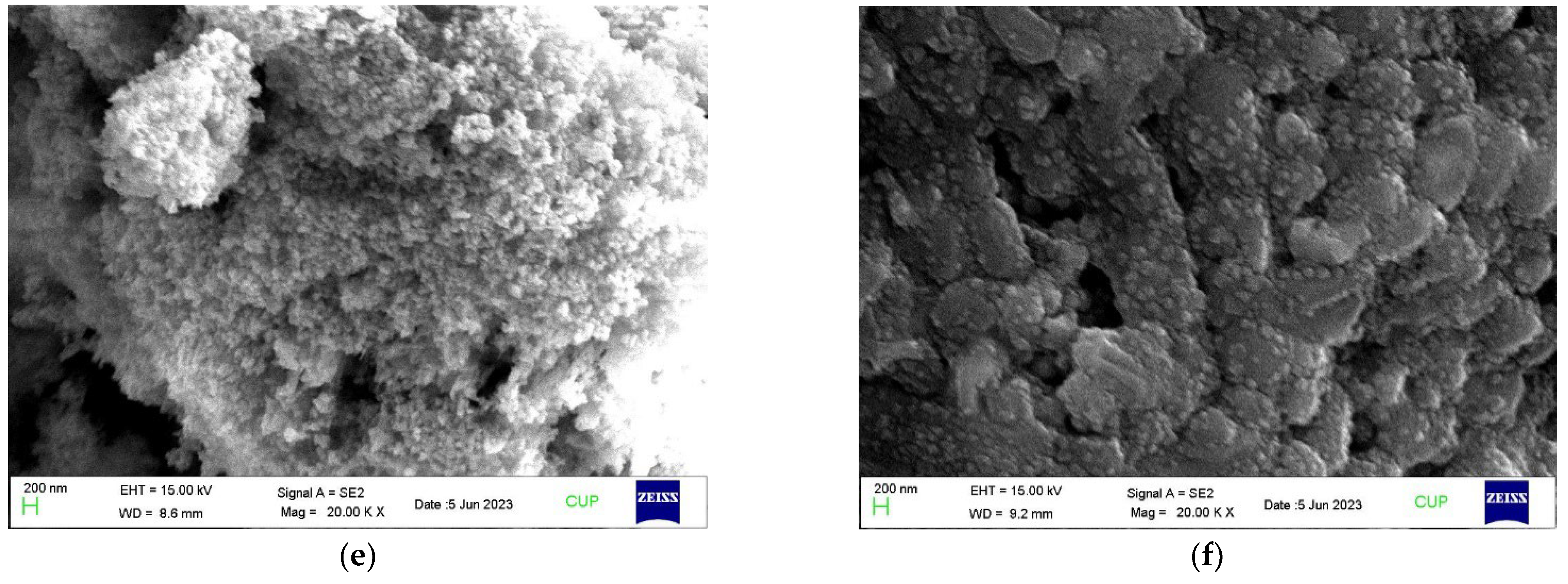
2.3.2. SEM Investigation of the Nanobiofertilizers
2.3.3. Viability of Nanoencapsulation
2.4. Crop Establishment and Treatment Application
2.5. Observed Responses and Measurement Methods
2.5.1. Agronomic and Physiological Traits
2.5.2. Nutrient Uptake
2.5.3. Soil Chemical Properties
2.5.4. Soil Enzyme Activity
2.6. Statistical and Correlation Analyses
3. Results
3.1. Plant Growth-Promoting Attributes of CP4 and AHP3
3.2. SEM Analysis of Nanoparticles and Nanobiofertilizers
3.3. Differential Effects of Biofertilizers, Nanofertilizers, and Nanobiofertilizers on Plant Growth Attributes and Biochemical and Physiological Parameters
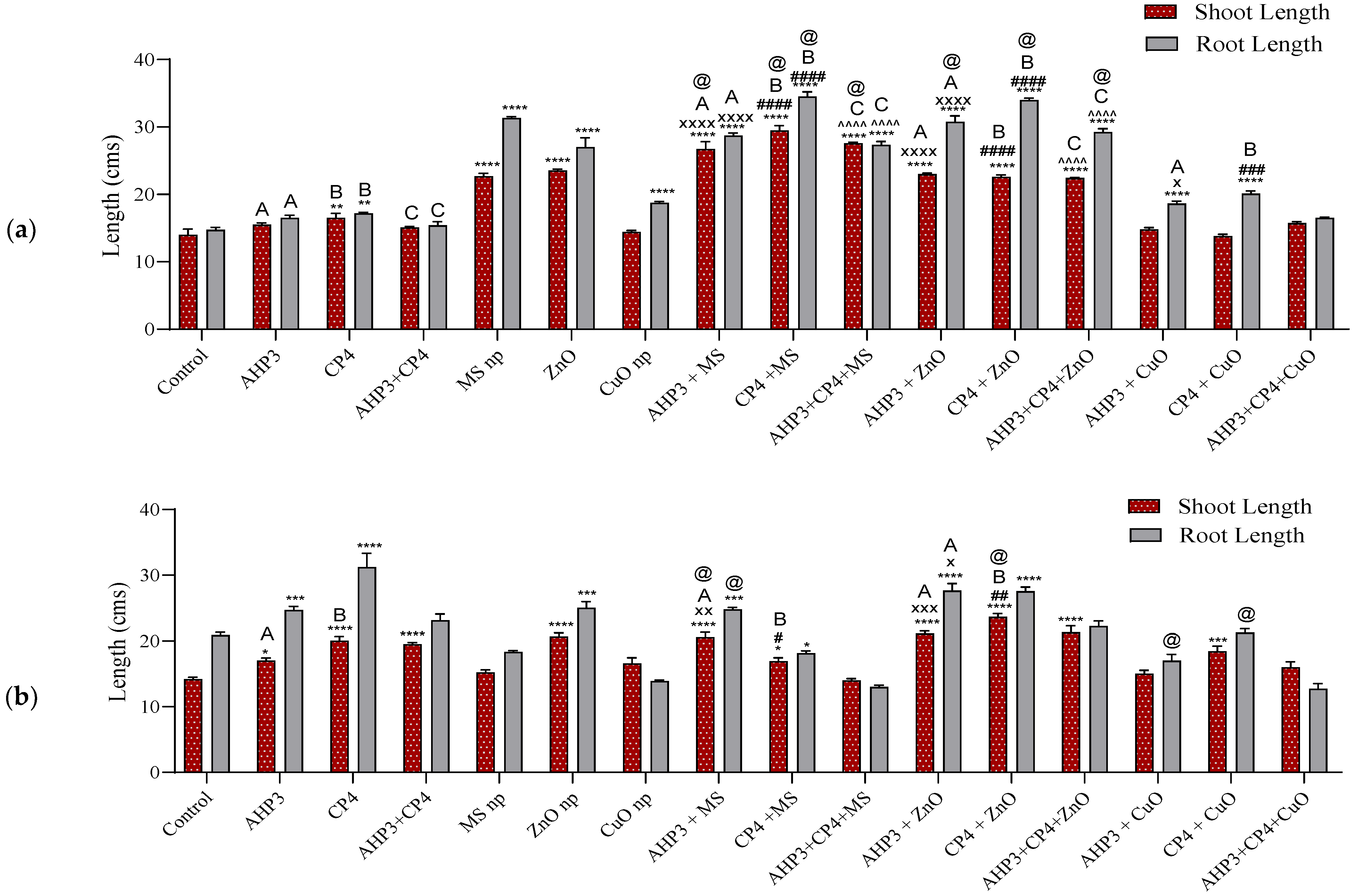
3.3.1. Shoot and Root Length
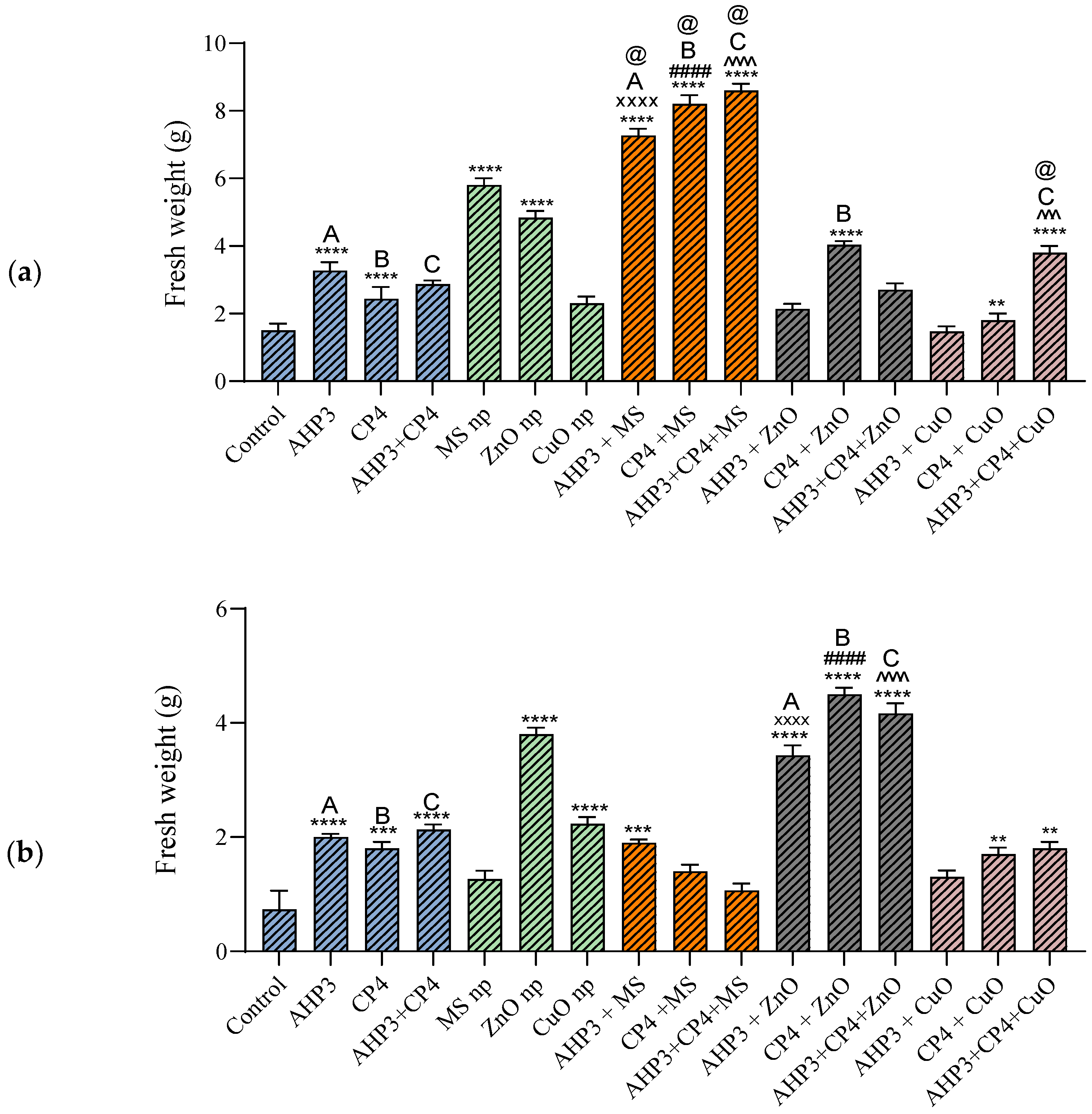
3.3.2. Plant Fresh Weight
3.3.3. Photosynthetic Pigments
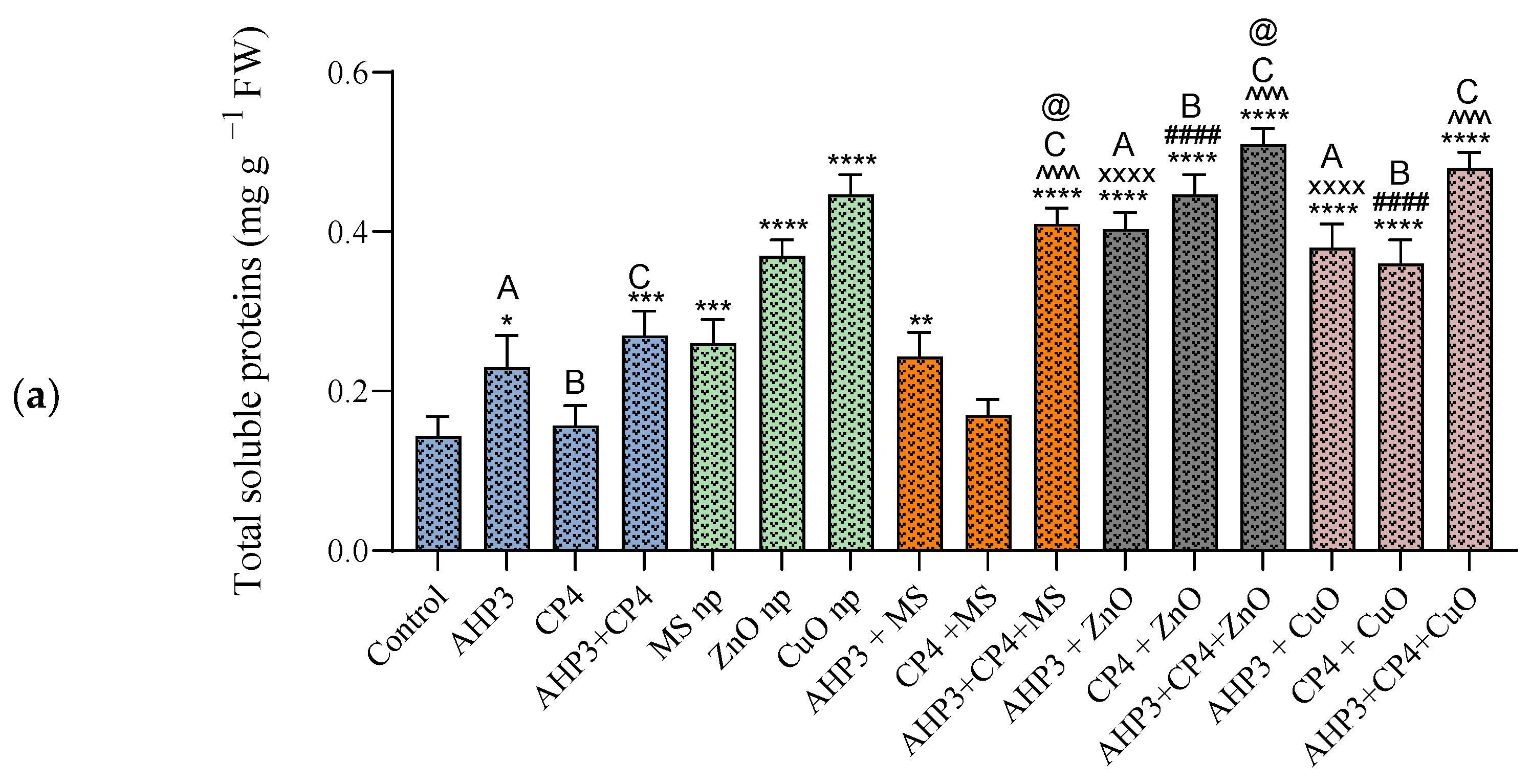
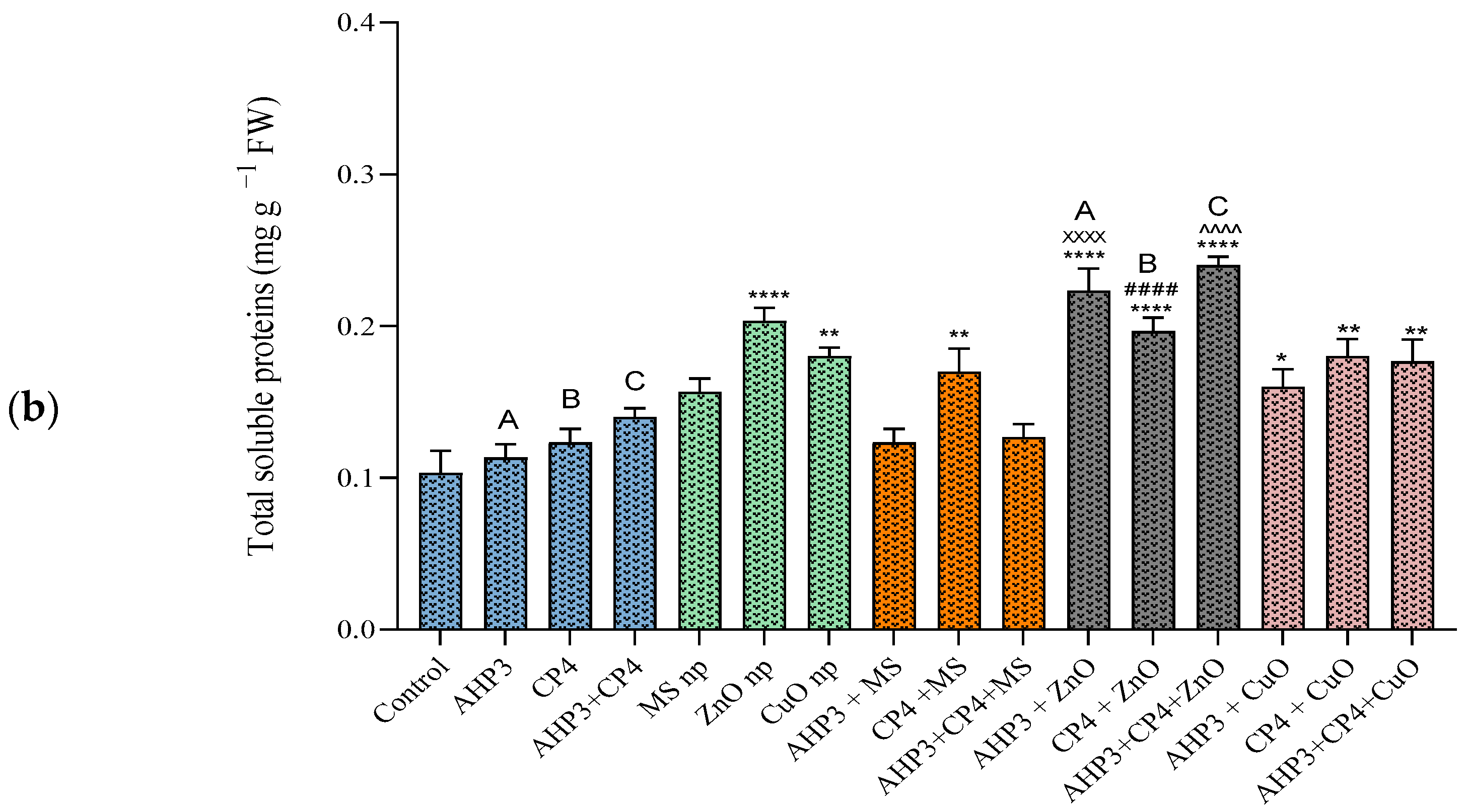
3.3.4. Total Soluble Protein Content
3.3.5. Proline Content
3.3.6. Nitrogen Uptake
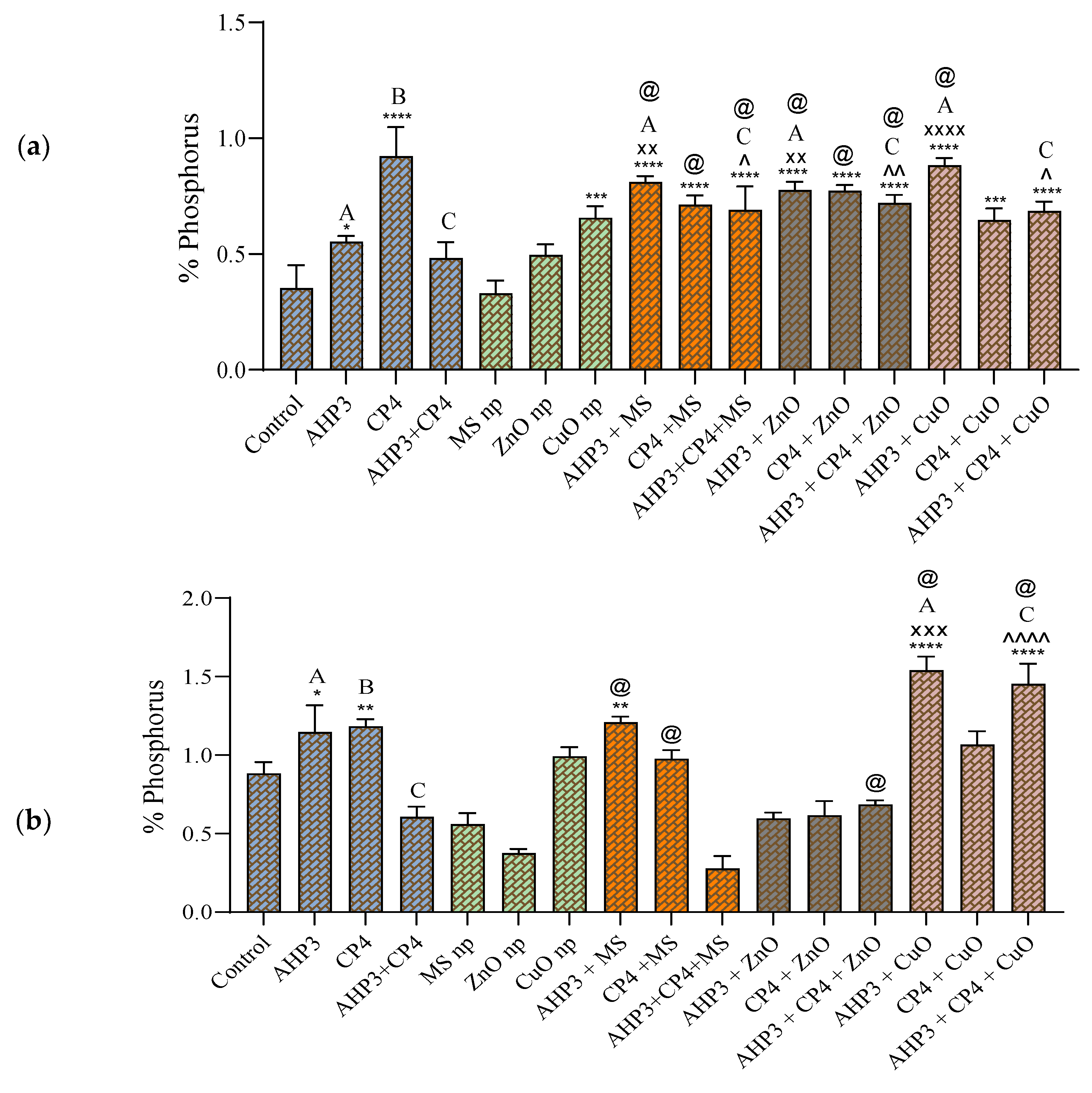
3.3.7. Phosphorus Uptake
3.4. ICP-MS Analysis Identified Enhancement of Macro- and Micronutrients
3.5. Post-Harvest Analysis Indicated Soil Health Improvement
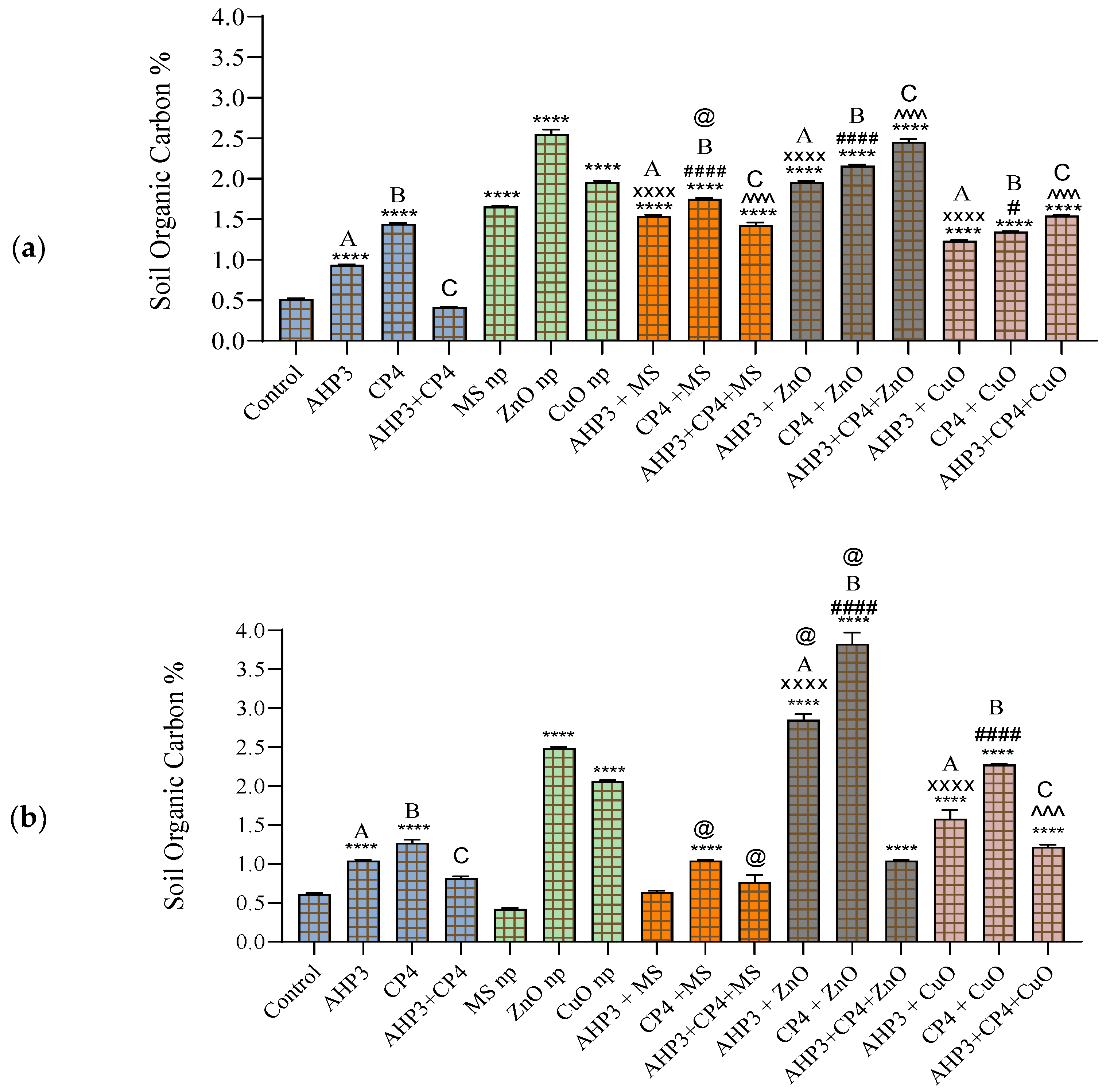
3.5.1. Soil Organic Carbon
3.5.2. Soil Invertase Activity
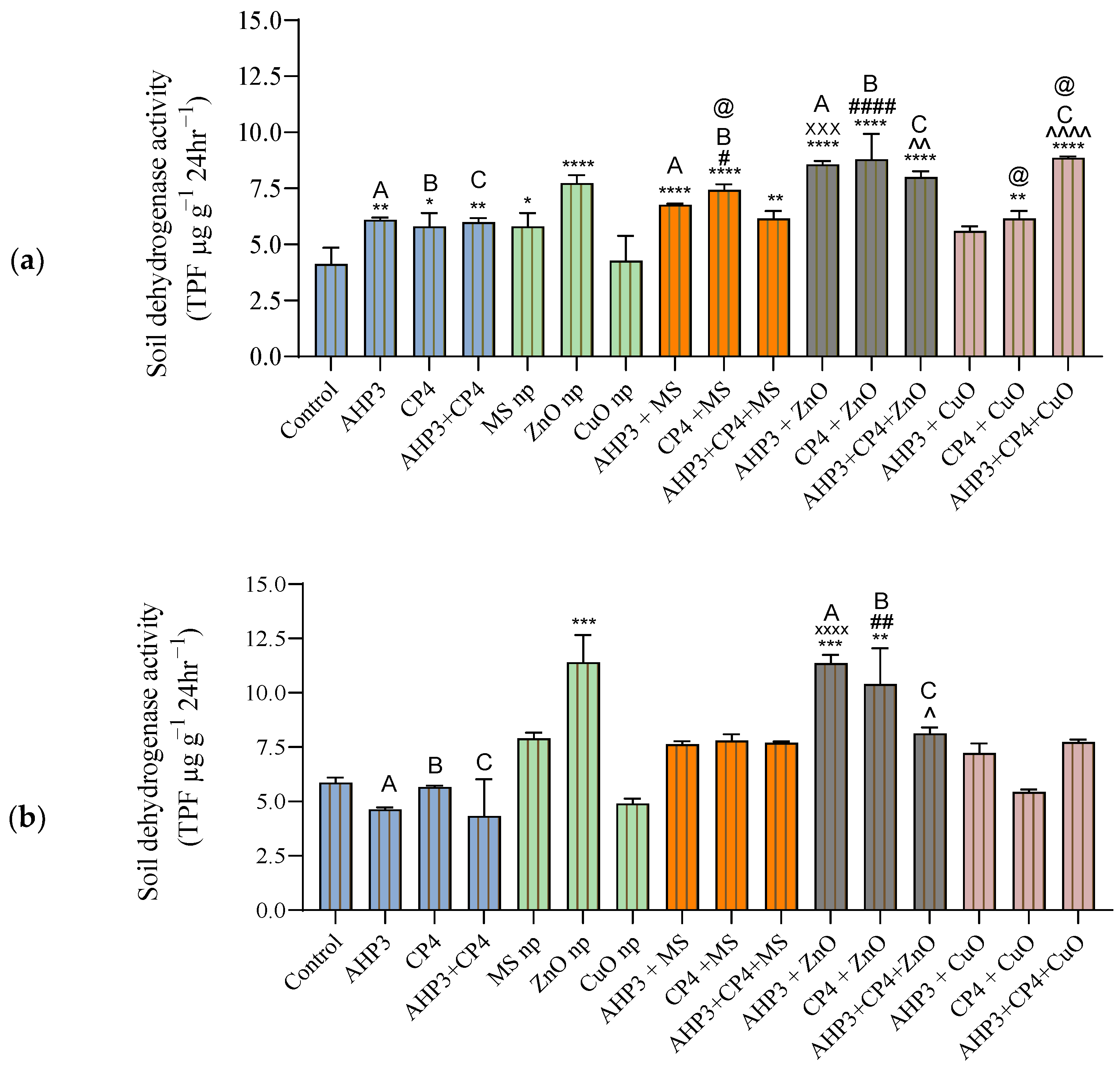
3.5.3. Soil Dehydrogenase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Diksha Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2015; p. 243. [Google Scholar]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Abebe, T.G.; Tamtam, M.R.; Abebe, A.A.; Abtemariam, K.A.; Shigut, T.G.; Dejen, Y.A.; Haile, E.G. Growing use and impacts of chemical fertilizers and assessing alternative organic fertilizer sources in Ethiopia. Appl. Environ. Soil Sci. 2022, 2022, 4738416. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Tsolakidou, M.-D.; Stringlis, I.A.; Fanega-Sleziak, N.; Papageorgiou, S.; Tsalakou, A.; Pantelides, I.S. Rhizosphere-enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs. FEMS Microbiol. Ecol. 2019, 95, fiz138. [Google Scholar] [CrossRef]
- Khan, M.; Salman, M.; Jan, S.A.; Shinwari, Z.K. Biological control of fungal phytopathogens: A comprehensive review based on Bacillus species. MedCrave Online J. Biol. Med. 2021, 6, 90–92. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Rathore, P.; Ramakrishna, W. Bacteria from native soil in combination with arbuscular mycorrhizal fungi augment wheat yield and biofortification. Plant Physiol. Biochem. 2020, 150, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers. New products for the industry? J. Agric. Food Chem. 2017, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.; Mashwani, Z.-u.-R.; Poczai, P. Improvement of plant responses by nanobiofertilizer: A step towards sustainable agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, D.P. Nano-biofertilizer: An emerging eco-friendly approach for sustainable agriculture. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 733–741. [Google Scholar] [CrossRef]
- de Moraes, A.C.P.; Ribeiro, L.d.S.; de Camargo, E.R.; Lacava, P.T. The potential of nanomaterials associated with plant growth-promoting bacteria in agriculture. 3 Biotech 2021, 11, 318. [Google Scholar] [CrossRef]
- Vedamurthy, A.; Bhattacharya, S.; Das, A.; Shruthi, S. Exploring nanomaterials with rhizobacteria in current agricultural scenario. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 487–503. [Google Scholar]
- Jogaiah, S.; Singh, H.B.; Fraceto, L.F.; De Lima, R. Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture: A Smart Delivery System for Crop Improvement; Woodhead Publishing: Sawston, UK, 2020; p. 650. [Google Scholar]
- Hayden, S.C.; Zhao, G.; Saha, K.; Phillips, R.L.; Li, X.; Miranda, O.R.; Rotello, V.M.; El-Sayed, M.A.; Schmidt-Krey, I.; Bunz, U.H. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc. 2012, 134, 6920–6923. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Srinivasan, V.; Srambikkal, H. Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 2012, 173–174, 19–27. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, K.; Vachet, R.W.; Xing, B. Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir 2010, 26, 18071–18077. [Google Scholar] [CrossRef] [PubMed]
- Abarzua, S.; Jakubowski, S. Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Tang, H.; Wang, A.; Liang, X.; Cao, T.; Salley, S.O.; McAllister, J.P., III; Ng, K.S. Effect of surface proteins on Staphylococcus epidermidis adhesion and colonization on silicone. Colloids Surf. B Biointerfaces 2006, 51, 16–24. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. CuO and ZnO nanoparticles differently affect the secretion of fluorescent siderophores in the beneficial root colonizer, Pseudomonas chlororaphis O6. Nanotoxicology 2012, 6, 635–642. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Zeng, J.; McLean, J.E.; Britt, D.W.; Zhan, J.; Anderson, A.J. Production of indole-3-acetic acid via the indole-3-acetamide pathway in the plant-beneficial bacterium Pseudomonas chlororaphis O6 is inhibited by ZnO nanoparticles but enhanced by CuO nanoparticles. Appl. Environ. Microbiol. 2012, 78, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Gao, X.; Avellan, A.; Spielman-Sun, E.; Xu, J.; Laughton, S.; Yun, J.; Zhang, Y.; Bland, G.D.; Zhang, Y.; et al. CuO nanoparticles alter the rhizospheric bacterial community and local nitrogen cycling for wheat grown in a calcareous soil. Environ. Sci. Technol. 2020, 54, 8699–8709. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Effect of Metalloid and Metal Oxide Nanoparticles on Fusarium Wilt of Watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef]
- Djamin, A.; Pathak, M.D. Role of silica in resistance to Asiatic rice borer, Chilo suppressalis (Walker), in rice varieties. J. Econ. Entomol. 1967, 60, 347–351. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Takeoka, Y.; Carlson, T.J.; Bigelow, W.C.; Jones, J.; Moore, P.; Ghosheh, N. Studies on silica deposition in sugarcane (Saccharum spp.) using scanning electron microscopy, energy-dispersive X-ray analysis, neutron activation analysis, and light microscopy. Phytomorphology 1979, 29, 185–193. [Google Scholar]
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar] [CrossRef]
- Gordienko, A.S.; Kurdish, I.K. Surface electrical properties of Bacillus subtilis cells and the effect of interaction with silicon dioxide particles. Biophysics 2007, 52, 217–219. [Google Scholar] [CrossRef]
- Hirota, R.; Hata, Y.; Ikeda, T.; Ishida, T.; Kuroda, A. The silicon layer supports acid resistance of Bacillus cereus spores. J. Bacteriol. 2010, 192, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Singh, G.P.; Prabhu, K.V.; Singh, P.K.; Singh, A.M.; Jain, N.; Ramya, P.; Sharma, J.B.; Kumar, J.; Siwasami, M.; Prasad, S.S.; et al. HD 3086: A new wheat variety for irrigated, timely sown condition of North Western Plains Zone of India. J. Wheat Res. 2014, 6, 179–180. [Google Scholar]
- Singh, K.; Madhusudanan, M.; Verma, A.K.; Kumar, C.; Ramawat, N. Engineered zinc oxide nanoparticles: An alternative to conventional zinc sulphate in neutral and alkaline soils for sustainable wheat production. 3 Biotech 2021, 11, 322. [Google Scholar] [CrossRef]
- Haider, H.I.; Zafar, I.; Ain, Q.u.; Noreen, A.; Nazir, A.; Javed, R.; Sehgal, S.A.; Khan, A.A.; Rahman, M.M.; Rashid, S.; et al. Synthesis and characterization of copper oxide nanoparticles: Its influence on corn (Z. mays) and wheat (Triticum aestivum) plants by inoculation of Bacillus subtilis. Environ. Sci. Pollut. Res. 2023, 30, 37370–37385. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Li, W.; Beta, T.; Sun, S.; Corke, H. Protein characteristics of Chinese black-grained wheat. Food Chem. 2006, 98, 463–472. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Sharma, N.; Pandey, A.K.; Garg, M. Physiological and molecular response of colored wheat seedlings against phosphate deficiency is linked to accumulation of distinct anthocyanins. Plant Physiol. Biochem. 2022, 170, 338–349. [Google Scholar] [CrossRef]
- Cappuccino, J.; Sherman, N. Microbiology: A Laboratory Manual; Benjamin/Cummings Publishing Co.: New York, NY, USA, 1992; pp. 125–179. [Google Scholar]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Reiner, K. Catalase Test Protocol; American Society for Microbiology: Washington, DC, USA, 2010; pp. 1–6. [Google Scholar]
- Sivolapov, P.; Myronyuk, O.; Baklan, D. Synthesis of Stober silica nanoparticles in solvent environments with different Hansen solubility parameters. Inorg. Chem. Commun. 2022, 143, 109769. [Google Scholar] [CrossRef]
- Nazir, S.; Zaka, M.; Adil, M.; Abbasi, B.H.; Hano, C. Synthesis, characterisation and bactericidal effect of ZnO nanoparticles via chemical and bio-assisted (Silybum marianum in vitro plantlets and callus extract) methods: A comparative study. IET Nanobiotechnol. 2018, 12, 604–608. [Google Scholar] [CrossRef]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Seyed Sharifi, R.; Khalilzadeh, R.; Pirzad, A.; Anwar, S. Effects of biofertilizers and nano zinc-iron oxide on yield and physicochemical properties of wheat under water deficit conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 2511–2524. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Beniwal, R.; Kumar, S.; Ramakrishna, W. Bacillus sp. and arbuscular mycorrhizal fungi consortia enhance wheat nutrient and yield in the second-year field trial: Superior performance in comparison with chemical fertilizers. J. Appl. Microbiol. 2022, 132, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.; Sanders, K. Biomacromolecular Applications of UV-Visible Absorption Spectroscopy. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 495–502. [Google Scholar]
- Guy, C.; Haskell, D.; Neven, L.; Klein, P.; Smelser, C. Hydration-state-responsive proteins link cold and drought stress in spinach. Planta 1992, 188, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.; Waldren, R.A.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Flindt, M.R.; Lillebø, A.I. Determination of total nitrogen and phosphorus in leaf litter. In Methods to Study Litter Decomposition; Graça, M.A., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 53–59. [Google Scholar]
- Lindner, R. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1944, 19, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Dogra, N.; Yadav, R.; Kaur, M.; Adhikary, A.; Kumar, S.; Ramakrishna, W. Nutrient enhancement of chickpea grown with plant growth promoting bacteria in local soil of Bathinda, Northwestern India. Physiol. Mol. Biol. Plants 2019, 25, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; Ecological Risk Assessment Support Center, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 2002; pp. 1–23. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Skujins, J.J.; Braal, L.; McLaren, A.D. Characterization of phosphatase in a terrestrial soil sterilized with an electron beam. Enzymologia 1962, 25, 125–133. [Google Scholar]
- Frankeberger, W.; Johanson, J. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Casida Jr, L.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2015, 2, 70. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Rathore, P.; Kumar, S.; Ramakrishna, W. Bacillus subtilis CP4, isolated from native soil in combination with arbuscular mycorrhizal fungi promotes biofortification, yield and metabolite production in wheat under field conditions. J. Appl. Microbiol. 2021, 131, 339–359. [Google Scholar] [CrossRef]
- Saleem, S.; Malik, A.; Khan, S.T. ZnO nanoparticles in combination with Zn biofertilizer improve wheat plant growth and grain Zn content without significantly changing the rhizospheric microbiome. Environ. Exp. Bot. 2023, 213, 105446. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, P.; Guo, Z.; Chetwynd, A.J.; Zhang, M.; Adeel, M.; Li, M.; Guo, K.; Gao, R.; Li, J.; et al. Different physiological responses of C3 and C4 plants to nanomaterials. Environ. Sci. Pollut. Res. 2021, 28, 25542–25551. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Gonzalez, D.J.; Haste, N.M.; Hollands, A.; Fleming, T.C.; Hamby, M.; Pogliano, K.; Nizet, V.; Dorrestein, P.C. Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry. Microbiology 2011, 157 Pt 9, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.; Khanif, Y.; Saleem, M. Role of zinc in plant nutrition-a review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.F.E.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostruct. 2018, 13, 315. [Google Scholar]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Exposure to weathered and fresh nanoparticle and ionic Zn in soil promotes grain yield and modulates nutrient acquisition in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2018, 66, 9645–9656. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.; Hortin, J.; Anderson, A.J.; Jacobson, A.R.; Britt, D.W.; Stewart, J.; McLean, J.E. Rhizosphere interactions between copper oxide nanoparticles and wheat root exudates in a sand matrix: Influences on copper bioavailability and uptake. Environ. Toxicol. Chem. 2018, 37, 2619–2632. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Guo, J.; An, J.; Tang, Z.; Wang, X.; Li, W.; Zhao, X.; Jin, L.; Xiang, Y.; Li, Z.; et al. Estimation of chlorophyll content in soybean crop at different growth stages based on optimal spectral index. Agronomy 2023, 13, 663. [Google Scholar] [CrossRef]
- Balashouri, P.; Prameeladevi, Y. Effect of zinc on germination, growth and pigment content and phytomass of Vigna radiata and Sorghum bicolor. J. Ecobiol. 1995, 7, 109–114. [Google Scholar]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Song, A.; Li, P.; Fan, F.; Li, Z.; Liang, Y. The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE 2014, 9, e113782. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Fernandes, G.C.; da Silva, E.C.; da Costa, K.N.; de Souza, J.S.; Leite, G.d.S.; Biagini, A.L.C.; Galindo, F.S.; Teixeira Filho, M.C.M. Integrated use of plant growth-promoting bacteria and nano-zinc foliar spray is a sustainable approach for wheat biofortification, yield, and zinc use efficiency. Front. Plant Sci. 2023, 14, 1146808. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mi, K.; Yuan, X.; Chen, J.; Pu, J.; Shi, X.; Yang, Y.; Zhang, H.; Zhang, H. Zinc oxide nanoparticles foliar application effectively enhanced zinc and aroma content in rice (Oryza sativa L.) grains. Rice 2023, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Ji, H.; Guo, Z.; Wang, G.; Wang, X.; Liu, H. Effect of ZnO and CuO nanoparticles on the growth, nutrient absorption, and potential health risk of the seasonal vegetable Medicago polymorpha L. PeerJ 2022, 10, e14038. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Peverill, K.I.; Sparrow, L.; Reuter, D.J. Soil Analysis: An Interpretation Manual; CSIRO Publishing: Clayton, Australia, 1999. [Google Scholar]
- Hortin, J.; Anderson, A.; Britt, D.; Jacobson, A.; McLean, J. Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: Influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. Nano 2020, 7, 2618–2631. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Mahawar, H.; Kumar, R.; Gupta, P.; Mathur, T.; Kaul, R.K.; Praveen-Kumar Kalia, A.; Gautam, R.; Singh, S.K.; et al. ZnO nanoparticles induced exopolysaccharide production by B. subtilis strain JCT1 for arid soil applications. Int. J. Biol. Macromol. 2014, 65, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Martineau, N.; McLean, J.E.; Dimkpa, C.O.; Britt, D.W.; Anderson, A.J. Components from wheat roots modify the bioactivity of ZnO and CuO nanoparticles in a soil bacterium. Environ. Pollut. 2014, 187, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Adeel, M.; Zain, M.; Rizwan, M.; Irshad, M.K.; Jilani, G.; Hameed, A.; Khan, A.; Arshad, M.; Raza, A.; et al. Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: A full life cycle study. J. Environ. Manag. 2020, 263, 110365. [Google Scholar] [CrossRef] [PubMed]





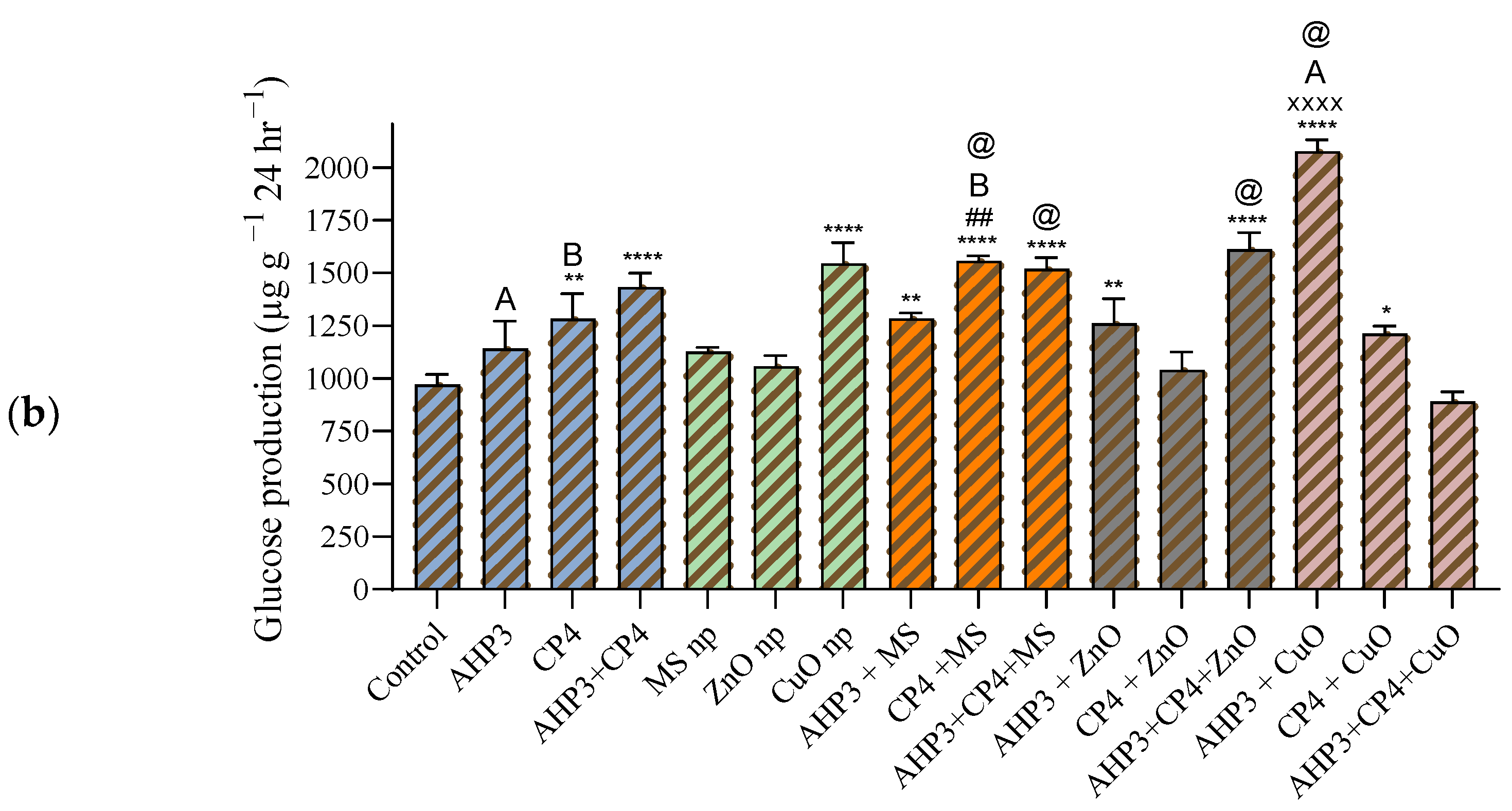
| Mg [mg kg−1] | Ca [mg kg−1] | Mn [mg kg−1] | Fe [mg kg−1] | Cu [mg kg−1] | Zn [mg kg−1] | Se [mg kg−1] | |
|---|---|---|---|---|---|---|---|
| Control | 1678 ± 0.8 | 112 ± 3 | 50 ± 1 | 506 ± 11 | 22 ± 0.5 | 131 ± 2 | 43 ± 2 |
| AHP3 | * 1786 ± 3 | * 260 ± 5 | 81 ± 2 | * 661 ± 22 | 10 ± 0.6 | * 237 ± 5 | ∞ 64 ± 4 |
| CP4 | * 1940 ± 21 | * 213 ± 3 | 92 ± 0.4 | * 609 ± 9 | 22 ± 0.4 | * 235 ± 6 | ∞ 78 ± 4 |
| AHP3 + CP4 | * 1978 ± 25 | 113 ± 3 | 91 ± 1 | 493 ± 22 | 5 ± 0.3 | * 540 ± 1 | 46 ± 1 |
| AHP3 + MS | * 2110 ± 9 x | * 188 ± 1 | 71 ± 0.3 | * 643 ± 11 | 5 ± 0.2 | * 219 ± 5 | ∞ 57 ± 3 |
| CP4 + MS | * 2265 ± 45 # | 165 ± 2 | 111 ± 2 | 531 ± 24 | 6 ± 0.1 | --- | 52 ± 3 |
| AHP3 + CP4 + MS | * 1795 ± 10 ^ | * 235 ± 4 ^ | 64 ± 1 | 317 ± 11 | 19 ± 0.4 | 47 ± 1 | ∞ 60 ± 5 |
| MS NP | * 2398 ± 13 | 146 ± 3 | 98 ± 1 | * 667 ± 12 | 11 ± 0.2 | * 518 ± 6 | 53 ± 1 |
| AHP3 + ZnO | * 5106 ± 50 x | 79 ± 2 | 94 ± 1 | 482 ± 29 | 17 ± 0.6 | 99 ± 2 | 48 ± 2 |
| CP4 + ZnO | * 6132 ± 71 # | 109 ± 6 | 96 ± 1 | * 636 ± 8 | 19 ± 0.4 | 94 ± 5 | ∞ 63 ± 4 |
| AHP3 + CP4 + ZnO | * 5756 ± 157 ^ | 115 ± 5 | 83 ± 1 | 5650 ± 10 | 13 ± 0.8 | 42 ± 4 | 52 ± 4 |
| ZnO NP | * 5555 ± 67 | 79 ± 4 | 108 ± 2 | 557 ± 9 | 18 ± 0.2 | 53 ± 3 | 54 ± 1 |
| AHP3 + CuO | * 7411 ± 84 x | 81 ± 3 | 117 ± 1 | * 599 ± 46 | ∞ 30 ± 0.4 | 126 ± 4 | 52 ± 3 |
| CP4 + CuO | * 5333 ± 45 # | * 199 ± 4 | 91 ± 0.8 | 541 ± 18 | ∞ 53 ± 0.4 | 180 ± 1 | ∞ 57 ± 4 |
| AHP3 + CP4 + CuO | * 8879 ± 154 ^ | --- | * 148 ± 1 | * 608 ± 17 ^ | ∞ 51 ± 0.2 | 154 ± 4 | 52 ± 2 |
| CuO NP | * 2688 ± 11 | * 304 ± 2 | 52 ± 1 | 500 ± 18 | 14 ± 0.5 | --- | ∞ 59 ± 1 |
| Mg [mg kg−1] | Ca [mg kg−1] | Mn [mg kg−1] | Fe [mg kg−1] | Cu [mg kg−1] | Zn [mg kg−1] | Se [mg kg−1] | |
|---|---|---|---|---|---|---|---|
| Control | 2478 ± 7 | 161 ± 0.4 | 67 ± 0 | 518 ± 13 | 11 ± 0.4 | 197 ± 1.5 | 46 ± 0.8 |
| AHP3 | * 2563 ± 22 | * 553 ± 2 | 68 ± 0.8 | * 1035 ± 96 | 10 ± 0.3 | --- | 104 ± 3 |
| CP4 | * 2633 ± 38 | * 261 ± 2 | 49 ± 0.4 | 489 ± 26 | 8 ± 0.3 | --- | 61 ± 3 |
| AHP3 + CP4 | * 2768 ± 40 | 187 ± 2 | 70 ± 1 | 532 ± 18 | ∞ 18 ± 0 | 44 ± 6 | 47 ± 2 |
| AHP3 + MS | * 3747 ± 88 x | 161 ± 0.4 | 57 ± 2 | 433 ± 18 | 9 ± 0.4 | 29 ± 2 | 46 ± 0.5 |
| CP4 + MS | * 8368 ± 101 # | --- | * 203 ± 7 # | * 1010 ± 26 # | ∞ 51 ± 1 | * 2277 ± 77 | 79 ± 3 |
| AHP3 + CP4 + MS | * 5341 ± 55 ^ | * 348 ± 6 ^ | 103 ± 1 | * 768 ± 18 ^ | ∞ 28 ± 0.8 | --- | 93 ± 6 |
| MS NP | * 4846 ± 69 | 152 ± 0.7 | 135 ± 2 | * 795 ± 20 | 16 ± 0.7 | 5 ± 5 | 48 ± 2 |
| AHP3 + ZnO | * 9125 ± 42 x | --- | * 180 ± 2 x | * 722 ± 12 | ∞ 33 ± 0.4 | 156 ± 6 | 54 ± 3 |
| CP4 + ZnO | * 7754 ± 76 # | 140 ± 2 | 110 ± 2 | * 622 ± 12 # | ∞ 22 ± 0.2 | 111 ± 4 | * 165 ± 4 # |
| AHP3 + CP4 + ZnO | * 4241 ± 33 ^ | 156 ± 4 | 89 ± 2 | 531 ± 12 | ∞ 22 ± 0.5 | 88 ± 6 | 53 ± 0.7 |
| ZnO NP | * 6523 ± 72 | 105 ± 4 | 90 ± 1 | 447 ± 28 | ∞ 22 ± 0.5 | 208 ± 13 | 47 ± 2 |
| AHP3 + CuO | --- | * 1160 ± 3 x | 20 ± 1 | --- | --- | --- | * 333 ± 0.4 x |
| CP4 + CuO | * 4299 ± 72 # | * 300 ± 3 | 86 ± 0.5 | 499 ± 33 | ∞ 31 ± 0.6 | * 459 ± 9 # | 74 ± 5 |
| AHP3 + CP4 + CuO | * 4591 ± 70 ^ | * 708 ± 5 ^ | 75 ± 2 | * 754 ± 17 ^ | ∞ 36 ± 1 | * 929 ± 25 ^ | * 150 ± 8 ^ |
| CuO NP | * 4551 ± 79 | 154 ± 4 | 76 ± 2 | 378 ± 4 | ∞ 31 ± 0.1 | 230 ± 9 | 54 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunakaran, A.; Fathima, Y.; Singh, P.; Beniwal, R.; Singh, J.; Ramakrishna, W. Next-Generation Biofertilizers: Nanoparticle-Coated Plant Growth-Promoting Bacteria Biofertilizers for Enhancing Nutrient Uptake and Wheat Growth. Agriculture 2024, 14, 517. https://doi.org/10.3390/agriculture14040517
Karunakaran A, Fathima Y, Singh P, Beniwal R, Singh J, Ramakrishna W. Next-Generation Biofertilizers: Nanoparticle-Coated Plant Growth-Promoting Bacteria Biofertilizers for Enhancing Nutrient Uptake and Wheat Growth. Agriculture. 2024; 14(4):517. https://doi.org/10.3390/agriculture14040517
Chicago/Turabian StyleKarunakaran, Anagha, Yaraa Fathima, Pallavi Singh, Rahul Beniwal, Jyoti Singh, and Wusirika Ramakrishna. 2024. "Next-Generation Biofertilizers: Nanoparticle-Coated Plant Growth-Promoting Bacteria Biofertilizers for Enhancing Nutrient Uptake and Wheat Growth" Agriculture 14, no. 4: 517. https://doi.org/10.3390/agriculture14040517
APA StyleKarunakaran, A., Fathima, Y., Singh, P., Beniwal, R., Singh, J., & Ramakrishna, W. (2024). Next-Generation Biofertilizers: Nanoparticle-Coated Plant Growth-Promoting Bacteria Biofertilizers for Enhancing Nutrient Uptake and Wheat Growth. Agriculture, 14(4), 517. https://doi.org/10.3390/agriculture14040517








