Abstract
Mycotoxins in sesame seeds pose a significant risk to both food safety and Ethiopia’s economy. The purpose of this study was to determine the presence and concentrations of mycotoxins in sesame seeds kept on farms in Ethiopia’s key sesame-producing areas. Purposive sampling was used to obtain 470 sesame seed samples from farmers′ storage facilities in five important districts. Total aflatoxin (AFT), ochratoxin A (OTA), total fumonisin (FUM), and deoxynivalenol (DON) were identified using both a lateral flow reader and an enzyme-linked immunosorbent assay (ELISA). The analysis revealed that all samples contained mycotoxins to varying degrees, with AFT and DON being particularly common. AFT levels varied between 2.5 and 27.8 parts per billion (μg/kg), averaging 13.8 μg/kg, while OTA concentrations were between 5.0 and 9.7 μg/kg, averaging 7.1 μg/kg. Total fumonisin levels spanned from 300 to 1300 μg/kg, averaging 800 μg/kg. DON was found in the range of 560 to 700 μg/kg. Notably, 96.8% of the samples fell within the safe range for AFT, FUM, and DON mean levels as defined by the Federal Drug Administration’s maximum limits. The co-occurrence rates of AFT-OTA, DON-OTA, AFT-FUM, FUM-DON, and FUM-OTA were observed at 44.0%, 38.3%, 33.8%, 30.2%, 29.8%, and 26.0%, respectively. Around 37.2% of the samples showed signs of fungal infection, and seed germination rates varied between 66.8% and 91.1%. The Limmu district exhibited higher total aflatoxin levels, greater kernel infection, and reduced germination rates compared to other districts. The Wollega sesame variety was more susceptible to kernel infection, had higher total aflatoxin levels, and lower germination rates compared to other varieties. Additionally, the age of the grain significantly affected (p < 0.05) both kernel infection and germination. Current storage practices in Ethiopia’s primarily sesame-growing districts are conducive to the growth of mycotoxin-producing fungi. Given the public health implications of mycotoxin levels in sesame, it is imperative for stakeholders to collaborate in identifying and implementing secure and effective storage solutions to preserve both the quantity and quality of sesame at the smallholder farmer level. This study underscores the necessity for improved storage technologies to safeguard sesame quality and diminish the risk of mycotoxin contamination.
1. Introduction
Sesame (Sesamum indicum L.) is an essential oilseed crop predominantly grown in tropical and semi-tropical regions [1]. It plays a crucial role in the agricultural economy of developing countries, especially in Africa and Asia, due to its high nutrient content, including proteins, unsaturated fats, vitamins, and minerals [2]. These seeds are not only a nutritional powerhouse, but they also provide health benefits, such as cholesterol reduction and heart health support, owing to their antioxidant and lignan content. Globally, major sesame producers like China, Ethiopia, India, Myanmar, Nigeria, Sudan, Uganda, and Tanzania significantly contribute to the global market [3,4].
In Ethiopia, sesame is second only to coffee (Coffea arabica) as an export commodity and is predominantly cultivated in the Amhara, Benishangul-Gumuz, and Tigray regions, mostly by smallholder and commercial farmers [5]. Most of the high-quality yield is exported, with a smaller local use for oil extraction and the by-products as animal feed [6].
Ethiopia grows various sesame varieties suited to its diverse climatic and soil conditions [7]. However, the crop, adaptable to Ethiopia’s diverse climate and soil, faces challenges like biotic and abiotic stressors, impacting yield and quality [1,8]. One of the critical issues in sesame cultivation is mold infestation, significantly affecting seed germination and mycotoxin levels [1]. Post-harvest handling presents additional challenges, particularly fungal contamination from strains of Aspergillus, Fusarium, and Penicillium [9,10,11,12]. These fungi are especially problematic due to the high oil content and respiration rate of sesame seeds, leading to diminished nutritional value and potential health risks. Specific strains of these fungi produce mycotoxins, which contaminate food products, including sesame seeds, posing health risks and creating trade barriers [13,14]. The focus on aflatoxins, ochratoxin A, fumonisins, and deoxynivalenol (DON) in sesame seeds arises from their distinct toxic properties, their prevalent occurrence in foodstuffs and animal feed, and their profound impact on both public health and economic wellbeing. These mycotoxins underscore the necessity for regulations grounded in rigorous scientific data, continuous investigative efforts, and global cooperation to safeguard public health and maintain food security. These mycotoxins contaminate food products, including sesame seeds, as evidenced by various studies [15,16,17,18,19,20,21]. Research by Ezekiel et al. [22] specifically identified several microbial metabolites in sesame samples from Nigeria. Mycotoxin accumulation in sesame seeds is influenced by factors like initial seed quality and storage conditions [23]. In Ethiopia, common practices such as field-drying and storing seeds in suboptimal conditions exacerbate the risk of mycotoxin contamination [23,24]. Sesame seeds are usually stored in woven polypropylene bags, making them susceptible to moisture-induced fungal growth [25]. Ethiopia’s economic reliance on sesame is challenged by inadequate storage and limited handling knowledge, exacerbated by an informal seed system with limited technological support, leading to increased risks of fungal infection and mycotoxin buildup. Grain moisture and temperature are key factors in the development of these mycotoxins [26], with multiple mycotoxins intensifying health risks [27]. Global regulatory measures, especially concerning aflatoxin exposure, impact trade and economic dynamics, often leading to the export of higher-quality sesame and the retention of lower-quality produce domestically [28,29,30]. Beyond economic implications, mycotoxins pose significant health hazards [31]. Despite global research on mycotoxins in crops, studies on mycotoxins in farmer-stored sesame in Ethiopia are limited, primarily focusing on single aflatoxins and small sample sizes [32].
Our study aims to fill this gap by assessing levels of total aflatoxin, total fumonisin, deoxynivalenol, and ochratoxin A in sesame seeds from various Ethiopian regions and varieties. The research hypotheses are as follows: (1) the prevalence and concentration of mycotoxins in sesame seeds vary significantly across different sesame-producing districts in Ethiopia, (2) sesame seed varieties exhibit different susceptibilities to mycotoxin contamination, (3) the age of sesame seeds significantly affects kernel infection rates and seed germination rates, and (4) current storage practices in Ethiopia’s main sesame-growing districts contribute to the growth of mycotoxin-producing fungi and subsequent seed contamination. Through this study, we aim to provide insights into the relationships between mold infection, mycotoxin levels, and seed germination in Ethiopia, thereby contributing to the broader context of agricultural and environmental health.
2. Materials and Methods
2.1. Description of the Study Areas
A field survey was conducted in July 2018 in six major districts in four areas of Ethiopia that produce sesame: Metema and Tech Armachiho in the Amhara region, Kafta Humera and Tsegede in Tigray, Limmu in Oromia, and Pawe in the Benishangul Gumuz region. Selection of these districts was based on their sesame production potential, ease of access for sampling, and the history of sesame cultivation in the area [7]. The survey covered 10 kebeles in six districts to efficiently target sesame-growing households: Adebay, Bereket, Mykadra, and Rawyan in Kafta Humera, Lelistu in Limmu, Kumer and Kokit in Metemma, Mender 46 in Pawe, Yayira in Tech Armachiho, and Lekatit in Tsegede. Data from these locations were collected and evaluated at the district level in order to create a more precise and targeted plan. Furthermore, complete geographical and agro-ecological information was rigorously recorded, including altitude, latitude, longitude (calculated with GPS), cultivar specifications, storage structure, and storage length. Table 1 and Figure 1 show the geographical location of these sample sites.

Table 1.
Geographical description of study sites.
Figure 1.
Sesame sample collection districts of Ethiopia in July 2018.
2.2. Sample Collection
A total of 470 samples were collected from randomly identified households, i.e., the 1st farmer’s house was sampled, followed by the 3rd farmer’s house, skipping the 2nd farmer’s house. Ten kebeles (villages) were considered to reach sesame-growing households in six districts: Adebay, Bereket, Mykadra, and Rawyan (Kafta Humera), Lelistu (Limmu), Kumer and Kokit (Metemma), Mender 46 (Pawe), Yayira (Tech Armachiho), and Lekatit (Tsegede). For each household, a composite (1kg) of grain was sampled by taking from the top, middle, and bottom parts of the farmers’ storage systems, put in labeled polyethylene bags, and transported to Mekelle University for laboratory analyses. All collected data were analyzed at the district level in order to develop a more targeted and focused approach. Furthermore, data on altitude, latitude, and longitude (recorded using GPS), as well as agro-ecological conditions, crops, and storage methods, were documented. Structured questionnaires were used to collect information on cultivars, storage structures, and storage duration. The moisture content and temperature of the samples were measured using a moisture meter in accordance with the manufacturer’s guidance prior to sub-sampling for laboratory testing.
2.3. Determination of Moisture Contents
In each sesame seed sample, the moisture content was determined using a cost-effective yet highly accurate moisture meter, following the protocol outlined by Armstrong et al. [33]. This measurement was carried out prior to extracting samples from each on-farm storage facility. The meter operates by assessing temperature and relative humidity to estimate the equilibrium moisture content. Notably, this method has been successfully validated for use with the black sesame variety, confirming its accuracy and reliability for our purposes. This crucial step was performed immediately upon sample collection from each on-farm storage facility to ensure the most accurate representation of the seeds’ condition at the time of storage.
2.4. Determination of Kernel Infection by Molds
To evaluate mold infection in sesame kernels, we collected kernels from various varieties and locations. Each kernel was initially surface-sterilized using a 1% sodium hypochlorite (NaOCl) solution for one minute. Following this, we rinsed each kernel thrice with sterile distilled water (SDW) to ensure thorough sterilization. Subsequently, 100 surface-disinfected kernels from each sample were arranged on potato dextrose agar (PDA) plates. We placed 25 kernels on each PDA plate with a diameter of 120 cm. These plates were then stored in multi-room incubators from WITEG Labortechnik, Wertheim, Germany, at a stable temperature of 27 °C. Seven days post-incubation, we began monitoring each plate and kernel for any signs of fungal growth. The proportion of infected kernels on each plate was calculated by counting the number of affected kernels, which helped in determining the infection rate in percentages. We used the following formula to quantify the prevalence of the fungus: frequency of fungus = (number of seeds with a specific fungus × 100)/total kernels used. For further analysis, fungi isolated from infected kernels on each plate were carefully transferred and preserved on agar slants. This step was crucial for the subsequent identification and classification of the fungal species.
2.5. Seed Germination Tests
To evaluate the germination capacity, 100 sesame kernels from each sample were selected at random. These kernels were then evenly distributed between two 215 × 215 mm rectangular plastic Petri dishes, with each dish containing 50 kernels. The kernels were placed on filter papers within the dishes, as per the methodology outlined by Altaf et al. [34] and Alemayehu et al. [35], and the papers were moistened with sterile distilled water to aid germination. The kernels were arranged to maintain equal spacing within each Petri dish. To ensure a comprehensive representation from each district, the sampling process incorporated three random replications. The Petri dishes were then placed in a germination chamber equipped with fluorescent lights. The chamber was set to a 12 h light/dark cycle at a consistent temperature of 25 °C. Daily, each plate received additional sterile distilled water to sustain the required moisture levels for optimal germination. Germination tracking for each sample began seven days after incubation. The count of sprouted kernels in each sample was recorded to ascertain the germination capacity. We calculated the germination percentage using the following formula to quantify the overall germination success rate of the seeds: (number of kernels germinated × 100)/total kernels used.
2.6. Preparation of Samples and Analysis of Mycotoxins
For mycotoxin analysis, we utilized 300 g of sesame grain from each 1 kg sample obtained from the households. Concentrations of total aflatoxin (AFT), total fumonisin (FUM), deoxynivalenol (DON), and ochratoxin (OTA) were determined using enzyme-linked immunosorbent assay (ELISA) tests, following the procedures outlined by Alemayehu et al. [35]. Initially, the samples were finely ground using an electric grinder, sieved through a 1 mm mesh size sieve, and then stored in sealed Ziplock bags at −20 °C to preserve their integrity.
The process of sample preparation, mycotoxin extraction, and analysis adhered to the guidelines provided by Romer Labs [36]. For the extraction of mycotoxins, we used methanol for ochratoxin A and distilled water for the remaining mycotoxins. The quantification of total aflatoxin, total fumonisin, and deoxynivalenol was carried out using a lateral flow AgraVision reader, which incorporated a calibration curve specific to each test kit lot (Table 2). The levels of ochratoxin A were determined using a Stat Fax 4700 Enzyme-Linked Immuno-Sorbent Assay (ELISA) reader. All the test kits utilized in this study for determining various toxin levels were USDA-GIPSA approved and sourced from Romer Labs, Union, Missouri, USA. Each kit had undergone validation with sesame samples containing known mycotoxin levels at Romer Lab in the USA. The quantitative results were recorded in parts per billion (μg/kg). The detection range for each mycotoxin was as follows: 2–4 μg/kg for ochratoxin A; 3.3–100 μg/kg for total aflatoxins; 200–5000 μg/kg for deoxynivalenol; and 300–5000 μg/kg for total fumonisins. In cases where readings exceeded the maximum limit, the extracts were diluted to achieve a concentration within the detection range, and this adjusted concentration was noted after applying the appropriate dilution factor. Additionally, we conducted an analysis to identify the co-occurrence of total aflatoxins, ochratoxin A, deoxynivalenol, and total fumonisins in positive samples, using Fisher’s exact test to determine the statistical significance.

Table 2.
Mycotoxin extraction solution, range of quantification, and recovery rate.
2.7. Data Analysis
In our study, we employed descriptive statistical methods to evaluate the occurrence of mycotoxins. This included calculating frequencies and central tendencies to understand the prevalence and distribution of mycotoxins in the samples. To identify variations in mycotoxin levels across different districts, grain ages, and sesame varieties, we applied one-way analysis of variance (ANOVA). Further, we utilized Fisher’s exact test to assess the relationship between the occurrence of mycotoxins in samples and variables like district locations and grain ages. For comparing the levels of aflatoxin, deoxynivalenol, fumonisin, and ochratoxin across the samples, the Kruskal–Wallis one-way ANOVA was used. This non-parametric test was particularly useful for analyzing data that did not follow a normal distribution. All statistical analyses were performed using R software, version 3.5.0. For visual representation, we created figures using Sigma Plot software, verison 12.5. Additionally, to summarize the data, we calculated means and standard deviations. Significant differences between various storage structures and districts were identified using one-way ANOVA, providing insights into the factors affecting mycotoxin levels in the samples.
3. Results
3.1. Moisture Content, Relative Humidity, and Temperature of Sesame Samples
Table 3 presents the mean values (with standard errors) of moisture content, relative humidity, and temperature across various districts, sesame varieties, and grain ages in Ethiopia. The statistical significance of differences is indicated by p-values, and means sharing the same letter within each variable category are not significantly different at a 0.05 significance level. Kafta Humera, Metemma, Tsegede, and Mixed variety samples show similar moisture content (around 8.6–8.7%), which is lower than that in Limmu and Pawe (above 10%). Relative humidity is highest in Limmu and Pawe (around 57.6–57.9%), while Tsegede reports the lowest (37.2%). The highest average temperature is observed in Tsegede (34.4 °C), and the lowest is observed in Limmu and Pawe (around 26.0–26.4 °C). The differences in these measurements across districts are statistically significant (p < 0.001).

Table 3.
Mean (±SE) moisture content (%), relative humidity (%), and temperature (°C) varieties and grain ages across districts in Ethiopia.
The Wollega and Limmu district samples show higher moisture content (around 10.4%) compared to other varieties. Relative humidity is highest for the Wollega variety (57.9%) and lowest for the Mixed and Setit-I varieties (around 36.4–36.9%). Temperature varies significantly among varieties, with Wollega having the lowest (26.4 °C) and Setit-I the highest (34.5 °C). Varietal differences in all three variables are statistically significant (p < 0.001). The moisture content increases with grain age, from 7.8% at 5 months to 9.0% at 8 months. Relative humidity is lowest for 5-month-old grains (36.7%) and highest for 8-month-old grains (43.6%). Temperature is highest in 7- and 8-month-old grains (around 31.9–32.3 °C) compared to 5-month-old grains (29.9 °C). These differences across grain ages are also statistically significant (p < 0.001). Overall, the table indicates significant variations in moisture content, relative humidity, and temperature based on the district location, sesame variety, and grain age. These factors are crucial in determining storage conditions and potential risk for mycotoxin contamination in sesame seeds.
3.2. Seed Germination and Fungal Infection
The seed germination and kernel infection (mean ± SD) percentages across districts and varieties are depicted below (Figure 2 and Figure 3). One-way ANOVA showed that there were significant (p ≤ 0.05) differences among the districts, varieties, and grain ages regardless of seed germination and kernel infection. Germination rates varied across districts, with an overall average of 84.3%. The highest germination rates were found in Tech Armachiho (91.1%), Tsegede (90.2%), and Metemma (87.6%), with the lowest in Pawe (73.3%) and Limmu (66.9%) (Figure 2a). Regarding sesame varieties, Setit-I exhibited the highest germination rate (91.9%), followed by the Gojam type (87.4%). The Wollega type had the lowest germination rate (65.7%) (Figure 2b).
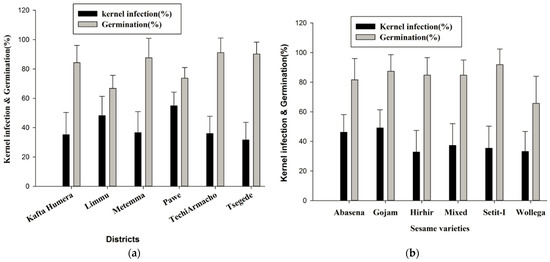
Figure 2.
Mean (±SD) sesame germination and kernel infection (%) across sesame-growing districts (a) and varieties (b).
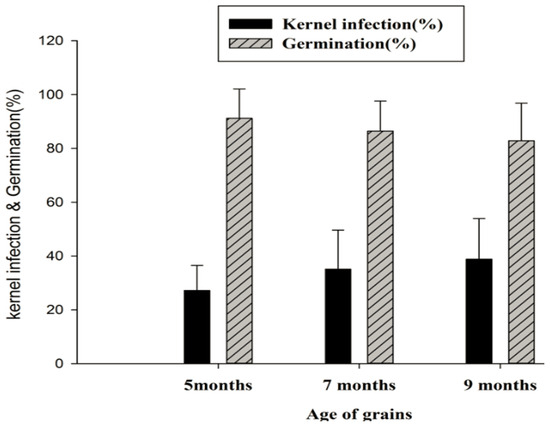
Figure 3.
Mean (±SD) sesame seed germination and kernel infection (%) across the ages and sampling districts of grains in Ethiopia.
On average, 37.2% (range 31.7 to 54.9%) of the sesame samples were infected with fungal species. Pawe (54.9%) and Limmu (48.2%) districts had the highest kernel infection rates, with no significant difference between them. Samples collected from the rest of the four districts had lower kernel infection (ranging from 31.7 ± 1 to 36.7 ± 14.3%) than the other two districts; however, kernel infection levels were not significantly (p > 0.05) different from one another (Figure 2a). Sesame varieties showed variability in infection rates, with Abasena and Wollega types having the highest infections, comparable to each other. The Mixed, Gojam type, Setit-I, and Hirhir varieties had lower infection rates, also comparable among them ((Figure 2b). The age of grains significantly influenced both kernel infection and germination rates. Kernel infection increased and germination decreased with the age of the grains. The highest kernel infection rate (39.0%) was observed in grains stored for eight months, while the lowest infection rate (27.2%) was associated with grains stored for five months (Figure 3).
Figure 4 comprises two graphical representations focusing on mycotoxin occurrences in different districts and sesame seed types. In Figure 4a, the data exhibit the levels of various mycotoxins—specifically, total aflatoxins, ochratoxin A, total fumonisins, and deoxynivalenol—across several districts such as Kafta Humera, Limmu, Metemma, Pawe, Tech Armachiho, and Tsegede, on a 0–80% scale. Meanwhile, Figure 4b delineates the prevalence of these mycotoxins within distinct sesame seed variants, namely Abasena, Gojam, Hirhir, Mixed, Setit-I, and Wollega, with the incidence rate measured on a 0–100% scale.
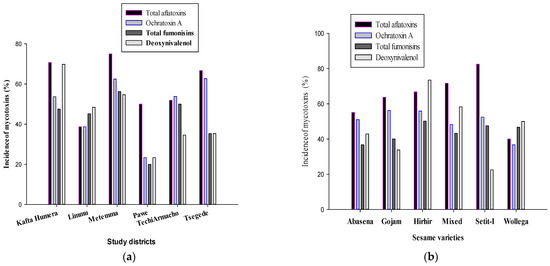
Figure 4.
Incidences of four important mycotoxins (%) across study districts (a) and sesame varieties (b).
3.3. Occurrence of Mycotoxins in Sesame across Varieties and Districts in Ethiopia
Table 4 presents the mean concentrations and standard errors (SE) of four types of mycotoxins (total aflatoxins, ochratoxin A, fumonisins, and deoxynivalenol) found in sesame samples collected from five different districts and various sesame varieties in Ethiopia.

Table 4.
Types and levels (mean ± SE) of mycotoxins present in samples collected from five districts and sesame varieties of Ethiopia (n = 470).
3.3.1. Total Aflatoxin
Total aflatoxins were found in 65.3% of the collected sesame samples across the districts. Total aflatoxin concentration varied across districts, with the highest being in Limmu (17.6 ± 1.4 μg/kg) and the lowest in Kafta Humera (12.8 ± 0.4 μg/kg). Among varieties, the Wollega variety showed the highest concentration (17.6 ± 1.3 μg/kg), while Hirhir and Mixed varieties had the lowest concentrations (13.1 ± 0.4 μg/kg and 12.5 ± 0.7 μg/kg, respectively). The differences in total aflatoxin concentrations among districts and varieties were statistically significant (p < 0.001).
3.3.2. Ochratoxin A
Ochratoxin A was detected in 52.5% of the sesame samples. Ochratoxin A concentration was found in Pawe (7.9 ± 0.4 μg/kg), and the lowest in Kafta Humera (6.7 ± 0.1 μg/kg). In terms of varieties, the highest concentration was observed in the Abasena variety (7.6 ± 0.2 μg/kg), and the lowest was observed in Hirhir (6.8 ± 0.1 μg/kg). The variations in ochratoxin A levels were statistically significant across districts (p < 0.001).
3.3.3. Total Fumonisins
Overall, 45.7% of samples contained total fumonisins, with concentrations ranging from 300 to 1300 μg/kg, with a mean of 830 μg/kg. Furthermore, Metema sesame samples had the highest incidence of total fumonisin, while Pawe district sesame samples had the lowest. However, there was no statistically significant (p > 0.05) variation in fumonisin concentration among growing districts or sesame varieties.
3.3.4. Deoxynivalenol
Deoxynivalenol was the second most prevalent toxin, detected in 23.3–69.8% of samples, with a mean incidence of 58.3%. Deoxynivalenol concentrations in detected sesame samples ranged from 300 to 900 μg/kg, with a mean value of 700 μg/kg. Among the five districts studied, sesame samples from Pawe (450 ± 0.1 μg/kg) had the lowest mean concentration of deoxynivalenol. There were no statistically significant differences among the mean deoxynivalenol concentrations in samples from the other four districts (Kafta Humera, Limmu, Metema, Tech Armachiho, and Tsegede). The current study that sesame samples from Pawe had lower levels of ochratoxin A, total fumonisin, and deoxynivalenol than samples from any of the other study districts.
Total aflatoxin (r = 0.5), fumonisins (r = 0.2), deoxynivalenol (r = 0.3), moisture (r = 0.3), and humidity (r = 0.3) were positively correlated with grain age but negatively correlated with kernel germination (Table 5). Kernel infection and all mycotoxin levels rose as sesame grain aged in farmer storage, with a decrease in seed germination percentage, followed by an increase in moisture and temperature. Moisture contents and relative humidity were positively correlated with total aflatoxin (r = 0.34), fumonisins (r = 0.12), and deoxynivalenol (r = 0.11) but negatively correlated with germination (Table 5).

Table 5.
The relationship between sesame grain age, mycotoxin, kernel infection, and physical environment.
3.4. Co-Occurrence of Mycotoxins
Mycotoxins continue to attract worldwide attention because of their impacts on human and animal health, agricultural losses, and the potential effects of climate change. The co-occurrence of the mycotoxins was also observed in the majority (74.7%) of the sesame samples (Figure 5). Among tested mycotoxins, only one of them was detected in 16.6% of sesame samples, while none of the mycotoxins were detected in 8.7% (n = 470) of samples. The co-occurrence of AFT-DON and AFT-OTA were the dominant ones observed in 44.0 and 38.3% of sesame samples, respectively. DON-OTA, AFT-FUM, FUM-DON, and FUM-OTA were found in 33.8, 30.2, 29.8, and 26% of sesame samples, respectively. The co-occurrence of all three mycotoxins, such as AFT-DON-OTA, AFT-FUM-DON, and FUM-DON-OTA was found in 25, 22.3, and 18.3% of sesame samples, respectively. The co-occurrence of all four mycotoxins was found in only 13.6% of sesame samples.
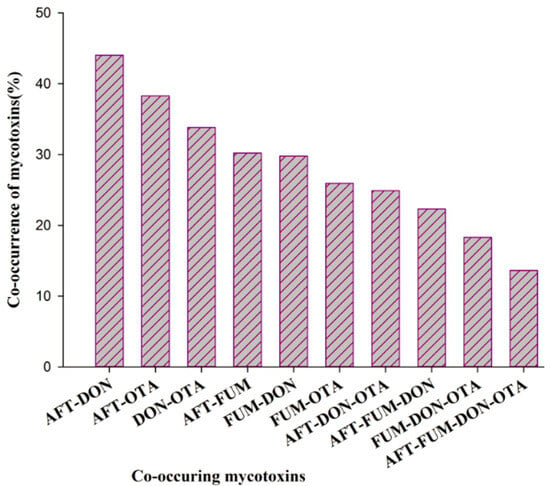
Figure 5.
Co-occurrence of mycotoxins in stored sesame samples (n = 470) of sample districts in Ethiopia.
3.5. Discussion
This research evaluated the occurrence of kernel infection, germination capacity, and the presence of four critical mycotoxins (aflatoxin, deoxynivalenol, fumonisin, and ochratoxin A) in sesame crops from five Ethiopian districts. The findings revealed a range of mycotoxin levels in sesame stored on farms, confirming previous studies conducted in diverse regions, including Uganda, Niger, Mississippi, and Thailand, where similar mycotoxins were detected in sesame samples [24,37,38,39]. A notable portion of the sesame samples (96.8%) had aflatoxin levels below the U.S. Food and Drug Administration’s (FDA) threshold of 20 μg/kg, indicating relatively safe levels for most samples. However, 3.2% of the samples exceeded this limit, and 64.9% surpassed the more stringent European Union (EU) limit of 4 μg/kg. This difference in compliance rates underscores the need for stricter quality control to meet diverse international standards. The aflatoxin levels observed in this study were lower than those reported in other African regions such as Niger and Uganda, possibly reflecting differences in grain types, climates, and storage methods [37,38,40]. In contrast, a study by Mimoune et al. [27] reported lower incidence but higher concentrations of aflatoxins in Chinese sesame samples, diverging from our findings of lower average levels but more widespread contamination. Regarding DON, all sesame samples adhered to the limits set by both the EU (750 μg/kg) and the FDA (1000 μg/kg). Similarly, total fumonisin concentrations complied with the FDA’s maximum allowable limit for human consumption (2000 μg/kg) [41,42]. However, ochratoxin levels exceeded the EU’s maximum permissible level (5 μg/kg) in 49.2% of the samples. This high rate of exceedance suggests that current storage practices in Ethiopia may not adequately prevent ochratoxin accumulation. The co-occurrence of different mycotoxins and the detected levels indicate that storage conditions in Ethiopia are conducive to the growth of mycotoxin-producing fungi. Factors such as moisture content, relative humidity, temperature, storage duration, and facility conditions, as well as the initial seed quality, significantly influence mycotoxin concentrations. Ensuring sesame seeds are dried to an optimal moisture content is crucial for inhibiting the growth of mycotoxin-producing fungi. Seeds should be dried to a moisture content of 6–8% before storage, as levels within this range significantly reduce the risk of fungal proliferation [35]. Utilizing hermetic storage methods can significantly reduce oxygen and moisture ingress, creating an unfavorable environment for fungal growth and mycotoxin production. These findings echo previous studies highlighting the critical role of these factors in mycotoxin development in agricultural products [32,43,44]. Research by Ezekiel et al. [45] and Mrema et al. [46] further emphasizes the impact of moisture, temperature, drying, and storage practices on mycotoxin formation. The duration of grain storage notably affected kernel infection and mycotoxin incidence in this study. Sesame grains stored for eight months exhibited higher levels of moisture, temperature, and relative humidity compared to those stored for shorter durations. This relationship suggests that prolonged storage under suboptimal conditions can significantly exacerbate mycotoxin risks, underscoring the need for improved storage technologies and practices. The study found that sesame seeds stored by farmers showed higher levels of kernel infection and mycotoxins, leading to poorer seed germination. This decline was influenced by elevated moisture content [35]. Table 5 shows that there is a negative association between kernel infection, mycotoxin levels, and seed germination potential. This conclusion is consistent with those of Alkahtani et al. [47] and Wang et al. [48], who reported that fungal invasions affect the integrity of the seed coat and reduce the availability of critical nutrients, impairing germination rates. Additionally, studies by Habib et al. [49] and Nayyar et al. [50] showed that mycotoxins disrupt the seed’s internal processes and hormonal balance, which harms germination. These findings have important implications for agricultural practices, food safety, and environmental health in Ethiopia. Enhancing storage methods and adopting practices that mitigate mycotoxin development can not only improve the safety and quality of sesame seeds but also have broader implications for public health and the sustainability of the agricultural sector. This study contributes valuable insights into the complex interactions between storage conditions, seed quality, and mycotoxin formation, offering guidance for future research and policy development in this area.
3.6. Conclusions
This study established that mycotoxin contamination, particularly aflatoxin, deoxynivalenol (DON), fumonisin, and ochratoxin A, is prevalent in sesame seeds stored across various districts in Ethiopia. This phenomenon is consistent with global observations, underscoring the widespread issue of mycotoxin presence in sesame seeds. Although a majority of the samples complied with the FDA’s limits for total aflatoxins, a significant proportion failed to meet the stricter EU standards, highlighting the need for more robust quality control measures in the context of international trade. The research has identified that storage methods and durations are key factors influencing mycotoxin development in sesame seeds. Traditional storage techniques, such as the use of polypropylene bags, are less effective in preventing contamination, compared to more advanced hermetic storage solutions. Additionally, prolonged storage is linked to heightened mycotoxin risks due to increased moisture, temperature, and relative humidity. Geographical factors, including climate and grain types, also contribute to regional variations in mycotoxin levels. The results in present samples, for instance, showed lower average aflatoxin levels than those reported in other African regions, suggesting the impact of local environmental conditions. The study highlights the importance of managing environmental factors during storage to mitigate mycotoxin risks. Even at low concentrations, the presence of multiple mycotoxins poses significant threats to food safety and public health, particularly in areas where sesame is a dietary staple or a major export commodity.
To address mycotoxin contamination, it is essential to adopt improved storage practices, ensure regulatory compliance, and regularly monitor environmental conditions during storage. Further research is warranted to develop more effective storage solutions and sesame varieties with enhanced resistance to fungal infections and mycotoxin production. In conclusion, this study provides valuable insights for stakeholders in agriculture, food safety, and public health, emphasizing the necessity for collaborative efforts to tackle mycotoxin contamination in sesame seeds.
Author Contributions
S.A. led the original draft preparation and carried out the investigation. F.A.A., J.H., and B.S. provided supervision and resources, enhanced the statistical analysis, and oversaw project administration. K.-M.A. also played a supervisory role. R.M. and J.U. contributed to the methodology and were involved in the review and editing of the manuscript. R.E. hosted the writing phase at Makerere University. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by American people through the United States Agency for International Development (USAID) under the Feed the Future initiative (www.feedthefuture.gov) with grant number AID-OAA-L-14-00002. The contents are the responsibility of the Innovation Lab for the Reduction of Post-harvest Loss (www.k-state.edu/phl) and do not necessarily reflect the views of USAID or the United States Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors affirm that the information regarding personal interests presented above is accurate to the best of their knowledge. They are conscious of their obligations to mitigate any real or perceived conflicts of interest in their public roles. All authors have substantially contributed to the development, analysis, and interpretation of the study and drafting and critical review of the manuscript for important intellectual content and have given their final approval of the version to be published. This manuscript has not been submitted to, nor is under review by, any other journal or publication platform. The authors declare no conflicts of interest concerning the publication of this article.
References
- UNCTAD (United Nations Conference on Trade and Development). National Green Export Review of Ethiopia: Leather and Sesame Seeds; UNCTAD: Geneva, Switzerland, 2018; p. 57. [Google Scholar]
- FAOSTAT (Food and Agriculture Organization/Statistics Database). Food and Agriculture Organization Statistical Databases. 2023. Available online: http://faostat.fao.org/ (accessed on 5 February 2023).
- Laurentin, H.E.; Karlovsky, P. Genetic relationship and diversity in a sesame (Sesamum indicum L.) germplasm collection using amplified fragment length polymorphism (AFLP). BMC Genet. 2006, 7, 1–10. [Google Scholar] [CrossRef]
- Teklu, D.H.; Shimelis, H.; Tesfaye, A.; Abady, S. Appraisal of the sesame production opportunities and constraints, and farmer-preferred varieties and traits, in eastern and southwestern Ethiopia. Sustainability 2021, 13, 11202. [Google Scholar] [CrossRef]
- Boere, A.; Rutgers, T.; Willems, D.; Kidane, D.; Dolfen, W. Business Opportunities Report: Oilseeds and pulses. In Proceedings of the Ethiopian Netherlands Business Event, Rijswijk, The Netherlands, 5–6 November 2015. [Google Scholar]
- Francom, M.G.; Counselor, A. Ethiopia’s Oilseed Sector Set to Expand; USDA: Washington, DC, USA, 2018; Volume 19, p. 15. [Google Scholar]
- Terefe, G.; Wakjira, A.; Berhe, M.; Tadesse, H. Sesame Production Manual; Ethiopian Institute of Agricultural Research, Embassy of the Kingdom of the Netherlands, EIAR: Addis Ababa, Ethiopia, 2012; p. 49. [Google Scholar]
- Zerihun, J. Sesame (Sesame indicum L.) crop production in Ethiopia: Trends, challenges, and future prospects. Sci. Technol. Arts Res. J. 2012, 1, 1–7. [Google Scholar] [CrossRef]
- Shephard, G.S. Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. 2008, 25, 1246–1256. [Google Scholar] [CrossRef]
- Jeyalakshmi, C.; Rettinassababady, C.; Nema, S. Integrated management of sesame diseases. J. Biopestic. 2013, 6, 68. [Google Scholar]
- Sabry, B.A.; Hathout, A.S.; Nooh, A.; Aly, S.E.; Shehata, M.G. The prevalence of aflatoxin and Aspergillus parasiticus in Egyptian sesame seeds. Int. J. ChemTech Res. 2016, 9, 308–319. [Google Scholar]
- Jimoh, K.O.; Kolapo, A.L. Mycoflora and aflatoxin production in market samples of some selected Nigerian foodstuffs. Res. J. Microbiol. 2008, 3, 169–174. [Google Scholar]
- Wu, F. Mycotoxin Risk Assessment for the Purpose of Setting International Regulatory Standards. Environ. Sci. Technol. 2004, 38, 4049–4055. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Sheir, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2019, 59, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Gulderen, Y.; Bucket, E.; Muzaffer, G.O.; Aysel, B.O. Determination of aflatoxins in peanut butter and sesame samples using a high-performance liquid chromatography method. Eur. Food Res. Technol. 2006, 224, 167–170. [Google Scholar] [CrossRef]
- Li, F.Q.; Li, Y.W.; Wang, Y.R.; Luo, X.Y. Natural occurrence of aflatoxins in Chinese peanut butter and sesame paste. J. Agric. Food Chem. 2009, 57, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Idris, Y.M.; Mariod, A.A.; Elnour, I.A.; Mohamed, A.A. Determination of aflatoxin levels in Sudanese edible oils. Food Chem. Toxicol. 2010, 48, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Hosseininia, A.R.; Vahabzadeh, M.; Rashedinia, M.; Riahi-Zanjani, B.; Karimi, G. A survey of aflatoxins in sesame seeds imported into Khorasan Province, Iran. Mycotoxin Res. 2014, 30, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Kollia, E.; Tsourouflis, K.; Markaki, P. Aflatoxin B1 in sesame seeds and sesame products from the Greek market. Food Addit. Contam. B 2016, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Fapohunda, S.O.; Anjorin, T.S.; Sulyok, M.; Krska, R. Profile of major and emerging mycotoxins in sesame and soybean grains in the Federal Capital Territory, Abuja, Nigeria. Eur. J. Biol. Res. 2018, 8, 121–130. [Google Scholar]
- Ezekiel, C.N.; Sulyok, M.; Warth, B.; Krska, R. Multi-microbial metabolites in fonio millet (acha) and sesame seeds in Plateau State, Nigeria. Eur. Food Res. Technol. 2012, 235, 285–293. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and post-harvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Abbas, H.K.; Ebelhar, M.W.; Bellaloui, N.; Mulvaney, M.J.; Stoner, G.R.D.; Kotowicz, J.K.; Little, N.S.; Accinelli, C.; Shier, W.T. Contamination of sesame seed grown in Mississippi with aflatoxin, fumonisin, and mycotoxin-producing fungi. World Mycotoxin J. 2019, 12, 123–132. [Google Scholar] [CrossRef]
- Bradford, K.J.; Dahal, P.; Van Asbrouck, J.; Kunusoth, K.; Bello, P.; Thompson, J.; Wu, F. The dry chain: Reducing post-harvest losses and improving food safety in humid climates. Trends Food Sci. Technol. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Mimoune, N.A.; Riba, A.; Verheecke, C.; Mathieu, F.; Sabaou, N. Fungal contamination and mycotoxin production by Aspergillus spp. In nuts and sesame seeds. J. Microb. Biotech. Food Sci. 2016, 5, 301–305. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Worldwide Regulations for Mycotoxins in Food and Feed. In FAO Food and Nutrition Paper 81; FAO/UN: Rome, Italy, 2004. [Google Scholar]
- WHO (World Health Organization). Evaluation of Certain Mycotoxins in Foods. Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; Technical Report No. 906; WHO: New York, NY, USA, 2002. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Amer. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Sahile, S.; Assefa, N. Evaluation of Aflatoxins and Storage Fungi in Sesame, Chickpea and Faba Bean Export Commodities from Gondar Town, North West Ethiopia. Adv. Res. J. Multidiscip. Discov. 2018, 25, 76–82. [Google Scholar]
- Armstrong, P.R.; Maghirang, E.B.; Bhadriraju, S.; McNeill, S.G. Equilibrium Moisture Content of Kabuli Chickpea, Black Sesame, and White Sesame Seeds. Appl. Eng. Agric. 2017, 33, 737–742. [Google Scholar] [CrossRef]
- Altaf, N.; Khan, S.A.; Ahmad, M.; Asghar, R.; Ahmed, R.A.; Shaheen, S.; Saqib, M. Seed borne mycoflora of sesame (Sesamum indicum L.) and their effects on germination and seedling. Pak. J. Biol. Sci. 2004, 7, 243–245. [Google Scholar] [CrossRef][Green Version]
- Alemayehu, S.; Abay, F.; Ayimut, K.M.; Assefa, D.; Chala, A.; Mahroof, R.; Subramanyam, B. Evaluating different hermetic storage technologies to arrest mold growth, prevent mycotoxin accumulation and preserve germination quality of stored chickpea in Ethiopia. J. Stored Prod. Res. 2020, 85, 26. [Google Scholar] [CrossRef]
- Romer Labs. Romer Labs Methods, Method: PI-000073-1, PI-000082-1, PI 000083-1, PI-000093-1; Romer Labs: Getzersdorf, Austria, 2007. [Google Scholar]
- Echodu, R.; Malinga, G.M.; Kaducu, J.M.; Ovuga, E.; Haesaert, G. Prevalence of aflatoxin, ochratoxin and deoxynivalenol in cereal grains in northern Uganda: Implication for food safety and health. Toxicol. Rep. 2011, 6, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Esan, A.O.; Fapohunda, S.O.; Ezekiel, C.N.; Sulyok, M.; Krska, R. Distribution of fungi and their toxic metabolites in melon and sesame seeds marketed in two major producing states in Nigeria. Mycotoxin Res. 2020, 36, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Pongpraket, M.; Poapolathep, A.; Wongpanit, K.; Tanhan, P.; Giorgi, M.; Zhang, Z.; Li, P.; Poapolathep, S. Exposure assessment of multiple mycotoxins in black and white sesame seeds consumed in Thailand. J. Food Prot. 2020, 83, 1198–1207. [Google Scholar] [CrossRef]
- Anthony, M.H.; Ojochenemi, A.D.; Yemi, A.H.R.; Tahir, N.A.G.A.G.O.; Okechukwu, O.J.; Saidu, M.A.; Ayobami, O.B. Determination of aflatoxins in sesame, rice, millet and acha from Nigeria using HPLC. Chem. Sci. Trans. 2014, 3, 1516–1524. [Google Scholar]
- US Food and Drug Administration. Guidance for industry: Action levels for poisonous or deleterious substances in human food and animal feed; USFDA: Washington, DC, USA, 2000. [Google Scholar]
- EU (European Commission). Commission Regulation (EU) 2023/915 of 1 June 2023 amending Regulation (EC) No 1881/2006; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Worku, A.F.; Merkuz, A.; Kalsa, K.K.; Tenagashaw, M.W.; Habtu, N.G. Occurrence of mycotoxins in farm-stored wheat in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 25. [Google Scholar] [CrossRef]
- Sauer, D.B. Effects of fungal deterioration on grain: Nutritional value, toxicity, germination. Int. J. Food Microbiol. 1988, 7, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Udom, I.E.; Frisvad, J.C.; Adetunji, M.C.; Houbraken, J.; Fapohunda, S.O.; Samson, R.A.; Atanda, O.O.; Agi-Otto, M.C.; Onashile, O.A. Assessment of aflatoxigenic Aspergillus and other fungi in millet and sesame from Plateau State, Nigeria. Mycology 2014, 5, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mrema, G.; Gumbe, L.O.; Chepete, H.J.; Agullo, J. Grain crop drying, handling and storage. In Rural Structures in the Tropics: Design and Development; FAO: Rome, Italy, 2011; Volume 3, pp. 363–411. [Google Scholar]
- Alkahtani, M.D.; Mazen, M.M.; El-Naggar, M.A.; Arfa, M.K. The relationship between some mycotoxins excretion and bean seed discoloration. J. Plant Sci. 2011, 6, 182. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Li, B.; Shang, H.; Ma, R.; Zhu, Y.; Yang, X.; Ju, S.; Zhao, W.; Sun, H.; Zhuang, J.; et al. Effective inhibition of fungal growth, deoxynivalenol biosynthesis and pathogenicity in cereal pathogen Fusarium spp. by cold atmospheric plasma. Chem. Eng. J. 2022, 437, 135307. [Google Scholar] [CrossRef]
- Habib, A.; Sahi, S.T.; Javed, N.; Ahmad, S. Prevalence of seed-borne fungi on wheat during storage and its impact on seed germination. Pak. J. Phytopathol. 2011, 23, 42–47. [Google Scholar]
- Nayyar, B.G.; Akram, A.; Akhund, S.; Rafiq, M. Seed viability test and pathogenecity assessment of most prevalent fungi infecting Sesamum indicum L. IOSR J. Pharm. Biol. Sci. (IOSR-JPBS) 2014, 9, 21–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).