Abstract
Combining the application of nitrogen and phosphorus is widely recognized as an effective measure to promote seedling growth. The purpose of this study was to investigate the effects of the combined application of nitrogen and phosphorus on the growth and nutrient status of C. camphora container seedlings. Exponential fertilization was applied to a range of fertilizers, including single nitrogen fertilizer, single phosphorus fertilizer, and combinations of nitrogen and phosphorus to C. camphora. Eight distinct fertilization levels of treatment (CK, N1, N2, N3, P, N1P, N2P, and N3P) were established. The height and ground diameter of the seedlings were determined periodically in each treatment group. Biomass, root system (including root length, root surface area, and root volume), and nutrient accumulation (content of soluble sugars, soluble proteins, and starch in the root system, stems, and leaves) were determined for each treatment group of seedlings. The N3P treatment (N: 9000 mg∙plant−1 + P: 6000 mg∙plant−1) had the most significant effect on the height and ground diameter of C. camphora container seedlings, while the N3 treatment (N: 9000 mg∙plant−1) proved to be the most beneficial for biomass accumulation among the eight different fertilizer levels. In addition, a single P fertilizer (P: 6000 mg∙plant−1) emerged as the most effective fertilizer for enhancing root morphology (root length, root surface area, root volume, and root average diameter) and accumulating nutrient composition (soluble sugar, soluble protein, and starch). Furthermore, it was discovered that a high level of nitrogen fertilization hindered the accumulation of nutrient composition in C. camphora container seedlings. Our comprehensive analysis revealed that nitrogen fertilizer promoted the morphological growth of C. camphora container seedlings, while phosphorus fertilizer proved to be the most beneficial for root growth and nutrient composition accumulation. Additionally, it was emphasized that controlling the quantity of nitrogen fertilizer is also a crucial measure.
1. Introduction
Cinnamomum camphora is an evergreen tree belonging to the Cinnamomum genus of the Lauraceae family. It is a tall tree with dense foliage and a beautiful tree shape, which can be used as street and shade tree that provides excellent afforestation [1]. C. camphor has a strong adaptive capacity and is widely distributed in areas to the south and southwest of the Yangtze River. As a species fond of light, C. camphor prefers warm and humid climates. It grows mainly on sunny slopes, valleys and riverbank flats with fertile soil. The plant is an economically valuable tree species with both direct cultivation and multi-objective management value [2,3]. Nowadays, urban greening has become an important part of urban construction. C. camphora is the main cultivated tree species for urban landscaping because of its usefulness as timber [4], ornamental [5], oil [6,7], afforestation [5] and other multifunctional values. It has become the first choice for many areas of landscaping in southern China. However, in accordance with the combined effects of global climate change and the destruction of ecological resources, the number of C. camphora trees is gradually decreasing, which results in more demand over supply in the market. Therefore, the protection and development of C. camphora resources should be strengthened to increase the number of C. camphora to meet the market demand.
High-quality seedlings are the basis of successful afforestation. Container seedlings often perform better than bare-root seedlings in unfavorable environmental conditions [8,9]. However, the gradual release of nutrients from the growth media necessitates the use of fertilization to improve plant growth and strengthen resistance to biotic and abiotic stresses. Fertilization is an important measure in seedling cultivation, which can promote seedling growth, soil fertility, and seedling quality [10]. At present, numerous scholars have begun to pay attention to the study of C. camphora seedlings, mainly from the aspects of landscaping [1,11], gene identification [12,13], pest control [7] and other aspects. However, there have been relatively few studies about the effect of fertilization on nutrient accumulation and the growth of C. camphora container seedlings. There are currently no specific fertilization regimes for the cultivation of the seedlings for various purposes. In this regard, it is necessary to study the effects of nitrogen-phosphorus synergy on the growth and nutrient accumulation of C. camphora container seedlings. In this experiment, one-year-old C. camphora seedlings were utilized as the test material and exponential fertilization involving the combined application of nitrogen and phosphorus was employed. The reasons for the appearance of various phenomena were analyzed by determining their growth and physiological indicators under different treatments. The purpose of this study is to reveal the C. camphora seedlings’ performances based on the functioning status of different fertilization, in order to establish an appropriate fertilizer quantity and provide a theoretical basis for effective cultivation in the future.
2. Materials and Methods
2.1. Experimental Materials
The experimental site was located in the seedling cultivation base of Nanjing Forest University’s Xiashu forest farm in Zhenjiang, Jiangsu province (32°7′29″ N, 119°13′9″ E). This location has a north subtropical monsoon climate, with four distinct seasons, abundant rainfall, and sufficient light. The mean annual precipitation is about 1058.8 mm, the annual average sunlight is 2157 h, the frost-free period is about 233 days, and the annual average temperature is 15.2 °C. It can provide good environmental conditions for the growth of northern subtropical tree species.
In early November 2021, the C. camphora seeds (Zhangshu, China) were collected in Zhangshu City, Jiangxi Province, and cleaned promptly. These seeds were then disinfected with 0.5% KMnO4 (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China) and stratified at a low temperature. In late February 2022, the stratified seeds were washed and seeded into white non-woven containers (20 cm × 20 cm, diameter × height) (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China). Non-woven bags are usually made from two materials: polypropylene (PP) or polyethylene terephthalate (PET, also known as polyester). Natural soil (Nanjing Forest University’s Xiashu Forest Farm, Zhenjiang, China) was used as the main substrate in the containers. The test substrate was a mixture of natural soil, peat (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China), and perlite (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China) in a ratio of 5:3:2 by volume. A total of 2–3 seeds were put into each non-woven container, the seeding hole was filled with the substrate and placed on the greenhouse seedling bed (Nanjing Forest University’s Xiashu forest farm, Zhenjiang, China). When the seedling height reached about 10 cm, the seedlings in the container were thinned properly. Weak seedlings were removed, strong seedlings were retained, and only one seedling was kept in each non-woven container.
2.2. Experimental Fertilization Design
The experiment was a single-factor randomized block design, a total of 8 treatments were conducted, including CK: no fertilization, N1: low-level nitrogen (N: 3000 mg∙plant−1), N2: medium-level nitrogen (N: 6000 mg∙plant−1), N3: high-level nitrogen (N: 6000 mg∙plant−1), P: single phosphorus (P: 6000 mg∙plant−1), N1P: low level of N + P (N: 3000 mg∙plant−1 + P: 6000 mg∙plant−1), N2P: medium level of N + P (N: 6000 mg∙plant−1 + P: 6000 mg∙plant−1), N3P: high level of N + P (N: 9000 mg∙plant−1 + P: 6000 mg∙plant−1). Three replicates were set up for each treatment with 15 seedlings per replicate, and a total of 45 seedlings per treatment. The nutrient status of the test substrate and the initial nutrient composition of the seedlings were observed, combined with the pre-experiment and a review of the relevant literature. Based on the analysis of previous studies, it was concluded that the optimal exponential P application rate for C. camphora seedlings is 6000 mg per seedling [14]. The optimal N application amount was also 6000 mg per seedling, which was set as a medium N level; 0.5 times (3000 mg per seedling) of medium N level was set as a low N level; and 1.5 times (9000 mg per seedling) of medium N level was set as a high N level [15]. Calcium superphosphate (12% P mass fraction) was chosen as P fertilizer (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China), and urea (46% N mass fraction) was used as N fertilizer (Nanjing Zebra Experimental Equipment Co., Ltd., Nanjing, China). (According to many studies conducted on C. camphora, it was found that the initial NPK content of the substrate varied considerably. Therefore, fertilizer experiments can only be conducted using fertilizer application amounts proposed by other scientists as a reference and in conjunction with existing conditions. It cannot be guaranteed that the initial NPK content of the selected substrate will be consistent with that in the existing literature).
The amount of fertilizer per application was calculated using the exponential fertilization formula [16] as shown in Table 1. The amount of fertilizer calculated in Table 1 for each application was dissolved into 900 mL of water. With the water-soluble regime, each seedling was applied with 20 mL solution at a time, and the specific nitrogen application amount was shown in Table 1. The fertilizer solution was thoroughly shaken before injection to ensure uniform distribution into each C. camphora container seedling. Based on the study of the annual growth rhythm of C. camphora seedlings, fertilization began on 29 June and ended in late September, during which fertilization was conducted once every 7 days, with a total of 14 times.

Table 1.
The design of fertilizer treatments.
2.3. Samplings and Determination
2.3.1. Seedling Height and Ground Diameter
The first-time measurement was conducted on 29 June (no fertilizer was applied at this time), ten C. camphora container seedlings were randomly selected for measurement. On 29 July, 29 August, 29 September, and 29 November, ten C. camphora container seedlings were randomly chosen for determination in each treatment. Seedling height and ground diameter were determined using a tape measure (0.1 cm accuracy) (Nanjing Forestry University, Nanjing, China) and vernier calipers (0.01 mm accuracy) (Nanjing Forestry University, Nanjing, China) for a total of 5 times, with consistent position for each measurement.
2.3.2. Root System Indexes
In late November, 9 container seedlings were chosen from each treatment (a total of 72 seedlings) and taken back to the laboratory. After cleaning the roots of the 72 seedlings with water, the WinRHIZO plant root scanning system (Regent Instruments Inc., Quebec, QC, Canada) was used to scan the roots. The total root length, total root surface area, total root volume, and average diameter of seedlings were analyzed.
2.3.3. Biomass
After washing with tap water and drying, seedlings were dissected into leaves, stems, and roots, respectively. These dissected seedlings were put in separate envelopes and placed into the oven (Yiheng, Shanghai, China). The oven temperature was initially set at 105 °C for 30 min to inactivate them and then adjusted to 70 °C to dry them to a constant weight subsequently. Eventually, an electronic balance (0.001 g accuracy) (Sartorius, Göttingen, Germany) was utilized to measure the dry weight of each part separately.
2.3.4. Nutrient Composition Contents
The container seedlings, which had their biomass measured previously, were ground into a powder form and sieved. The roots, stems, and leaf samples (0.2 g) from 9 seedlings were separately collected to determine soluble sugar content and starch content by Anthrone colorimetric method [17]. In addition, the 0.2 g of roots, stems, and leaf samples were selected to measure soluble protein content by Coomassie brilliant blue [18].
2.4. Data Statistics and Analysis
The measurement results are expressed as mean ± standard deviation. Microsoft Excel 2010 was adopted for the data processing, analysis, and drawing of various indicator changes. SPSS 23.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis of variance and Duncan multiple comparisons (p < 0.05 indicates a significant difference).
3. Results
3.1. Seedling Height and Ground Diameter
The effects of the application of N and P combined on the height and ground diameter of C. camphora container seedlings are shown in Table 2 and Table 3. Fertilization has a significant effect on the growth of height and ground diameter in C. camphora container seedlings. At the beginning of fertilization (29 June), the height and ground diameter of C. camphora container seedlings were basically 12.94 cm and 1.85 mm, respectively, and both morphological indexes reached their maximum on 29 November. Comparing the height and ground diameter of C. camphora container seedlings by various fertilizer treatments, it was found that the height and diameter of the N2, N3, and N3P treatments were significantly higher than the other treatments. Among them, seedling height (47.43 cm) and ground diameter (5.27 mm) were highest with the N3P treatment on 29 November, respectively, which were significantly different from CK (p < 0.05). This finding demonstrated that the combined application of N and P can promote the growth of height and ground diameter of C. camphora container seedlings, especially high N level + P fertilizer application is more conducive to seedlings growth.

Table 2.
Effects of N and P combined application on height of C. camphora container seedlings. CK, N1, N2, N3, P, N1P, N2P and N3P indicate that there is no fertilization, low-level nitrogen, medium-level nitrogen, high-level nitrogen, phosphorus only, low level of N + P, medium level of N + P, and high level of N + P, respectively (The same as below).

Table 3.
Effects of N and P combined application on and ground diameter of C. camphora container seedlings.
Each value represents the means ± standard deviation. The lowercase letters a, b, c, and d in Table 2 and Table 3 represent the differences between each treatment, respectively. In addition, different lowercase letters in the same column represent significant difference between treatments (p < 0.05) and the same letter indicates no significant difference in the different treatments (p > 0.05). (The same as below) Among them, 6.29, 29 June; 7.29, 29 July; 8.29, 29 August; 9.29, 29 September; and 11.29, 29 November.
3.2. Root System
Table 4 indicates the effects of the combined application of N and P on the root growth of C. camphora container seedlings. Various fertilization treatments have different effects on the root system of C. camphora. As general knowledge, fertilization can enhance the integral growth of its root system. In this experiment, root length (341.57 cm), root surface area (180.68 cm2), root volume (8.29 cm3), and mean root diameter (2.36 mm) were the largest under single P fertilizer treatment, and there were apparent discrepancies between all of them and CK (p < 0.05). It indicates that single P application has a significant promoting effect on the growth of root system of C. camphora container seedlings.

Table 4.
Effects of combined N and P application on root growth of container C. camphora seedlings.
3.3. Biomass
The impacts of combined N and P applications on the biomass of C. camphora container seedlings are presented in Figure 1. Various fertilization treatments had different impacts on biomass in each part of C. camphora. The biomass of various parts was ranked from largest to smallest as follows: leaves > stems > roots. The underground part (roots: 1.86 g) of the biomass under single P treatment was the maximum. Compared to the control group (CK), the root biomass of single P treatment elevated by 138.99% and showed a significant difference (p < 0.05). N3 treatment was the most beneficial for the growth of the aboveground part (stems: 3.73 g, leaves: 5.92 g), which was higher by 468.34% and 273.28%, respectively, compared to CK. Evident differences occurred between N3 treatments and CK (p < 0.05). From Figure 1, it can be seen that biomass in all parts of C. camphora container seedlings increased significantly with elevating levels of N. In general, the biomass of the aboveground part (stems and leaves) benefited the most at the high N level (N3), and the single P treatment most profited the underground part (roots).
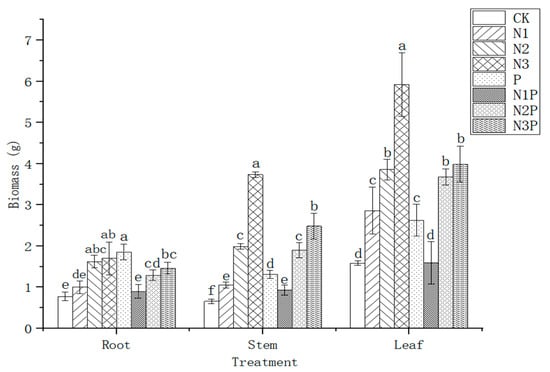
Figure 1.
Effects of combined N and P application on the biomass of C. camphora container seedlings. The error line in the figure is the mean ± standard error, and different lowercase letters in the same treatment indicate significant differences at the 0.05 level.
3.4. Nutrient Composition Contents
As shown in Figure 2, N and P combined application affect soluble sugar content, soluble protein content and starch content of C. camphora container seedlings. We found that soluble sugar, soluble protein and starch contents in the leaves are the highest, indicating that nutrient composition is mainly concentrated in the leaves. Comparing different treatments, the content of soluble sugar, soluble protein and starch in the leaves of C. camphora with single P fertilizer reached the highest values of 205.23 mg∙g−1 FW, 3.14 mg∙g−1 FW and 83.13 mg∙g−1 FW, respectively. They were significantly different from CK (p < 0.05). It is demonstrated that P fertilizer had the most significant facilitating effect on the accumulation of soluble sugar, soluble protein and starch in C. camphora seedlings leaves. In addition, it was also found that the nutrient composition content of all parts of C. camphora gradually decreased with increasing levels of nitrogen application. And N2, N2P, N3, and N3P treatments were significantly lower than those of CK (p < 0.05). The results indicated that the accumulation of soluble sugar, soluble protein and starch contents of all organs decreased with the increase of N fertilizer content. Therefore, it is suggested that N fertilizer should be applied in controlled amounts. Excessive N fertilizer not only restrains the accumulation of nutrient composition in C. camphora container seedlings but also inhibits their growth.
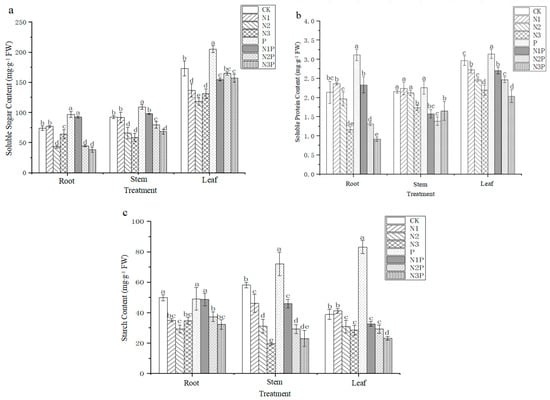
Figure 2.
Effects of combined N and P application on soluble sugar (a), soluble protein (b), and starch (c) content of C. camphora container seedlings. Note: The error line in the figure is the mean ± standard error, and different lowercase letters in the same treatment indicate significant differences at the 0.05 level.
4. Discussion
4.1. Morphological Growth
Nitrogen and phosphorus are key factors in plant growth that have a critical impact on plant development and nutrient accumulation. Plant growth and root morphology are important parameters for evaluating the nutrient supply [19]. This is mainly reflected in seedling height increase, ground diameter thickening, root growth, and biomass accumulation. Nitrogen and phosphorus, as essential nutrients, are supplied to plants through fertilization to meet their growth requirements, as well as to improve seedling quality and biomass accumulation [20]. Therefore, nitrogen and phosphorus fertilization has been a research hotspot for many scholars around the world. The study of Roy et al. [21] found that the growth-related parameters (seedling height, ground diameter, etc.) of Tamarix chinensis improved significantly with a high concentration of water + N + P additions. Souza et al. [22] found that high levels of N and P combined applications were most effective in promoting the growth of Peltophorum dubium seedling height and ground diameter. The above conclusions are consistent with the present experimental results. In this study, the combined application of N and P can significantly promote the height and ground diameter growth in C. camphora container seedlings. The height and ground diameter of C. camphora container seedlings increased gradually over time, with N3P treatment showing the most significant enhancement. The results showed that nitrogen fertilizer enhanced seedling growth more obviously compared to phosphorus fertilizer. The higher the concentration of N fertilizer, the more beneficial it was to the growth of the seedlings.
Biomass is considered an important indicator that reflects plant productivity, whereas nitrogen fertilizer affects biomass distribution in various parts of the plant [23]. Previous studies discovered that N fertilizer resulted in a significant improvement in the biomass content of the entire Pinus koraiensis seedlings [24] and in parts of the stems and leaves of Guindilia trinervis [25]. The findings of this research align with the above findings. In the present experiment, it was found that the leaves of C. camphora container seedlings accumulated the highest biomass content, with the biomass accumulation of various parts of seedlings ranking from largest to smallest: leaves > stems > roots. This may be attributed to the ability of N fertilizer to enhance the chlorophyll content in the leaves, thereby accelerating the production and operation of photosynthetic products. In particular, the biomass content of all parts of C. camphora container seedlings treated with N3 treatment was the highest. This indicates that the application of N fertilizer significantly promoted the accumulation of biomass in C. camphora container seedlings, and single-factor fertilization was more effective than the combined application of N and P.
The root system is an essential organ for the absorption of water and nutrients in plants. Root length, root surface area, root volume, and root average diameter play a decisive role in nutrient uptake. Accordingly, studying the morphological characteristics of the root system is extremely important [24]. Previous studies have shown that phosphorus plays an important role in lateral root morphology and root branching [26], affecting not only root development but also nutrient availability [27]. Existing studies indicated that P fertilizers have a more pronounced role in promoting root growth [28]. Some studies have found that phosphorus application significantly increased the average root diameter and total root length of legumes [29] and herbs [27], and that root length, area, volume, and biomass growth of exponential-fertilized seedlings were significantly better than those of conventional fertilization [30]. This is similar to the results of this study. In the current experiment, root length, root surface area, root volume, and root average diameter grew best under single P fertilizer treatment. It indicates that P fertilizer is more beneficial for the formation and growth of the early root system of the plant, which can improve its ability to adapt to the external environment.
4.2. Nutrient Status
Soluble sugar and starch are nutrient compositions stored in plant tissues that reflect the nutrient status of the plant. It can also provide energy and metabolic intermediates for plant growth [31,32]. Soluble proteins are important osmoregulatory substances that play a protective role in vital cellular substances and biological membranes [33]. Several studies have shown that fertilization can promote the accumulation of nutrients in plants, which in turn enhances plant growth and improves stress tolerance [34,35]. For example, fertilization was able to facilitate soluble sugar synthesis and chlorophyll content increase in Tsoongiodendron odorum leaves [36]. It can improve soluble protein content in Berberidopsis corallina and enhance its growth [37]. Nitrogen fertilization could also increase the starch concentration in Picea rubens leaves [38]. In this study, the accumulation of soluble sugar, soluble protein and starch contents in C. camphora container seedlings was most significant with a single application of P fertilizer. Previous studies have found that phosphorus fertilization facilitated an increase in soluble sugar content in fruits of the citrus species [39], non-structural carbohydrate content in Pinus elliottii [40], and soluble protein content in Vigna radiata [41] and Solanum tuberosum [42]. The above studies were similar to the results of this research. However, it has also been observed that the addition of N and P inhibits the accumulation of soluble sugars, soluble proteins, and starch in various plants, including understory woody species Salvinia minima, herbaceous species such as Stipa grandis and Leymus chinensis [43,44,45]. This diverse accumulation of nutrient composition accumulation in plants could be attributed to differences in plant species, the quantity and type of fertilizer applied as well as the concentration of the fertilizer. In addition, some studies have shown that the non-structural carbohydrate content in Moringa oleifera [46] and Pinus armandii [47] was greatly reduced with increasing levels of fertilization. This is similar to the results of the present study. In this experiment, soluble sugar content, soluble protein content and starch content gradually decreased with the increase of nitrogen fertilizer content. When excessive fertilizer is applied, it can inhibit nutrient accumulation and plant growth instead. Therefore, it suggests that the amount of fertilizer should be controlled to promote plant growth.
5. Conclusions
In summary, the application of fertilizer led to an increase in the height, ground diameter, biomass, root morphology, and nutrient status of C. camphora container seedlings. Results indicated that the N3P treatment was optimal for enhancing seedling height and ground diameter, while the N3 treatment proved to be most beneficial for biomass accumulation. Furthermore, the application of single P fertilizer emerged as the most effective treatment for improving root length, surface area, volume, average diameter, and the content of soluble sugar, soluble protein, and starch in leaves. Therefore, the combined application of N and P promoted the morphological growth of C. camphora seedlings, especially at high N levels, whereas the use of single P fertilizer is more conducive to root system development and nutrient accumulation.
Author Contributions
Conceptualization, X.M.; methodology, X.M. and G.Z.; software, X.M.; validation, F.Y. and Z.L.; formal analysis, X.M.; investigation, X.M.; resources, F.H.; data curation, X.M. and G.Z.; writing—original draft preparation, X.M.; writing—review and editing, F.Y. and Z.L.; visualization, F.H.; supervision, F.Y.; project administration, F.H.; funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangxi Provincial Innovative Project for Forest Sciences and Technology (202116).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
Thanks to Fangyuan Yu for supporting this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhatta, S.P.; Sharma, K.P.; Balami, S. Variation in carbon storage among tree species in the planted forest of Kathmandu, Central Nepal. Curr. Sci. India 2018, 115, 274–282. [Google Scholar] [CrossRef]
- Roszaini, K.; Nor Azah, M.A.; Mailina, J.; Zaini, S.; Mohammad, F.Z. Toxicity and antitermite activity of the essential oils from Cinnamomum camphora, Cymbopogon nardus, Melaleuca cajuputi and Dipterocarpus sp. against Coptotermes curvignathus. Wood Sci. Technol. 2013, 47, 1273–1284. [Google Scholar] [CrossRef]
- Li, Y.Y.; Qi, Y.Q. Study on the dyeing properties of Cinnamomum camphora fruit dyes on wood veneer. J. For. Eng. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.M.; Yan, H.G.; Qi, W.Y.; Lin, J.G.; Li, J.Q. Emission of Volatile Camphor Compounds from Cinnamomum camphora wood. Wood Res. 2020, 65, 663–674. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W.D. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2016, 63, 1049–1061. [Google Scholar] [CrossRef]
- Bhandari, U.; Kumar, A.; Lohani, H.; Chauhan, N. Chemical composition of essential oil of camphor tree (Cinnamomum camphora) leaves grown in Doon Valley of Uttarakhand. J. Essent. Oil Bear. Plants 2022, 25, 548–554. [Google Scholar] [CrossRef]
- Guo, S.S.; Geng, Z.F.; Zhang, W.J.; Liang, J.Y.; Wang, C.F.; Deng, Z.W.; Du, S.S. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; El-Kassaby, Y.A. Bareroot versus container stocktypes: A performance comparison. New For. 2016, 47, 1–51. [Google Scholar] [CrossRef]
- Stoven, A.A.; Mathers, H.M.; Struve, D.K. Fertilizer application method affects growth, nutrient, and water use efficiency of container-grown shade tree whips. Hortscience 2006, 41, 1206–1212. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.H.; Sun, F.B.; Wang, Y.J.; Wu, H.T.; Hu, Z.W.; Zhang, B.B.; Yu, L.; Yan, H.; Shao, F. Capacity of Landscaping Plants to Accumulate Airborne Particulate Matter in Hangzhou, China. Pol. J. Environ. Stud. 2020, 29, 153–161. [Google Scholar] [CrossRef]
- Gong, X.; Shen, T.F.; Li, X.Q.; Lin, H.B.; Chen, C.H.; Li, H.H.; Wu, Z.X.; Liu, Q.L.; Xu, M.; Zhang, B.; et al. Genome-Wide Characterization and Analysis of bHLH Transcription Factors Related to Anthocyanin Biosynthesis in Cinnamomum camphora (‘Gantong 1’). Int. J. Mol. Sci. 2023, 24, 3498. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jia, G.L.; Xin, G.L.; Cai, X. The complete chloroplast genome of Cinnamomum camphora (L.) Presl., a unique economic plant to China. Mitochondrial DNA Part B 2019, 4, 2511–2512. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Shu, X.; Li, X.Q.; Li, X.W.; Huang, F.X.; Li, P. Effects of fertilization on growth and nutrient distribution of Cinnamomum camphora seedlings. Guihaia 2015, 35, 213–220. (In Chinese) [Google Scholar]
- Shu, X. Effects of Fertilization on Growth and Photosynthetic Physiology of Cinnamomum camphora Seedlings; Sichuan Agricultural University: Ya’an, China, 2014. (In Chinese) [Google Scholar]
- Ni, M.; Gao, Z.Z.; Chen, H.; Chen, C.; Yu, F.Y. Exponential fertilization regimes improved growth and nutrient status of Quercus nuttallii container seedlings. Agronomy 2022, 12, 669. [Google Scholar] [CrossRef]
- Fairbairn, N.J. A modified anthrone reagent. Chem. Ind. 1953, 4, 86. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.L.; Salahuddin. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef]
- Valinger, E.; Sjögren, H.; Nord, G.; Cedergren, J. Effects on stem growth of Scots pine 33 years after thinning and/or fertilization in northern Sweden. Scand. J. For. Res. 2019, 34, 33–38. [Google Scholar] [CrossRef]
- Roy, R.; Wang, J.X.; Mostofa, M.G.; Fornara, D. Optimal water and fertilizer applications improve growth of Tamarix chinensis in a coal mine degraded area under arid conditions. Physiol. Plant. 2020, 172, 371–390. [Google Scholar] [CrossRef]
- Souza, N.H.; Marchetti, M.E.; Carnevali, T.D.; Ramos, D.D.; Scalon, S.D.Q.; Silva, E.F. Nutrition study of canafístula (I): Initial growth and seedlings quality of peltophorum dubium in response to fertilization with nitrogen and phosphorus. Rev. Árvore 2013, 37, 717–724. [Google Scholar] [CrossRef]
- Wu, J.S.; Tong, G.P.; Guo, R.; Ye, Z.H.; Jin, J.; Lin, H.P. N-Exponential fertilization could affect the growth and nitrogen accumulation of Metasequoia glyptostroboides seedling in a greenhouse environment. Phyton-Int. J. Exp. Bot. 2022, 91, 2211–2220. [Google Scholar] [CrossRef]
- Huang, H.; Wu, H.B.; Lopez, R.; Yin, D.S.; Shen, H.L.; Zhang, P. Effects of pre-hardening and autumn fertilization on biomass allocation and root morphology of Pinus koraiensis Seedlings. Forest 2023, 14, 59. [Google Scholar] [CrossRef]
- Prehn, D.; Bonomelli, C.; San Martin, R. Effect of fertilization on Guindilia trinervis in its natural habitat and in the greenhouse. Bosque 2013, 34, 243–252. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, G.H.; Liu, X.; Pan, X.; Herbert, S.J. Phosphorus application affects the soybean root response to water deficit at the initial flowering and full pod stages. Soil Sci. Plant Nutr. 2005, 51, 953–960. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.I.; Turner, N.C.; Chen, Z.; Liu, H.Y.; Wang, X.L.; Siddique, K.H.; Li, F.M. Phosphorus application increases root growth, improves daily water use during the reproductive stage, and increases grain yield in soybean subjected to water shortage. Environ. Exp. Bot. 2019, 166, 103816. [Google Scholar] [CrossRef]
- Xu, B.C.; Niu, F.R.; Duan, D.P.; Xu, W.Z.; Huang, J. Root morphological characteristics of Lespedeza davurica (L.) intercropped with Bothriochloa ischaemum (L.) Keng under water stress and P application conditions. Pak. J. Bot. 2012, 44, 1857–1864. [Google Scholar]
- Wang, R.; Li, J.Y.; Zhang, F.Q.; Zhu, B.Z.; Pan, W. Growing dynamic root system of Aquilaria malaccensis and Aquilaria sinensis seedlings in response to different fertilizing types. Acta Ecol. Sin. 2011, 31, 98–106. [Google Scholar]
- Han, H.; He, H.; Wu, Z.; Cong, Y.; Zong, S.; He, J.; Fu, Y.; Liu, K.; Sun, H.; Li, Y.; et al. Non-Structural Carbohydrate Storage strategy explains the spatial distribution of treeline species. Plants 2020, 9, 384. [Google Scholar] [CrossRef]
- Geiger, D.R.; Servaites, J.C.; Fuchs, M.A. Role of starch in carbon translocation and partitioning at the plant level. Aust. J. Plant Physiol. 2000, 27, 571–582. [Google Scholar] [CrossRef]
- Liu, D.H. Aging of plant leaves. Plant Physiol. J. 1983, 14–19. (In Chinese) [Google Scholar] [CrossRef]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. Plant responses to nutrient addition experiments conducted in tropical forests. Ecol. Monogr. 2019, 89, 1382–1399. [Google Scholar] [CrossRef]
- Tang, X.Y.; Kang, Y.C.; Liang, X.X.; Ma, D.C.; Wang, L.H. Effects of N, P and K proportional fertilization on the physiological and photosynthetic characteristics of Tsoongiodendron odorum seedlings. J. NW For. Univ. 2022, 37, 37–42. (In Chinese) [Google Scholar]
- Latsague, M.; Saez, P.; Mora, M. Effect of the fertilization with nitrogen, phosphorus and potassium, on the foliar content of carbohydrates, proteins and photosynthetic pigments in plants of Berberidopsis corallina Hook. f. Gayana Bot. 2014, 71, 37–42. [Google Scholar] [CrossRef]
- Schaberg, P.G.; DeHayes, D.H.; Hawley, G.J.; Murakami, P.F.; Strimbeck, G.R.; McNulty, S.G. Effects of chronic N fertilization on foliar membranes, cold tolerance, and carbon storage in montane red spruce. Can. J. For. Res. 2002, 32, 1351–1359. [Google Scholar] [CrossRef]
- Wu, S.W.; Li, M.; Zhang, C.M.; Tan, Q.L.; Yang, X.Z.; Sun, X.Z.; Pan, Z.Y.; Deng, X.X.; Hu, C.X. Effects of phosphorus on fruit soluble sugar and citric acid accumulations in citrus. Plant Physiol. Biochem. 2021, 160, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Fang, X.M.; Yuan, Y.; Fu, Y.X.; Yi, M.; Yuan, S.G.; Guo, S.M.; Lai, M.; Xie, J.W.; Zhang, L. Phosphorus addition alter the pine resin flow rate by regulating tree growth and non-structural carbohydrates in a subtropical slash pine plantation. Ind. Crop. Prod. 2023, 199, 116782. [Google Scholar] [CrossRef]
- Chesti, M.H.; Ali, T.; Bhat, M.A. Effect of organic and inorganic phosphorus sources on quality of green gram (Vigna radiata L.) under temperate conditions of Jammu and Kashmir. Legume Res. 2012, 35, 47–49. [Google Scholar]
- Shi, Q.S. Effect of Canola Intercropped with Potato and Phosphorus Rate; Huazhong Agricultural University (People’s Republic of China): Wuhan, China, 2009. (In Chinese) [Google Scholar]
- Mo, Q.F.; Chen, Y.Q.; Yu, S.Q.; Fan, Y.X.; Peng, Z.T.; Wang, W.J.; Li, Z.A.; Wang, F.M. Leaf nonstructural carbohydrate concentrations of understory woody species regulated by soil phosphorus availability in a tropical forest. Ecol. Evol. 2020, 10, 8429–8438. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdani, S.H.; Sirna, C.B. Physiological Responses of Salvinia minima to Different Phosphorus and Nitrogen Concentrations. Am. Fern J. 2008, 98, 71–82. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Yan, C.; Luo, W.; Wang, R.; Han, X.; Jiang, Y.; Li, M.H. Responses and sensitivity of N, P and mobile carbohydrates of dominant species to increased water, N and P availability in semi-arid grasslands in northern China. J. Plant Ecol. 2017, 10, 486–496. [Google Scholar] [CrossRef]
- Peng, Z.T.; Chen, M.; Huang, Z.; Zou, H.; Qin, X.; Yu, Y.; Bao, Y.; Zeng, S.; Mo, Q. Non-Structural carbohydrates regulated by nitrogen and phosphorus fertilization varied with organs and fertilizer levels in Moringa oleifera seedlings. J. Plant Growth Regul. 2021, 40, 1777–1786. [Google Scholar] [CrossRef]
- Feng, W.; Chen, S.; Shan, C.D.; Liu, J.M.; Huang, X.S.; Fu, L.S.; Kong, H.P.; Liu, G.A.; Yao, Z.Y. Growth, nutritional status, and nonstructural carbohydrates of Armand pine (Pinus armandii) seedlings in response to fertilization regimes and levels. J. For. Res. 2023, 28, 1341–6979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).