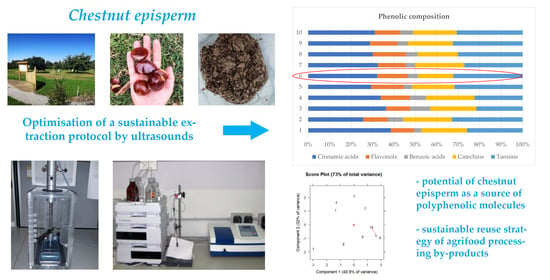
Chestnut Episperm as a Promising Natural Source of Phenolics from Agri-Food Processing by-Products: Optimisation of a Sustainable Extraction Protocol by Ultrasounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Chestnut Samples
2.3. Optimisation of the Extraction Protocol
2.4. Spectrophotometric Analysis
2.5. Chromatographic Analysis
2.6. Principal Component Analysis (PCA)
2.7. Data Analysis
3. Results and Discussion
3.1. Total Polyphenolic Content and Antioxidant Capacity
3.2. Phenolic Composition by HPLC
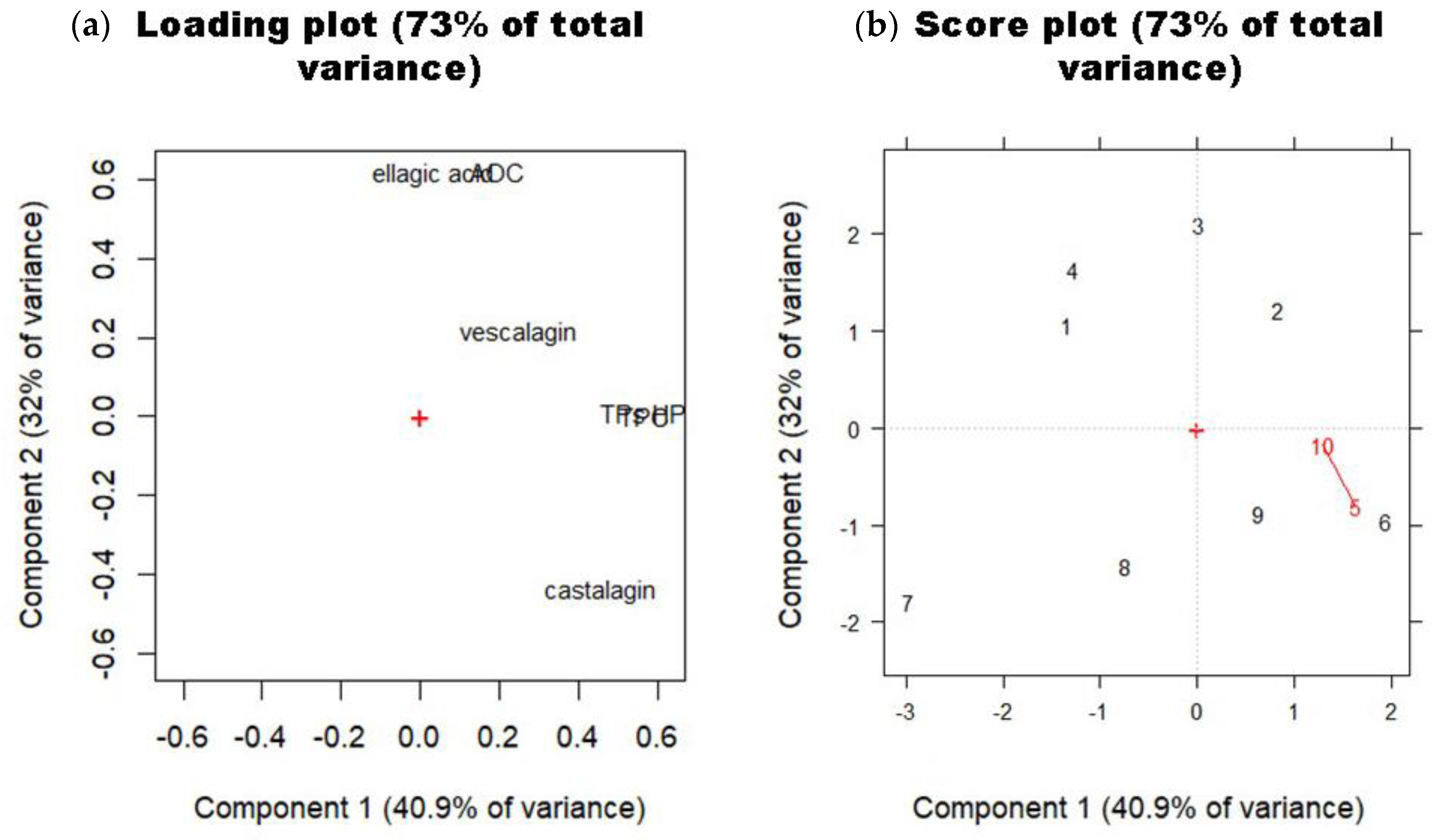
3.3. Multivariate Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Scarlat, N.; Fahl, F.; Lugato, E.; Monforti-Ferrario, F.; Dallemand, J. Integrated and spatially explicit assessment of sustainable crop residues potential in europe. Biomass-Bioenergy 2019, 122, 257–269. [Google Scholar] [CrossRef]
- Conidi, C.; Donato, L.; Algieri, C.; Cassano, A. Valorization of chestnut processing by-products: A membrane-assisted green strategy for purifying valuable compounds from shells. J. Clean. Prod. 2022, 378, 134564. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Vella, F.M.; De Masi, L.; Calandrelli, R.; Morana, A.; Laratta, B. Valorization of the agro-forestry wastes from italian chestnut cultivars for the recovery of bioactive compounds. Eur. Food Res. Technol. 2019, 245, 2679–2686. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Carini, V.; Bergamasco, E.; Gamba, G.; Beccaro, G.L.J.A. Application of traditional cooking methods in chestnut processing: Effects of roasting and boiling on secondary metabolites and antioxidant capacity in Castanea spp. Fruits. Agriculture 2023, 13, 530. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits—A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar] [CrossRef]
- Donno, D.; Fabro, M.; Mellano, M.G.; Gamba, G.; Fioccardi, A.; Beccaro, G.L. Integrating traditional wheat-based foods with high health value flours: Castanea spp. Agro-biodiversity in bakery products. Agriculture 2022, 12, 946. [Google Scholar] [CrossRef]
- Šoronja-Simović, D.; Pajin, B.; Šubarić, D.; Dokić, L.; Šereš, Z.; Nikolić, I. Quality, sensory and nutritional characteristics of cookies fortified with chestnut flour. J. Food Process. Preserv. 2017, 41, e12887. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- Ferrara, E.; Pecoraro, M.T.; Cice, D.; Piccolella, S.; Formato, M.; Esposito, A.; Petriccione, M.; Pacifico, S. A joint approach of morphological and uhplc-hrms analyses to throw light on the autochthonous ‘verdole’ chestnut for nutraceutical innovation of its waste. Molecules 2022, 27, 8924. [Google Scholar] [CrossRef]
- de Vasconcelos, M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crop. Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- de Vasconcelos, M.; Nunes, F.; Viguera, C.G.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Industrial processing effects on chestnut fruits (Castanea sativa mill.) 3. Minerals, free sugars, carotenoids and antioxidant vitamins. Int. J. Food Sci. Technol. 2010, 45, 496–505. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. Agrobiodiversity conservation: Genotype influence on chemical and sensorial traits of cultivars grown on the same clonal rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, C.; Marengo, A.; Sgorbini, B.; Rubiolo, P. Green strategies for analysis of natural products. In Green Approaches for Chemical Analysis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 85–125. [Google Scholar]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Paraíso, C.M.; dos Santos, S.S.; Correa, V.G.; Magon, T.; Peralta, R.M.; Visentainer, J.V.; Madrona, G.S. Ultrasound assisted extraction of hibiscus (Hibiscus sabdariffa L.) bioactive compounds for application as potential functional ingredient. J. Food Sci. Technol. 2019, 56, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Capelo-Martínez, J.-L. Ultrasound in Chemistry: Analytical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P.J.M. Optimization of the ultrasonic-assisted extraction of phenolic compounds from oryza sativa l.‘Violet nori’and determination of the antioxidant properties of its caryopses and leaves. Molecules 2018, 23, 844. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Donno, D.; Hassani, S.; Sofoini, T.; Mellano, M.G.; Riondato, I.; Gamba, G.; Beccaro, G.L. Traditional foods and sustainable rural development: Exploiting the case of the comoros tea as a potential source of bioactive compounds. Sustainability 2021, 13, 5815. [Google Scholar] [CrossRef]
- Crawley, M. The r Book, 2nd ed.; John Wiley & Sons. Ltd.: Chichester UK, 2013. [Google Scholar]
- Leardi, C.M.R.; Polotti, G. Cat (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 20 September 2023).
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut (Castanea sativa mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P. Promising new applications of castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin e profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Medica 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-assisted extraction as a green technology approach to recover polyphenols from castanea sativa shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate-squeezed marcs. Eur. Food Res. Technol. 2020, 246, 273–285. [Google Scholar] [CrossRef]
- Vázquez, G.; Pizzi, A.; Freire, M.S.; Santos, J.; Antorrena, G.; González-Álvarez, J. Maldi-tof, hplc-esi-tof and 13c-nmr characterization of chestnut (Castanea sativa) shell tannins for wood adhesives. Wood Sci. Technol. 2013, 47, 523–535. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin c and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]

| Extraction Parameters | Experimental CCD Matrix | |
|---|---|---|
| Extraction Time (min) ID Code: x1 | Sample/Solvent Ratio (w/v) ID Code: x2 | Levels |
| 10 | 1/30 | −1 |
| 20 | 1/20 | 0 |
| 30 | 1/10 | +1 |
| Extraction Time (min) | Sample/Solvent Ratio (w/v) | |||
|---|---|---|---|---|
| Extract ID Code | Experimental Values | CCD Matrix Levels | Experimental Values | CCD Matrix Levels |
| 1 | 10 | −1 | 1/30 | −1 |
| 2 | 20 | 0 | 1/30 | −1 |
| 3 | 30 | 1 | 1/30 | −1 |
| 4 | 10 | −1 | 1/20 | 0 |
| 5 | 20 | 0 | 1/20 | 0 |
| 6 | 30 | 1 | 1/20 | 0 |
| 7 | 10 | −1 | 1/10 | 1 |
| 8 | 20 | 0 | 1/10 | 1 |
| 9 | 30 | 1 | 1/10 | 1 |
| 10 | 20 | 0 | 1/20 | 0 |
| TPC | AOC | |
|---|---|---|
| Extract ID Code | (mg GAE/g DW) | (mmol Fe2+/Kg DW) |
| 1 | 101.45 ± 0.09 c | 459.30 ± 57.96 c |
| 2 | 119.63 ± 1.86 e | 490.68 ± 72.72 c |
| 3 | 132.53 ± 0.73 g | 505.12 ± 57.38 c |
| 4 | 96.93 ± 1.10 b | 356.36 ± 28.07 b |
| 5 | 122.35 ± 0.13 f | 351.81 ± 35.72 b |
| 6 | 142.04 ± 1.12 h | 342.10 ± 46.03 b |
| 7 | 90.88 ± 1.31 a | 162.95 ± 44.90 a |
| 8 | 113.92 ± 0.17 d | 173.97 ± 46.70 a |
| 9 | 124.84 ± 0.53 fg | 206.92 ± 46.01 a |
| 10 | 128.40 ± 0.39 fg | 340.45 ± 59.69 b |
| Cinnamic Acids | Flavonols | Benzoic Acids | Catechins | Tannins | TPs | |
|---|---|---|---|---|---|---|
| Extract ID Code | (mg/100 g DW) | (mg/100 g DW) | (mg/100 g DW) | (mg/100 g DW) | (mg/100 g DW) | (mg/100 g DW) |
| 1 | 137.80 ± 4.69 d | 38.28 ± 1.27 a | 12.61 ± 1.25 a | 76.07 ± 6.58 b | 92.60 ± 6.42 ab | 357.35 ± 6.40 ab |
| 2 | 101.77 ± 6.73 a | 44.73 ± 1.67 b | 29.37 ± 1.19 c | 89.50 ± 3.55 d | 130.54 ± 3.03 bc | 395.92 ± 9.86 d |
| 3 | 137.59 ± 4.62 d | 42.41 ± 2.05 b | 35.96 ± 1.30 d | 81.56 ± 6.44 bc | 82.01 ± 6.42 a | 379.53 ± 9.74 c |
| 4 | 129.72 ± 4.03 c | 52.09 ± 1.80 c | 29.31 ± 1.68 c | 86.75 ± 5.94 c | 85.96 ± 7.92 ab | 383.83 ± 5.24 c |
| 5 | 129.49 ± 5.00 c | 67.00 ± 1.75 d | 17.07 ± 1.21 b | 87.66 ± 5.34 c | 140.00 ± 6.51 c | 441.21 ± 8.73 e |
| 6 | 136.49 ± 5.95 d | 60.47 ± 1.72 cd | 20.19 ± 1.16 b | 69.61 ± 4.44 a | 137.10 ± 7.43 c | 423.87 ± 5.14 e |
| 7 | 103.60 ± 5.08 ab | 41.42 ± 2.17 b | 14.33 ± 1.32 a | 73.06 ± 5.54 b | 86.43 ± 7.04 ab | 318.84 ± 5.78 a |
| 8 | 111.67 ± 5.98 b | 57.09 ± 3.13 cd | 20.16 ± 1.31 b | 70.01 ± 4.56 a | 111.33 ± 7.00 b | 370.26 ± 6.38 b |
| 9 | 115.44 ± 5.33 b | 51.94 ± 2.35 c | 19.90 ± 1.23 b | 84.18 ± 6.64 c | 130.51 ± 5.50 bc | 401.98 ± 8.05 d |
| 10 | 131.29 ± 3.53 c | 50.25 ± 4.64 c | 24.76 ± 0.57 bc | 85.99 ± 3.96 c | 129.57 ± 5.04 bc | 421.86 ± 10.64 e |
| MLR Model | TPC y1 | TPs HPLC y2 | AOC y3 | Castalagin y5 | Vescalagin y6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | coeff. | p-value | coeff. | p-value | coeff. | p-value | coeff. | p-value | coeff. | p-value | |||||
| b0 | 124.9 | 429.0 | 346.7 | 70.5 | 64.2 | ||||||||||
| ET | 18.4 | 0.00 | *** | 24.2 | 0.00 | ** | 12.6 | 0.17 | 13.9 | 0.12 | 0.2 | 0.91 | |||
| S/S R | −4.0 | 0.09 | −7.0 | 0.16 | −151.9 | 0.00 | *** | 7.6 | 0.34 | −3.8 | 0.15 | ||||
| ET × S/S R | 0.7 | 0.76 | 15.2 | 0.04 | * | −0.5 | 0.96 | −1.5 | 0.87 | 15.2 | 0.00 | ** | |||
| ET^2 | −5.0 | 0.15 | −22.5 | 0.03 | * | 2.0 | 0.87 | −19.9 | 0.15 | −3.3 | 0.39 | ||||
| S/S R^2 | −7.7 | 0.05 | −43.3 | 0.00 | ** | −14.9 | 0.29 | −11.8 | 0.36 | −1.9 | 0.60 | ||||
| E.V.% | 92.9 | 92.5 | 97.8 | 35.5 | 79.2 | ||||||||||
| SD of the residuals | 4.4 | 9.9 | 18.4 | 17.3 | 5.2 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donno, D.; Turrini, F.; Farinini, E.; Mellano, M.G.; Boggia, R.; Beccaro, G.L.; Gamba, G. Chestnut Episperm as a Promising Natural Source of Phenolics from Agri-Food Processing by-Products: Optimisation of a Sustainable Extraction Protocol by Ultrasounds. Agriculture 2024, 14, 246. https://doi.org/10.3390/agriculture14020246
Donno D, Turrini F, Farinini E, Mellano MG, Boggia R, Beccaro GL, Gamba G. Chestnut Episperm as a Promising Natural Source of Phenolics from Agri-Food Processing by-Products: Optimisation of a Sustainable Extraction Protocol by Ultrasounds. Agriculture. 2024; 14(2):246. https://doi.org/10.3390/agriculture14020246
Chicago/Turabian StyleDonno, Dario, Federica Turrini, Emanuele Farinini, Maria Gabriella Mellano, Raffaella Boggia, Gabriele Loris Beccaro, and Giovanni Gamba. 2024. "Chestnut Episperm as a Promising Natural Source of Phenolics from Agri-Food Processing by-Products: Optimisation of a Sustainable Extraction Protocol by Ultrasounds" Agriculture 14, no. 2: 246. https://doi.org/10.3390/agriculture14020246
APA StyleDonno, D., Turrini, F., Farinini, E., Mellano, M. G., Boggia, R., Beccaro, G. L., & Gamba, G. (2024). Chestnut Episperm as a Promising Natural Source of Phenolics from Agri-Food Processing by-Products: Optimisation of a Sustainable Extraction Protocol by Ultrasounds. Agriculture, 14(2), 246. https://doi.org/10.3390/agriculture14020246