Abstract
Weed responses in disturbance-prone agroecosystems are linked to specific plant traits that enable their persistence. Understanding how weeds adapt to thrive in these systems in response to herbicide application is important for farmers to improve weed management for enhanced crop productivity. In this study, we investigated the functional traits and types of weed species able to persist within fields of glyphosate-tolerant maize in the Oliver Tambo District of the Eastern Cape Province, South Africa. This was accomplished by exploring the abundance patterns, composition, and richness of specific weed traits and functional types. Frequency measures (%) were used to identify indicator species. A data set comprising 42 indicator weed species and 11 predefined disturbance traits from 28 fields of glyphosate-tolerant maize was considered for functional analysis. Clusters were identified according to the grouping of weed species based on their trait scores, which revealed ten plant functional types (PFTs). Disturbances associated with post-emergence (after ploughing, sowing, and herbicide application) act as filters that select for weed species with traits such as life span, life form, growth form, photosynthetic pathway, carbon storage, and nitrogen-fixing ability to colonise fields. Trait richness did not differ significantly across maize fields. Our results highlighted the functional types and traits that are favourable to weed resistance and survival, and these need to be considered when developing different herbicide application protocols. By understanding which traits are favourable for weed survival post-emergence, farmers can apply targeted weed management to safeguard maize productivity. In addition, successful control of weeds will contribute to landscape-targeted herbicide applications that are less harmful to the environment.
1. Introduction
Weeds and their associated traits have been the subject of research because of their impact in both the agriculture and horticultural industries [1,2]. Weeds occur across many areas and landscapes, often with variable densities, usually grow fast, and reproduce relatively quickly [2]. Weed research has focussed on the origins of weedy species and the evolution of certain characteristics and traits, to a point where they adapt and become tolerant or even resistant to various means of control and management [1].
Weed resistance to herbicides can arise when there is selection for different genotypes or when selection pressure forces mutation processes that might result in a beneficial characteristic that allows biotypes to survive [3,4]. Mutations due to herbicide resistance depend on the genetic background and environmental conditions. The detrimental effects of herbicide resistance mutations on plant fitness may arise as a direct impact on fitness-related traits, with changes in other life history traits that ultimately may lead to fitness costs under ecological conditions [4]. Traits are morphological, phenological or physiological features that impact the fitness of individual species. The specific plant functional traits of a weed, therefore, assist us to understand their responses to ecosystem dynamics [5,6,7,8,9]. Traits are morphological, phenological, or physiological features that impact the fitness of individual species. However, principles of functional trait ecology have not been widely applied to inform agricultural research and management [10]. Functional traits are known to be affected by agricultural activities that act as filters to select for specific adaptive traits that allow weeds to persist in a community [11,12,13]. As a result, plant functional traits may be used to group species in functional types with similar features, as they are likely to respond similarly to climatic conditions and/or disturbances [8,14].
The transformation of natural rangelands to cultivated agroecosystems has been shown to reduce plant diversity and alter composition [15,16,17]. For the maintenance of diverse and functional ecosystems within farmlands, it has become important to understand the potential effects that farming activities and practices might have on surrounding plant community composition and diversity [18]. Therefore, farmers need to manage their land for specific plant functional types to maintain the required ecosystem services.
In agroecosystems, specific management practices need to be applied to maximise crop production levels. For crop production, farmers may adopt various methods of pest and weed control that can be either chemical or mechanical [19,20,21]. Adoption and application of methods vary for different crops, farming practices, and regional requirements, and are influenced by abiotic and biotic components. As pest management mechanisms evolved, so did genetic engineering tools [22]. It has been more than 25 years since the introduction and use of Genetically Modified (GM) crops [23] to improve pest management and alleviate crop failures, while also addressing other climatic and environmental challenges [24,25].
Globally, cotton, canola, maize, and soybean remain the big four biotech crops, even though other new crops have entered the market [23]. These crops continue to offer, with improvements, traits (single and stacked) that confer insect resistance and herbicide resistance [22]. In South Africa, maize is one of the most important grain crops and is cultivated in all nine provinces [26]. South Africa produces approximately 1.1 million hectares of yellow maize and 1.3 million hectares of white maize for commercial purposes annually [27]. About 85–95% of maize grown in South Africa is GM, with the major traits being that of insect resistance, herbicide tolerance, and/or a stacked combination of both [28].
The use of herbicide-tolerant crops has not been a complete solution, as weeds have been shown to have varying levels of natural tolerance to different herbicides in Australia, Brazil, Canada, China, Europe, South Africa and United States of America [29,30], which is further influenced by regional climatic conditions, site-specific herbicide application, and grazing intensities [30]. Fried et al. [31] found that in France most abundant weeds in maize crops were species that germinate in spring to summer, flower late, and are likely to have the same life cycle as maize. Weeds therefore share traits that are related to high colonisation capacity, involving wind dispersal and seed longevity, which are beneficial to species exposed to exogenous disturbances such as ploughing. Traits related to specific seasonal adaptation (such as late flowering) and the C4 photosynthetic pathway, are better adapted to endogenous disturbances such as herbicide use. Traits that predict the ability of weeds to persist after agricultural disturbance include growth form [32], life span [33], flowering phenology, pollination mode, flowering season [34], N-fixating ability [30], and photosynthetic pathway [31].
In plant communities, it is often those with high functional trait diversity that are more stable and resilient after a disturbance. This large pool of traits allows plant species to respond across a wider range of disturbance [35]. To understand weed dynamics, it is therefore important to know which weed traits play an important role in species’ ability to cope with the disturbances associated with agricultural activities, as some functional traits are more beneficial than others [36]. For example, functional traits that are more resilient to agricultural activities may serve as indicators in land-use monitoring studies to assess the transformation of the natural landscape into agricultural systems [36]. From a biodiversity management and conservation approach, the protection of field margins enhances those species with trait diversity that provide a wide array of ecosystem services [37]. At the same time, it is important to assess, monitor, and guard against some species in these marginal areas with traits that can contribute to their weediness or the ability to develop resistance towards various types of chemical control methods. This is important to monitor, as weed species with such traits can threaten ecosystems and their functions should they become herbicide-resistant [38,39].
To develop a better understanding of the plant traits that allow species to overcome agricultural disturbances, we considered changes/shifts in weed functionality in an agroecosystem exposed to ploughing and herbicide application, by testing the following hypotheses: Firstly, we expected that weeds in different fields of glyphosate-tolerant maize would share similar functional traits in response to similar disturbances; and secondly, we predicted that dominant weeds of the same field of glyphosate-tolerant maize would, before and after two rounds of herbicide application, not share similar traits. To test these hypotheses, we aimed to identify and assess the functional traits that enable weed species to persist in glyphosate-tolerant maize fields that followed a specific herbicide application protocol (pre- and post-maize emergence applications). The main objectives were to: (1) identify indicator weed species that persist after herbicide application; (2) classify weed species into functional types using disturbance traits; (3) compare the composition of weed traits before and after herbicide applications; (4) compare the richness of weed traits before and after herbicide applications; and (5) compare if plant functional types (PFTs) increase from before to after herbicide application. These objectives allowed us to assess and establish whether there are specific functional traits that allow weed species to persist in glyphosate-tolerant maize and determine which species are likely to develop resistance to herbicides. This would enable us to address the knowledge gap regarding weed resistance to herbicides in maize agroecosystems of South Africa.
2. Results
2.1. Indicator Weed Species
Forty-two indicator weed species were identified (Supplementary Table S1) and the dominant families were Asteraceae (11 species), Cyperaceae (4), and Fabaceae (5). Twenty-five native species and seventeen alien species were identified as indicator species. Indicator species with the highest IndVal, greater or equal to 0.3, were Amaranthus viridis L. with the highest value of 0.558 and highest frequency of occurrence in 114 plots, followed by other exotic weeds, namely, Bidens bipinnata L., Chenopodium carinatum R.Br., Cirsium vulgare (Savi) Ten., Conyza bonariensis (L.) Cronquist, Daucus carota L., Ipomoea purpurea (L.) Roth, Portulaca oleracea L., and Sonchus nanus Sond. ex Harv.
2.2. Plant Functional Types
Twenty-four indicator weed species were annual (Figure 1), whereas eighteen species were perennial (Figure 2) to provide a 4:3 split. Full names for abbreviated weed species names can be found in (Supplementary Table S1). Ten PFTs were identified for the 42 weed indicator species based on 11 disturbance resistance traits, which are growth form, life form, perennial or biennial, monocotyledon or dicotyledon, prostrate or erect growing, nitrogen- or non-nitrogen-fixing, spinescent or non-spinescent, assisted or unassisted seed dispersal, specified flowering season, specialised carbon storage organs, and C3 and C4 photosynthetic pathway.
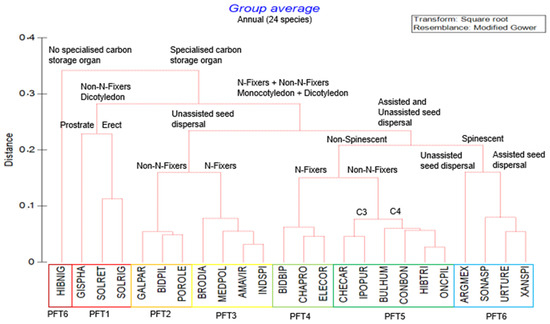
Figure 1.
Cluster analysis Unweighted Pair Group Method with Arithmetic Mean (UPGMA) based on Gower distance measure proposing six major plant functional types (PFTs) from the trait composition of annual species recorded in fields of glyphosate-tolerant maize. Red lines in the dendrogram indicate branching with significant structure, as determined by Similarity Profile Analysis (SIMPROF).
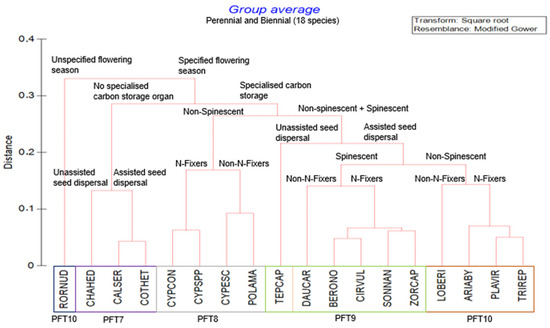
Figure 2.
Cluster analysis Unweighted Pair Group Method with Arithmetic Mean (UPGMA) based on Gower distance measure proposing four major plant functional types (PFTs) from the trait composition for perennial species recorded in fields of glyphosate-tolerant maize. Red lines in the dendrogram indicate branching with significant structure, as determined by Similarity Profile Analysis (SIMPROF).
Six PFTs were identified for annual species (Figure 1). The pairs PFT 2 and 3, and PFT 4 and 5, shared similar traits, with the exception that one of each pair was a nitrogen fixer. An outlier species Hibiscus nigricaulis Baker f. was grouped with PFT 6 because it shared most of its traits with this group, such as being a spinescent, N-fixing species with assisted seed dispersal (Figure 1). This indicates that a carbon storage organ was an important trait for all but one annual species.
Four groupings of PFTs were identified for perennial species (Figure 2). An outlier species, Rorippa nudiuscula Thell., was grouped with PFT 10 because it shared some of the traits such as a specialised carbon storage organ, assisted seed dispersal without spinescence, and nitrogen-fixing ability (Figure 2). Therefore, all but one perennial species had a specified flowering season, and this highlights the importance thereof. All PFTs (Table 1), except PFT 7, had specialised carbon storage organs as the main trait.

Table 1.
Description of the ten plant functional types (PFTs) identified by means of cluster analysis. The detailed descriptions of Functional Traits are in (Supplementary Table S2).
2.3. Weed Trait Assemblages
Weed assemblages differed significantly among most sites (p < 0.005), except Tsolo (T), which showed similarity to some Baziya sites (Supplementary Table S3). These differences were supported by the non-metric multidimensional scaling (NMDS), which indicated strong clustering. However, the variation in trait composition per field at Baziya Makaula (MV) (elongated clustering—substantial species turnover) made it different from all the other sites (Figure 3). Although trait composition before and after herbicide applications (Figure 4) did not separate conspicuously into clear groups, significant differences in composition were revealed by permutational multivariate analysis of variance (PERMANOVA) (p = 0.003). A stress value of 0.1 was an acceptable representation of weed trait composition in ordination space to support the significant differences in composition.
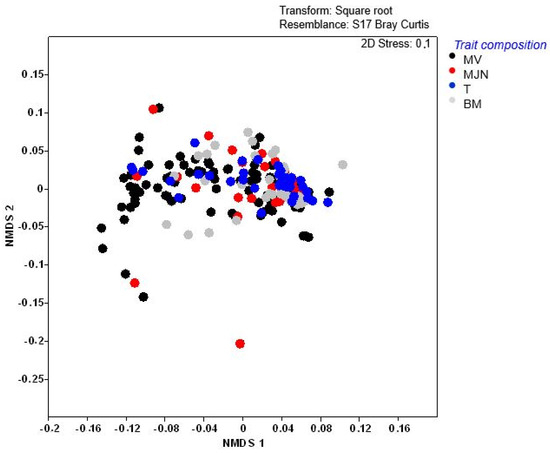
Figure 3.
Non-metric multi-dimensional scaling (NMDS) ordination of weed functional trait composition across maize field sites MV = Baziya Makaula; MJN = Baziya Jojweni; T = Tsolo; BM = Baziya Mission. Permutational multivariate analysis of variance (PERMANOVA): p = 0.001. (Bray–Curtis resemblance on square root-transformed functional trait data).
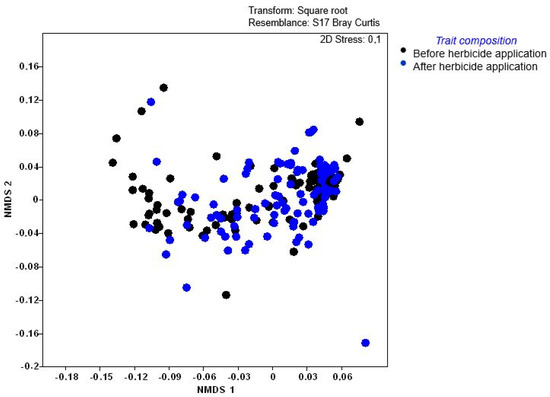
Figure 4.
Non-metric multi-dimensional scaling (NMDS) ordination of weed functional trait composition before and after herbicide applications. Permutational multivariate analysis of variance (PERMANOVA): p = 0.003. (Bray–Curtis resemblance on fourth root-transformed functional trait data).
2.4. Weed Trait Richness
Weed trait richness among maize field sites differed significantly (p < 0.05) between MV and T sites only, with the latter significantly higher than MV, which had the lowest trait richness (Figure 5). In terms of functional trait richness, site T showed similarity to the other sites, particularly sites Baziya Mission (BM) and Baziya Jojweni (MJN), whereas no differences in trait richness were observed before and after herbicide applications (Figure 6).

Figure 5.
Weed trait richness of maize field sites MV = Baziya Makaula; MJN = Baziya Jojweni; T = Tsolo; BM = Baziya Mission. Significant differences at p < 0.05 are indicated by means of different lowercase letters. Symbol (*) expresses a relationship between mean values.
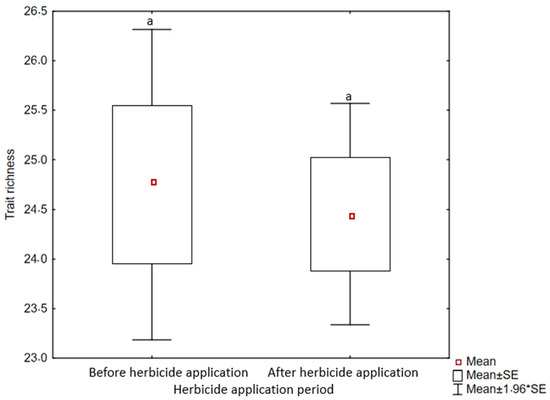
Figure 6.
Weed trait richness of maize fields before and after herbicide applications. Significant differences at p < 0.05 are indicated by means of lowercase letters. Symbol (*) expresses a relationship between mean values.
2.5. Weed Trait Responses to Disturbance
Redundancy analysis (RDA) of the ten PFTs in response to herbicide application revealed a strong correlation of PFTs 9 and 10 before herbicide application (Figure 7). The longest vectors (associated with after-herbicide applications) were those of PFTs 3 and 5 (Figure 7), which could be expected, as these groups were constituted of annual species. Moreover, both these PFTs had specialised carbon storage organs with unassisted seed dispersal traits. PFTs 9 and 10 were closely related and consisted of perennial species with specialised carbon storage organs and assisted seed dispersal as the main shared traits for fields before herbicide application.
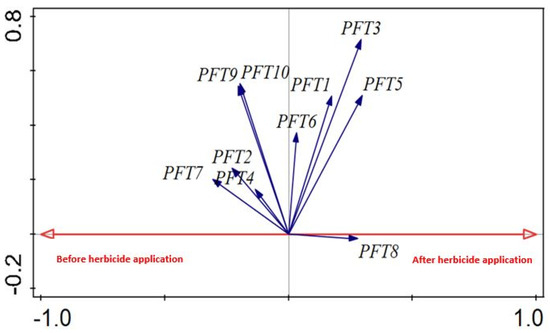
Figure 7.
Redundancy analysis (RDA) of plant functional type data in response to before- and after-herbicide applications.
Trait and environmental data used to compile the RDA (Figure 7) had response and explanatory variables of 10 and 2, respectively, with functional type composition and general analyses. The results showed a total variation of 5827.91 and explanatory variables accounted for 5.8% (Table 2). The test values on all axes revealed pseudo-F = 12.7 and p = 0.002, which shows a significant difference in plant functional types before and after herbicide applications.

Table 2.
Eigenvalues for axis 1 and axis 2, with explained variation and correlation for both axes.
To better unpack the effect of herbicide control on weeds, the abundances of species in each PFT were compared before and after herbicide applications. Annual plant species count was substantially higher for PFTs 1 (p = 0.009), 3 (p = 0.017), and 5 (p = 0.003), and lower for PFT 2 (p = 0.008) after herbicide applications (Table 3). However, perennial weed species counts showed that PFT 8 had greater number of individuals after herbicide applications, although non-significant (Table 4). All the other perennial PFTs showed a non-significant decrease in weed abundance. The species of PFTs 1, 3, 5 and possibly 8, can therefore be considered to hold traits that enable them to overcome both ploughing and herbicide application (Table 4).

Table 3.
Plant functional types of t-test results before and after herbicide applications.

Table 4.
T-tests of the increase or decrease in species abundances of plant functional types from before- and after-herbicide applications.
Ten weed indicator species with highest abundances could be considered in terms of three important traits (carbon storage, nitrogen-fixing ability, and life span) known to be significant in crop fields (Table 5). Amaranthus viridis had the highest average sum of 62.77, followed by Chenopodium carinatum with an average sum of 36.83 (after herbicide applications). When the average sums before- and after-herbicide applications were compared, eight out of the ten most dominant weed species showed an increase after herbicide applications. These provisionally flagged as herbicide tolerant (Table 5).

Table 5.
Top ten most abundant weed species after herbicide applications in glyphosate-tolerant maize fields and their associated traits.
3. Discussion
3.1. Weed Traits Composition and Richness
Ecosystem functions are known to be affected by species composition [40,41], functional trait composition of the community, and trait diversity [30,42]. We found weed species trait composition of maize fields to differ before and after herbicide applications (Figure 4). This trend is consistent with Hejda et al. [43], who found disturbance to be one of the main influences on species trait composition. Trait variation can maintain diversity in communities by reducing competition [44], but these responses are spatially and temporally dynamic, as shown in our study. For example, some sites were relatively similar in trait composition and richness, despite being located far apart (Tsolo, which is 40 km away from Baziya), and were expected to differ, considering the different soil and climate conditions (Figure 3) [43]. Field history in terms of use and weed management may also contribute to this difference [3,45], even though these were not fully known and explored in our study. We related our findings to the herbicide protocol that was successfully applied and efficient, and generally seemed to override the environmental parameters, because although soil and climate were different between sites, these sites still harboured plant traits that were similar compositionally. This suggests that weeds of fields, be it before or after herbicide application conditions, had specific, shared sets of traits that enabled persistence in fields despite the herbicide disturbance effect or locality.
Additionally, assemblages differed in trait composition among sites in proximity although they used similar herbicides and had similar soil and climate conditions. The weeds of these maize fields were expected to have similar traits to persist, given their exposure to similar environmental conditions and herbicide applications. A plausible explanation for this dissimilar composition could be that of incorrect and/or overuse of herbicides applied in the individual fields in terms of litres per hectare [45,46,47]. Moreover, various factors such as the mixing ratio, time of day for application, and weather conditions have been shown to have an influence when it comes to the response of weeds towards herbicides [46,48]. In this study, these were some of the factors that we had no control over as they were the farmer’s responsibility. Therefore, any such variation between sites could have led to the observations reported here. The careful notation of exact times of application and weather conditions needs to be documented to achieve similar weed control.
Weed composition differed before and after herbicide application, as the former was less disturbed (before ploughing), and no herbicides were applied. Therefore, weeds with traits associated with a more stable ecosystem would be present [49]. Weeds that appear after ploughing and seed sowing are responding to intense disturbances and colonise fields with seed or vegetative means after herbicide application. There is a selection for species with either tolerance (vegetative) [49] or rapid colonisation ability (seeding) [50]. In the case of cropland disturbance, weed species traits enabling weeds to persist after herbicide application would be the most beneficial, but detrimental to the farmer, as they persist and thereby enable a shift in trait composition in the fields [51]. It is for this reason that the herbicide application protocol of this study included non-selective herbicides, to better manage those species often hard to control—especially where mechanical control is not possible.
Management strategies to counter herbicide resistance should consider species traits that indicate persistence before herbicide application takes place. Herbicides are still the best choice for overall weed control [52], but to cater to the different functional groups of weeds, it is becoming important to be more proactive with weed control and post-emergence herbicides by seeking advice from crop advisors and weed specialists to assist with long-term planning initiatives [53]. The need for advice is supported by the lack of differences in trait richness that was observed between before- and after-herbicide application (Figure 6), suggesting that the two dominant weed assemblages before and after herbicide application are pre-adapted to the changes brought about. The idea should be to target those species within a particular functional plant response that are pre-adapted to successfully survive in frequently disturbed environments.
3.2. Plant Functional Types
We reported a higher number of functional types for annual than for perennial weeds (Table 1). This is because perennials are slower growing and not well-adapted to being periodically disturbed by ploughing and herbicides and are continuously removed and replaced by fast-reproducing annual species that colonise in abundance [50]. Annual weeds are adapted to various disturbance scenarios and bloom continuously throughout the season, but only live (and reproduce) when conditions are favourable [54]. Hence, they have a wider range of functional types and are dominant in herbicide-disturbed fields because over short time spans, they use resources efficiently, flower quickly, set seed, and disperse between ploughing and herbicide application events [55].
Before a weed can germinate and grow, its seeds must reach and remain viable in the soil seed bank of a specific area. The soil seed bank contains seeds from previous weed generations within the region and time within the soil can represent a stage in the life cycle of a weed [56]. At this point, weeds may have a chance to germinate or remain dormant within the seed bank for many seasons. Farmers use tillage, crop rotation, as well as herbicides to reduce and inhibit weed seeds that germinate [47]. Therefore, understanding weed seed characteristics and requirements for germination in particular crop systems remains essential in developing sustainable weed control strategies [45].
Botha et al. [30] also found that maize fields favoured annual species with a long-range dispersal trait. These findings are further supported by that of Siebert et al. [57], that reported the annual, nitrogen-fixing ability of forbs as favourable after abiotic disturbance such as fire, herbivory, and drought stress. In addition, Botha et al. [30] found that maize fields were characterised by a greater relative abundance of herbaceous growth forms without nitrogen-fixing ability compared to the higher numbers of nitrogen-fixers in marginal and rangeland vegetation. The differentiation in the relative abundance of nitrogen-fixing taxa may be the result of fertiliser application in maize fields, which overrides the competitive advantage that would favour nitrogen-fixers [30].
Most weed species that persisted in fields had carbon storage organs. Carbon storage organs are important to aid survival in response to frequent disturbances in fields. Shen et al. [58] demonstrated that disturbance contributed to a diversity of traits associated with carbon storage.
Assisted seed dispersal is one of the traits that was found to be common in our analysis and possibly the reason PFTs 1, 3, 4, 5, and 8 maintained high abundances after herbicide applications (Table 3 and Table 4). Maize fields are dominated by weeds that are known to spread their seeds through wind dispersal [31], which is typical for plants to overcome high disturbance levels [59]. Tainton [60] found that wind dispersal is a characteristic of pioneer species that often occupy disturbed sites. This is an adaptive strategy which evolved to rapidly colonise open systems, which is ideally mimicked by the intensive management associated with maize agriculture, including agro-chemical application [36]. Petit et al. [61] concluded that weed dispersal mechanisms are now globally driven by agricultural management at multiple scales.
Predominant weed species traits in maize fields revealed a positive relationship between regional frequency and local abundance [62]. Our results here indicated maize crop fields to be dominated by weeds that follow the C4 photosynthetic pathway (Supplementary Table S1). This agrees with the findings by Fried et al. [31], as the majority of our weed indicator species followed C4 photosynthesis, such as Bulbostylis humilis (Kunth) C.B.Clarke, Erigeron bonariensis, Hibiscus trionum L., and Oncosiphon pilulifer (L.f.) Källersjö. In high light and temperature environments, C4 plants tend to be more productive. Some agrotypes are associated with a specific crop, and such association can evolve a system of mimicry, where the weed resembles the crop at specific stages during its life cycle [63]. It would then appear that the herbicide-resistant mechanisms are easier in C4 weeds. It has also been reported that herbicide usage of glyphosate with higher concentrations than recommended levels has led to the evolution of herbicide resistance in both C3 and C4 [64].
Plant species of the Poaceae, and those with triazine-resistant populations, were documented to be more abundant in maize crop fields [31]. Although grass species richness was high, we recorded only two indicator weeds from the Poaceae, namely, Bromus diandrus Roth and E. coracana. Flowering period or season is another key trait of weeds for their adaptation and survival [65]. Lososová et al. [66] reported specified flowering season as one of the main weed traits in cultivated fields of Czech Republic. We also found perennial PFTs to have specified flowering seasons throughout the year (Table 1), and this is further supported by Charbonneau et al. [65] in their findings on perennials. It means that if weeds flower, they will produce seeds in favourable conditions specific to the species and result in the effective dispersal of the seeds, which makes the unaligned reproduction periods of weeds difficult to control.
3.3. Herbicide-Resistant Weeds
Fried et al. [31] reported five species, Amaranthus retroflexus L., Chenopodium album L., Echinochloa crus-galli (L.) P. Beauv., Persicaria maculosa Gray, and P. lapathifolia (L.) Delarbre to have traits resistant to herbicides. Although these weed species were not recorded in this study, their plant genera and families were well represented. The families were Amaranthaceae, Poaceae, and Polygonaceae, and their associated traits correspond with that of species we reported on for maize fields. The common traits include annual life form, C4 photosynthetic pathway, and specified flowering season.
Certain traits were identified for dominant annual weeds after herbicide applications, and distinguished between monocotyledonous and dicotyledonous, prostrate, and erect-growing, nitrogen-fixing and non-nitrogen-fixing with specialised carbon storage organs with a C3 and C4 photosynthetic pathway, non-spinescent and following an assisted or unassisted seed dispersal. These traits are associated with related to dominant weeds: Argemone mexicana L., Amaranthus viridis L., Bromus diandrus Roth, Bulbostylis humilis (Kunth) C.B.Clarke, Chenopodium carinatum R.Br., Erigeron bonariensis L., Gisekia pharnacioides L., Hibiscus nigricaulis Baker f., Hibiscus trionum L., Ipomoea purpurea (L.) Roth, Indigofera spicata ForsskForsk., Medicago polymorpha L., Oncosiphon pilulifer (L.f.) Källersjö, Sonchus asper (L.) Hill, Solanum humile Lam., Solanum retroflexum Dunal, Urtica urens L., Xanthium spinosum L. (Table 1) [2,67,68]. Since these weed species have traits that are pre-adapted to herbicide disturbance and can persist after herbicide application, it is therefore necessary that they are further investigated for their potential herbicide tolerance under different agricultural practices and landscape management approaches. They also have the potential to become problematic weeds, possibly with invasive traits, that may pose a threat to various ecosystems and their functions.
4. Materials and Methods
4.1. Generated Plant List of Weed Species
The list of weed species (Supplementary Table S4) was generated from surveys conducted in four study sites of the Oliver Tambo District Municipality, Eastern Cape Province, South Africa (see Kwinda et al. [69] where the comprehensive sampling criteria and procedures are explained, including the identification process). The weed surveys in fields of glyphosate-tolerant maize were carried out across three growing seasons, namely, 2017/18, 2018/19, and 2019/20. Overall, a total of 74 weed species were recorded within 208 quadrats within fields. Glyphosate-tolerant maize NK603XMON810 (2017/18) and MON89034 (2018/19 and 2019/20) were planted by the farmers. Two different herbicide protocols were administered to the maize fields in the three seasons, between the summer months of December and February/March. The herbicide protocols applied are detailed in Table 6. The herbicide spraying protocol consisted of a pre-emergence herbicide that was sprayed between three and seven days after planting, followed by post-emergence spraying five to six weeks after germination of the maize crop [69]. The herbicide protocol consisted of both selective and non-selective herbicides, ensuring that a wide variety of weeds were controlled/managed successfully. Surveys took place before ploughing and herbicide application and then again after ploughing and two herbicide applications (pre- and post-emergence).

Table 6.
Detailed listing of pre- and post-emergence herbicides applied in fields planted with glyphosate-tolerant maize over three seasons (2017/18; 2018/19; 2019/20). Insecticides in italics.
4.2. Functional Trait Identification and Adaptation for Herbicide Disturbance Analysis
The indicator value index (IndVal) proposed by Dufrene and Legendre [70] was calculated from quadrat data to determine the indicator weed species in glyphosate-tolerant maize fields. Overall abundance of all weed species was recorded from maize fields before ploughing and herbicide applications. Frequency measures (%) were used for indicator species analysis performed in RStudio using the IndVal function under the labdsv package version 2.5-7, and significance levels were considered at p < 0.05.
Functional traits that have known positive responses to agricultural disturbance were selected based upon reported and published data from different databases [32,71]. To detect groupings of indicator weed species based on their trait scores, hierarchical clustering analysis with an Unweighted Pair Group Method with Arithmetic Mean (UPGMA)-clustering algorithm and Gower distance measure appropriate for mixed (categorical and binary) data types was created in PRIMER 6 [30]. The Gower distance measure was used because it is appropriate for mixed data types [30]. A Similarity Profile (SIMPROF) test was also applied as an objective method for the identification of significant groupings compared to subjective cut-off levels. Each life form (annual versus perennial) was analysed separately to identify and describe the plant functional types across glyphosate-tolerant maize fields. Since life form remains the strongest indicator of agricultural disturbance [72], the effects of underlying important disturbance traits may be weakened when PFTs of life forms are analysed collectively [5]. PFT clusters were identified at different hierarchical levels, following the approach of Linstädter et al. [8].
Redundancy analysis (RDA) in Canoco 5 [73] was applied to the complete species–trait matrix to test for clustering based on specific traits in ordinal space. Non-metric multi-dimensional scaling (NMDS) ordinations of weed functional traits were compiled using PAST software version 4 to determine the functional trait composition of indicator species. A trait–species matrix of the 42 indicator weed species (determined by IndVal), and 11 functional traits (from the literature review) were multiplied with the species–abundance matrix using the MMult function in Microsoft Excel to produce a matrix from which trait diversity index calculations (richness) was performed in PRIMER 6 [74]. Prior to detailed statistical analyses, the normality of trait richness data was tested in Statistica version 64 and transformed accordingly to adhere to assumptions for normality. Variables that were transformed were appropriately back-transformed to generate visual representations. Significant differences in weed species diversity among the sites, and for before-herbicide application, were tested in Statistica. T-tests were performed using PFTs from before- and after-herbicide applications to test for differences between the means of the two data groups collected at different times in the same maize fields. The top ten most abundant weed indicator species were selected manually from the IndVal results and their average sum for the before-herbicide application survey was calculated in Microsoft Excel. These species were selected according to the highest total number of individuals to determine whether the total number of individuals per species increased or decreased when comparing the selected traits per PFT before and after herbicide applications.
5. Conclusions
Maize fields are agroecosystems that are subjected to a diverse range of disturbances through various management practices. Our study identified specific weed traits which are associated with species that can persist in fields planted with glyphosate-tolerant maize. We successfully documented specific patterns of plant functional types and specific weed functional traits associated with maize fields before and after herbicide applications. Our findings revealed that weed species in fields with glyphosate-tolerant maize share similar traits in response to similar disturbances (first hypothesis supported). We further showed that weed traits before herbicide application differed compositionally from those after herbicide application (second hypothesis supported). In addition, specific traits (e.g., seed dispersal) might be important for some species, but not others. Therefore, functional types dictated species persistence and how they react to measures of control and/or disturbance such as herbicide application in maize fields. Overall, we determined a set of plant traits that might lead to herbicide resistance in weeds. As these traits can be linked to specific plant functional groups of species after herbicide applications, this knowledge will enable farmers to develop and adapt spray protocols and apply specific herbicides to target these problematic weeds. To delay development of herbicide tolerance, farmers need to adopt protocols that allow for rotation, as both annuals and perennials have different adaptive strategies towards herbicide tolerance—ultimately resistance.
Even though we detailed some of the reasons behind our findings and various mechanisms behind some of the functional traits, they were still unclear for other observed trends relating to weed responses to the agricultural disturbances. This is where a factor such as the soil seed bank could have had an impact which needs to be considered for further investigation. Other factors of interest could also include those on genetic variability, the evolution of reproductive organs, and other survival lifespan traits. Some maize fields shared traits with those not in proximity, whereas those in proximity had differences in traits. Although we assume this to result from various ecological and management factors, it calls for further investigation and close monitoring of the herbicide protocols administered, the monitoring of environmental variables, especially on spraying days (including time of day and weather conditions), and the need for additional sampling seasons. This approach will benefit the formulation of future management practices in glyphosate-tolerant maize cultivation, while enhancing our knowledge of biodiversity and conservation priorities within these grassland agroecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14020223/s1, Table S1: Indicator weed species with their functional traits in fields planted with herbicide tolerant (Glyphosate) maize. Table S2: Description of plant functional traits. Table S3: Dissimilarity in weed trait assemblages across maize field sites at p < 0.05. Table S4: List of species recorded for maize fields and margins in Baziya and Tsolo.
Author Contributions
Conceptualisation, M.K. and T.S.M.; methodology, T.S.M.; validation, M.K., T.S.M., S.J.S. and H.V.C.; formal analysis, H.V.C.; investigation, M.K.; resources, M.K., T.S.M. and S.J.S.; data curation, M.K. and T.S.M.; writing—original draft preparation, M.K.; writing—review and editing, M.K., T.S.M., S.J.S. and H.V.C.; visualisation, M.K.; supervision, S.J.S. and T.S.M.; project administration, M.K.; funding acquisition, T.S.M. and S.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South African National Biodiversity Institute (SANBI), National Research Foundation (NRF) and North-West University (NWU) (Potchefstroom).
Institutional Review Board Statement
The study was approved by the Faculty of Natural and Agricultural Sciences Ethics Committee of North-West University Senate Committee for Research Ethics (NWU-01405-20-A9) on 01 February 2020.
Data Availability Statement
All data supporting the findings of this research are available within the paper and Supplementary Materials.
Acknowledgments
We would like to thank the Mthatha GrainSA staff, the chieftaincy, farmers of Baziya, the community, and the farmers of Tsolo for allowing us access to their fields during entire sampling seasons. Appreciation is extended to field assistants and Nanette van Staden for assisting with RStudio data interpretation. Many thanks to the Compton Herbarium, South African National Biodiversity Institute (SANBI), and AP Goossens Herbarium, North-West University (NWU), for plant identification and curation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Głowacka, A. Dominant weeds in maize (Zea mays L.) cultivation and their competitiveness under conditions of various methods of weed control. Acta Agrobot. 2012, 64, 119–126. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Life History of Colonizing Plants: Some Demographic, Genetic, and Physiological Features. In Ecology of Biological Invasions of North America and Hawaii; Ecological Studies Series; Mooney, H.A., Drake, J.A., Eds.; Springer: New York, NY, USA, 1986; Volume 58, pp. 96–110. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M. Fitness of herbicide-resistant weeds: Current knowledge and implications for management. Plants 2019, 8, 469. [Google Scholar] [CrossRef]
- Lavorel, S.; McIntyre, S.; Landsberg, J.; Forbes, T. Plant functional classifications: From general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997, 12, 474–478. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing—A global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Linstädter, A.; Schellberg, J.; Brüser, K.; Moreno García, C.A.; Oomen, R.J.; Du Preez, C.C.; Ruppert, J.C.; Ewert, F. Are there consistent grazing indicators in drylands? Testing plant functional types of various complexity in South Africa’s grassland and savanna biomes. PLoS ONE 2014, 9, e104672. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.; Funk, J.L.; Spasojevic, M.J.; Suding, K.N.; Parker, S.; Goulden, M.L. Can functional traits predict plant community response to global change? Ecosphere 2016, 7, e01602. [Google Scholar] [CrossRef]
- Martin, A.; Isaac, M. Plant functional traits in agroecosystems: A blueprint for research. J. Appl. Ecol. 2015, 52, 1425–1435. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. Int. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. J. Sci. 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. N. Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Vegetation classification by reference to strategy. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Muller, M.; Siebert, F.; Ntloko, B.R.; Siebert, S.J. A floristic assessment of grassland diversity loss in South Africa. Bothalia 2021, 51, 1–9. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Moonen, A.C. Field margins in northern Europe: Their functions and interactions with agriculture. Agric. Ecosyst. Environ. 2002, 89, 5–21. [Google Scholar] [CrossRef]
- Wessels, K.J.; Reyers, B.; van Jaarsveld, A.S.; Rutherford, M.C. Identification of potential conflict areas between land transformation and biodiversity conservation in north-eastern South Africa. Agric. Ecosyst. Environ. 2003, 95, 157–178. [Google Scholar] [CrossRef]
- Dale, V.; Polasky, S. Measures of the effects of agricultural practices on ecosystem services. Ecol. Econ. 2007, 64, 286–296. [Google Scholar] [CrossRef]
- Abate, T.; van Huis, A.; Ampofo, J.K.O. Pest management strategies in traditional agriculture: An African perspective. Annu. Rev. Entomol. 2000, 45, 631–659. [Google Scholar] [CrossRef]
- Banik, D.; Jha, M.K. Chapter 8: ‘Weed Management’. In Research Trends in Crop and Weed; Nand, V., Ed.; ND University of Agriculture and Technology: Kumarganj, India, 2020; p. 131. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Nitin, K.S.; Masehela, T.S.; Chakravarthy, A.K.; Geerts, S. Management of pests using genetic tools in Africa. In Genetic Methods and Tools for Managing Crop Pests; Springer: Berlin/Heidelberg, Germany, 2022; pp. 303–326. [Google Scholar] [CrossRef]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA Brief No. 55; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Morton, J.F. The impact of climate change on smallholder and subsistence agriculture. Proc. Natl. Acad. Sci. USA 2007, 104, 19680–19685. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Tack, J. Is another genetic revolution needed to offset climate change impacts for US maize yields? Environ. Res. Lett. 2018, 13, 124009. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- BFAP. Baseline Agricultural Outlook. Available online: https://www.sagis.org.za/BFAPBaseline-2019.pdf (accessed on 11 April 2020).
- Masehela, T.S.; Rhodes, J.I.; Groenewald, H.; Poole, C.J.; Van den Berg, J.; Gouse, M.; Skowno, A.L.; Barros, E.; Seymour, C.L.; Mandivenyi, W.G.; et al. An Initial Assessment of Impacts on Biodiversity from GMOs Released into the Environment in South Africa; South African National Biodiversity Institute: Pretoria, South Africa, 2021. [Google Scholar]
- Ofosu, R.; Agyemang, E.; Marton, A.; Pasztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Botha, M.; Siebert, S.J.; Van den Berg, J.; Ellis, S.; Dreber, N. Plant functional types differ between the grassland and savanna biomes along an agro-ecosystem disturbance gradient in South Africa. S. Afr. J. Bot. 2017, 113, 308–317. [Google Scholar] [CrossRef]
- Fried, G.; Chauvel, B.; Munoz, F.; Reboud, X. Which traits make weeds more successful in maize crops? Insights from a three-decade monitoring in France. Plants 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchman, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Martínková, J.; Klimeš, A.; Puy, J.; Klimešová, J. Response of clonal versus non clonal herbs to disturbance: Different strategies revealed. Perspect. Plant Ecol. Evol. Syst. 2020, 44, 125529. [Google Scholar] [CrossRef]
- Crayn, D.M.; Smith, J.A.C.; Winter, K. Carbon-isotope ratios and photosynthetic pathways in the neotropical family Rapateaceae. Plant Biol. 2001, 3, 569–576. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Liira, J.; Schmidt, T.; Aavik, T.; Arens, P.; Augenstein, I.; Bailey, D.; Billeter, R.; Bukáče, R.; Burel, F.; Blust, G. Plant functional group composition and large-scale species richness in European agricultural landscapes. J. Veg. Sci. 2008, 19, 3–14. [Google Scholar] [CrossRef]
- Wratten, S.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Nasim, G.; Shabbir, A. Invasive weed species—A threat to sustainable agriculture. In Crop Production for Agricultural Improvement; Springer: Berlin/Heidelberg, Germany, 2012; pp. 523–556. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Kumar, L.; Solhjouy-fard, S.; Tehrany, M.S.; Shabani, F.; Kalantar, B.; Esmaeili, A. Invasive weed species’ threats to global biodiversity: Future scenarios of changes in the number of invasive species in a changing climate. Ecol. Indic. 2020, 116, 106436. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.S.; Niklaus, P.A.; Bruelheide, H. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. Royal Soc. 2018, 285, 20181240. [Google Scholar] [CrossRef]
- Carrick, P.; Forsythe, K. The species composition-ecosystem function relationship: A global meta-analysis using data from intact and recovering ecosystems. PLoS ONE 2020, 15, e0236550. [Google Scholar] [CrossRef]
- Liu, X.; Swenson, N.G.; Lin, D.; Mi, X.; Umaña, M.N. Linking individual-level functional traits to tree growth in a subtropical forest. Ecology 2016, 97, 2396–2405. [Google Scholar] [CrossRef]
- Hejda, M.; Štajerová, K.; Pergl, J.; Pyšek, P. Impacts of dominant plant species on trait composition of communities: Comparison between the native and invaded ranges. Ecosphere 2019, 10, e02880. [Google Scholar] [CrossRef]
- Kraft, N.; Godoy, O.; Levine, J. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef]
- Sharma, N.; Rayamajhi, M. Different aspects of weed management in maize (Zea mays L.): A brief review. Adv. Agric. 2022, 2022, 7960175. [Google Scholar] [CrossRef]
- Gage, K.; Krausz, R.; Walters, S. Emerging challenges for weed management in herbicide-resistant crops. Agriculture 2019, 9, 180. [Google Scholar] [CrossRef]
- Loddo, D.; Scarabe, L.; Sattin, M.; Pederzoli, A.; Morsiani, C.; Canestrale, R.; Tommasini, M.G. Combination of herbicide band application and inter-row cultivation provides sustainable weed control in maize. Agronomy 2019, 10, 20. [Google Scholar] [CrossRef]
- Silva, M.R.; Galon, L.; Rosseto, E.R.O.; Silva, A.F.; Favretto, E.L.; Brunetto, L.; Gallin, A.; Silva, A.M.L.; Tonin, R.J. Weed management in glyphosate-resistant maize. Arq. Do Inst. Biológico 2020, 87, e0862019. [Google Scholar] [CrossRef]
- Vencill, W.; Nichols, R.; Webster, T.; Soteres, J.; Mallory-Smith, C.; Burgos, N. Herbicide Resistance: Toward an understanding of resistance development and the impact of herbicide-resistant crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Franzén, M.; Dieker, P.; Schrader, J.; Helm, A. Rapid plant colonization of the forelands of a vanishing glacier is strongly associated with species traits. Arct. Antarct. Alp. Res. 2019, 51, 366–378. [Google Scholar] [CrossRef]
- Guerra, J.G.; Cabello, F.; Quintanilla, C.F.; Pena, J.M.; Dorado, J. Plant functional diversity is affected by weed management through processes of trait convergence and divergence. Front. Plant Sci. 2022, 13, 993051. [Google Scholar] [CrossRef] [PubMed]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed control research and SAFENER technology: The path to modern agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef]
- Espig, M.; Henwood, R.J. The social foundations for re-solving herbicide resistance in Canterbury, New Zealand. PLoS ONE 2023, 18, e0286515. [Google Scholar] [CrossRef] [PubMed]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Owen, M.D. Diverse approaches to herbicide-resistant weed management. Weed Sci. 2016, 64, 570–584. [Google Scholar] [CrossRef]
- Colbach, N. The functional role of the soil seed bank in agricultural ecosystems. In Seeds: The Ecology of Regeneration in Plant Communities; CABI: Wallingford, UK, 2013; pp. 235–262. [Google Scholar] [CrossRef]
- Siebert, F.; Van Staden, N.; Komape, D.M.; Swemmer, A.M.; Siebert, S.J. Effects of land-use on herbaceous vegetation in a semi-arid Mopaneveld savanna. Bothalia 2021, 51, 1–26. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Lian, J.; Shen, H.; Cao, H.; Lu, H.; Ye, W. Tree aboveground carbon storage correlates with environmental gradients and functional diversity in a tropical forest. Sci. Rep. 2016, 6, 25304. [Google Scholar] [CrossRef]
- Heijting, S.; Van Der Werf, W.; Kropff, M.J. Seed dispersal by forage harvester and rigid-tine cultivator in maize. Weed Res. 2009, 49, 153–163. [Google Scholar] [CrossRef]
- Tainton, N.M. Veld Management in South Africa; University of Natal Press: Pietermaritzburg, South Africa, 1999. [Google Scholar]
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant, G.N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef]
- Siebert, F.; Klem, J.; Van Coller, H. Forb community responses to an extensive drought in two contrasting land-use types of a semi-arid Lowveld savanna. Afr. J. Range Forage Sci. 2020, 37, 53–64. [Google Scholar] [CrossRef]
- Barrett, S.H. Crop mimicry in weeds. Econ. Bot. 1983, 37, 255–282. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide resistant. Pest Manag. Sci. 2013, 70, 1306–1315. [Google Scholar] [CrossRef]
- Charbonneau, A.; Tack, D.; Lale, A.; Goldston, J.; Caple, M.; Conner, E.; Barazani, O.; Ziffer-Berger, J.; Dworkin, I.; Conner, J.K. Weed evolution: Genetic differentiation among wild, weedy, and crop radish. Evol. Appl. 2018, 11, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Lososová, Z.; Chytry, M.; Kuhn, I. Plant attributes determining the regional abundance of weeds on central European arable land. J. Biogeogr. 2008, 35, 177–187. [Google Scholar] [CrossRef]
- Chipomho, J.; Mupeti, S.; Chipomho, C.; Mashavakure, N.; Mashingaidze, A.B. Evaluation of a pre-formulated post-emergence herbicide mixture of topramezone and dicamba on annual weeds and Bermuda grass in maize in a sub-tropical agro-ecology. Heliyon 2019, 5, e01712. [Google Scholar] [CrossRef]
- Bogale, A. Reducing weed impacts and yield losses by application of herbicides in summer-grown maize. Int. J. Agric. Sci. Food Technol. 2023, 9, 49–53. [Google Scholar] [CrossRef]
- Kwinda, M.; Siebert, S.J.; Van Coller, H.; Masehela, T.S. Composition and diversity patterns of weeds in herbicide tolerant maize fields and margins in the Eastern Cape, South Africa. Biodiversitas 2023, 24, 399–414. [Google Scholar] [CrossRef]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Aronson, M.F.; Handel, S.N.; Clemants, S.E. Fruit type, life form and origin determine the success of woody plant invaders in an urban landscape. Biol. Invasions. 2007, 9, 465–475. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis. A Practical Approach; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, MA, USA, 2006; Available online: http://primer-e.com (accessed on 4 May 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).