Biochar and Flooding Increase and Change the Diazotroph Communities in Tropical Paddy Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Experimental Setup
2.3. Soil Physicochemical Properties
2.4. Soil DNA Extraction and Real-Time PCR
2.5. High-Throughput Sequencing Analysis
2.6. Data Analysis
3. Results
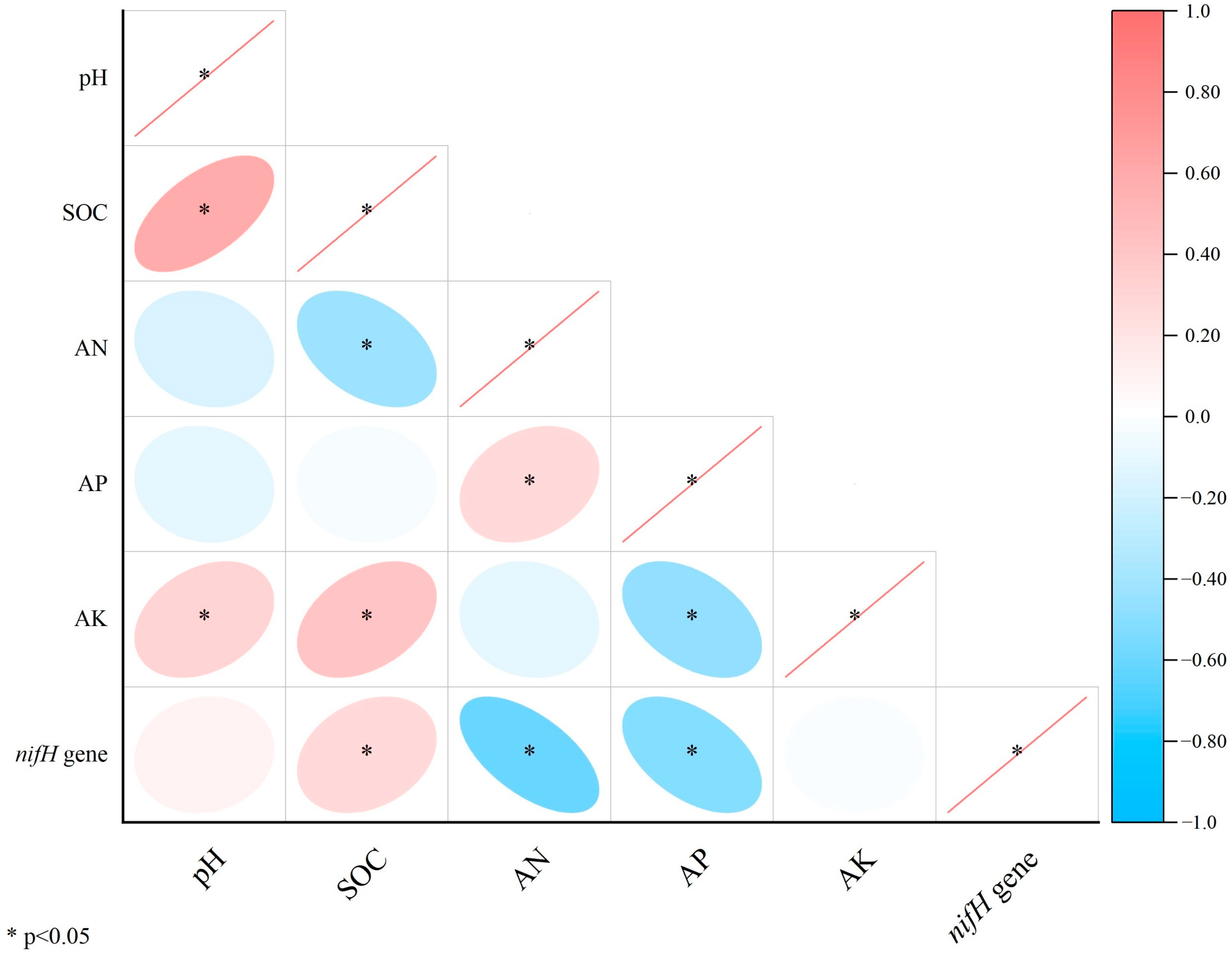
3.1. Soil Physicochemical Properties
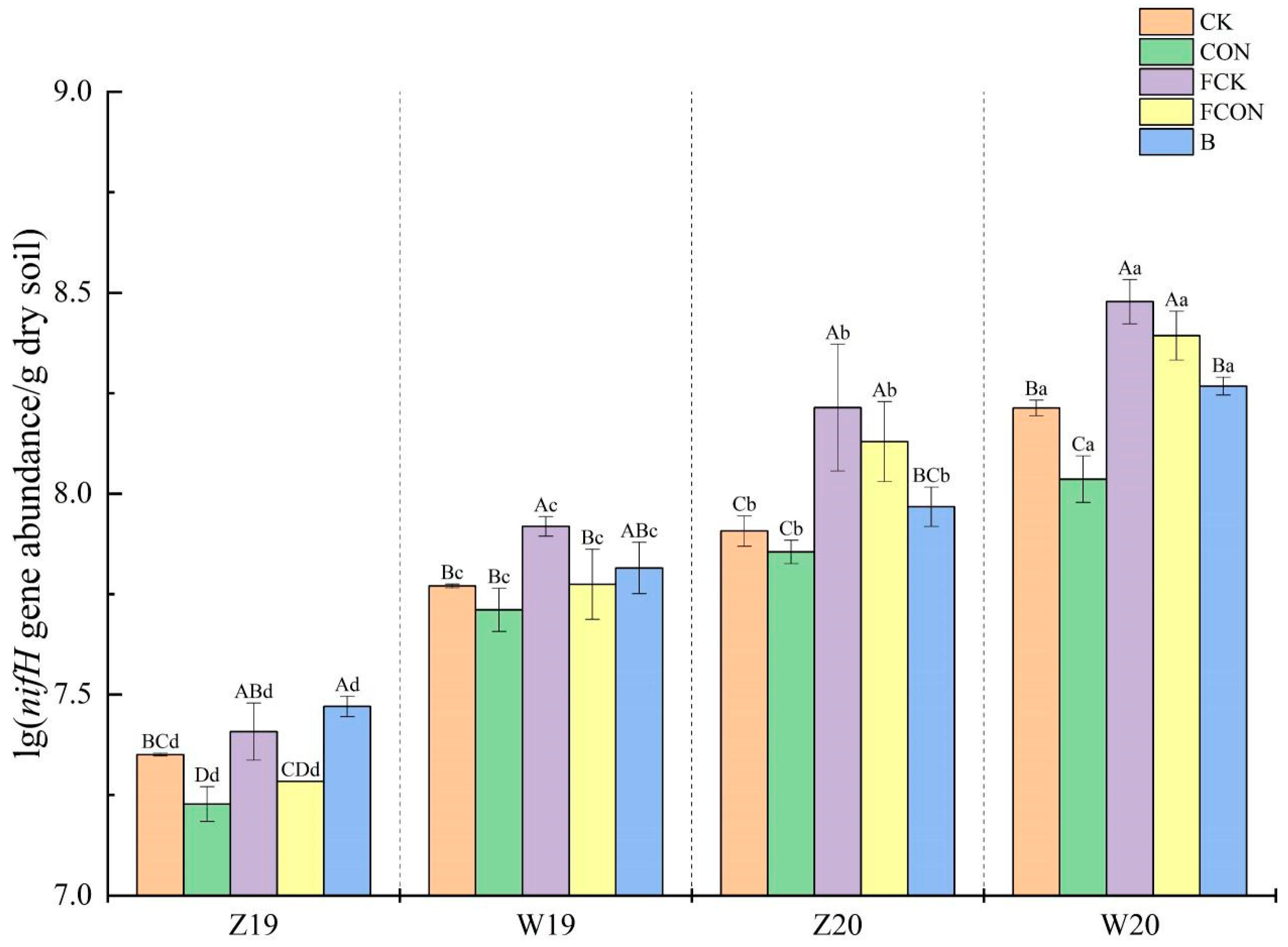
3.2. nifH Gene Abundance
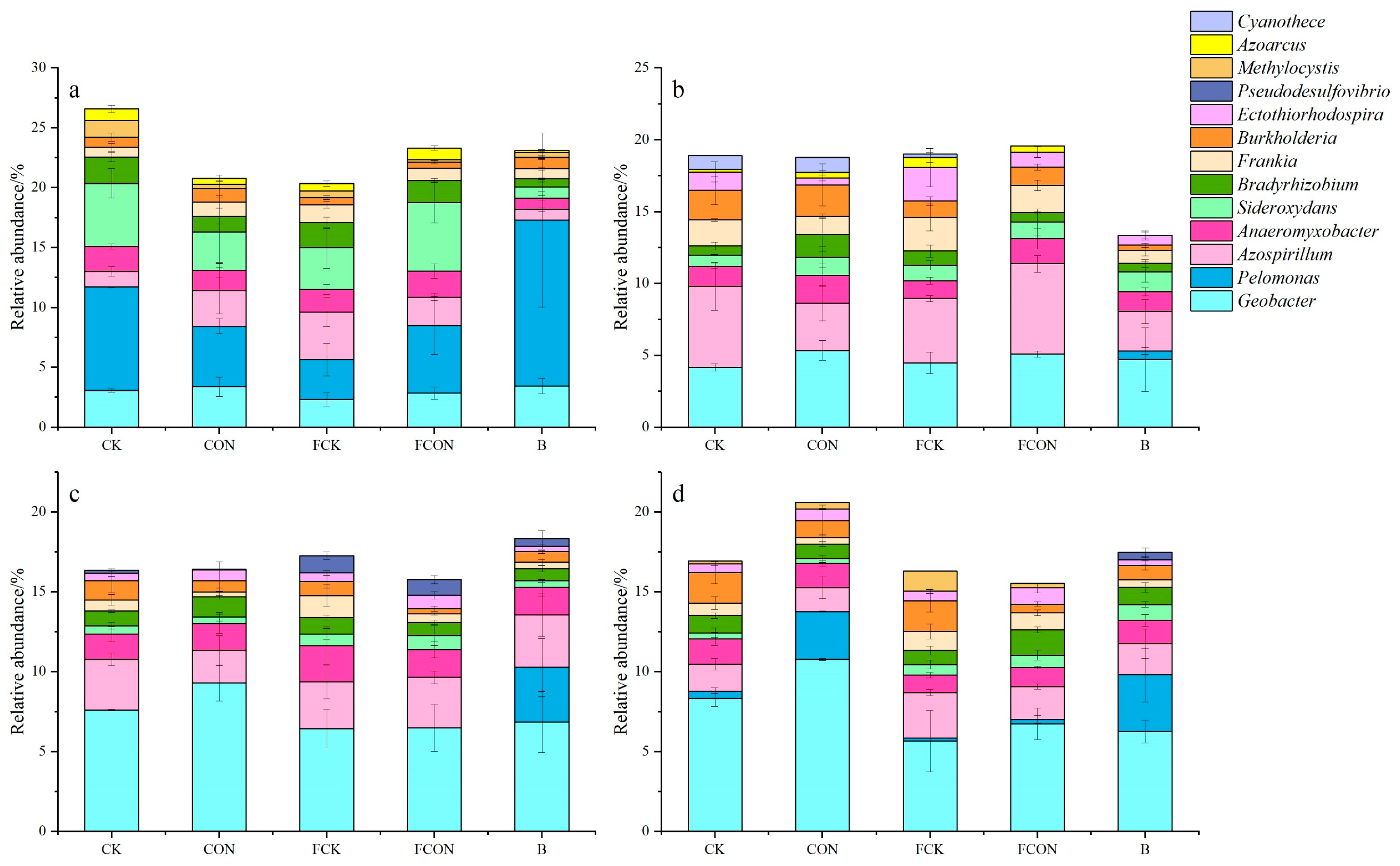
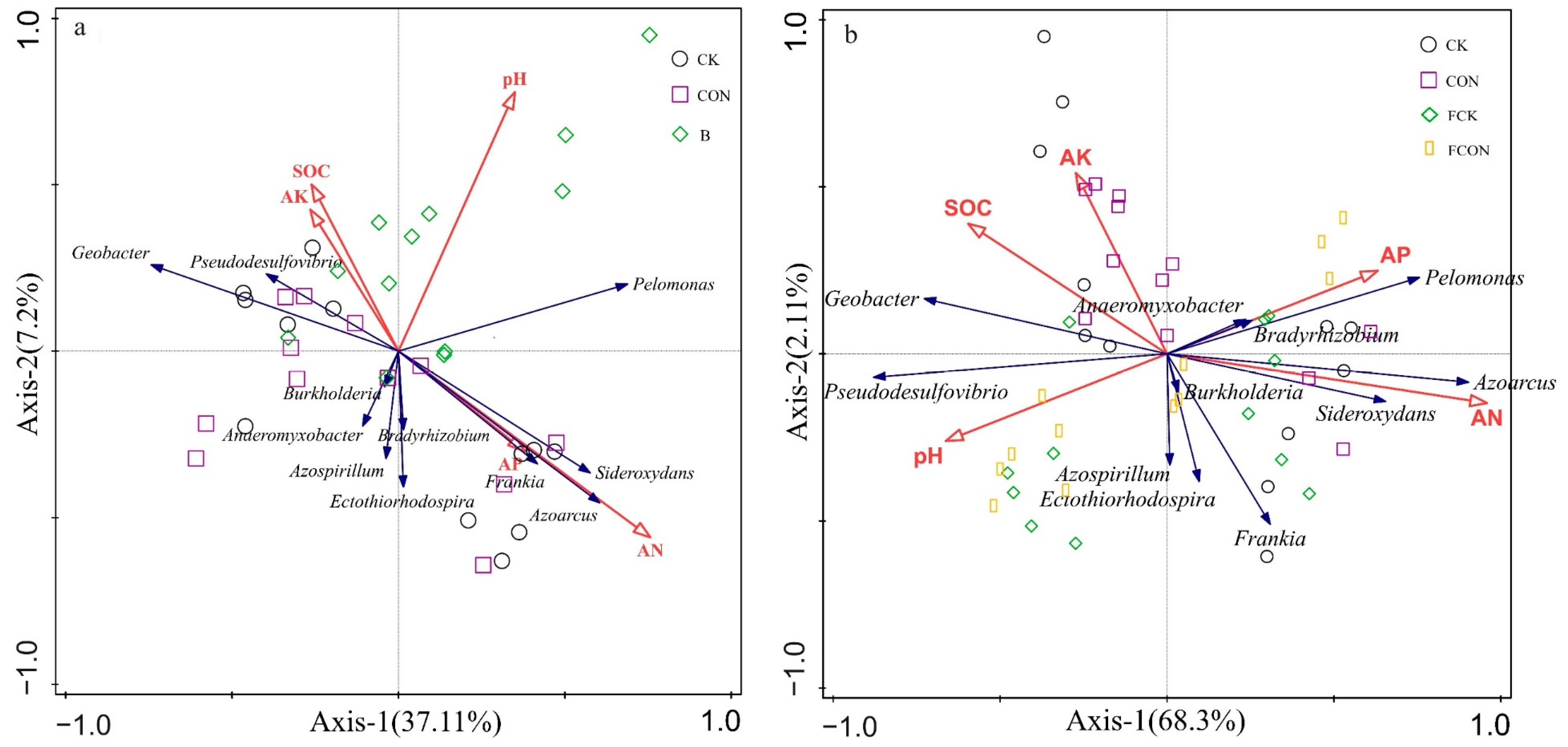
3.3. Effects of Different Treatments on Community Structure of nifH Gene-Related Microorganisms
4. Discussion
4.1. Effects of Flooding and Biochar Amendment on nifH Gene Abundance
4.2. Effects of Flooding and Biochar on the Community Structure of Diazotrophic Microorganisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, C.; Wright, A.L.; Ma, L.; He, X.; Xie, D.; Jiang, X. Improving nitrogen-use efficiency by using ridge tillage in rice paddy soils. Soil Use Manag. 2022, 38, 528–536. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China—Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, J.; Wang, J.; Cai, M.; Li, C.; Cao, C. Emissions of N2O and NH3, and nitrogen leaching from direct seeded rice under different tillage practices in central China. Agric. Ecosyst. Environ. 2011, 140, 164–173. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisher, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; Von Fischer, J.C.; Elseroad, A. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645. [Google Scholar] [CrossRef]
- Davies-Barnard, T.; Friedlingstein, P. The Global Distribution of Biological Nitrogen Fixation in Terrestrial Natural Ecosystems. Global Biogeochem. Cycles 2020, 34, e2019GB006387. [Google Scholar] [CrossRef]
- Roger, P.A.; Watanabe, I. Technologies for utilizing biological nitrogen fixation in wetland rice: Potentialities, current usage, and limiting factors. In Nitrogen Economy of Flooded Rice Soils. Developments in Plant and Soil Sciences; De Datta, S.K., Patrick, W.H., Eds.; Springer: Dordrecht, The Netherlands, 1986; Volume 26. [Google Scholar] [CrossRef]
- Ueda, T.; Suga, Y.; Yahiro, N.; Matsuguchi, T. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J. Bacteriol. 1995, 177, 1414–1417. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Boujenna, A.; del Moral, L.F.G. Biotechnological approaches to develop nitrogen-fixing cereals: A review. Span. J. Agric. Res. 2021, 19, 12. [Google Scholar] [CrossRef]
- Islam, M.R.; Sultana, T.; Cho, J.C.; Joe, M.M.; Sa, T.M. Diversity of free-living nitrogen-fixing bacteria associated with Korean paddy fields. Ann. Microbiol. 2012, 62, 1643–1650. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Zheng, M.M.; Li, W.X.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Fertilization practices affect biological nitrogen fixation by modulating diazotrophic communities in an acidic soil in southern China. Pedosphere 2023, 33, 301–311. [Google Scholar] [CrossRef]
- Masuda, Y.; Shiratori, Y.; Ohba, H.; Ishida, T.; Takano, R.; Satoh, S.; Shen, W.S.; Gao, N.; Itoh, H.; Senoo, K. Enhancement of the nitrogen-fixing activity of paddy soils owing to iron application. Soil Sci. Plant Nutr. 2021, 67, 243–247. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, H.; Zhu, H.; Xu, S.; Zhang, D.; Lv, W. Rice Rotation System Affects the Spatial Dynamics of the Diazotrophic Community in Paddy Soil of the Yangtze Delta, China. Eurasia Soil Sci. 2019, 52, 696–706. [Google Scholar] [CrossRef]
- Wu, C.; Wei, X.; Hu, Z.; Liu, Y.; Hu, Y.; Qin, H.; Chen, X.; Wu, J.; Ge, T.; Zhran, M.; et al. Diazotrophic Community Variation Underlies Differences in Nitrogen Fixation Potential in Paddy Soils across a Climatic Gradient in China. Microb. Ecol. 2021, 81, 425–436. [Google Scholar] [CrossRef]
- Ge, T.; Luo, Y.; He, X. Quantitative and mechanistic insights into the key process in the rhizodeposited carbon stabilization, transformation and utilization of carbon, nitrogen and phosphorus in paddy soil. Plant Soil 2019, 445, 1–5. [Google Scholar] [CrossRef]
- Mårtensson, L.; Díez, B.; Wartiainen, I.; Zheng, W.; El-Shehawy, R.; Rasmussen, U. Diazotrophic diversity, nifH gene expression and nitrogenase activity in a rice paddy field in Fujian, China. Plant Soil 2009, 325, 207–218. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Li, F.; Su, Z.; Zhang, H. Community Composition and Co-Occurrence Patterns of Diazotrophs along a Soil Profile in Paddy Fields of Three Soil Types in China. Microb. Ecol. 2021, 82, 961–970. [Google Scholar] [CrossRef]
- Peng, J.; Wu, X.; Ni, S.; Wang, J.; Song, Y.; Cai, C. Investigating intra-aggregate microstructure characteristics and influencing factors of six soil types along a climatic gradient. Catena 2022, 210, 105867. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Ma, J.; Zhang, Y.; Hu, T.; Zhang, H.; Feng, Y.; Pan, H.; Xu, Z.; Liu, G.; et al. Soil aluminum oxides determine biological nitrogen fixation and diazotrophic communities across major types of paddy soils in China. Soil Biol. Biochem. 2019, 131, 81–89. [Google Scholar] [CrossRef]
- Mergel, A.; Kloos, K.; Bothe, H. Seasonal fluctuations in the population of denitrifying and N2-fixing bacteria in an acid soil of a Norway spruce forest. Plant Soil 2001, 230, 145–160. [Google Scholar] [CrossRef]
- Bentley, B.L. Nitrogen Fixation by Epiphylls in a Tropical Rainforest. Ann. Mo. Bot. Gard. 1987, 74, 234–241. [Google Scholar] [CrossRef]
- Pereira, E.S.M.C.; Schloter-Hai, B.; Schloter, M.; van Elsas, J.D.; Salles, J.F. Temporal dynamics of abundance and composition of nitrogen-fixing communities across agricultural soils. PLoS ONE 2013, 8, e74500. [Google Scholar]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To Fix or Not to Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef] [PubMed]
- Said-Pullicino, D.; Cucu, M.A.; Sodano, M.; Birk, J.J.; Glaser, B.; Celi, L. Nitrogen immobilization in paddy soils as affected by redox conditions and rice straw incorporation. Geoderma 2014, 228, 44–53. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharyya, P.; Adhya, T.K. Impact of elevated CO2, flooding, and temperature interaction on heterotrophic nitrogen fixation in tropical rice soils. Biol. Fertil. Soils 2011, 47, 25–30. [Google Scholar] [CrossRef]
- Rao Rajaramamohan, V.; Rao, J.L.N. Nitrogen fixation (C2H2 reduction) in soil samples from rhizosphere of rice grown under alternate flooded and nonflooded conditions. Plant Soil 1984, 81, 111–118. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Mosharrof, M. Rice Growth Performance, Nutrient Use Efficiency and Changes in Soil Properties Influenced by Biochar under Alternate Wetting and Drying Irrigation. Sustainability 2022, 14, 7977. [Google Scholar] [CrossRef]
- Wu, J.; Ma, C.; Li, F. Microbial community structure and function in paddy soil as affected by water-saving irrigation mode. Eur. J. Soil Biol. 2022, 113, 103450. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Xue, C.; Gao, W.; Wang, G.; Liu, X. Changes in N2-fixation activity, abundance and composition of diazotrophic communities in a wheat field under elevated CO2 and canopy warming. Appl. Soil Ecol. 2021, 165, 104017. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Wang, H.; Xiao, Q.; Liu, X. Molecular diversity of nitrogen-fixing bacteria from the Tibetan Plateau, China. FEMS Microbiol. Lett. 2006, 260, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Shen, C.; Yang, F.; Wang, J.; Ge, Y. Significant dose effects of fertilizers on soil diazotrophic diversity, community composition, and assembly processes in a long-term paddy field fertilization experiment. Land Degrad. Dev. 2020, 32, 420–429. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Yao, H. Fertilization with inorganic and organic nutrients changes diazotroph community composition and N-fixation rates. J. Soils Sediment 2018, 18, 1076–1086. [Google Scholar] [CrossRef]
- Masuda, Y.; Satoh, S.; Miyamoto, R.; Takano, R.; Ishii, K.; Ohba, H.; Shiratori, Y.; Senoo, K. Biological nitrogen fixation in the long-term nitrogen-fertilized and unfertilized paddy fields, with special reference to diazotrophic iron-reducing bacteria. Arch. Microbiol. 2023, 205, 291. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Zhang, M.M.; Chen, A.L.; Zhang, W.Z.; Wei, W.X.; Sheng, R. Impact of fertilization regimes on diazotroph community compositions and N-fixation activity in paddy soil. Agric. Ecosyst. Environ. 2017, 247, 1–8. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Gao, W.; Xue, C.; Guo, Z.; Jiang, L.; Li, F.; Liu, Y. Impact of biochar amendment on the abundance and structure of diazotrophic community in an alkaline soil. Sci. Total Environ. 2019, 688, 944–951. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Li, Y.; Xiao, P.; Liu, S.; Shao, J.; Cai, Y.; Yan, X.; Fan, L. Biochar amendment reduces biological nitrogen fixation and nitrogen use efficiency in cadmium-contaminated paddy fields. J. Environ. Manag. 2023, 344, 118338. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Sun, P.; Wang, Q.; Ruan, Y. Effects of biochar and chemical fertilizer amendment on diazotrophic abundance and community structure in rhizosphere and bulk soils. Environ. Sci. Pollut. Res. Int. 2022, 29, 62361–62370. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Shen, J.; Li, Y.; Chen, D.; Bolan, N.; Li, Y.; Wu, J. Biochar amendment increases the abundance and alters the community composition of diazotrophs in a double rice cropping system. Biol. Fertil. Soils 2023, 59, 873–886. [Google Scholar] [CrossRef]
- Lv, R.; Wang, Y.; Wang, Q.; Wen, Y.; Shang, Q. Rice straw biochar alters inorganic nitrogen availability in paddy soil mainly through abiotic processes. J. Soils Sed. 2022, 23, 568–581. [Google Scholar] [CrossRef]
- Duan, P.; Zhang, X.; Zhang, Q.; Wu, Z.; Xiong, Z. Field-aged biochar stimulated N2O production from greenhouse vegetable production soils by nitrification and denitrification. Sci. Total Environ. 2018, 642, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Chen, K.; Li, N.; Zhang, S.; Liu, N.; Yang, J.; Zhan, X.; Han, X. Biochar-induced changes in the soil diazotroph community abundance and structure in a peanut field trial. Biochar 2022, 4, 26. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Hu, T.L.; Wang, H.; Jin, H.Y.; Liu, Q.; Lin, Z.B.; Liu, B.J.; Liu, H.T.; Chen, Z.; Lin, X.W.; et al. How do different nitrogen application levels and irrigation practices impact biological nitrogen fixation and its distribution in paddy system? Plant Soil 2021, 467, 329–344. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Loeppert, R.; Inskeep, W.; Sparks, D. Methods of Soil Analysis. Part 3. Chemical Methods; ACSESS: Mississauga, ON, Canada, 1996; Volume 677. [Google Scholar]
- Rosch, C.; Mergel, A.; Bothe, H. Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl. Environ. Microbiol. 2002, 68, 3818–3829. [Google Scholar] [CrossRef]
- Gihring, T.M.; Green, S.J.; Schadt, C.W. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ. Microbiol. 2012, 14, 285–290. [Google Scholar] [CrossRef]
- Horel, A.; Bernard, R.J.; Mortazavi, B. Impact of crude oil exposure on nitrogen cycling in a previously impacted Juncus roemerianus salt marsh in the northern Gulf of Mexico. Environ. Sci. Pollut. Res. Int. 2014, 21, 6982–6993. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Chen, L.; Chen, Z.; Chu, J.; Li, Y. Comparison of the abundance and community structure of ammonia oxidizing prokaryotes in rice rhizosphere under three different irrigation cultivation modes. World J. Microbiol. Biotechnol. 2016, 32, 85. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Yang, S.; Qi, S.; Jiang, Z.; Dai, H.; Zhou, J. Soil nitrogen functional transformation microbial genes response to biochar application in different irrigation paddy field in southern China. Environ. Sci. Pollut. Res. Int. 2023, 30, 7770–7785. [Google Scholar] [CrossRef]
- Fan, H.; Jia, S.; Yu, M.; Chen, X.; Shen, A.; Su, Y. Long-term straw return increases biological nitrogen fixation by increasing soil organic carbon and decreasing available nitrogen in rice–rape rotation. Plant Soil 2022, 479, 267–279. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Shi, L.; Xiao, X.; Cheng, K.; Wen, L.; Li, W. Effect of different long-term fertilizer managements on soil nitrogen fixing bacteria community in a double-cropping rice paddy field of southern China. PLoS ONE 2021, 16, e0256754. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Li, Y.; Chen, D.; Ao, J.; Zhou, W.; Shen, D.; Li, Q.; Huang, Z.; Jiang, Y. Influence of nitrogen and phosphorus additions on N(2)-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation. Sci. Total Environ. 2018, 619–620, 1530–1537. [Google Scholar] [CrossRef]
- Chakraborty, A.; Islam, E. Temporal dynamics of total and free-living nitrogen-fixing bacterial community abundance and structure in soil with and without history of arsenic contamination during a rice growing season. Environ. Sci. Pollut. Res. Int. 2017, 25, 4951–4962. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Orr, C.H.; Leifert, C.; Cummings, S.P.; Cooper, J.M. Impacts of organic and conventional crop management on diversity and activity of free-living nitrogen fixing bacteria and total bacteria are subsidiary to temporal effects. PLoS ONE 2012, 7, e52891. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Ming, C.; Li, Y.; Wang, X.; Jiang, C. Assessing the difference of biochar and aged biochar to improve soil fertility and cabbage (Brassica oleracea var. capitata) productivity. J. Soils Sediment 2022, 23, 606–618. [Google Scholar] [CrossRef]
- Yang, L.; Bai, J.; Zeng, N.; Zhou, X.; Liao, Y.; Lu, Y.; Rees, R.M.; Nie, J.; Cao, W. Diazotroph abundance and community structure are reshaped by straw return and mineral fertilizer in rice-rice-green manure rotation. Appl. Soil Ecol. 2019, 136, 11–20. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Song, W.; Wen, S.; Wang, B.; Zhu, C.; Shen, R. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 2017, 113, 240–249. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Zhang, R.; Chen, M.; Yang, W.; Li, J.; Fang, Z.; Wang, B.; Qiu, Y.; Zheng, M. N2 fixation impacted by carbon fixation via dissolved organic carbon in the changing Daya Bay, South China Sea. Sci. Total Environ. 2019, 674, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, J.; Boorboori, M.R.; Li, D.; Chen, S.; Ma, X.; Cheng, P.; Zhang, H. Effect of biochar application rate on changes in soil labile organic carbon fractions and the association between bacterial community assembly and carbon metabolism with time. Sci. Total Environ. 2023, 855, 158876. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Agnelli, A.E.; Pastorelli, R.; Pallara, G.; Rasse, D.P.; Silvennoinen, H. Past water management affected GHG production and microbial community pattern in Italian rice paddy soils. Soil Biol. Biochem. 2016, 93, 17–27. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Olivares, F.L.; Pereira Passaglia, L.M.; Selbach, P.A.; de Sá, E.L.S.; de Camargo, F.A.O. Characterization of diazotrophic bacteria associated with maize: Effect of plant genotype, ontogeny and nitrogen-supply. World J. Microbiol. Biotechnol. 2006, 22, 967–974. [Google Scholar] [CrossRef]
- Röling, W.F.M. The Family Geobacteraceae. In The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 157–172. [Google Scholar]
- Chen, S.; Xiang, X.; Ma, H.; Penttinen, P.; Zhao, J.; Li, H.; Gao, R.; Zheng, T.; Fan, G. Straw Mulching and Nitrogen Fertilization Affect Diazotroph Communities in Wheat Rhizosphere. Front. Microbiol. 2021, 12, 658668. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ye, G.; Liu, D.; Ledgard, S.; Luo, J.; Fan, J.; Yuan, J.; Chen, Z.; Ding, W. Long-term application of lime or pig manure rather than plant residues suppressed diazotroph abundance and diversity and altered community structure in an acidic Ultisol. Soil Biol. Biochem. 2018, 123, 218–228. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, J.; Li, X. The diversity and co-occurrence patterns of diazotrophs in the steppes of Inner Mongolia. Catena 2017, 157, 130–138. [Google Scholar] [CrossRef]
- Roy, M.L.; Srivastava, R.C. Changes in Diazotrophic Population in Paddy Soil on N-Fertilisation Alone or in Integration with Certain Growth Promoting Rhizobacteria. Vegetos 2013, 26, 357–361. [Google Scholar] [CrossRef]
- Feng, M.; Du, Y.; Li, X.; Li, F.; Qiao, J.; Chen, G.; Huang, Y. Insight into universality and characteristics of nitrate reduction coupled with arsenic oxidation in different paddy soils. Sci. Total Environ. 2023, 866, 161342. [Google Scholar] [CrossRef] [PubMed]
- Cassan, F.; Coniglio, A.; Lopez, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xu, Y.; Rodgers, T.F.M.; Bao, M.; Tan, F. Uptake, translocation, bioaccumulation, and bioavailability of organophosphate esters in rice paddy and maize fields. J. Hazard. Mater. 2023, 446, 130640. [Google Scholar] [CrossRef]
- Bao, Z.; Sasaki, K.; Okubo, T.; Ikeda, S.; Anda, M.; Hanzawa, E.; Kakizaki, K.; Sato, T.; Mitsui, H.; Minamisawa, K. Impact of Azospirillum sp. B510 Inoculation on Rice-Associated Bacterial Communities in a Paddy Field. Microbes Environ. 2013, 28, 487–490. [Google Scholar] [CrossRef]
- Ishii, S.; Ohno, H.; Tsuboi, M.; Otsuka, S.; Senoo, K. Identification and isolation of active N2O reducers in rice paddy soil. ISME J. 2011, 5, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
| Season | Field Management | 2019 | 2020 | Nitrogenous Fertilizer/Urea: N 46% | Phosphate Fertilizer/Superphosphate: P2O5 16% | Potassium Fertilizer /Potassium Chloride: K2O 60% |
|---|---|---|---|---|---|---|
| Early rice season | Seedling transplanting, Base fertilizer | 20 April 2019 | 1 May 2020 | 60 kg·ha−1 | 90 kg·ha−1 | 90 kg·ha−1 |
| Tillering fertilizer | 13 May 2019 | 8 May 2020 | 36 kg·ha−1 | |||
| Ear fertilizer | 13 June 2019 | 14 June 2020 | 24 kg·ha−1 | |||
| harvest | 20 July 2019 | 5 August 2020 | ||||
| Late rice season | Seedling transplanting, Base fertilizer | 30 July 2019 | 14 August 2020 | 90 kg·ha−1 | 120 kg·ha−1 | |
| Tillering fertilizer | 12 August 2019 | 22 August 2020 | 90 kg·ha−1 | |||
| Ear fertilizer | 9 September 2019 | 21 September 2020 | 60 kg·ha−1 | |||
| harvest | 5 November 2019 | 8 November 2020 |
| Season | Treatments | pH | SOC/g·kg−1 | Available N/mg·kg−1 | Available P/mg·kg−1 | Available K/mg·kg−1 |
|---|---|---|---|---|---|---|
| Z19 | CK | 5.5 ± 0.19 b † | 16.3 ± 0.40 b | 216.8 ± 14.31 a | 101.1 ± 17.05 ab | 29.8 ± 2.39 b |
| CON | 5.4 ± 0.09 b | 15.4 ± 0.40 b | 199.8 ± 25.71 a | 120.4 ± 12.40 a | 20.5 ± 1.64 c | |
| FCK | 5.3 ± 0.06 b | 15.6 ± 0.34 b | 186.8 ± 4.09 a | 89.7 ± 5.30 b | 35.0 ± 5.85 ab | |
| FCON | 5.2 ± 0.13 b | 15.8 ± 0.11 b | 217.0 ± 4.86 a | 94.3 ± 2.50 b | 37.1 ± 4.10 ab | |
| B | 6.2 ± 0.48 a | 19.0 ± 0.90 a | 128.5 ± 8.52 b | 111.8 ± 8.53 ab | 40.4 ± 4.99 a | |
| W19 | CK | 5.7 ± 0.09 b | 17.6 ± 0.20 ab | 189.5 ± 27.42 ab | 113.9 ± 5.49 a | 30.2 ± 8.92 b |
| CON | 5.5 ± 0.01 ab | 17.6 ± 1.0 ab | 113.5 ± 6.19 d | 121.7 ± 18.10 a | 26.8 ± 1.12 b | |
| FCK | 5.4 ± 0.16 b | 15.4 ± 1.15 b | 217.3 ± 16.98 a | 64.9 ± 1.27 b | 51.2 ± 6.42 a | |
| FCON | 5.3 ± 0.10 b | 15.3 ± 0.24 b | 136.5 ± 2.35 dc | 81.9 ± 4.96 b | 27.5 ± 0.49 b | |
| B | 6.5 ± 0.44 a | 19.2 ± 2.10 a | 165.2 ± 16.74 bc | 82.6 ± 11.42 b | 35.6 ± 6.47 b | |
| Z20 | CK | 5.8 ± 0.04 b | 19.9 ± 0.30 a | 81.4 ± 12.12 c | 56.2 ± 3.21 a | 148.6 ± 12.02 a |
| CON | 5.6 ± 0.07 bc | 16.8 ± 0.70 b | 114.1 ± 6.64 b | 48.3 ± 2.71 a | 99.0 ± 12.61 b | |
| FCK | 5.7 ± 0.23 bc | 16.3 ± 1.14 b | 92.6 ± 1.79 c | 52.0 ± 8.88 a | 31.6 ± 1.99 c | |
| FCON | 5.5 ± 0.16 c | 16.5 ± 1.18 b | 84.9 ± 2.43 c | 54.3 ± 1.90 a | 25.6 ± 1.40 c | |
| B | 6.2 ± 0.18 a | 20.4 ± 1.10 a | 141.8 ± 8.12 a | 50.4 ± 8.36 a | 153.5 ± 21.84 a | |
| W20 | CK | 5.6 ± 0.09 b | 18.3 ± 0.80 b | 83.7 ± 3.56 b | 105.0 ± 14.66 a | 29.8 ± 2.39 b |
| CON | 5.3 ± 0.05 b | 18.3 ± 0.70 b | 73.7 ± 1.78 c | 115.9 ± 11.96 a | 20.5 ± 1.64 d | |
| FCK | 5.6 ± 0.24 b | 18.0 ± 0.36 b | 93.8 ± 2.92 a | 32.3 ± 4.46 b | 39.4 ± 2.49 a | |
| FCON | 5.7 ± 0.10 ab | 18.3 ± 0.85 b | 82.5 ± 5.99 b | 37.5 ± 15.76 b | 36.0 ± 4.39 b | |
| B | 5.9 ± 0.11 a | 19.5 ± 0.40 a | 82.3 ± 10.82 c | 111.2 ± 3.18 a | 24.7 ± 2.37 c |
| Treatments | pH | SOC/g·kg−1 | Available N/mg·kg−1 | Available P/mg·kg−1 | Available K/mg·kg−1 | lg (nifH Gene Abundance/Dry Soil) |
|---|---|---|---|---|---|---|
| CK | 5.6 ± 0.18 a † | 18.0 ± 1.39 a | 142.8 ± 63.33 a | 94.0 ± 25.22 a | 59.6 ± 51.96 a | 7.8 ± 0.34 b |
| CON | 5.5 ± 0.12 b | 17.0 ± 1.34 b | 125.3 ± 48.00 b | 101.6 ± 33.29 a | 41.7 ± 33.77 b | 7.7 ± 0.33 b |
| FCK | 5.5 ± 0.25 b | 16.3 ± 1.20 b | 146.6 ± 57.27 a | 59.7 ± 21.58 b | 39.3 ± 8.73 b | 8.0 ± 0.48 a |
| FCON | 5.4 ± 0.21 b | 16.5 ± 1.36 b | 130.2 ± 54.71 b | 67.0 ± 23.88 b | 31.5 ± 5.92 c | 7.9 ± 0.42 a |
| N | ** | ** | ** | * | ** | * |
| S | ** | ** | ** | ** | ** | ** |
| W | ns | ** | ns | ** | ** | ** |
| N × S | ns | ** | ** | ns | ** | ns |
| N × W | ns | * | ns | ns | * | ns |
| S × W | * | ** | * | ** | ** | * |
| N × S × W | ns | ns | ** | ns | ** | ns |
| Treatments | pH | SOC/g·kg−1 | Available N/mg·kg−1 | Available P/mg·kg−1 | Available K/mg·kg−1 | lg (nifH Gene Abundance/Dry Soil) |
|---|---|---|---|---|---|---|
| CK | 5.6 ± 0.18 b † | 18.0 ± 1.39 b | 142.8 ± 63.33 a | 94.0 ± 25.22 ab | 59.6 ± 51.96 a | 7.8 ± 0.34 b |
| CON | 5.5 ± 0.12 b | 17.0 ± 1.34 c | 125.3 ± 48.00 ab | 101.6 ± 33.29 a | 41.7 ± 33.77 b | 7.7 ± 0.33 b |
| B | 6.2 ± 0.39 a | 19.5 ± 1.38 a | 129.4 ± 32.38 b | 89.0 ± 26.60 b | 63.6 ± 53.52 a | 7.9 ± 0.31 a |
| S | ns | ** | ** | ** | ** | ** |
| F | ** | ** | ns | ns | ** | ** |
| S × F | ns | ns | ** | ns | ** | ns |
| Season | Treatments | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| Z19 | CK | 694.3 ± 9.12 bc † | 701.9 ± 6.28 c | 0.89 ± 0.01 ab | 5.4 ± 0.10 b |
| CON | 674.4 ± 4.82 c | 670.2 ± 5.83 d | 0.88 ± 0.01 b | 5.1 ± 0.02 c | |
| FCK | 711.7 ± 0.06 b | 725.3 ± 1.50 b | 0.91 ± 0.01 a | 5.9 ± 0.03 a | |
| FCON | 752.9 ± 10.08 a | 754.3 ± 9.59 a | 0.91 ± 0.01 a | 5.8 ± 0.15 a | |
| B | 675.7 ± 18.68 c | 684.3 ± 18.32 cd | 0.81 ± 0.01 c | 4.4 ± 0.12 d | |
| W19 | CK | 757.8 ± 17.1 a | 770.2 ± 16.81 a | 0.89 ± 0.01 bc | 5.1 ± 0.25 b |
| CON | 697.6 ± 14.58 c | 730.8 ± 29.20 a | 0.89 ± 0.01 bc | 5.6 ± 0.21 ab | |
| FCK | 727.8 ± 7.15 b | 762.7 ± 19.69 a | 0.92 ± 0.01 a | 6.0 ± 0.11 a | |
| FCON | 700.3 ± 1.52 c | 664.9 ± 5.29 b | 0.92 ± 0.02 ab | 5.8 ± 0.09 ab | |
| B | 773.9 ± 10.58 a | 766.2 ± 2.02 a | 0.87 ± 0.00 c | 5.5 ± 0.19 ab | |
| Z20 | CK | 803.2 ± 14.13 a | 822.1 ± 9.35 b | 0.84 ± 0.01 b | 5.4 ± 0.03 ab |
| CON | 686.5 ± 7.41 d | 727.8 ± 15.85 d | 0.86 ± 0.02 b | 5.2 ± 0.08 b | |
| FCK | 832.5 ± 23.59 a | 864.7 ± 16.97 a | 0.84 ± 0.02 b | 5.4 ± 0.34 ab | |
| FCON | 718.5 ± 12.48 c | 734.1 ± 0.95 d | 0.90 ± 0.01 a | 5.7 ± 0.04 a | |
| B | 766.8 ± 0.99 b | 786.2 ± 11.00 c | 0.88 ± 0.01 ab | 5.7 ± 0.04 a | |
| W20 | CK | 778.3 ± 21.4 bc | 794.5 ± 29.40 bc | 0.84 ± 0.01 a | 5.2 ± 0.07 b |
| CON | 746.0 ± 18.54 d | 753.5 ± 11.63 c | 0.85 ± 0.03 a | 4.8 ± 0.03 c | |
| FCK | 856.9 ± 7.16 a | 862.0 ± 0.23 a | 0.88 ± 0.01 a | 5.6 ± 0.02 a | |
| FCON | 817.9 ± 47.01 ab | 832.8 ± 43.07 ab | 0.87 ± 0.01 a | 5.7 ± 0.16 a | |
| B | 787.2 ± 3.65 bc | 808.7 ± 4.76 abc | 0.84 ± 0.01 a | 5.2 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhao, Y.; Wang, Y.; Liu, X.; Qin, X.; Chen, Q.; Zhu, Q.; Elrys, A.S.; Wu, Y.; Meng, L. Biochar and Flooding Increase and Change the Diazotroph Communities in Tropical Paddy Fields. Agriculture 2024, 14, 211. https://doi.org/10.3390/agriculture14020211
Chen Y, Zhao Y, Wang Y, Liu X, Qin X, Chen Q, Zhu Q, Elrys AS, Wu Y, Meng L. Biochar and Flooding Increase and Change the Diazotroph Communities in Tropical Paddy Fields. Agriculture. 2024; 14(2):211. https://doi.org/10.3390/agriculture14020211
Chicago/Turabian StyleChen, Yunzhong, Yan Zhao, Yuqin Wang, Xinrui Liu, Xiaofeng Qin, Qiqi Chen, Qilin Zhu, Ahmed S. Elrys, Yanzheng Wu, and Lei Meng. 2024. "Biochar and Flooding Increase and Change the Diazotroph Communities in Tropical Paddy Fields" Agriculture 14, no. 2: 211. https://doi.org/10.3390/agriculture14020211
APA StyleChen, Y., Zhao, Y., Wang, Y., Liu, X., Qin, X., Chen, Q., Zhu, Q., Elrys, A. S., Wu, Y., & Meng, L. (2024). Biochar and Flooding Increase and Change the Diazotroph Communities in Tropical Paddy Fields. Agriculture, 14(2), 211. https://doi.org/10.3390/agriculture14020211






