Performance, Carcass Composition, and Meat Quality during Frozen Storage in Male Layer-Type Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds and Housing
2.2. Slaughtering, Carcass Analysis, Sampling, and Storage
2.3. Economic Evaluation
Methodology of Calculation of the Costs:
2.4. Analysis of Meat Quality
2.4.1. Measurement of Meat pH and Color
2.4.2. Free Amino Groups
2.4.3. Lipid Oxidation
2.5. Statistical Evaluation
3. Results
3.1. Growth Performance and Carcass Composition
3.2. Economic Evaluation of the Rearing of Male Layer-Type Chickens
3.3. Meat Quality
3.3.1. pH and Color
3.3.2. Proteolysis in Meat
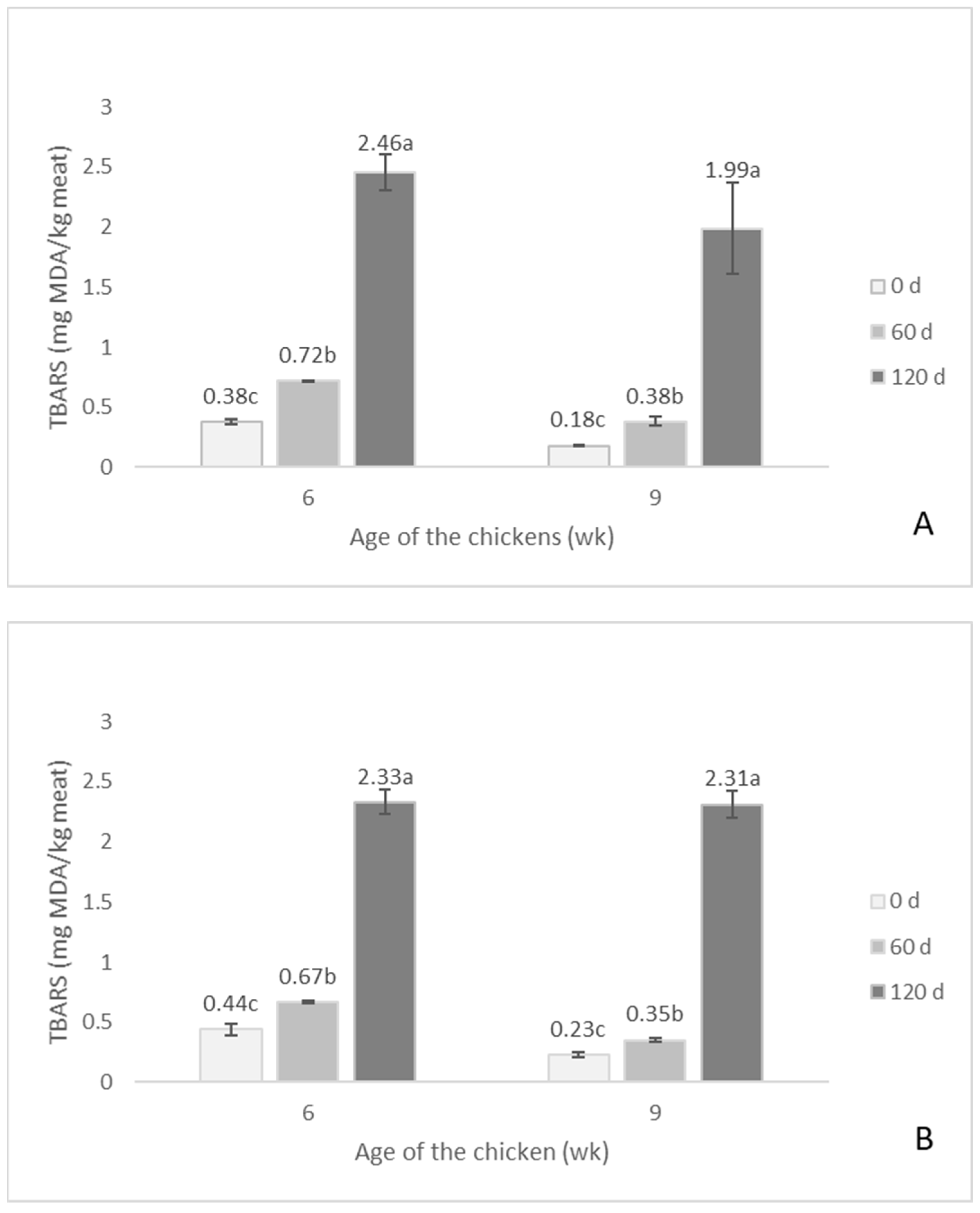
3.3.3. Lipid Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, P.M.C.C.; Vicente, A.F.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Papp, R.E.; Hasenegger, V.; Ekmekcioglu, C.; Schwingshackl, L. Association of poultry consumption with cardiovascular diseases and all-cause mortality: A systematic review and dose response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 2366–2387. [Google Scholar] [CrossRef]
- Bonpoor, J.; Petermann-Rocha, F.; Parra-Soto, S.; Pell, J.P.; Gray, S.R.; Celis-Morales, C.; Ho, F.K. Types of diet, obesity, and incident type 2 diabetes: Findings from the UK Biobank prospective cohort study. Diabetes Obes. Metab. 2022, 24, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, D.B.; Warberg, C.K.; Würtz, A.M.L.; Overvad, K.; Dahm, C.C. Substitution of red meat with poultry or fish and risk of type 2 diabetes: A Danish cohort study. Eur. J. Nutr. 2019, 58, 2705–2712. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Hollenbeck, A.R.; Park, Y.; Sinha, R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev. Res. 2011, 4, 1903–1911. [Google Scholar] [CrossRef]
- Lo, J.J.; Park, Y.-M.M.; Sinha, R.; Sandler, D.P. Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int. J. Cancer 2020, 146, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 November 2023).
- Valenta, J.; Chodová, D.; Tůmová, E.; Ketta, M. Carcass characteristics and breast meat quality in fast-, medium- and slow-growing chickens. Czech J. Anim. Sci. 2022, 67, 286–294. [Google Scholar] [CrossRef]
- Sirri, F.; Castellini, C.; Bianchi, M.; Petracci, M.; Meluzzi, A.; Franchini, A. Effect of fast-, medium- and slow-growing strains on meat quality of chickens reared under the organic farming method. Animal 2011, 5, 312–319. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ignatova, M.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S.; Kolev, N. Male layer-type chickens—An alternative source for high quality poultry meat: A review on the carcass composition, sensory characteristics and nutritional profile. Braz. J. Poult. Sci. 2022, 24, 1–10. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ignatova, M.; Dragoev, S.; Vlahova-Vangelova, D.; Balev, D.; Kolev, N. Growth Performance, carcass composition and tenderness of meat in male layer-type chickens slaughtered at different age. C. R. Acad. Bulg. Sci. 2023, 76, 156–164. [Google Scholar]
- Angelovičová, M.; Angelovič, M.; Čapla, J.; Zajác, P.; Folvarčíková, P.; Čurlej, J. The effect of oregano essential oil on chicken meat lipid oxidation and peroxidation. Potravin. S. J. Food Sci. 2021, 15, 1056–1068. [Google Scholar] [CrossRef]
- Lichovníková, M.; Jandásek, J.; Jùzl, M.; Draèková, E. The meat quality of layer males from free range in comparison with fast growing chickens. Czech J. Anim. Sci. 2009, 54, 490–497. [Google Scholar] [CrossRef]
- Mueller, S.; Kreuzer, M.; Siegrist, M.; Mannale, K.; Messikommer, R.E.; Gangnat, I.D.M. Carcass and meat quality of dual-purpose chickens (Lohmann Dual, Belgian Malines, Schweizerhuhn) in comparison to broiler and layer chicken types. Poult. Sci. 2018, 97, 3325–3336. [Google Scholar] [CrossRef]
- Mueller, S.; Taddei, L.; Albiker, D.; Kreuzer, M.; Siegrist, M.; Messikommer, R.E.; Gangnat, I.D.M. Growth, carcass, and meat quality of 2 dual-purpose chickens and a layer hybrid grown for 67 or 84 D compared with slow-growing broilers. J. Appl. Poult. Res. 2020, 29, 185–196. [Google Scholar] [CrossRef]
- Choo, Y.K.; Oh, S.T.; Lee, K.W.; Kang, C.W.; Kim, H.W.; Kim, C.J.; Kim, E.J.; Kim, H.S.; An, B.K. The growth performance, carcass characteristics, and meat quality of egg-type male growing chicken and White-Mini Broiler in comparison with commercial broiler (Ross 308). Korean J. Food Sci. Anim. Resour. 2014, 34, 622–629. [Google Scholar] [CrossRef]
- Evaris, E.F.; Sarmiento-Franco, L.; Sandoval-Castro, C.A. Meat and bone quality of slow-growing male chickens raised with outdoor access in tropical climate. J. Food Compos. Anal. 2021, 98, 103802. [Google Scholar] [CrossRef]
- Khan, A. Extraction and fractionation of proteins in fresh chicken muscle. J. Food Sci. 1962, 27, 430–434. [Google Scholar] [CrossRef]
- Vassilev, K.; Ivanov, G.; Balev, D.; Dobrev, G. Protein changes of chicken light and dark muscles during chilled storage. J. EcoAgriTourism 2012, 8, 263–268. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 7, 421–424. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- JMP, version 7; SAS Institute Inc.: Cary, NC, USA, 2007.
- Popova, T.; Petkov, E.; Ignatova, M.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S.; Kolev, N.; Dimov, K. Meat quality of male layer-type chickens slaughtered at different ages. Agriculture 2023, 13, 624. [Google Scholar] [CrossRef]
- Habig, C.; Bayerbach, M.; Kember, N. Comparative analyses of layer males, dual purpose males and mixed sex broilers kept for fattening purposes regarding their floor space covering, weight-gain and several animal health traits. Arc. Geflügelkd. 2016, 80, 1–10. [Google Scholar]
- Koenig, M.; Hahn, G.; Damme, K.; Schmutz, M. Utilization of laying-type cockerels as coquelets: Influence of genotype and diet characteristics on growth performance and carcass composition. Arc. Geflügelkd. 2012, 76, 197–202. [Google Scholar]
- Putra, W.P.B.; Riaz, R.; Gunawan, A.A.; Orman, A. Comparison of growth curve in male layer chickens. J. Res. Vet. Med. 2021, 40, 49–53. [Google Scholar] [CrossRef]
- Evaris, F.E.; Sarmiento, F.L.; Sandoval, C.C.; Segura, C.J.; Caamal, M.J.A. Male Layer chicken’s response to dietary Moringa oleifera meal in a tropical climate. Animals 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.; Wickramasinghe, Y.; Kalubowila, D. Growth performance and carcass quality of layer type cockerels and broiler chicken. Iran. J. Appl. Anim. Sci. 2016, 6, 429–433. [Google Scholar]
- Petkov, E.; Popova, T.; Ignatova, M. Carcass and meat composition in f1 crosses of two lines of slow-growing chickens reared in conventional or alternative system with access to pasture. Int. J. Innov. Approaches Agric. Res. 2018, 2, 359–374. [Google Scholar] [CrossRef]
- Petkov, E.; Popova, T.; Ignatova, M.; Sharkova, V.; Dimov, K. Development of dual-purpose cross for meat and egg production I. Growth performance and carcass composition of the crossbred chickens in comparison to the parent lines. Arch. Zootech. 2022, 25, 119–129. [Google Scholar] [CrossRef]
- Murawska, D.; Bochno, R. Comparison of the slaughter quality of Layer-type cockerels and broiler chicken. J. Poult. Sci. 2007, 44, 105–110. [Google Scholar] [CrossRef]
- Sarica, M.; Yamak, U.S.; Boz, M.A.; Erensoy, K.; Cilavdaroglu, E.; Noubandiguim, M. Performance of fast, medium and slow growing broilers in indoor and free-range production systems. S. Afr. J. Anim. Sci. 2019, 49, 1127–1138. [Google Scholar] [CrossRef]
- Wereńska, M.; Okruszek, A. Impact of frozen storage on some functional properties and sensory evaluation of goose meat. Poult. Sci. 2023, 102, 102894. [Google Scholar] [CrossRef] [PubMed]
- Śmiecińska, K.; Hnatyk, N.; Daszkiewicz, T.; Kubiak, D.; Matusevičius, P. The effect of frozen storage on the quality of vacuum-packaged Turkey meat. Vet. Zootech. 2015, 71, 61–66. [Google Scholar]
- Kluth, I.K.; Teuteberg, V.; Ploetz, M.; Krischek, C. Effects of freezing temperatures and storage times on the quality and safety of raw turkey meat and sausage products. Poult. Sci. 2021, 100, 101305. [Google Scholar] [CrossRef]
- Wei, R.; Wang, P.; Han, M.; Chen, T.; Xu, X.; Zhou, G. Effect of freezing on electrical properties and quality of thawed chicken breast meat. Asian-Australas J. Anim. Sci. 2017, 30, 569–575. [Google Scholar] [CrossRef]
- Saewa, S.; Khidhir, Z.; Al Bayati, M. The impact of storage duration and conditions on the formation of biogenic amines and microbial content in poultry meat. Iraqi J. Vet. Sci. 2021, 35, 183–188. [Google Scholar] [CrossRef]
- Medić, H.; Kušec, I.D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Saha, A.; Xiong, R.; Owens, C.M.; Meullenet, J.F. Changes in broiler breast fillet tenderness water-holding capacity and colour attributes during long-term frozen storage. J. Food Sci. 2008, 73, 162–168. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Hanus, P.; Sokołowicz, Z.; Kačániová, M. Assessment of technological characteristics and microbiological quality of marinated turkey meat with the use of dairy products and lemon juice. Anim. Biosci. 2021, 34, 2003–2011. [Google Scholar] [CrossRef]
- Ali, S.; Rajput, N.; Li, C.; Zhang, W.; Zhou, G. Effect of Freeze-thaw cycles on lipid oxidation and myowater in broiler chickens. Braz. J. Poult. Sci. 2016, 18, 35–40. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Park, T.S.; Kim, G.D. Proteolysis and changes in meat quality of chicken pectoralis major and iliotibialis muscles in relation to muscle fiber type distribution. Poult. Sci. 2022, 108, 102185. [Google Scholar] [CrossRef]
- Teye, G.A.; Okutu, I. Effect of ageing under tropical conditions on the eating qualities of beef. Afr. J. Food Agric. Nutr. Dev. 2010, 9, 1901–1913. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Lai, M.M.C.; Zhang, H.A.; Kitts, D.D. Ginseng prong added to broiler diets reduces lipid peroxidation in refrigerated and frozen stored poultry meats. Molecules 2021, 26, 4033. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Haug, A.; Nyquist, N.F.; Egelandsdal, B. Hydroperoxide formation in different lean meats. Food Chem. 2013, 141, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Garcia, C.C.M.; Sena, F.; Freitas, F.P.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Lipid hydroperoxide-induced and hemoglobin-enhanced oxidative damage to colon cancer cells. Free Radic. Biol. Med. 2011, 51, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Talbot, G. The stability and shelf life of fats and oils. In The Stability and Shelf Life of Food; Subramaniam, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 461–503. [Google Scholar]
- Wereńska, M.; Okruszek, A.; Haraf, G.; Wołoszyn, J.; Goluch, Z. Impact of frozen storage on oxidation changes of some components in goose meat. Poult Sci. 2022, 101, 101517. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Czauderna, M.; Rozbicka-Wieczorek, A.; Smulikowska, S. The effect of dietary fat, vitamin E and selenium concentrations on the fatty acid profile and oxidative stability of frozen stored broiler meat. J. Anim. Feed Sci. 2015, 24, 244–251. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Inês lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef]
- Seong, P.N.; Seo, H.W.; Kim, J.H.; Kang, G.H.; Cho, S.H.; Chae, H.S.; Park, B.Y.; Van Ba, H. Assessment of frozen storage duration effect on quality characteristics of various horse muscles. Asian-Australas J. Anim. Sci. 2017, 30, 1756–1763. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef] [PubMed]




| Trait | BWG, g | FI, g/Bird | FI Cumulative g/Bird | FCR Feed/Gain |
|---|---|---|---|---|
| 1 week | 30.52 (3.16) | 65.00 (1.20) | 65.00 (1.20) | 2.15 (0.25) |
| 2 week | 55.40 (1.73) | 74.32 (2.62) | 139.32 (3.06) | 1.34 (0.06) |
| 3 week | 76.79 (1.12) | 183.07(2.47) | 322.38 (4.47) | 2.38 (0.04) |
| 4 week | 111.32 (3.98) | 250.04 (4.21) | 572.43 (8.14) | 2.25 (0.10) |
| 5 week | 117.25 (7.74) | 349.40 (2.68) | 921.83 (8.46) | 2.99 (0.18) |
| 6 week | 181.10 (11.04) | 369.00 (0.99) | 1290.83 (7.53) | 2.04 (0.13) |
| 7 week | 151.79 (12.67) | 464.79 (16.55) | 1755.61 (15.71) | 3.07 (0.15) |
| 8 week | 166.73 (20.75) | 597.96 (21.28) | 2353.57 (35.63) | 3.63 (0.42) |
| 9 week | 188.60 (22.11) | 618.92 (30.37) | 2972.49 (63.31) | 3.31 (0.31) |
| Group of Costs | Parameters | Costs (EUR/kg Live Weight) | ||
|---|---|---|---|---|
| 6 Weeks Old | 9 Weeks Old | Difference | ||
| FC (Fixed costs) | Preparing the premises | 0.43 | 0.22 | 0.21 |
| Rent | 0.76 | 1.07 | −0.31 | |
| VC1 (Variable costs 1) | Price of one day chicken | 0.29 | 0.14 | 0.16 |
| Energy | 0.76 | 0.70 | 0.06 | |
| Labor | 0.96 | 1.26 | −0.30 | |
| Feed | 1.49 | 1.41 | 0.07 | |
| Medication | 0.35 | 0.16 | 0.18 | |
| TC (Total costs) | 5.03 | 4.96 | 0.07 | |
| Live weight (kg) | 304.698 | 237.440 | ||
| Group of Costs | Parameters | Cost (EUR/kg Product Weight) | ||
|---|---|---|---|---|
| 6 Weeks Old | 9 Weeks Old | Difference | ||
| FC (Fixed costs) | Preparation of premises | 0.74 | 0.33 | 0.41 |
| Rent | 1.32 | 1.64 | −0.32 | |
| VC1 (Variable costs 1) | Price of one-day-old chick | 0.51 | 0.21 | 0.30 |
| Energy | 1.32 | 1.07 | 0.25 | |
| Labor | 1.66 | 1.93 | −0.27 | |
| Feed | 2.58 | 2.16 | 0.42 | |
| Medication | 0.60 | 0.25 | 0.35 | |
| VC2 (Variable costs 2) | Transport | 0.73 | 0.83 | −0.10 |
| Slaughter | 1.34 | 0.56 | 0.78 | |
| Package | 0.17 | 0.14 | 0.03 | |
| TC (Total costs) | 10.97 | 9.13 | 1.84 | |
| Product weight (kg) | 175.439 | 154.770 | ||
| Trait | Age (Weeks) | Storage (Days) | Significance (p) | ||
|---|---|---|---|---|---|
| Breast | 0 | 60 | 120 | ||
| pH | 6 | 6.06 (0.004) b | 6.13 (0.02) a | 5.87 (0.06) c | <0.0001 |
| 9 | 5.87 (0.04) b | 5.94 (0.07) b | 6.24 (0.03) a | <0.0001 | |
| L* | 6 | 58.51 (1.25) b | 61.86(1.81) a | 58.53 (1.99) b | 0.0001 |
| 9 | 56.51 (0.28) b | 59.76 (0.65) a | 54.96 (1.20) c | <0.0001 | |
| a* | 6 | 13.60 (0.75) a | 13.19 (0.99) a | 9.54 (1.29) b | <0.0001 |
| 9 | 14.44 (0.17) a | 7.66 (0.89) b | 13.70 (1.36) a | <0.0001 | |
| b* | 6 | 8.46 (0.41) b | 10.90 (0.68) a | 6.19 (0.65) c | <0.0001 |
| 9 | 7.62 (0.19) a | 6.76 (0.43) b | 6.49 (0.85) b | 0.0003 | |
| Thigh | |||||
| pH | 6 | 6.55 (0.007) b | 6.67 (0.03) a | 6.54 (0.04) b | <0.0001 |
| 9 | 6.42 (0.03) b | 6.41 (0.02) b | 6.59 (0.008) a | <0.0001 | |
| L* | 6 | 52.06 (0.90) a | 52.97 (1.60) a | 47.91 (2.32) b | <0.0001 |
| 9 | 47.95 (1.42) b | 53.10 (2.88) a | 49.06 (1.52) b | <0.0001 | |
| a* | 6 | 16.52 (0.23) | 16.01 (1.26) | 16.26 (1.25) | 0.5501 |
| 9 | 18.49 (0.81) a | 13.55 (1.49) c | 16.96 (1.36) b | <0.0001 | |
| b* | 6 | 5.23 (0.67) c | 8.65 (0.84) a | 6.89 (0.88) b | <0.0001 |
| 9 | 4.86 (0.75) b | 4.22 (0.95) b | 7.76 (0.83) a | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, T.; Petkov, E.; Dimov, K.; Vlahova-Vangelova, D.; Kolev, N.; Balev, D.; Dragoev, S.; Ignatova, M. Performance, Carcass Composition, and Meat Quality during Frozen Storage in Male Layer-Type Chickens. Agriculture 2024, 14, 185. https://doi.org/10.3390/agriculture14020185
Popova T, Petkov E, Dimov K, Vlahova-Vangelova D, Kolev N, Balev D, Dragoev S, Ignatova M. Performance, Carcass Composition, and Meat Quality during Frozen Storage in Male Layer-Type Chickens. Agriculture. 2024; 14(2):185. https://doi.org/10.3390/agriculture14020185
Chicago/Turabian StylePopova, Teodora, Evgeni Petkov, Krasimir Dimov, Desislava Vlahova-Vangelova, Nikolay Kolev, Desislav Balev, Stefan Dragoev, and Maya Ignatova. 2024. "Performance, Carcass Composition, and Meat Quality during Frozen Storage in Male Layer-Type Chickens" Agriculture 14, no. 2: 185. https://doi.org/10.3390/agriculture14020185
APA StylePopova, T., Petkov, E., Dimov, K., Vlahova-Vangelova, D., Kolev, N., Balev, D., Dragoev, S., & Ignatova, M. (2024). Performance, Carcass Composition, and Meat Quality during Frozen Storage in Male Layer-Type Chickens. Agriculture, 14(2), 185. https://doi.org/10.3390/agriculture14020185










