Physio-Biochemical Mechanisms of Arbuscular Mycorrhizal Fungi Enhancing Plant Resistance to Abiotic Stress
Abstract
1. Introduction
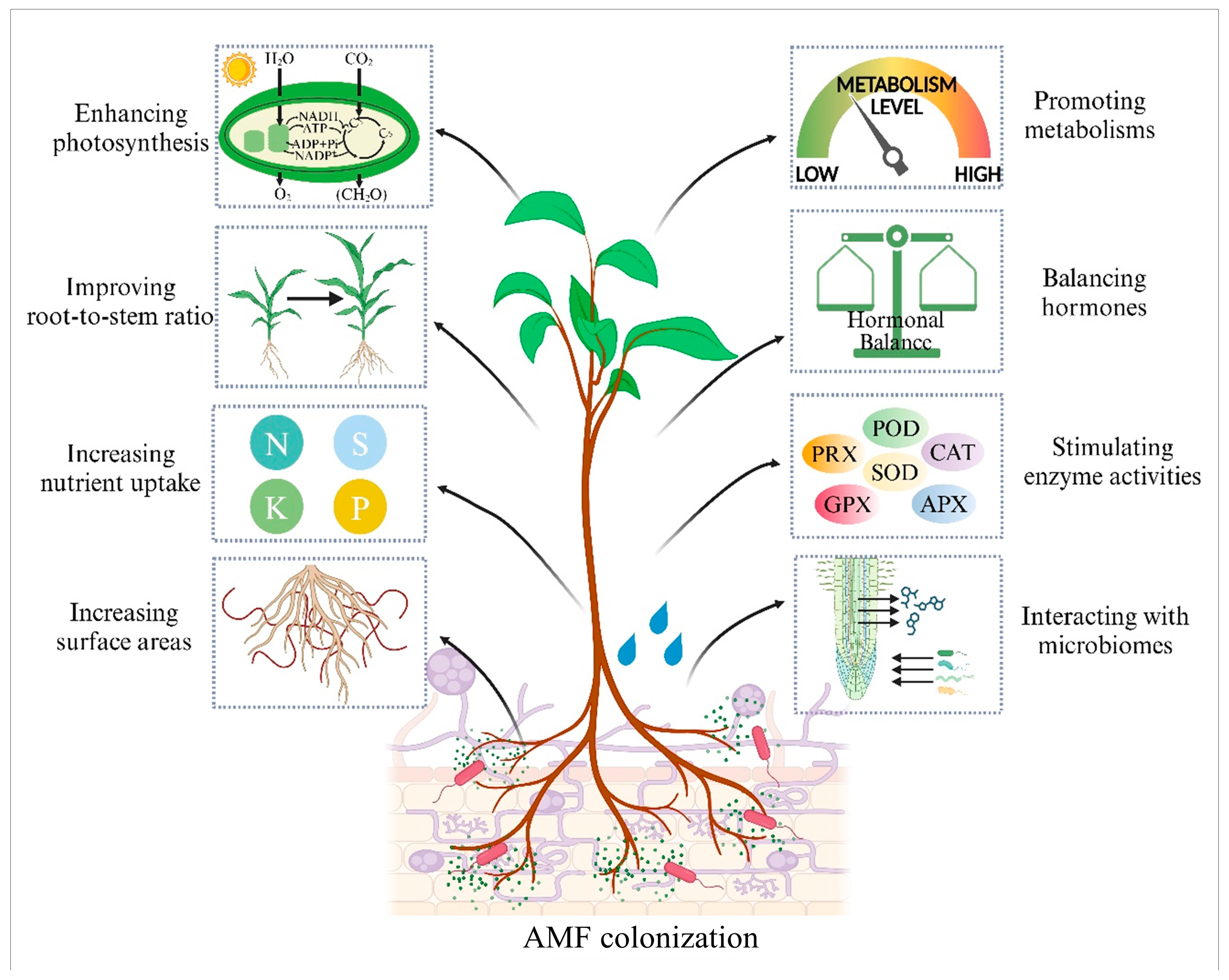
2. Physio-Biochemical Mechanisms of AM Fungi Enhancing Plant Resistance to Abiotic Stress
2.1. Mechanisms for AM Fungi Enhancing Nutrient Uptake
2.2. Mechanisms for AM Fungi Enhancing Water Absorption Under Abiotic Stress
2.3. AM Fungi Promote Osmotic Regulation Under Drought Stress
2.4. AM Fungi Promote Stress-Related Gene Expression Under Abiotic Stress
2.5. AM Fungi Enhancing Antioxidant Activity in Response to Abiotic Stress
2.6. AM Fungi Modulating Hormonal Crosstalk Under Abiotic Stress
2.7. Hyphosphere Regulates Fungal Functioning to Abiotic Stress
3. Limitations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, S.R.; Isbell, F.; Arce-Plata, M.I.; Di Marco, M.; Harfoot, M.; Johnson, J.; Lerman, S.B.; Miller, B.W.; Morelli, T.L.; Mori, A.S.; et al. Biodiversity loss reduces global terrestrial carbon storage. Nat. Commun. 2024, 15, 4354. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Bardgett, R.D.; Farfan, M.; Montanarella, L.; Six, J.; Wall, D.H. Soil biodiversity needs policy without borders soil health laws should account for global soil connections. Science 2023, 379, 32–34. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Searchinger, T.D.; Zhou, M.; Pan, D.; Yang, J.; Wu, L.; Cui, Z.; Zhang, W.; Zhang, F.; et al. Air quality, nitrogen use efficiency and food security in China are improved by cost-effective agricultural nitrogen management. Nat. Food 2020, 1, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, X.; Lam, S.K.; Yu, Y.; van Grinsven, H.J.M.; Zhang, S.; Wang, X.; Bodirsky, B.L.; Wang, S.; Duan, J.; et al. Cost-effective mitigation of nitrogen pollution from global croplands. Nature 2023, 613, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Marris, E. A world without soil: The past, present, and precarious future of the Earth beneath our feet. Nature 2022, 601, 503–504. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- de Lange, E.; Sze, J.S.; Allan, J.; Atkinson, S.; Booth, H.; Fletcher, R.; Khanyari, M.; Saif, O. A global conservation basic income to safeguard biodiversity. Nat. Sustain. 2023, 6, 1016–1023. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Food and Biodiversity. Science 2011, 333, 1231–1232. [Google Scholar] [CrossRef]
- Li, R.; Ren, C.; Wu, L.; Zhang, X.; Mao, X.; Fan, Z.; Cui, W.; Zhang, W.; Wei, G.; Shu, D. Fertilizing-induced alterations of microbial functional profiles in soil nitrogen cycling closely associate with crop yield. Environ. Res. 2023, 231, 116194. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, X.; Huang, S.; Linquist, B.; Kuzyakov, Y.; Wassmann, R.; Minamikawa, K.; Martinez-Eixarch, M.; Yan, X.; Zhou, F.; et al. Greenhouse gas emissions and mitigation in rice agriculture. Nat. Rev. Earth Environ. 2023, 4, 716–732. [Google Scholar] [CrossRef]
- Steel, D.; DesRoches, C.T.; Mintz-Woo, K. Climate change and the threat to civilization. Proc. Natl. Acad. Sci. USA 2022, 119, e2210525119. [Google Scholar] [CrossRef]
- Webb, P.; Benton, T.G.; Beddington, J.; Flynn, D.; Kelly, N.M.; Thomas, S.M. The urgency of food system transformation is now irrefutable. Nat. Food 2020, 1, 584–585. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Zhu, X.; Zhang, Y.; Yang, H.; Dobbie, S.; Zhang, X.; Deng, A.; Qian, H.; Zhang, W. Effects of arbuscular mycorrhizal fungi on crop growth and soil N2O emissions in the legume system. Agric. Ecosyst. Environ. 2021, 322, 107641. [Google Scholar] [CrossRef]
- Wong-Bajracharya, J.; Singan, V.R.; Monti, R.; Plett, K.L.; Ng, V.; Grigoriev, I.V.; Martin, F.M.; Anderson, I.C.; Plett, J.M. The ectomycorrhizal fungus Pisolithus microcarpus encodes a microRNA involved in cross-kingdom gene silencing during symbiosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2103527119. [Google Scholar] [CrossRef] [PubMed]
- Ledford, W.C.; Silvestri, A.; Fiorilli, V.; Roth, R.; Rubio-Somoza, I.; Lanfranco, L. A journey into the world of small RNAs in the arbuscular mycorrhizal symbiosis. New Phytol. 2023, 237, e19394. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Lutz, S.; Bodenhausen, N.; Hess, J.; Valzano-Held, A.; Waelchli, J.; Deslandes-Hérold, G.; Schlaeppi, K.; van der Heijden, M.G.A. Soil microbiome indicators can predict crop growth response to large-scale inoculation with arbuscular mycorrhizal fungi. Nat. Microbiol. 2023, 8, 2277–2289. [Google Scholar] [CrossRef]
- Fan, K.; Chu, H.; Eldridge, D.J.; Gaitan, J.J.; Liu, Y.-R.; Sokoya, B.; Wang, J.-T.; Hu, H.-W.; He, J.-Z.; Sun, W.; et al. Soil biodiversity supports the delivery of multiple ecosystem functions in urban greenspaces. Nat. Ecol. Evol. 2023, 7, 113–126. [Google Scholar] [CrossRef]
- Durant, E.; Hoysted, G.A.; Howard, N.; Sait, S.M.; Childs, D.Z.; Johnson, D.; Field, K.J. Herbivore-driven disruption of arbuscular mycorrhizal carbon-for-nutrient exchange is ameliorated by neighboring plants. Curr. Biol. 2023, 33, 2566–2573.e4. [Google Scholar] [CrossRef]
- Müller, L.M. Underground connections: Arbuscular mycorrhizal fungi influence on interspecific plant-plant interactions. Plant Physiol. 2021, 187, 1270–1272. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Cargill, R.I.M.; Van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal mycelium as a global carbon pool. Curr. Biol. 2023, 33, 560–573. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; George, T.S.; Feng, G. A core microbiome in the hyphosphere of arbuscular mycorrhizal fungi has functional significance in organic phosphorus mineralization. New Phytol. 2023, 238, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Yang, X.; Zhang, S.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alleviate phosphorus limitation by reducing plant N:P ratios under warming and nitrogen addition in a temperate meadow ecosystem. Sci. Total Environ. 2019, 686, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Ren, Y.; Chen, A.; Yang, C.; Zheng, Q.; Chen, J.; Wang, D.; Li, Y.; Hu, S.; Xu, G. Plant nitrogen nutrition: The roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 2022, 269, 153591. [Google Scholar] [CrossRef] [PubMed]
- Moreno Jiménez, E.; Ferrol, N.; Corradi, N.; Peñalosa, J.M.; Rillig, M.C. The potential of arbuscular mycorrhizal fungi to enhance metallic micronutrient uptake and mitigate food contamination in agriculture: Prospects and challenges. New Phytol. 2024, 242, 1441–1447. [Google Scholar] [CrossRef]
- Xu, Y.; Lambers, H.; Feng, J.; Tu, Y.; Peng, Z.; Huang, J. The role of arbuscular mycorrhizal fungi in micronutrient homeostasis and cadmium uptake and transfer in rice under different flooding intensities. Ecotoxicol. Environ. Saf. 2024, 284, 116978. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, R.; Li, X.; Zhang, J. Potential of arbuscular mycorrhizal fungi for soil health: A review. Pedosphere 2024, 34, 279–288. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Yan, F.; Zheng, X.; Fang, X.; Bai, Y.; Zhao, J.; Ma, X.; Ma, C.; Cai, X.; et al. Global research trends on the effects of arbuscular mycorrhizal fungi on the soil carbon cycle: A bibliometric analysis. Ecol. Indic. 2024, 158, 111543. [Google Scholar] [CrossRef]
- He, T.; Lin, W.; Yang, S.; Du, J.; Giri, B.; Feng, C.; Gilliam, F.S.; Zhang, F.; Zhang, X.; Zhang, X. Arbuscular mycorrhizal fungi reduce soil N2O emissions by altering root traits and soil denitrifier community composition. Sci. Total Environ. 2024, 933, 173065. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, B. Arbuscular mycorrhizal fungi reduce soil nitrous oxide emission. Geoderma 2021, 402, 115179. [Google Scholar] [CrossRef]
- Zhang, H.; Powell, J.R.; Power, S.A.; Churchill, A.C.; Plett, J.M.; Macdonald, C.A.; Jacob, V.; Kim, G.W.; Pendall, E.; Tissue, D.T.; et al. Arbuscular mycorrhizal fungal-mediated reductions in N2O emissions were not impacted by experimental warming for two common pasture species. Pedobiologia 2021, 87, 150744. [Google Scholar] [CrossRef]
- Storer, K.; Coggan, A.; Ineson, P.; Hodge, A. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol. 2018, 220, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Man, J.; Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal fungi benefit plants in response to major global change factors. Ecol. Lett. 2023, 26, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Arbuscular mycorrhizal fungi inoculation and phosphorus application improve growth, physiological traits, and grain yield of rice under alternate wetting and drying irrigation. J. Plant Physiol. 2022, 278, 153829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bi, S.; Meng, J.; Liu, T.; Li, P.; Yu, C.; Peng, X. Arbuscular mycorrhizal fungi enhanced rice proline metabolism under low temperature with nitric oxide involvement. Front. Plant Sci. 2022, 13, 962460. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-c.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Tan, Q.; Guo, Q.; Wei, R.; Zhu, G.; Du, C.; Hu, H. Influence of arbuscular mycorrhizal fungi on bioaccumulation and bioavailability of As and Cd: A meta-analysis. Environ. Pollut. 2023, 316, e120619. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Souza, T.; Barros, I.C.; da Silva, L.J.R.; Laurindo, L.K.; dos Santos Nascimento, G.; de Lucena, E.O.; Martins, M.; dos Santos, V.B. Soil microbiota community assembling in native plant species from Brazil’s legal Amazon. Symbiosis 2022, 86, 93–109. [Google Scholar] [CrossRef]
- Dar, M.H.; Razvi, S.M.; Singh, N.; Mushtaq, A.; Dar, S.; Hussain, S. Arbuscular mycorrhizal fungi for salinity stress: Anti-stress role and mechanisms. Pedosphere 2023, 33, 212–224. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Wang, H.; Xing, S.; Yin, R.; Fu, W.; Rillig, M.C.; Chen, B.; Zhu, Y. Arbuscular mycorrhizal fungi can inhibit the allocation of microplastics from crop roots to aboveground edible parts. J. Agric. Food Chem. 2023, 71, 18323–18332. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Feng, L.; Bergmann, J.; Wulf, A.; Rillig, M.C. Microplastic fiber and drought effects on plants and soil are only slightly modified by arbuscular mycorrhizal fungi. Soil Ecol. Lett. 2022, 4, 32–44. [Google Scholar] [CrossRef]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Coito, J.L.; Cardoso, H.; da Silva, J.M.; Pereira, H.S.; Viegas, W.; Nogales, A. Dynamic Regulation of Grapevine’s microRNAs in Response to Mycorrhizal Symbiosis and High Temperature. Plants 2023, 12, e982. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Alamri, S.A.; Siddiqui, M.H.; Rastogi, A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates the Adverse Effects of High Temperature in Soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Mathura, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind Crops Prod 2020, 143, 11934. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Song, F.-B.; Liu, S.-Q.; Liu, T.-D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Hua, J.; Zhu, C.; Guo, S. Arbuscular mycorrhizal fungi enhanced resistance to low-temperature weak-light stress in snapdragon (Antirrhinum majus L.) through physiological and transcriptomic responses. Front Plant Sci. 2024, 15, 1330032. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of arbuscular mycorrhizal fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, H.; Lu, G.; Zhou, X.; Wang, J.; Li, J.; Zheng, K.; Fan, Y.; Zhou, H.; Wang, J.; et al. Non-targeted metabolomics analysis reveals the mechanism of arbuscular mycorrhizal symbiosis regulating the cold-resistance of Elymus nutans. Front. Microbiol. 2023, 14, 1134585. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrà, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics of maize under low temperature stress. Yingyong Shengtai Xuebao 2010, 21, 470–475. [Google Scholar] [PubMed]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.; Yang, G.; He, C.; Shi, Y.; Li, C.; Liu, S.; Wang, Y.; Wang, Z.; Chen, L.; et al. Variation of arbuscular mycorrhizal fungi communities in the rhizosphere soil of Eucalyptus plantations based on different stand ages and its effect on phosphorus fractionation. Appl. Soil Ecol. 2023, 189, 104908. [Google Scholar] [CrossRef]
- Qin, Z.; Peng, Y.; Yang, G.; Feng, G.; Christie, P.; Zhou, J.; Zhang, J.; Li, X.; Gai, J. Relationship between phosphorus uptake via indigenous arbuscular mycorrhizal fungi and crop response: A 32P-labeling study. Appl. Soil Ecol. 2022, 180, 104624. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Chen, G.; Yao, M.; Liu, Z.; Li, X.; Yang, X.; Yang, Y.; Cai, D.; Tuerxun, Z.; et al. Arbuscular mycorrhizal fungi enhance drought resistance and alter microbial communities in maize rhizosphere soil. Environ. Technol. Innov 2025, 37, 103947. [Google Scholar] [CrossRef]
- Sun, R.-T.; Zhang, Z.-Z.; Zhou, N.; Srivastava, A.K.; KuCa, K.; Abd-allah, E.F.; Hashem, A.; Wu, Q.-S. A review of the interaction of medicinal plants and arbuscular mycorrhizal fungi in the rhizosphere. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, e12454. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Lu, T.; Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant Soil 2018, 423, 125–140. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, e3101. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant-soil interaction. Sustainability 2022, 14, e7840. [Google Scholar] [CrossRef]
- Etesami, H.; Li, Z.; Maathuis, F.J.M.; Cooke, J. The combined use of silicon and arbuscular mycorrhizas to mitigate salinity and drought stress in rice. Environ. Exp. Bot. 2022, 201, e104955. [Google Scholar] [CrossRef]
- Fileccia, V.; Ruisi, P.; Ingraffia, R.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Arbuscular mycorrhizal symbiosis mitigates the negative effects of salinity on durum wheat. PLoS ONE 2017, 12, e0184158. [Google Scholar] [CrossRef]
- Yin, R.; Hao, Z.; Yuan, X.; Wang, M.; Li, S.; Zhang, X.; Chen, B. Arbuscular mycorrhizal symbiosis alleviates ozone injury in ozone-tolerant poplar clone but not in ozone-sensitive poplar clone. Sci. Total Environ. 2023, 894, e165023. [Google Scholar] [CrossRef]
- Yin, R.; Hao, Z.; Zhou, X.; Wu, H.; Feng, Z.; Yuan, X.; Chen, B. Ozone does not diminish the beneficial effects of arbuscular mycorrhizas on Medicago sativa L. in a low phosphorus soil. Mycorrhiza 2022, 32, 33–43. [Google Scholar] [CrossRef]
- Sun, S.; Feng, Y.; Huang, G.; Zhao, X.; Song, F. Rhizophagus irregularis enhances tolerance to cadmium stress by altering host plant hemp Cannabis sativa L. photosynthetic properties. Environ. Pollut. 2022, 314, e120309. [Google Scholar] [CrossRef]
- Li, W.; Zhai, Y.-L.; Xing, H.-S.; Xing, L.-J.; Guo, S.-X. Arbuscular mycorrhizal fungi promote photosynthesis in Antirrhinum majus L. under low-temperature and weak-light conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13012. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal Fungi Influence Soil Structure. In Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds, D.D., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2000; pp. 3–18. [Google Scholar]
- Leifheit, E.F.; Lehmann, A.; Rillig, M.C. Potential effects of microplastic on arbuscular mycorrhizal fungi. Front. Plant Sci. 2021, 12, e626709. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Rillig, M.C. Chapter 14—Mycorrhizas and Soil Aggregation. In Mycorrhizal Mediation of Soil; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 241–262. [Google Scholar]
- Gehring, C.A. Chapter 13—Introduction: Mycorrhizas and Soil Structure, Moisture, and Salinity. In Mycorrhizal Mediation of Soil; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 235–240. [Google Scholar]
- Singh, G.; Pankaj, U. Arbuscular Mycorrhizal Fungi-Assisted Phytoextraction of Toxic Metals by Zea mays L. From Tannery Sludge. Soil Sediment Contam. Int. J. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Chen, B.D.; Zhu, Y.G.; Duan, J.; Xiao, X.Y.; Smith, S.E. Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ. Pollut. 2007, 147, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential contribution of arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 2006, 65, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Zhao, B. Arbuscular mycorrhizal fungi mediated uptake of lanthanum in Chinese milk vetch (Astragalus sinicus L.). Chemosphere 2007, 68, 1548–1555. [Google Scholar] [CrossRef]
- Sudová, R.; Vosátka, M. Differences in the effects of three arbuscular mycorrhizal fungal strains on P and Pb accumulation by maize plants. Plant Soil 2007, 296, 77–83. [Google Scholar] [CrossRef]
- Radka, S.; Pavla, D.; Miroslav, V. Mycorrhizal association of Agrostis capillaris and Glomus intraradices under heavy metal stress: Combination of plant clones and fungal isolates from contaminated and uncontaminated substrates. Appl. Soil Ecol. 2008, 40, 19–29. [Google Scholar] [CrossRef]
- Azcón, R.; Perálvarez, M.d.C.; Biró, B.; Roldán, A.; Ruíz-Lozano, J.M. Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy-metal multicontaminated soil amended with treated lignocellulosic agrowaste. Appl. Soil Ecol. 2009, 41, 168–177. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Xiao, Z.; Ban, Y.; Belvett, N. Positive effects of Funneliformis mosseae inoculation on reed seedlings under water and TiO2 nanoparticles stresses. World J. Microbiol. Biotechnol. 2019, 35, 81. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lou, X.; Tang, M. Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ. Exp. Bot. 2019, 164, 10–19. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead-zinc mine wasteland. Int. J. Phytoremed. 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Guangjuan, C.; Shaoying, A.; Kang, C.; Xiurong, W. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremed. 2019, 21, 663–671. [Google Scholar] [CrossRef]
- Ould Amer, S.; Aliat, T.; Kucher, D.E.; Bensaci, O.A.; Rebouh, N.Y. Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.). Agriculture 2023, 13, 552. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Tisarum, R.; Samphumphuang, T.; Phisalaphong, M.; Cha-um, S. Integrated strength of osmotic potential and phosphorus to achieve grain yield of rice under water deficit by arbuscular mycorrhiza fungi. Sci. Rep. 2023, 13, 5999. [Google Scholar] [CrossRef]
- Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Fu, W.; Chen, B.; Jansa, J.; Wu, H.; Ma, W.; Luo, W.; Xu, C.; Hao, Z.; Wu, H.; Yu, Q.; et al. Contrasting community responses of root and soil dwelling fungi to extreme drought in a temperate grassland. Soil Biol. Biochem. 2022, 169, 108670. [Google Scholar] [CrossRef]
- Battini, F.; Gronlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef]
- Jajoo, A.; Mathur, S. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol. Mol. Biol. Plants 2021, 27, 2589–2603. [Google Scholar] [CrossRef]
- De La Providencia, I.E.; De Souza, F.A.; Fernández, F.; Delmas, N.S.; Declerck, S. Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol. 2005, 165, 261–271. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Sbrana, C. Functional significance of anastomosis in arbuscular mycorrhizal networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 41–67. [Google Scholar]
- Liu, M.; Wang, H.; Lin, Z.; Ke, J.; Zhang, P.; Zhang, F.; Ru, D.; Zhang, L.; Xiao, Y.; Liu, X. Arbuscular mycorrhizal fungi inhibit necrotrophic, but not biotrophic, aboveground plant pathogens: A meta-analysis and experimental study. New Phytol. 2024, 241, 1308–1320. [Google Scholar] [CrossRef]
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.; Zhang, L. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef]
- Rozmoš, M.; Bukovská, P.; Hršelová, H.; Kotianová, M.; Dudáš, M.; Gančarčíková, K.; Jansa, J. Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J. 2022, 16, 676–685. [Google Scholar] [CrossRef]
- Faghihinia, M.; Jansa, J.; Halverson, L.J.; Staddon, P.L. Hyphosphere microbiome of arbuscular mycorrhizal fungi: A realm of unknowns. Biol. Fertil. Soils 2023, 59, 17–34. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and sifferent approaches to alleviate its adverse effects. Plants 2021, 10, 10020259. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Pantha, S.; Kilian, B.; Özkan, H.; Zeibig, F.; Frei, M. Physiological and biochemical changes induced by drought stress during the stem elongation and anthesis stages in the Triticum genus. Environ. Exp. Bot. 2024, 228, 106047. [Google Scholar] [CrossRef]
- Tominaga, J.; Shimada, H.; Kawamitsu, Y. Direct measurement of intercellular CO2 concentration in a gas-exchange system resolves overestimation using the standard method. J. Exp. Bot. 2018, 69, 1981–1991. [Google Scholar] [CrossRef]
- Cramer, P. Organization and regulation of gene transcription. Nature 2019, 573, 45–54. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Saxena, B.; Sharma, K.; Kapoor, R.; Wu, Q.S.; Giri, B. Insights into the molecular aspects of salt stress tolerance in mycorrhizal plants. World J. Microbiol. Biotechnol. 2022, 38, 253. [Google Scholar] [CrossRef]
- Tao, J.; Dong, F.; Wang, Y.; Chen, H.; Tang, M. Arbuscular mycorrhizal fungi enhance photosynthesis and drought tolerance by regulating MAPK genes expressions. Physiol. Plant. 2022, 174, e13829. [Google Scholar] [CrossRef]
- Begum, N.; Xiao, Y.; Wang, L.; Li, D.; Irshad, A.; Zhao, T. Arbuscular mycorrhizal fungus Rhizophagus irregularis alleviates drought stress in soybean with overexpressing the GmSPL9d gene by promoting photosynthetic apparatus and regulating the antioxidant system. Microbiol. Res. 2023, 273, 127398. [Google Scholar] [CrossRef]
- Zayova, E.; Stancheva, I.; Geneva, M.; Hristozkova, M.; Dimitrova, L.; Petrova, M.; Sichanova, M.; Salamon, I.; Mudroncekova, S. Arbuscular mycorrhizal fungi enhance antioxidant capacity of in vitro propagated garden thyme (Thymus vulgaris L.). Symbiosis 2018, 74, 177–187. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Xu, F.-Q.; Meng, L.-L.; Kuča, K.; Wu, Q.-S. The mechanism of arbuscular mycorrhizal fungi-alleviated manganese toxicity in plants: A review. Plant Physiol. Biochem. 2024, 213, 108808. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Y.; Tao, J.; Xu, T.; Tang, M. Arbuscular mycorrhizal fungi affect the expression of PxNHX gene family, improve photosynthesis and promote Populus simonii×P. nigra growth under saline-alkali stress. Front. Plant Sci. 2023, 14, 1104095. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Chen, D.; Ren, A.; Gao, T.; Zhao, M. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2015, 82, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Qausain, S.; Basheeruddin, M. Unraveling the Peroxidase Activity in Peroxiredoxins: A Comprehensive Review of Mechanisms, Functions, and Biological Significance. Cureus 2024, 16, e66117. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, A.; Yadav, S. Thioredoxins as Molecular Players in Plants, Pests, and Pathogens. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology: Chemical Ecology; Singh, I.K., Singh, A., Eds.; Springer: Singapore, 2021; pp. 107–125. [Google Scholar]
- Chandrasekaran, M.; Paramasivan, M. Arbuscular mycorrhizal fungi and antioxidant enzymes in ameliorating drought stress: A meta-analysis. Plant Soil 2022, 480, 295–303. [Google Scholar] [CrossRef]
- Marín, R.; Abad, C.; Rojas, D.; Chiarello, D.I.; Alejandro, T.-G. Chapter Four—Biomarkers of oxidative stress and reproductive complications. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 113, pp. 157–233. [Google Scholar]
- Liu, R.; Yang, L.; Zou, Y.; Wu, Q. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, X.; Zhang, Y.; Tang, M. The synergy of arbuscular mycorrhizal fungi and exogenous abscisic acid benefits Robinia pseudoacacia L. growth through altering the distribution of Zn and endogenous abscisic acid. J. Fungi 2021, 7, e34436210. [Google Scholar] [CrossRef]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Ocampo Bote, J.A.; García Garrido, J.M. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef]
- Tominaga, T.; Miura, C.; Takeda, N.; Kanno, Y.; Takemura, Y.; Seo, M.; Yamato, M.; Kaminaka, H. Gibberellin Promotes Fungal Entry and Colonization during Paris-Type Arbuscular Mycorrhizal Symbiosis in Eustoma grandiflorum. Plant Cell Physiol. 2019, 61, 565–575. [Google Scholar] [CrossRef]

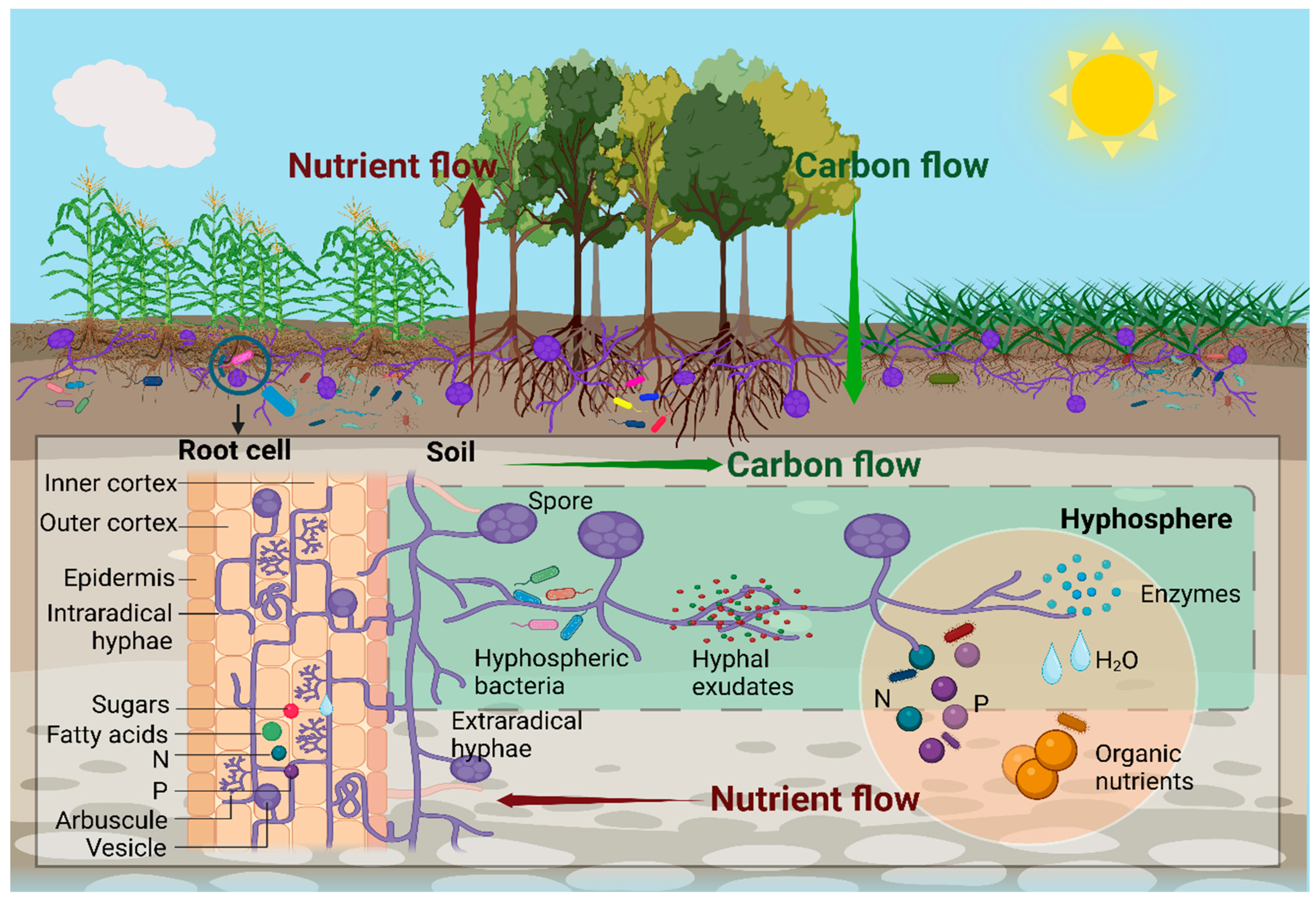

| Stress | Impacts of Abiotic Stress | AM Fungi-Induced Functional Effects | AM Fungal Species | Host Plant Species | Refer. |
|---|---|---|---|---|---|
| Extreme high temp. | Excessive heat amplifies existing conditions and results in premature death | Promoting miRNAs isomiRs expression in mycorrhizal plants via upregulating homeobox-leucine zipper proteins and auxin receptors | Rhizophagus intraradices; Funneliformis mosseae; Funneliformis geosporum | Zea mays; Acacia pachyceras; Pennisetum divisum; Glycine max | [53,54,55,56,57] |
| Cold stress | Causing cell membrane to rupture and lead to cell death | Enhancing stomatal conductance, maximal photochemical efficiency, promoting activities of antioxidant enzymes, and K+: Na+ ratio while lowering leaf relative electrolyte conductivity | Rhizophagus irregularis; Rhizophagus clarus;Rhizophagus intraradices;Glomus lamellose; Funneliformis mosseae | Arachis hypogaea L.; Hordeum vulgare | [58,59,60,61,62] |
| Low-P soil environment | Affecting the expected life of an organism | It helps acquire P and other nutrients from organic matter decomposition, alters bacterial and fungal communities, and enhances soil microbial diversity. | Funneliformiscaledonium; Funneliformisgeosporum; Gigaspora margarita | Zea mays L.; Triticum aestivum L. | [63,64,65] |
| High salinity | Affecting reactive oxygen species, damaging biomolecules | Helping hormone balance, stomatal conductance, and osmotic adjustment via chlorophyll biosynthesis; Improving Mg2+ concentration; enhancing antioxidant enzyme activity | Rhizophagus irregularis; Funneliformis message; | Arachis hypogaea L.; Oryza sativa L.; Cucumis sativus | [49,66,67,68,69,70,71,72,73] |
| Enriched ozone (O3) | Entering plant cells, upsetting the antioxidant defense system, reducing photosynthesis | Mediating antioxidant enzyme (superoxide dismutase and peroxidase) activity and reducing the plant O3 sensitivity, thus minimizing species-dependent O3 injury in plants. | Rhizophagus irregularis; Septoglomus viscosum; Claroideoglomushanlinii, Claroideoglomus claroideum | Medicago sativa L.; Populus spp. | [74,75] |
| Weak light | Weak light affects the growth and flower quality of horticultural plants | Enhancing net photosynthetic rate, stomatal conductance, and chlorophyll content, the potential activity of photosystem II; reduced the intercellular CO2 concentration | Rhizophagus irregularis; Funneliformis mosseae; Glomus versiforme | Cannabis sativa L.; Antirrhinum majus L. | [58,76,77] |
| Poor soil structure | Low infiltration, poor nutrient provision, poor formation of aggregates | Hyphal decomposition provides nucleation sites for micro aggregate coating C; absorptive mycelium provides the ’backbone’ of a stabilizing network on aggregates affecting hydrophobicity | Rhizophagus irregularis; Septoglomus viscosum; Claroideoglomushanlinii; R. fasciculatus | A variety of crops | [67,78,79,80,81,82] |
| Heavy metal (Cu, Cd, Cr, Pb, Zn, Mo, Mn, Al, As, Ni, Ar, Cr, Pb, Zn) pollution | Pollution in water bodies, soils, and food products affecting human health | Alleviating heavy metals in host plant; improving nutrient uptake and antioxidants in host plant; remediating heavy metal by accumulating large part of heavy metals in fungal structures | Rhizophagus irregularis; Funneliformis mosseae; Glomus versiforme; Glomus deserticola; Glomusclaroideum | Brassica indica; Coreopsis drummondii; Pteris vittate; Eucalyptus globulus; Glycine max; Medicago sativa | [83,84,85,86,87,88,89,90,91] |
| Microplastics | Threatening soil health: the risk of entering food products | Enhancing antagonistic interactions with soil properties and microbial communities. Helping microplastics biodegradable | Glomus lamellosu; Paraglomus occultum; | Oryza sativa L.; Zea mays L.; Affium cepa | [92] |
| Nutrientdeficiency | Reducing plant growth, lowering yields | Increasing N, K, Ca, and P uptake; increasing antioxidant activity (CAT, SOD, etc.); improving soil moisture | Glomus intraradices | Zea mays | [93] |
| Water logging | Damaging root systems | Upregulating aquaporins, increasing entry points of fungi to increase nutrient uptake, maintaining ion and cellular homeostasis | Funneliformismosseae | Prunus persica | [94] |
| Water deficit | Influencing plant–microbiome interaction, hormone balance, stomatal conductance, and osmotic adjustment | Increased height of aerial part, length of internodes, length of ear, plant dry weight, and chlorophyll content; increased phosphorus content and osmotic potential in plants | Funneliformis mosseae; Rhizophagus intraradices; Funneliformis geosporus; Claroideoglomus etunicatum; Glomus aggregatum | Triticum durumO. sativa | [95,96] |
| Prolonged drought | Threatening plant growth and development, thus the socio-economic and ecological environment | Increasing AMF richness, strengthening plant–microbiome interaction, improving hormone balance, stomatal conductance, osmotic adjustment, and photosynthetic activity | Rhizophagus intraradices; Glomus proliferum; Glomus etunicatum; Glomus diaphanum; Glomus constricted; Rhizophagusirregularis | Ceratonia siliqua L.; Triticum durum; Glycine max | [55,97,98] |
| Type of Crop | Type of Study | ||||||
|---|---|---|---|---|---|---|---|
| Field (95% CI) | n | Lab (95% CI) | n | P | Overall (95% CI) | n | |
| All | 0.127 (0.084, 0.168) | 218 | 0.195 (0.132, 0.265) | 174 | 0.05 | 0.185 (0.146, 0.230) | 402 |
| Barely | −0.003 (−0.058, 0.055) | 11 | −0.016 (−0.097, 0.061) | 17 | 0.81 | ||
| Maize | 0.133 (0.042, 0.229) | 82 | 0.262 (0.094, 0.432) | 14 | 0.32 | ||
| Millet | 0.162 (0.045, 0.297) | 3 | 0.295 (0.116, 0.494) | 5 | 0.21 | ||
| Rice | 0.182 (0.132, 0.242) | 29 | 0.312 (0.094, 0.539) | 48 | 0.34 | ||
| Wheat | 0.107 (0.061, 0.155) | 83 | 0.166 (0.113, 0.219) | 88 | 0.11 | ||
| Intervention | Type of Crop | ||
|---|---|---|---|
| All | Maize | Wheat | |
| Inoculation (95% CI) | 0.143 (0.113, 0.17) | 0.093 (0.05, 0.13) | 0.160 (0.11, 0.207) |
| n | 145 | 44 | 58 |
| Rotation (95% CI) | 0.077 (−0.023, 0.173) | 0.077 (−0.06, 0.203) | −0.067 (−0.163, 0.03) |
| n | 43 | 10 | 13 |
| Tillage (95% CI) | −0.033 (−0.15, 0.08) | 0.003 (−0.123, 0.133) | 0.127 (−0.15, 0.417) |
| n | 28 | 24 | 2 |
| P | 0.001 | 0.18 | 0.02 |
| Overall (95% CI) | 0.130 (0.086, 0.176) | ||
| n | 218 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Shang, X.; Cao, H.; Lee, S.-J.; Wang, L.; Gan, Y.; Feng, S. Physio-Biochemical Mechanisms of Arbuscular Mycorrhizal Fungi Enhancing Plant Resistance to Abiotic Stress. Agriculture 2024, 14, 2361. https://doi.org/10.3390/agriculture14122361
Sun D, Shang X, Cao H, Lee S-J, Wang L, Gan Y, Feng S. Physio-Biochemical Mechanisms of Arbuscular Mycorrhizal Fungi Enhancing Plant Resistance to Abiotic Stress. Agriculture. 2024; 14(12):2361. https://doi.org/10.3390/agriculture14122361
Chicago/Turabian StyleSun, Dandi, Xiaoqian Shang, Hanwen Cao, Soon-Jae Lee, Li Wang, Yantai Gan, and Shoujiang Feng. 2024. "Physio-Biochemical Mechanisms of Arbuscular Mycorrhizal Fungi Enhancing Plant Resistance to Abiotic Stress" Agriculture 14, no. 12: 2361. https://doi.org/10.3390/agriculture14122361
APA StyleSun, D., Shang, X., Cao, H., Lee, S.-J., Wang, L., Gan, Y., & Feng, S. (2024). Physio-Biochemical Mechanisms of Arbuscular Mycorrhizal Fungi Enhancing Plant Resistance to Abiotic Stress. Agriculture, 14(12), 2361. https://doi.org/10.3390/agriculture14122361









