Review on Photocatalytic Applications for Deodorization in Livestock and Poultry Farms
Abstract
1. Introduction
2. Characteristics of Odorous Gasses from Livestock and Poultry Farms
2.1. Compositions of Odorous Gasses from Animal Feeding
| Odor Component | Concentration | Olfactory Threshold | References | ||
|---|---|---|---|---|---|
| Livestock Farm | Poultry Farm | ||||
| NH3 | 0.66–61.93 ppmv | 51.9 ± 40.7 ppmv | 1.347 ppmv | [31,41,42] | |
| H2S | 43.6–367.3 ppbv | 2–401 ppbv | 17.8 ppmv | [1,36,43] | |
| VOCs | Methanethiol (ME) | 0.28–1.12 ppbv | 56 ppbv | 1.05 ppbv | [43,44] |
| Dimethyl sulfide (DMS) | 4.9 ppbv | 4.33 ppbv | 2.24 ppbv | [34,43,44] | |
| Dimethyl disulfide (DMDS) | 22 ppbv | 53.63 ppbv | 12.3 ppbv | [34,43,44] | |
| Trimethylamine (TMA) | 3.0–64.7 ppbv | - | 2.4 ppbv | [36,43] | |
| Acetic acid (AA) | 209.5 ppbv | 315.28 ppbv | 0.145 ppmv | [31,43,45] | |
| Propionic acid (PA) | 13 | 157 | 0.0355 ppmv | [43] | |
| Butyric acid (BA) | 60 ppbv | 16.92 ppbv | 3.89 ppbv | [34,43] | |
| Acetaldehyde | 4 ppbv | 20.36 ppbv | 0.186 ppmv | [36,43] | |
| Xylene | 0.9 ppbv | 1.39 ppbv | 0.1568 ppmv | [34] | |
| Phenol | 2 ppbv | - | 0.11 ppmv | [43] | |
| p-Cresol | 4.76–57.41 ppbv | 422 | 1.86 ppbv | [37,43] | |
| Indole | 0.12 ppbv | 3.6 ppbv | 0.0316 ppbv | [31,34,43] | |
| Skatole | 7.52 ppbv | 0.718 ppbv | 0.562 ppbv | [34,43] | |
| PM | PM2.5 | 39–56 μg/m3 | 0.01–4.51 μg/m3 | - | [46,47,48] |
| PM10 | 9.0–74.3 μg/m3 | 415 ± 44–761 ± 60 μg/m3 | - | ||
| TSP | 191–200 μg/m3 | 234–2090 μg/m3 | - | ||
2.2. Current Strategies for Abating Odors in Livestock and Poultry Farms
3. Fundamentals of Photocatalytic Deodorization Systems
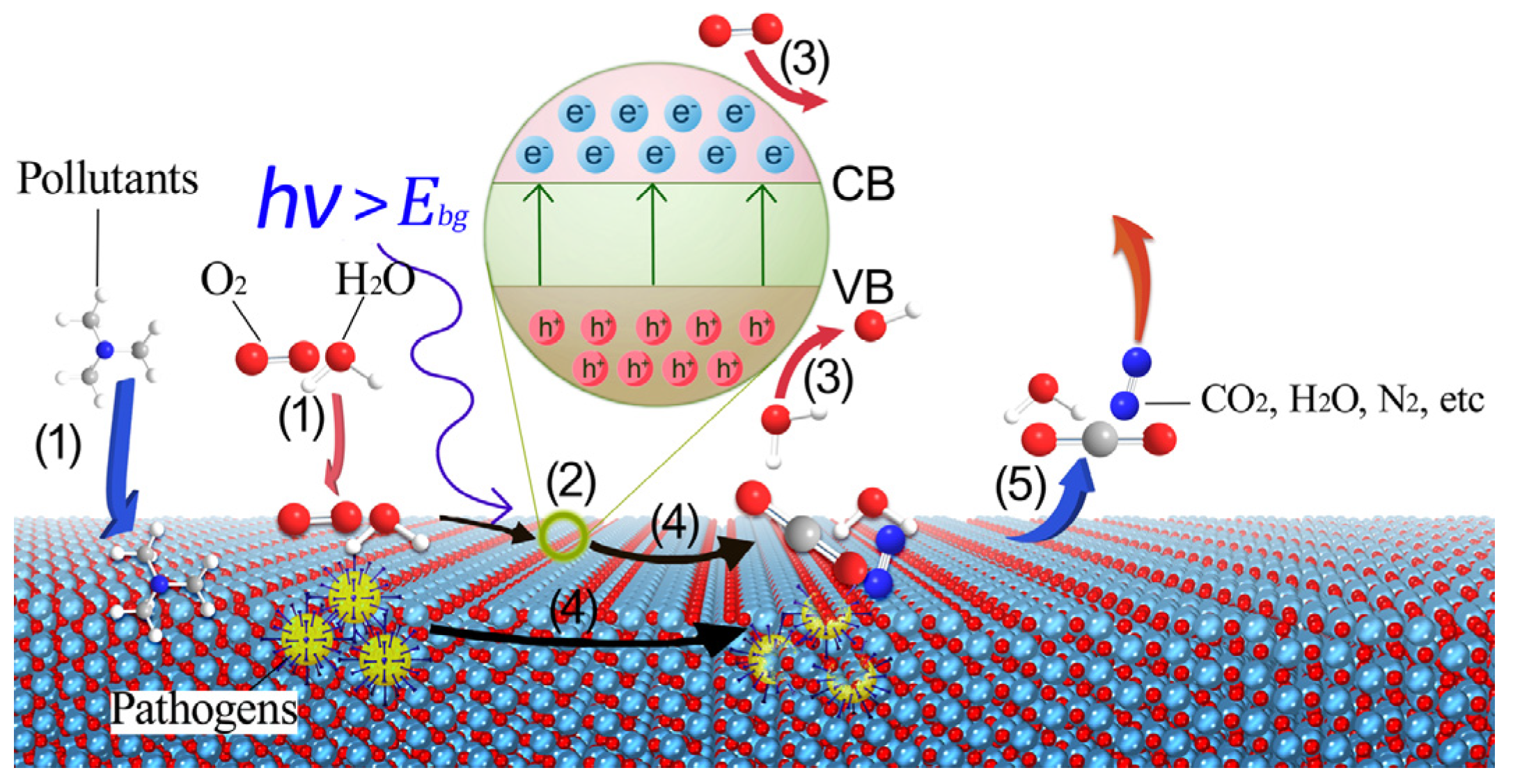
3.1. Basic Principles
3.2. Photocatalysts for Odor Mitigation
3.3. Light Sources
3.4. Photocatalytic Reactors
4. Application of Photocatalytic Air Treatment in Livestock and Poultry Farms
4.1. Degradation of NH3
4.2. Degradation of H2S
4.3. Degradation of VOCs
4.4. Degradation of PM and Airborne Pathogens
5. Combined Photocatalytic Deodorization Methods
5.1. Photocatalysis Combined with Non-Thermal Plasma
| Experimental Conditions Temp/RH | Experimental Scale | Year | Reactor | Treatment Time | Photocatalyst (Dose) | Combined Technology | Pollutant | Removal Efficiency | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Photocatalysis | Combined Method | Synergistic Method | |||||||||
| 20 °C/50% | Pilot-scale | 2014 | Rectangular planar reactor made of polymethyl methacrylate (PMMA) material with a size of 135 mm × 135 mm × 1 m | NR | Coated glass fiber tissue (6.5 g/m2) | Plasma surface discharge barrier dielectric (SDBD) | Trimethylamine | ~25%, ~20%, and 18% with flow rate of 4, 6, and 10 m3/h, respectively | ~35%, ~32%, and 28% with flow rate of 4, 6, and 10 m3/h, respectively | ~74%, ~63%, and 59% with flow rate of 4, 6, and 10 m3/h, respectively | [172] |
| Room temperature/50% | Pilot-scale | 2017 | NR | Coated glass fiber tissue containing colloidal silica and TiO2 (13 g/m2) | DBD plasma | BUTY | 18% | 28% | 53% | [163] | |
| DMDS | 28% | 47% | 70% | ||||||||
| Mixture of BUTY and DMDS | 19% for BUTY and 5% for DMDS | 45% for BUTY and 10% for DMDS | 55% for BUTY and 15% for DMDS | ||||||||
| 20 °C/5–85% | Pilot-scale | 2023 | Tubular reactor formed of two concentric Pyrex tubes | NR | Coated glass fiber tissue containing colloidal silica and TiO2 (13 g/m2) | DBD plasma | NH3 | 29% | 37% | 72% | [52] |
| Propionaldehyde | 36% | 42% | 83% | ||||||||
| Industrial-scale | NH3 | 30–43% | 26–34% | 59–96.81% | |||||||
| Ambient temperature/NR | Pilot-scale | 2013 | Rectangular tunnel photoreactor containing pleated photocatalytic media | NR | TiO2 glass fiber tissue (6.5 g/m2) | DBD plasma | Isovaleraldehyde | ~38% | ~20% | ~68% | [143] |
| 32.4 °C/53% | Industrial-scale | Two similar rectangular tunnel photoreactors connected in series | NR | TiO2 glass fiber tissue (13 g/m2) | Isobutyraldehyde/ | ~20% | ~28% | ~65% | |||
| Isovaleraldehyde | ~22% | ~13% | ~55% | ||||||||
| 2-methyl butyraldehyde | ~23% | ~36% | ~74% | ||||||||
| DMDS | NR | ~37% | ~24% | ||||||||
| 20 °C/60% | Pilot-scale | 2015 | Tubular reactor formed of two concentric Pyrex tubes | NR | Coated glass fiber tissue containing colloidal silica and TiO2 (13 g/m2) | DBD plasma | Trimethylamine | 20–35% | 30–40% | 59–91% | [130] |
| 20 °C/5% | Pilot-scale | 2023 | Cylindrical reactor with two concentric cylindrical Pyrex glass tubes | 10.05 s | TiO2-loaded glass fiber fabric (NR) | Double dielectric barrier discharge (D-DBD) plasma | Chlorobenzene | 18% | ~30% | ~75% | [167] |
| 20 ± 2 °C/NR | Lab-scale | 2013 | Tubular reactor with two coaxial quartz tubes | 0.4–0.8 s | TiO2-coated attapulgite at mass ratio of 3:1 (NR) | DBD plasma | CS2 | NR | ~30–65% | ~60–70% | [171] |
| 100 °C/NR | Field-scale | 2014 | Coil-shaped reactors | NR | TiO2-impregnated Ti-mesh filter (TMiPTM) (NR) | DBD plasma | TSP | NR | NR | ~98.5% | [173] |
| TVOC | 97.3–43.8% | ||||||||||
| 30 °C/NR | Lab-scale | 2017 | Photoreactor: cylindrical reactor constructed of PVC material (pretreatment) Bio-reactor: acrylic column filled with Raschig rings | 6 s (photocatalysis) 20 s (biotrickling) 24 s (combined system) | TiO2 (80% anatase and 20% rutile, NR) | Biotrickling filter | NH3 | 40.9% | NR | 97% | [129] |
| 32.0 ± 3.0 °C/68.8 ± 4.4 | Pilot-scale | 2012 | Photoreactor: monolithic reactor Bio-reactor: rectangular reactor mainly containing a biofiltration bed and a circulating nutrient unit (pretreatment) | 7.2 s (photocatalysis) 10.8 s (biotrickling) 18 s (combined system) | Foam nickel coated with P25 (5.19 g/m2) | Biotrickling filter | EA | ~75% | ~90% | ~99% | [174] |
| Toluene | ~87% | ~63% | ~98% | ||||||||
| EB | ~80% | ~72% | ~98% | ||||||||
| Xylene | ~77% | ~76% | ~96% | ||||||||
| ET | ~88% | ~86% | ~99% | ||||||||
| TMB | ~85% | ~88% | ~99% | ||||||||
| TVOC | 85.8–95.1% | 70.4–89.3% | 95.8–99.5% | ||||||||
| NR/NR | Pilot-scale | 2023 | Photoreactor: photocatalytic scrubber Bio-reactor: biological scrubber a | 4.3 s (both individual and combined systems) | 25 nm TiO2 (NR) | Bioscrubber | NH3 | 89.1% | 88.3% | 82.9% | [120] |
| H2S | >95% | >95% | >95% | ||||||||
| DMDS | NR | NR | ~100% | ||||||||
| DMS | 91.2% | ~8% | ~100% | ||||||||
| Butyraldehyde, | ~−10% | ~15% | ~90% | ||||||||
| Acetaldehyde | −51% | −18.2% | ~30% | ||||||||
| NR/NR | Pilot-scale | 2023 | Photoreactor: photocatalytic box with a glass roof and stainless steel walls Bio-reactor: two equal parts of Plexiglas | 22–45 s (photocatalysis) 45–90 s (biotrickling) | Foam nickel coated with TiO2 nanoparticles (NR) | Biotrickling filter | m-xylene | NR | ~30–75% | ~60–91% | [175] |
| 20–28 °C/~70% | Lab-scale | 2012 | Photoreactor: annular photoreactor Bio-reactor: methacrylate biofilter using peat as filter materials | 2.7 s (photocatalysis) 44.5 s (biofiltration) | Glass wool-supported TiO2 | Biofilter | Toluene | 6% | 65% | >90% | [145] |
| NR/60% | Lab-scale | 2021 | Bio-reactor: continuous stirred tank bioreactor | 27.6 s (photocatalysis) 44.5 s (biological treatment) | TiO2 (<10 g/m2) | Biodegradation | Dichloromethane | ~65% | 96.65% | 99.2% | [176] |
5.2. Photocatalysis Combined with Biomethods
6. Modeling Methods for Scaling up Photocatalytic Systems
6.1. Kinetic Modeling
6.2. Irradiation Transport Modeling
6.3. Computational Fluid Dynamics Modeling
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamon, L.; Andrès, Y.; Dumont, E. Aerial Pollutants in Swine Buildings: A Review of Their Characterization and Methods to Reduce Them. Environ. Sci. Technol. 2012, 46, 12287–12301. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, B.; Jia, Y.; He, F.; Chen, W. Mitigation Strategies of Air Pollutants for Mechanical Ventilated Livestock and Poultry Housing—A Review. Atmosphere 2022, 13, 452. [Google Scholar] [CrossRef]
- Cao, T.; Zheng, Y.; Dong, H. Control of Odor Emissions from Livestock Farms: A Review. Environ. Res. 2023, 225, 115545. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Chiarello, G.L.; Selli, E.; Guarino, M. Effects of TiO2 Based Photocatalytic Paint on Concentrations and Emissions of Pollutants and on Animal Performance in a Swine Weaning Unit. J. Environ. Manag. 2012, 96, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent Advances in Photocatalytic Removal of Organic and Inorganic Pollutants in Air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Tyndall, J.; Sauer, T.; Hernandez-Ramirez, G.; Pfeiffer, R.; Hatfield, J. Odorous Compounds Sources and Transport from a Swine Deep-Pit Finishing Operation: A Case Study. J. Environ. Manag. 2019, 233, 12–23. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Geretto, M.; Pulliero, A.; Izzotti, A. Odor Emissions: A Public Health Concern for Health Risk Perception. Environ. Res. 2022, 204, 112121. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Li, P.; Jenks, W.S. Mitigation of Air Pollutants by UV-A Photocatalysis in Livestock and Poultry Farming: A Mini-Review. Catalysts 2022, 12, 782. [Google Scholar] [CrossRef]
- Wi, J.; Lee, S.; Kim, E.; Lee, M.; Koziel, J.; Ahn, H. Evaluation of Semi-Continuous Pit Manure Recharge System Performance on Mitigation of Ammonia and Hydrogen Sulfide Emissions from a Swine Finishing Barn. Atmosphere 2019, 10, 170. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Pu, S.; Long, D.; Liu, Z.; Yang, F.; Zhu, J. Preparation of RGO-P25 Nanocomposites for the Photocatalytic Degradation of Ammonia in Livestock Farms. Catalysts 2018, 8, 189. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A Review on Heterogeneous Photocatalysis for Environmental Remediation: From Semiconductors to Modification Strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Witek-Krowiak, A.; Chojnacka, K. Recent Innovations in Various Methods of Harmful Gases Conversion and Its Mechanism in Poultry Farms. Environ. Res. 2022, 214, 113825. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic Materials and Technologies for Air Purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Macedo, N.; Li, P.; Chen, B.; Jenks, W.S.; Zimmerman, J.; Paris, R.V. Mitigation of Particulate Matter and Airborne Pathogens in Swine Barn Emissions with Filtration and UV-A Photocatalysis. Catalysts 2021, 11, 1302. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.; Chen, B.; Li, P.; Banik, C. Field-Scale Testing of Mobile Laboratory for Mitigation of Gaseous Emissions from the Swine Farm with UV-A Photocatalysis. In Proceedings of the 2021 ASABE Annual International Virtual Meeting, Online, 12–16 July 2021; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2021. [Google Scholar]
- Lee, M.; Li, P.; Koziel, J.A.; Ahn, H.; Wi, J.; Chen, B.; Meiirkhanuly, Z.; Banik, C.; Jenks, W.S. Pilot-Scale Testing of UV-A Light Treatment for Mitigation of NH3, H2S, GHGs, VOCs, Odor, and O3 Inside the Poultry Barn. Front. Chem. 2020, 8, 613. [Google Scholar] [CrossRef]
- Lei, D.; Xie, X.; Xiang, Y.; Huang, X.; Xiao, F.; Cao, J.; Li, G.; Leung, D.Y.C.; Huang, H. An Efficient Process for Aromatic VOCs Degradation: Combination of VUV Photolysis and Photocatalytic Oxidation in a Wet Scrubber. Chemosphere 2022, 309, 136656. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A Review on Photocatalysis for Air Treatment: From Catalyst Development to Reactor Design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Oliveira de Brito Lira, J.; Riella, H.G.; Padoin, N.; Soares, C. An Overview of Photoreactors and Computational Modeling for the Intensification of Photocatalytic Processes in the Gas-Phase: State-of-Art. J. Environ. Chem. Eng. 2021, 9, 105068. [Google Scholar] [CrossRef]
- Escobedo, S.; de Lasa, H. Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses. Catalysts 2020, 10, 966. [Google Scholar] [CrossRef]
- Weon, S.; He, F.; Choi, W. Status and Challenges in Photocatalytic Nanotechnology for Cleaning Air Polluted with Volatile Organic Compounds: Visible Light Utilization and Catalyst Deactivation. Environ. Sci.-Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic Air Cleaners and Materials Technologies—Abilities and Limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Li, Y.-W.; Ma, W.-L. Photocatalytic Oxidation Technology for Indoor Air Pollutants Elimination: A Review. Chemosphere 2021, 280, 130667. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic Systems as an Advanced Environmental Remediation: Recent Developments, Limitations and New Avenues for Applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical Identification and Biological Origin of Key Odor Components in Livestock Waste. J. Anim. Sci. 1998, 76, 1331. [Google Scholar] [CrossRef]
- Guarino, M.; Costa, A.; Porro, M. Photocatalytic TiO2 Coating—To Reduce Ammonia and Greenhouse Gases Concentration and Emission from Animal Husbandries. Bioresour. Technol. 2008, 99, 2650–2658. [Google Scholar] [CrossRef]
- Blunden, J.; Aneja, V.P.; Westerman, P.W. Measurement and Analysis of Ammonia and Hydrogen Sulfide Emissions from a Mechanically Ventilated Swine Confinement Building in North Carolina. Atmos. Environ. 2008, 42, 3315–3331. [Google Scholar] [CrossRef]
- Ni, J.-Q. Factors Affecting Toxic Hydrogen Sulfide Concentrations on Swine Farms—Sulfur Source, Release Mechanism, and Ventilation. J. Clean. Prod. 2021, 322, 129126. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile Organic Compounds at Swine Facilities: A Critical Review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef]
- Ngwabie, N.M.; Schade, G.W.; Custer, T.G.; Linke, S.; Hinz, T. Abundances and Flux Estimates of Volatile Organic Compounds from a Dairy Cowshed in Germany. J. Environ. Qual. 2008, 37, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Coggon, M.M.; Koss, A.R.; Warneke, C.; Eilerman, S.; Peischl, J.; Aikin, K.C.; Ryerson, T.B.; de Gouw, J.A. Emissions of Volatile Organic Compounds (VOCs) from Concentrated Animal Feeding Operations (CAFOs): Chemical Compositions and Separation of Sources. Atmos. Chem. Phys. 2017, 17, 4945–4956. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Li, H.; Burns, R.; Xin, H.; Hatfield, J. Speciation of Volatile Organic Compounds from Poultry Production. Atmos. Environ. 2010, 44, 3538–3546. [Google Scholar] [CrossRef]
- Van Huffel, K.; Hansen, M.J.; Feilberg, A.; Liu, D.; Van Langenhove, H. Level and Distribution of Odorous Compounds in Pig Exhaust Air from Combined Room and Pit Ventilation. Agric. Ecosyst. Environ. 2016, 218, 209–219. [Google Scholar] [CrossRef]
- Seo, S.-C.; Lee, W.-J.; Kim, D.-Y.; Kim, K.-Y. Temporal Distribution Characteristics of Odorous Compounds in Swine Houses of South Korea. Air Qual. Atmos. Health 2023, 16, 2003–2017. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Koziel, J.A.; Cai, L.; Jenks, W.S.; Laor, Y.; Leeuwen, J.H.V.; Hoff, S.J. Improved Quantification of Livestock Associated Odorous Volatile Organic Compounds in a Standard Flow-through System Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2015, 1414, 31–40. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Ma, C. Characterization of Odorous Pollution and Health Risk Assessment of Volatile Organic Compound Emissions in Swine Facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne Particulate Matter from Livestock Production Systems: A Review of an Air Pollution Problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Yang, X.; Lorjaroenphon, Y.; Cadwallader, K.R.; Wang, X.; Zhang, Y.; Lee, J. Analysis of Particle-Borne Odorants Emitted from Concentrated Animal Feeding Operations. Sci. Total Environ. 2014, 490, 322–333. [Google Scholar] [CrossRef]
- Chai, L.; Ni, J.-Q.; Diehl, C.A.; Kilic, I.; Heber, A.J.; Chen, Y.; Cortus, E.L.; Bogan, B.W.; Lim, T.T.; Ramirez-Dorronsoro, J.-C.; et al. Ventilation Rates in Large Commercial Layer Hen Houses with Two-Year Continuous Monitoring. Br. Poult. Sci. 2012, 53, 19–31. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor Air Pollutants in Office Environments: Assessment of Comfort, Health, and Performance. J. Hyg. Environ. Health 2013, 216, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Bennett, J.L.; Raymer, J.H. Quantification of Odors and Odorants from Swine Operations in North Carolina. Agric. For. Meteorol. 2001, 108, 213–240. [Google Scholar] [CrossRef]
- Feilberg, A.; Liu, D.; Adamsen, A.P.S.; Hansen, M.J.; Jonassen, K.E.N. Odorant Emissions from Intensive Pig Production Measured by Online Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2010, 44, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile Organic Compounds (VOCs) Removal by Photocatalysts: A Review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Huang, S.; Zhou, Y.; Xu, B.; Peng, H.; Qin, P.; Wu, G. Concentrations and Emissions of Particulate Matter and Ammonia from Extensive Livestock Farm in South China. Environ. Sci. Pollut. Res. 2019, 26, 1871–1879. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Chai, L.; Chen, L.; Bogan, B.W.; Wang, K.; Cortus, E.L.; Heber, A.J.; Lim, T.-T.; Diehl, C.A. Characteristics of Ammonia, Hydrogen Sulfide, Carbon Dioxide, and Particulate Matter Concentrations in High-Rise and Manure-Belt Layer Hen Houses. Atmos. Environ. 2012, 57, 165–174. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, Z.; Li, H.; Buser, M.D.; Wanjura, J.D.; Downey, P.M.; Zhang, C.; Craige, C.; Torrents, A.; McConnell, L.L.; et al. Assessment of Particulate Matter and Ammonia Emission Concentrations and Respective Plume Profiles from a Commercial Poultry House. Environ. Pollut. 2018, 238, 10–16. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Demeyer, P.; Volcke, E.I.P. Mitigating Emissions from Pig and Poultry Housing Facilities through Air Scrubbers and Biofilters: State-of-the-Art and Perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Hwang, O.; Lee, S.-R.; Cho, S.; Ro, K.S.; Spiehs, M.; Woodbury, B.; Silva, P.J.; Han, D.-W.; Choi, H.; Kim, K.-Y.; et al. Efficacy of Different Biochars in Removing Odorous Volatile Organic Compounds (VOCs) Emitted from Swine Manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Jafari, M.J.; Matin, A.H.; Rahmati, A.; Azari, M.R.; Omidi, L.; Hosseini, S.S.; Panahi, D. Experimental Optimization of a Spray Tower for Ammonia Removal. Atmos. Pollut. Res. 2018, 9, 783–790. [Google Scholar] [CrossRef]
- Saoud, W.A.; Belkessa, N.; Azzaz, A.A.; Rochas, V.; Mezino, V.; Presset, M.-A.; Lechevin, S.; Genouel, A.; Rouxel, S.; Monsimert, D.; et al. Pilot Scale Investigation of DBD-Plasma Photocatalysis for Industrial Application in Livestock Building Air: Elimination of Chemical Pollutants and Odors. Chem. Eng. J. 2023, 468, 143710. [Google Scholar] [CrossRef]
- Xia, D.; Li, Z.; Xie, Y.; Zhang, X. Kinetic Simulations of Volatile Organic Compounds Decomposition by Non-Thermal Plasma Treatment. Water Air Soil. Poll. 2016, 227, 463. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xie, R.; Huang, H.; Leung, M.K.H.; Li, J.; Leung, D.Y.C. Photocatalytic Oxidation for Volatile Organic Compounds Elimination: From Fundamental Research to Practical Applications. Environ. Sci. Technol. 2022, 56, 16582–16601. [Google Scholar] [CrossRef]
- Mazhar, S.I.; Shafi, H.Z.; Shah, A.; Asma, M.; Gul, S.; Raffi, M. Synthesis of Surface Modified Hydrophobic PTFE-ZnO Electrospun Nanofibrous Mats for Removal of Volatile Organic Compounds (VOCs) from Air. J. Polym. Res. 2020, 27, 222. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Škapin, A.S.; Seabra, M.P.; Labrincha, J.A. Visible Light Activated Photocatalytic Behaviour of Rare Earth Modified Commercial TiO2. Mater. Res. Bull. 2014, 50, 183–190. [Google Scholar] [CrossRef]
- Humayun, M.; Wang, C.; Luo, W. Recent Progress in the Synthesis and Applications of Composite Photocatalysts: A Critical Review. Small Methods 2022, 6, 2101395. [Google Scholar] [CrossRef]
- Truong, P.L.; Kidanemariam, A.; Park, J. A Critical Innovation of Photocatalytic Degradation for Toxic Chemicals and Pathogens in Air. J. Ind. Eng. Chem. 2021, 100, 19–39. [Google Scholar] [CrossRef]
- Fermoso, J.; Sánchez, B.; Suarez, S. Air Purification Applications Using Photocatalysis. In Nanostructured Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–128. ISBN 978-0-12-817836-2. [Google Scholar]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent Progress in Enhancing Photocatalytic Efficiency of TiO2-Based Materials. Appl. Catal. A-Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Priya, A.K.; Suresh, R.; Kumar, P.S.; Rajendran, S.; Vo, D.-V.N.; Soto-Moscoso, M. A Review on Recent Advancements in Photocatalytic Remediation for Harmful Inorganic and Organic Gases. Chemosphere 2021, 284, 131344. [Google Scholar] [CrossRef]
- Che, J.; Bae, N.; Noh, J.; Kim, T.; Yoo, P.J.; Shin, T.J.; Park, J. Poly(3-Hexylthiophene) Nanoparticles Prepared via a Film Shattering Process and Hybridization with TiO2 for Visible-Light Active Photocatalysis. Macromol. Res. 2019, 27, 427–434. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Evaluation of TiO2 Based Photocatalytic Treatment of Odor and Gaseous Emissions from Swine Manure with UV-A and UV-C. Animals 2021, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Grčić, I.; Marčec, J.; Radetić, L.; Radovan, A.-M.; Melnjak, I.; Jajčinović, I.; Brnardić, I. Ammonia and Methane Oxidation on TiO2 Supported on Glass Fiber Mesh under Artificial Solar Irradiation. Environ. Sci. Pollut. Res. 2021, 28, 18354–18367. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Zhu, L.; Burda, C. Considerations to Improve Adsorption and Photocatalysis of Low Concentration Air Pollutants on TiO2. Catal. Today 2014, 225, 24–33. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite–TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B-Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Wei, S.; Wang, J.; Liu, Y.; Li, M.; Liu, R. Selective Removal of H2S from Biogas Using a Regenerable Hybrid TiO2/Zeolite Composite. Fuel 2015, 157, 183–190. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Mao, H.; Fei, Z.; Bian, C.; Yu, L.; Chen, S.; Qian, Y. Facile Synthesis of High-Performance Photocatalysts Based on Ag/TiO2 Composites. Ceram. Int. 2019, 45, 12586–12589. [Google Scholar] [CrossRef]
- Liu, G.; Ji, J.; Hu, P.; Lin, S.; Huang, H. Efficient Degradation of H2S over Transition Metal Modified TiO2 under VUV Irradiation: Performance and Mechanism. Appl. Surf. Sci. 2018, 433, 329–335. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent Progress on Ag/TiO2 Photocatalysts: Photocatalytic and Bactericidal Behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Li, F. AgNO3-Induced Photocatalytic Degradation of Odorous Methyl Mercaptan in Gaseous Phase: Mechanism of Chemisorption and Photocatalytic Reaction. Environ. Sci. Technol. 2008, 42, 4540–4545. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, A.; Ghafourian, H.; Yadegarian, L.; Lari, K.; Sadatipour, M.T. Removal of Volatile Organic Compounds (VOCs) from Waste Air Stream Using Ozone Assisted Zinc Oxide (ZnO) Nanoparticles Coated on Zeolite. J. Environ. Health Sci. 2021, 19, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Siva, P.; Balu, K.S.; Suriyaprabha, R.; Rajendran, V.; Maaza, M. Acalypha Indica–Mediated Green Synthesis of ZnO Nanostructures under Differential Thermal Treatment: Effect on Textile Coating, Hydrophobicity, UV Resistance, and Antibacterial Activity. Adv. Powder Technol. 2017, 28, 3184–3194. [Google Scholar] [CrossRef]
- Shalahuddin Al Ja’farawy, M.; Kusumandari; Purwanto, A.; Widiyandari, H. Carbon Quantum Dots Supported Zinc Oxide (ZnO/CQDs) Efficient Photocatalyst for Organic Pollutant Degradation—A Systematic Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar] [CrossRef]
- Venkata Laxma Reddy, P.; Kim, K.-H.; Kim, Y.-H. A Review of Photocatalytic Treatment for Various Air Pollutants. Asian J. Atmos. Environ. 2011, 5, 181–188. [Google Scholar] [CrossRef]
- Kim, Y.; Irie, H.; Hashimoto, K. A Visible Light-Sensitive Tungsten Carbide/Tungsten Trioxde Composite Photocatalyst. Appl. Phys. Lett. 2008, 92, 182107. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Murugananthan, M.; Zhang, Y.; Zhang, L. Electrochemically Self-Doped WO3/TiO2 Nanotubes for Photocatalytic Degradation of Volatile Organic Compounds. Appl. Catal. B-Environ. 2020, 260, 118205. [Google Scholar] [CrossRef]
- Jansson, I.; Yoshiiri, K.; Hori, H.; García-García, F.J.; Rojas, S.; Sánchez, B.; Ohtani, B.; Suárez, S. Visible Light Responsive Zeolite/WO3–Pt Hybrid Photocatalysts for Degradation of Pollutants in Air. Appl. Catal. A-Gen. 2016, 521, 208–219. [Google Scholar] [CrossRef]
- Ning, X.; Lu, G. Photocorrosion Inhibition of CdS-Based Catalysts for Photocatalytic Overall Water Splitting. Nanoscale 2020, 12, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, M.; Zhu, N.; Cheng, Y.; Lam, S.S.; Chen, J.; Gao, Y.; Zhao, J. Bi-Based Visible Light-Driven Nano-Photocatalyst: The Design, Synthesis, and Its Application in Pollutant Governance and Energy Development. Nano Today 2022, 43, 101432. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic Oxidation for Indoor Air Purification: A Literature Review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; Van Grieken, R.; Marugán, J. Influence of Light Distribution on the Performance of Photocatalytic Reactors: LED vs Mercury Lamps. Appl. Catal. B-Environ. 2017, 215, 1–7. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Vilar, V.J.P. Strategies for the Intensification of Photocatalytic Oxidation Processes towards Air Streams Decontamination: A Review. Chem. Eng. J. 2020, 391, 123531. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Walsh, D.J.; Schneider, T.N.; Olsen, B.D.; Jensen, K.F. Design and Simulation of a Uniform Irradiance Photochemical Platform. React. Chem. Eng. 2023, 8, 416–423. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase—A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Zhu, X.; Hong, B.; Wang, W.; Qi, Z.; Xie, W.; Ding, J.; Bao, J.; Sun, S.; et al. Effect of Surface Modification with H2S and NH3 on TiO2 for Adsorption and Photocatalytic Degradation of Gaseous Toluene. Appl. Catal. B-Environ. 2015, 170–171, 215–224. [Google Scholar] [CrossRef]
- Wang, C.; Bai, H.; Yi, N.; Kang, X. Multi-Dimensional Optimization for a Novel Photocatalytic Reactor Incorporating the Decolorization of Azo Dye and Thermal Management of Ultraviolet Light-Emitting Diode Arrays. Energ. Convers. Man-X 2023, 17, 100344. [Google Scholar] [CrossRef]
- McMillan, T.J.; Leatherman, E.; Ridley, A.; Shorrocks, J.; Tobi, S.E.; Whiteside, J.R. Cellular Effects of Long Wavelength UV Light (UVA) in Mammalian Cells. J. Pharm. Pharmacol. 2010, 60, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-Art and Current Challenges for TiO2/UV-LED Photocatalytic Degradation of Emerging Organic Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Rasoulifard, M.H.; Fazli, M.; Eskandarian, M.R. Performance of the Light-Emitting-Diodes in a Continuous Photoreactor for Degradation of Direct Red 23 Using UV-LED/S2O82−Process. J. Ind. Eng. Chem. 2015, 24, 121–126. [Google Scholar] [CrossRef]
- Kneissl, M.; Seong, T.-Y.; Han, J.; Amano, H. The Emergence and Prospects of Deep-Ultraviolet Light-Emitting Diode Technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Roibu, A.; Morthala, R.B.; Leblebici, M.E.; Koziej, D.; Van Gerven, T.; Kuhn, S. Design and Characterization of Visible-Light LED Sources for Microstructured Photoreactors. React. Chem. Eng. 2018, 3, 849–865. [Google Scholar] [CrossRef]
- Yusuf, A.; Garlisi, C.; Palmisano, G. Overview on Microfluidic Reactors in Photocatalysis: Applications of Graphene Derivatives. Catal. Today 2018, 315, 79–92. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Avila, P.; Bahamonde, A.; Blanco, J.; Sánchez, B.; Cardona, A.I.; Romero, M. Gas-Phase Photo-Assisted Mineralization of Volatile Organic Compounds by Monolithic Titania Catalysts. Appl. Catal. B-Environ. 1998, 17, 75–88. [Google Scholar] [CrossRef]
- Jacobs, M.; Meir, G.; Hakki, A.; Thomassen, L.C.J.; Kuhn, S.; Leblebici, M.E. Scaling up Multiphase Photochemical Reactions Using Translucent Monoliths. Chem. Eng. Process 2022, 181, 109138. [Google Scholar] [CrossRef]
- Bouzaza, A.; Vallet, C.; Laplanche, A. Photocatalytic Degradation of Some VOCs in the Gas Phase Using an Annular Flow Reactor. J. Photochem. Photobiol. A Chem. 2006, 177, 212–217. [Google Scholar] [CrossRef]
- Vincent, G.; Marquaire, P.M.; Zahraa, O. Abatement of Volatile Organic Compounds Using an Annular Photocatalytic Reactor: Study of Gaseous Acetone. J. Photochem. Photobiol. A Chem. 2008, 197, 177–189. [Google Scholar] [CrossRef]
- Tong, K.; Yang, L.; Du, X.; Yang, Y. Review of Modeling and Simulation Strategies for Unstructured Packing Bed Photoreactors with CFD Method. Renew. Sustain. Energy Rev. 2020, 131, 109986. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.; Song, C. Adsorptive Desulfurization of Jet Fuels over TiO2-CeO2 Mixed Oxides: Role of Surface Ti and Ce Cations. Catal. Today 2021, 371, 265–275. [Google Scholar] [CrossRef]
- Zou, L.; Luo, Y.; Hooper, M.; Hu, E. Removal of VOCs by Photocatalysis Process Using Adsorption Enhanced TiO2–SiO2 Catalyst. Chem. Eng. Process. 2006, 45, 959–964. [Google Scholar] [CrossRef]
- Alvarado, A.C.; Predicala, B.Z. Control of Gas and Odor Levels in Swine Facilities Using Filters with Zinc Oxide Nanoparticles. Trans. ASABE 2017, 60, 943–956. [Google Scholar] [CrossRef]
- Assadi, H.; Armaghan, F.; Taheri, R.A. Photocatalytic Oxidation of Ketone Group Volatile Organic Compounds in an Intensified Fluidized Bed Reactor Using Nano-TiO2/UV Process: An Experimental and Modeling Study. Chem. Eng. Process. 2021, 161, 108312. [Google Scholar] [CrossRef]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic Degradation of Air Pollutant by Modified Nano Titanium Oxide (TiO2)in a Fluidized Bed Photoreactor: Optimizing and Kinetic Modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef]
- Zhang, M.; An, T.; Fu, J.; Sheng, G.; Wang, X.; Hu, X.; Ding, X. Photocatalytic Degradation of Mixed Gaseous Carbonyl Compounds at Low Level on Adsorptive TiO2/SiO2 Photocatalyst Using a Fluidized Bed Reactor. Chemosphere 2006, 64, 423–431. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.-H.; Thakur, S.; Nguyen, C.C.; Kim, S.Y.; Le, Q.V.; et al. An Overview on Recent Progress in Photocatalytic Air Purification: Metal-Based and Metal-Free Photocatalysis. Environ. Res. 2022, 214, 113995. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A Review of Intensification of Photocatalytic Processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Matter, F.; Niederberger, M. The Importance of the Macroscopic Geometry in Gas-Phase Photocatalysis. Adv. Sci. 2022, 9, 2105363. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-J.; Hong, Y.-Y. Removal of Hydrogen Sulfide Using a Photocatalytic Livestock Biogas Desulfurizer. Renew. Energy 2020, 149, 181–188. [Google Scholar] [CrossRef]
- Mosleh, S.; Ghaedi, M. Photocatalytic Reactors: Technological Status, Opportunities, and Challenges for Development and Industrial Upscaling. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 761–790. ISBN 978-0-12-818806-4. [Google Scholar]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Fonken, B.; Storjohann, R.; Chen, B.; Li, P.; Banik, C.; Wahe, L.; et al. Design and Testing of Mobile Laboratory for Mitigation of Gaseous Emissions from Livestock Agriculture with Photocatalysis. Int. J. Environ. Res. Public Health 2021, 18, 1523. [Google Scholar] [CrossRef] [PubMed]
- Calvet, S.; Cambra-López, M.; Blanes-Vidal, V.; Estellés, F.; Torres, A.G. Ventilation Rates in Mechanically-Ventilated Commercial Poultry Buildings in Southern Europe: Measurement System Development and Uncertainty Analysis. Biosyst. Eng. 2010, 106, 423–432. [Google Scholar] [CrossRef]
- Pu, S.; Wang, H.; Zhu, J.; Li, L.; Long, D.; Jian, Y.; Zeng, Y. Heterostructure Cu2O/(001)TiO2 Effected on Photocatalytic Degradation of Ammonia of Livestock Houses. Catalysts 2019, 9, 267. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, J.; Hua, W.; Li, K.; Zhang, X.; Xiao, L.; Zhang, W.; Ni, Y.; Zhang, J. Effect of a New Tungsten Trioxide-Based Bactericide on the Environment of Piggeries and Piglet Health. Environ. Technol. Innov. 2022, 28, 102628. [Google Scholar] [CrossRef]
- Cao, T.; Zheng, Y.; Dong, H.; Wang, S.; Zhang, Y.; Cong, Q. A New Air Cleaning Technology to Synergistically Reduce Odor and Bioaerosol Emissions from Livestock Houses. Agric. Ecosyst. Environ. 2023, 342, 108221. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Li, Y.; Zhang, C.; He, H. Photocatalytic Oxidation of Gaseous Ammonia over Fluorinated TiO2 with Exposed (001) Facets. Appl. Catal. B-Environ. 2014, 152–153, 82–87. [Google Scholar] [CrossRef]
- Lee, M.; Wi, J.; Koziel, J.A.; Ahn, H.; Li, P.; Chen, B.; Meiirkhanuly, Z.; Banik, C.; Jenks, W. Effects of UV-A Light Treatment on Ammonia, Hydrogen Sulfide, Greenhouse Gases, and Ozone in Simulated Poultry Barn Conditions. Atmosphere 2020, 11, 283. [Google Scholar] [CrossRef]
- Liu, Z.; Murphy, P.; Maghirang, R. Mitigation of Air Emissions from Swine Buildings through the Photocatalytic Technology Using UV/TiO2. In Proceedings of the 2015 ASABE International Meeting, New Orleans, LA, USA, 26–29 July 2015; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2015. [Google Scholar]
- Wu, L.-C.; Kuo, C.-L.; Chung, Y.-C. Removal of High Concentrations of NH3 by a Combined Photoreactor and Biotrickling Filter System. J. Environ. Sci. Health Part A 2011, 46, 1675–1682. [Google Scholar] [CrossRef]
- Maxime, G.; Aymen Amine, A.; Abdelkrim, B.; Dominique, W. Removal of Gas-Phase Ammonia and Hydrogen Sulfide Using Photocatalysis, Nonthermal Plasma, and Combined Plasma and Photocatalysis at Pilot Scale. Environ. Sci. Pollut. Res. 2014, 21, 13127–13137. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Feilberg, A. Characterisation of Photocatalytic Degradation of Odorous Compounds Associated with Livestock Facilities by Means of PTR-MS. Chem. Eng. J. 2015, 277, 341–351. [Google Scholar] [CrossRef]
- Zhu, W.; Koziel, J.; Maurer, D. Mitigation of Livestock Odors Using Black Light and a New Titanium Dioxide-Based Catalyst: Proof-of-Concept. Atmosphere 2017, 8, 103. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A. On-Farm Pilot-Scale Testing of Black Ultraviolet Light and Photocatalytic Coating for Mitigation of Odor, Odorous VOCs, and Greenhouse Gases. Chemosphere 2019, 221, 778–784. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Mitigation of Odor and Gaseous Emissions from Swine Barn with UV-A and UV-C Photocatalysis. Atmosphere 2021, 12, 585. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Lemasle, M.; Wolbert, D. Acceleration of Trimethylamine Removal Process Under Synergistic Effect of Photocatalytic Oxidation and Surface Discharge Plasma Reactor. Can. J. Chem. Eng. 2015, 93, 1239–1246. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Soutrel, I.; Petit, P.; Medimagh, K.; Wolbert, D. A Study of Pollution Removal in Exhaust Gases from Animal Quartering Centers by Combining Photocatalysis with Surface Discharge Plasma: From Pilot to Industrial Scale. Chem. Eng. Process 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Li, X.Z.; Hou, M.F.; Li, F.B.; Chua, H. Photocatalytic Oxidation of Methyl Mercaptan in Foul Gas for Odor Control. Ind. Eng. Chem. Res. 2006, 45, 487–494. [Google Scholar] [CrossRef]
- Yang, X.; Koziel, J.A.; Cutler, T.; van Leeuwen, H.; Zhang, S.; Hoff, S.J.; Jenks, W.; Zimmerman, J. Treatment of Livestock Odor and Pathogens with Ultraviolet Light. In Proceedings of the 2008 ASABE Annual International Meeting, Providence, RI, USA, 29 June–2 July 2008; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008. [Google Scholar]
- Yang, X.; Koziel, J.A.; Laor, Y.; Zhu, W.; van Leeuwen, J.H.; Jenks, W.S.; Hoff, S.J.; Zimmerman, J.; Zhang, S.; Ravid, U.; et al. VOC Removal from Manure Gaseous Emissions with UV Photolysis and UV-TiO2 Photocatalysis. Catalysts 2020, 10, 607. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Dong, F.; Giannakoudakis, D.A. Photocatalytic Platforms for Removal of Ammonia from Gaseous and Aqueous Matrixes: Status and Challenges. ACS Catal. 2020, 10, 8683–8716. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective Photocatalytic Oxidation of Gaseous Ammonia at Ppb Level over Pt and F Modified TiO2. Appl. Catal. B-Environ. 2022, 300, 120688. [Google Scholar] [CrossRef]
- Abdi, M.; Alinezhad, E.; Akbari Sene, R.; Haghighi, M.; Keshizadeh, H.; Naddafi, K. Evaluation of a Pilot-Scale Scrubber for the Mitigation of NH3 Emissions from Laboratory Animal House in the Presence of Different Oxidants. J. Environ. Chem. Eng. 2020, 8, 103708. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic Reactors and It’s Comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Kolinko, P.A.; Kozlov, D.V. Products Distribution during the Gas Phase Photocatalytic Oxidation of Ammonia over the Various Titania Based Photocatalysts. Appl. Catal. B-Environ. 2009, 90, 126–131. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Lo Porto, C.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-Based Coatings for Environmental Applications. Catal. Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Schreck, M.; Niederberger, M. Photocatalytic Gas Phase Reactions. Chem. Mater. 2019, 31, 597–618. [Google Scholar] [CrossRef]
- Alonso-Tellez, A.; Robert, D.; Keller, N.; Keller, V. A Parametric Study of the UV-A Photocatalytic Oxidation of H2S over TiO2. Appl. Catal. B Environ. 2012, 115–116, 209–218. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, T.; Zheng, L.; Yu, J. Photocatalytic Degradation of Hydrogen Sulfide Using TiO2 Film under Microwave Electrodeless Discharge Lamp Irradiation. Chem. Eng. J. 2013, 225, 9–15. [Google Scholar] [CrossRef]
- Sopyan, I. Kinetic Analysis on Photocatalytic Degradation of Gaseous Acetaldehyde, Ammonia and Hydrogen Sulfide on Nanosized Porous TiO2 Films. Sci. Technol. Adv. Mater. 2007, 8, 33–39. [Google Scholar] [CrossRef]
- Palau, J.; Penya-Roja, J.M.; Gabaldón, C.; Álvarez-Hornos, F.J.; Martínez-Soria, V. Effect of Pre-treatments Based on UV Photocatalysis and Photo-oxidation on Toluene Biofiltration Performance. J. Chem. Tech. Biotech. 2012, 87, 65–72. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for Treatment of VOCs and Odours—A Review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on Oxidation Mechanisms of Organic Compounds by TiO2 in Heterogeneous Photocatalysis. J. Photoch Photobio C 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Photocatalytic Transformations of Organic Sulfur Compounds and H2S. Russ. Chem. Rev. 2008, 77, 909–926. [Google Scholar] [CrossRef]
- Biard, P.-F.; Bouzaza, A.; Wolbert, D. Photocatalytic Degradation of Two Volatile Fatty Acids in Monocomponent and Multicomponent Systems: Comparison between Batch and Annular Photoreactors. Appl. Catal. B-Environ. 2007, 74, 187–196. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Photocatalytic Oxidation of Trimethylamine and Isovaleraldehyde in an Annular Reactor: Influence of the Mass Transfer and the Relative Humidity. J. Photochem. Photobiol. A Chem. 2012, 236, 61–69. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Naldoni, A.; Pirola, C. Photocatalytic Degradation of Toluene in the Gas Phase: Relationship between Surface Species and Catalyst Features. Environ. Sci. Technol. 2008, 42, 6671–6676. [Google Scholar] [CrossRef]
- Khunphonoi, R.; Grisdanurak, N. Mechanism Pathway and Kinetics of P-Cresol Photocatalytic Degradation over Titania Nanorods under UV–Visible Irradiation. Chem. Eng. J. 2016, 296, 420–427. [Google Scholar] [CrossRef]
- Héquet, V.; Raillard, C.; Debono, O.; Thévenet, F.; Locoge, N.; Le Coq, L. Photocatalytic Oxidation of VOCs at Ppb Level Using a Closed-Loop Reactor: The Mixture Effect. Appl. Catal. B-Environ. 2018, 226, 473–486. [Google Scholar] [CrossRef]
- Chen, K.-N.; Sari, F.N.I.; Ting, J.-M. Multifunctional TiO2/Polyacrylonitrile Nanofibers for High Efficiency PM2.5 Capture, UV Filter, and Anti-Bacteria Activity. Appl. Surf. Sci. 2019, 493, 157–164. [Google Scholar] [CrossRef]
- Sohara, K.; Yamauchi, K.; Sun, X.; Misawa, K.; Sekine, Y. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons in Fine Particulate Matter (PM2.5) Collected on TiO2-Supporting Quartz Fibre Filters. Catalysts 2021, 11, 400. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A Review of the Mechanisms and Modeling of Photocatalytic Disinfection. Appl. Catal. B-Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Curri, M.L.; Comparelli, R. TiO2-Based Nanomaterials Assisted Photocatalytic Treatment for Virus Inactivation: Perspectives and Applications. Curr. Opin. Chem. Eng. 2021, 34, 100716. [Google Scholar] [CrossRef]
- Zhao, Y.; Aarnink, A.J.A.; Xin, H. Inactivation of Airborne Enterococcus Faecalis and Infectious Bursal Disease Virus Using a Pilot-Scale Ultraviolet Photocatalytic Oxidation Scrubber. J. Air Waste Manag. 2014, 64, 38–46. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on Heterogeneous Photocatalytic Disinfection of Waterborne, Airborne, and Foodborne Viruses: Can We Win against Pathogenic Viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Misawa, K.; Sekine, Y.; Kusukubo, Y.; Sohara, K. Photocatalytic Degradation of Atmospheric Fine Particulate Matter (PM2.5) Collected on TiO2 Supporting Quartz Fibre Filter. Environ. Technol. 2020, 41, 1266–1274. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, M.; Zhang, X.; Gal, C.; Chen, X.; Liu, L.; Pan, S.; Wu, J.; Tang, L.; Clements-Croome, D. A Review of Air Filtration Technologies for Sustainable and Healthy Building Ventilation. Sustain. Cities Soc. 2017, 32, 375–396. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Soutrel, I.; Wolbert, D.; Rtimi, S. Study of Synergetic Effect, Catalytic Poisoning and Regeneration Using Dielectric Barrier Discharge and Photocatalysis in a Continuous Reactor: Abatement of Pollutants in Air Mixture System. Appl. Catal. B-Environ. 2017, 213, 53–61. [Google Scholar] [CrossRef]
- Khezami, L.; Nguyen-Tri, P.; Saoud, W.A.; Bouzaza, A.; El Jery, A.; Duc Nguyen, D.; Gupta, V.K.; Assadi, A.A. Recent Progress in Air Treatment with Combined Photocatalytic/Plasma Processes: A Review. J. Environ. Manag. 2021, 299, 113588. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, T.; An, X. Combining Non-Thermal Plasma Technology with Photocatalysis: A Critical Review. Phys. Chem. Chem. Phys. 2023, 25, 1538–1545. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Du, L.; Zhang, Q.; Wang, W. Synergistic Effect and Mechanism of Non-Thermal Plasma Catalysis System in Volatile Organic Compounds Removal: A Review. Catal. Sci. Technol. 2016, 6, 73–80. [Google Scholar] [CrossRef]
- Kone, N.A.; Belkessa, N.; Serhane, Y.; Coulibaly, S.L.; Kamagate, M.; Mouni, L.; Loganathan, S.; Coulibaly, L.; Bouzaza, A.; Amrane, A.; et al. Chlorobenzene Mineralization Using Plasma/Photocatalysis Hybrid Reactor: Exploiting the Synergistic Effect. Catalysts 2023, 13, 431. [Google Scholar] [CrossRef]
- Belkessa, N.; Bouzaza, A.; Assadi, A.A. Understanding of the Synergy Effect of DBD Plasma Discharge Combined to Photocatalysis in the Case of Ethylbenzene Removal: Interaction between Plasma Reactive Species and Catalyst. J. Environ. Chem. Eng. 2023, 11, 110640. [Google Scholar] [CrossRef]
- Rafique, M.S.; Tahir, M.B.; Rafique, M.; Shakil, M. Photocatalytic Nanomaterials for Air Purification and Self-Cleaning. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–219. ISBN 978-0-12-821192-2. [Google Scholar]
- Zhang, Y.; Zhu, Y.; Tao, S.; Zhang, Z.; Chen, M.; Jiang, Z.; Shangguan, W. Plasma-Coupled Catalysis in VOCs Removal and CO2 Conversion: Efficiency Enhancement and Synergistic Mechanism. Catal. Commun. 2022, 172, 106535. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Huang, Q.; Huang, L.; Xie, J.; Qing, C.; Chen, T. Removal of Gaseous Carbon Bisulfide Using Dielectric Barrier Discharge Plasmas Combined with TiO2 Coated Attapulgite Catalyst. Chem. Eng. J. 2013, 225, 567–573. [Google Scholar] [CrossRef]
- Assadi, A.A.; Palau, J.; Bouzaza, A.; Penya-Roja, J.; Martinez-Soriac, V.; Wolbert, D. Abatement of 3-Methylbutanal and Trimethylamine with Combined Plasma and Photocatalysis in a Continuous Planar Reactor. J. Photochem. Photobiol. A Chem. 2014, 282, 1–8. [Google Scholar] [CrossRef]
- Ochiai, T.; Ichihashi, E.; Nishida, N.; Machida, T.; Uchida, Y.; Hayashi, Y.; Morito, Y.; Fujishima, A. Field Performance Test of an Air-Cleaner with Photocatalysis-Plasma Synergistic Reactors for Practical and Long-Term Use. Molecules 2014, 19, 17424–17434. [Google Scholar] [CrossRef]
- He, Z.; Li, J.; Chen, J.; Chen, Z.; Li, G.; Sun, G.; An, T. Treatment of Organic Waste Gas in a Paint Plant by Combined Technique of Biotrickling Filtration with Photocatalytic Oxidation. Chem. Eng. J. 2012, 200–202, 645–653. [Google Scholar] [CrossRef]
- Du, Q.; Zhong, Z.; Zhang, T.; Xu, Y.; Zhang, G.; Wu, S.; Xu, J. Synergistic Degradation Performance of UV/TiO2 Photocatalysis and Biotrickling Filtration Inoculated with Aspergillus sp. S1 for Gaseous m-xylene. J. Chem. Technol. Biotechnol. 2023, 98, 498–505. [Google Scholar] [CrossRef]
- Almomani, F.; Rene, E.R.; Veiga, M.C.; Bhosale, R.R.; Kennes, C. Treatment of Waste Gas Contaminated with Dichloromethane Using Photocatalytic Oxidation, Biodegradation and Their Combinations. J. Hazard. Mater. 2021, 405, 123735. [Google Scholar] [CrossRef]
- Han, M.-F.; Hu, X.-R.; Wang, Y.-C.; Tong, Z.; Wang, C.; Cheng, Z.-W.; Feng, K.; Qu, M.-M.; Chen, J.-M.; Deng, J.-G.; et al. Comparison of Separated and Combined Photodegradation and Biofiltration Technology for the Treatment of Volatile Organic Compounds: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1325–1355. [Google Scholar] [CrossRef]
- Dobslaw, D.; Ortlinghaus, O. Biological Waste Air and Waste Gas Treatment: Overview, Challenges, Operational Efficiency, and Current Trends. Sustainability 2020, 12, 8577. [Google Scholar] [CrossRef]
- Rene, E.R.; Veiga, M.C.; Kennes, C. Combined Biological and Physicochemical Waste-Gas Cleaning Techniques. J. Environ. Sci. Health Part A 2012, 47, 920–939. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-R.; Han, M.-F.; Wang, C.; Yang, N.-Y.; Wang, Y.-C.; Duan, E.-H.; Hsi, H.-C.; Deng, J.-G. A Short Review of Bioaerosol Emissions from Gas Bioreactors: Health Threats, Influencing Factors and Control Technologies. Chemosphere 2020, 253, 126737. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main Parameters Influencing the Design of Photocatalytic Reactors for Wastewater Treatment: A Mini Review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Santoro, D.; Crapulli, F.; Turolla, A.; Antonelli, M. Detailed Modeling of Oxalic Acid Degradation by UV-TiO2 Nanoparticles: Importance of Light Scattering and Photoreactor Scale-Up. Water Res. 2017, 121, 361–373. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Liu, W.; Wang, Q.; Cao, W. Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2. Catalysts 2018, 8, 326. [Google Scholar] [CrossRef]
- Shie, J.-L.; Lee, C.-H.; Chiou, C.-S.; Chang, C.-T.; Chang, C.-C.; Chang, C.-Y. Photodegradation Kinetics of Formaldehyde Using Light Sources of UVA, UVC and UVLED in the Presence of Composed Silver Titanium Oxide Photocatalyst. J. Hazard. Mater. 2008, 155, 164–172. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Dai, Y.; Dong, W.; Zhang, S.; Chen, J. Dimethyl Sulfide Photocatalytic Degradation in a Light-Emitting-Diode Continuous Reactor: Kinetic and Mechanistic Study. Ind. Eng. Chem. Res. 2011, 50, 7977–7984. [Google Scholar] [CrossRef]
- Salvadó-Estivill, I.; Hargreaves, D.M.; Li Puma, G. Evaluation of the Intrinsic Photocatalytic Oxidation Kinetics of Indoor Air Pollutants. Environ. Sci. Technol. 2007, 41, 2028–2035. [Google Scholar] [CrossRef]
- Visan, A.; Van Ommen, J.R.; Kreutzer, M.T.; Lammertink, R.G.H. Photocatalytic Reactor Design: Guidelines for Kinetic Investigation. Ind. Eng. Chem. Res. 2019, 58, 5349–5357. [Google Scholar] [CrossRef]
- Salvadores, F.; Alfano, O.M.; Ballari, M.M. Kinetic Study of Air Treatment by Photocatalytic Paints under Indoor Radiation Source: Influence of Ambient Conditions and Photocatalyst Content. Appl. Catal. B-Environ. 2020, 268, 118694. [Google Scholar] [CrossRef]
- Tong, K.; Yang, L.; Du, X. Modelling of TiO2-Based Packing Bed Photocatalytic Reactor with Raschig Rings for Phenol Degradation by Coupled CFD and DEM. Chem. Eng. J. 2020, 400, 125988. [Google Scholar] [CrossRef]
- Malayeri, M.; Haghighat, F.; Lee, C.-S. Modeling of Volatile Organic Compounds Degradation by Photocatalytic Oxidation Reactor in Indoor Air: A Review. Build. Environ. 2019, 154, 309–323. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Ang, M.; Pareek, V. Some Aspects of Photocatalytic Reactor Modeling Using Computational Fluid Dynamics. Chem. Eng. Sci. 2013, 101, 764–784. [Google Scholar] [CrossRef]
- Meng, X.; Yun, N.; Zhang, Z. Recent Advances in Computational Photocatalysis: A Review. Can. J. Chem. Eng. 2019, 97, 1982–1998. [Google Scholar] [CrossRef]
- Jiang, S.; Li, F.; Xie, F. Nonrelativistic Limit of the Compressible Navier--Stokes--Fourier--P1 Approximation Model Arising in Radiation Hydrodynamics. Siam J. Math. Anal. 2015, 47, 3726–3746. [Google Scholar] [CrossRef]
- Mueses, M.A.; Machuca-Martinez, F.; Hernández-Ramirez, A.; Li Puma, G. Effective Radiation Field Model to Scattering—Absorption Applied in Heterogeneous Photocatalytic Reactors. Chem. Eng. J. 2015, 279, 442–451. [Google Scholar] [CrossRef]
- Claes, T.; Dilissen, A.; Leblebici, M.E.; Van Gerven, T. Translucent Packed Bed Structures for High Throughput Photocatalytic Reactors. Chem. Eng. J. 2019, 361, 725–735. [Google Scholar] [CrossRef]
- De Oliveira, G.X.; Kuhn, S.; Riella, H.G.; Soares, C.; Padoin, N. Combining Computational Fluid Dynamics, Photon Fate Simulation and Machine Learning to Optimize Continuous-Flow Photocatalytic Systems. React. Chem. Eng. 2023, 8, 2119–2133. [Google Scholar] [CrossRef]
- Peralta Muniz Moreira, R.; Li Puma, G. Multiphysics Computational Fluid-Dynamics (CFD) Modeling of Annular Photocatalytic Reactors by the Discrete Ordinates Method (DOM) and the Six-Flux Model (SFM) and Evaluation of the Contaminant Intrinsic Kinetics Constants. Catal. Today 2021, 361, 77–84. [Google Scholar] [CrossRef]
- Van Walsem, J.; Verbruggen, S.W.; Modde, B.; Lenaerts, S.; Denys, S. CFD Investigation of a Multi-Tube Photocatalytic Reactor in Non-Steady-State Conditions. Chem. Eng. J. 2016, 304, 808–816. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Dai, Y.; Dong, W.; Zhang, S.; Chen, J. CFD Modeling of a UV-LED Photocatalytic Odor Abatement Process in a Continuous Reactor. J. Hazard. Mater. 2012, 215–216, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent Developments and Challenges in Zeolite-Based Composite Photocatalysts for Environmental Applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Amano, H.; Collazo, R.; Santi, C.D.; Einfeldt, S.; Funato, M.; Glaab, J.; Hagedorn, S.; Hirano, A.; Hirayama, H.; Ishii, R.; et al. The 2020 UV Emitter Roadmap. J. Phys. D Appl. Phys. 2020, 53, 503001. [Google Scholar] [CrossRef]




| Mitigating Strategies | Mitigation Category | Target Pollutants | Merit | Demerit | Reference |
|---|---|---|---|---|---|
| Adsorption and Masking | Source-based/End-of-pipe | NH3, H2S, VOCs | Easy to operate Readily available raw materials Effective in adsorbing various odor compounds | High regeneration costs, difficult to handle waste Limited capacity for high flow or low gas concentration exhaust | [50] |
| Wet scrubber | End-of-pipe | NH3, H2S, VOCs, PM | Large exhaust treatment flow Efficient for NH3, and other hydrophilic substances | Difficult to degrade hydrophobic VOCs High water consumption and wastewater generation | [3,51] |
| Non-thermal plasma | End-of-pipe | VOCs, PM | Strong removal effect on VOCs in livestock and poultry farms Significantly reduce PM and airborne aerosol concentrations | Formation of harmful by-products and intermediates High electric consumption | [52,53] |
| Biological methods | End-of-pipe | NH3, VOCs, PM | Low energy consumption and no secondary pollution | Hard to control moisture and pH High pressure drop Deterioration of the filter bed during long-term operation | [10] |
| Photocatalysis | Source-based/End-of-pipe | NH3, H2S, VOCs | Safe and non-toxic High removal efficiency Operate under mild ambient conditions | Dust in livestock and poultry farms can reduce photocatalytic efficiency Potential generation of toxic by-products and intermediates | [54] |
| Photoreactor | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Monolithic reactor | High throughput and low pressure drop Large surface to volume ratio High photon flux utilization | Low light efficiency with significant gradient through monolithic materials | [111,115] |
| Annular reactor | Advantageous for determining reaction kinetic parameters High irradiance uniformity with a central light source Easy to quantify reactor configuration parameters | Low gas throughput Low surface to volume ratio | [20,111] |
| Packed-bed reactor | Simple structure, easy operation, and low cost High surface to volume ratio Good recycling and high stability | High pressure drop Easy to form channel flow resulting in low contact between catalysts and pollutants | [111,115] |
| Fluidized-bed reactor | High throughput and low pressure drop Good contact of catalyst-light and catalyst-reactants | Catalyst loss Hard to control | [20,110] |
| Experimental Conditions Temp/RH | Experimental Scale | Year | Light Source | Irradiance (mW/cm2) | UV Dose (mJ/cm2) | Reactor or Reaction Place | Treatment Time | Photocatalyst (Dose) | Pollutant/RE | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 18.9–27.30 °C/53.6% | Farm-scale in farrowing rooms | 2008 | UV-A (315–400 nm) | 0–0.144 | NR | The whole farrowing barn | NR | TiO2 (70 g/m2) | NH3/30.50% | [28] |
| 15 °C/75% | Lab-scale | 2018 | UV-A (365 nm) | NRs | NR | Tubular photocatalytic reactor | NR | RGO-P25(NR) | NH3/97.39% | [11] |
| 18 °C/NR | Lab-scale | 2019 | Xeon light | NR | NR | Tubular photocatalytic reactor | NR | Polyester fiber supported TiO2 (0.18 g) | NH3/90% | [118] |
| 22–28 °C/55–63% | Pilot-scale | 2020 | UV-A-LED (365 nm) | 0–4.85 | 0–824.5 | Rectangular tunnel photoreactor | 170 s | TiO2 (10 μg/cm2) | NH3/8.7% | [18] |
| 22–28 °C/55–63% | Pilot-scale | UV-A-fluorescent light (365 nm) | 0–0.44 | 0–74.8 | 170 s | TiO2 (10 μg/cm2) | NH3/5.2% | |||
| NR/50% | Lab-scale | 2021 | UV-A + UV-B | 2.45 (UV-A) 1.35 (UV-B) | NR | Annular reactor, mini-photocatalytic wind tunnel, photocatalytic wind tunnel | NR | Glass fiber cloth supported TiO2 (1.7 ± 0.1 μg/cm2) | NH3/43%, 50% (for mini-photocatalytic wind tunnel and photocatalytic wind tunnel, respectively) | [65] |
| 11 ± 3 °C/34 ± 6% | Pilot-scale | 2021 | UV-A-LED (367 nm) | 0.41 | 3.90 | Photocatalytic mobile laboratory (serpentine tunnel reactor) | 9.5 s | TiO2 (10 μg/cm2) | NH3/9% | [116] |
| 0.10 | 5.81 | 57 s | TiO2 (10 μg/cm2) | NH3/11% | ||||||
| NR/NR | Pilot-scale | 2022 | UV-A (315–400 nm) | NA | NA | The whole piglets barn | NA | WO3 (NR) | NH3/30.5% | [119] |
| NR/NR | Pilot-scale | 2023 | UV-C (185–254 nm) | NR | NR | Photocatalytic scrubber | NR | 25 nm-TiO2 (NR) | NH3/89.1% | [120] |
| NR/NR | Lab-scale | 2014 | UV-A (365 nm) | 0.46 | NR | Black-colored box | NR | F-TiO2 (4.2 mg/cm2) | NH3/35% | [121] |
| 25 ± 3 °C/12% | Lab-scale | 2020 | UV-A-LED (365 nm) | 4.85 | 970 | Annular reactor | 200 s | TiO2 (10 μg/cm2) | NH3/18.7% | [122] |
| NR/NR | Lab-scale | 2015 | UV-C (185–254 nm) | 2.2 | 8.8 | Multi-stage honeycomb photocatalytic reactor | 4 s | P25 (182 m2/m3) | NH3/53% | [123] |
| NR/NR | Farm-scale in nursery swine building | UV-C (185–254 nm) | 2.2 | 0.0396 | 0.018 s | P25 (182 m2/m3) | NH3/10% | |||
| 30 °C/NR | Lab-scale | 2011 | UV-A (365 nm) | 11.6 a | NR | Annular PVC photoreactor with aluminum foil | 10 s | TiO2 (3.3 mg/cm2) | NH3/47% | [124] |
| 12 s | TiO2 (3.3 mg/cm2) | NH3/54% | ||||||||
| 20 °C/60% | Lab-scale | 2023 | UV lamp (wavelength not reported) | NR | NR | Annular formed of two concentric Pyrex tubes | <4.76 s a | TiO2 Glass Fiber Tissue (13 g/m2) | NH3/29% | [52] |
| 23.6 °C/86% | Pilot-scale | NH3/30–43% | ||||||||
| NR/8.65, 25.9, 43.2, 69.2% b | Pilot-scale | 2014 | UV-A (355 nm) | 4.2 | 6.552–16.338 | Rectangular tunnel reactor | 1.56–3.89 s c | Glass fiber tissue (6.5 g/m2) | NH3/~4.6–32% | [125] |
| Experimental Conditions Temp/RH | Experimental Scale | Year | Light Source | Irradiance (mW/cm2) | UV Dose (mJ/cm2) | Reactor or Reaction Place | Treatment Time | Photocatalyst (Dose) | Pollutant/RE | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| NR/NR | Pilot-scale | 2021 | UV-A (365 nm) | NR | NR | Photocatalytic mobile laboratory (serpentine tunnel reactor) | NR | TiO2 (10 μg/cm2) | H2S/40% | [17] |
| 25 ± 3 °C/12% | Lab-scale | 2020 | UV-A-LED (365 nm) | 4.85 | 970 | Annular reactor | 200 s | TiO2 (10 μg/cm2) | H2S/~3% | [122] |
| 20.1 ± 1.4 °C/51.4 ± 2.0% | Lab-scale | 2015 | UV-A (368 nm) | 2.32–55.9 | 0.6–1.3 | Honeycomb monolith photocatalytic reactor | 0.23 s | TiO2 (NR) | H2S/14% | [126] |
| NR/NR | Lab-scale | 2015 | UV-C (185–254 nm) | 2.2 | 8.8 | Multi-stage honeycomb photocatalytic reactor | 4 s | P25 (182 m2/m3) | H2S/49% | [123] |
| NR/NR | Farm-scale in nursery swine building | UV-C (185–254 nm) | 2.2 | 0.0396 | 0.018 s | P25 (182 m2/m3) | H2S/24% | |||
| Lab-scale (150 ppm H2S gas) | Lab-scale | 2018 | VUV lamp (<200 nm) | NR | NR | Tubular quartz photoreactor loaded with catalysts | NR | M-TiO2 (M = Mn, Cu, Ni, Co) (1 g) | H2S/89.9% for Mn-TiO2 | [73] |
| Experimental Conditions Temp/RH | Experimental Scale | Year | Light Source | Irradiance (mW/cm2) | UV Dose (mJ/cm2) | Reactor | Treatment Time | Photocatalyst (Dose) | Pollutant/RE | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 20.1 ± 1.4 °C/51.4 ± 2.0% | Lab-scale | 2015 | UV-A (368 nm) | 2.32–5.59 | 0.6–1.3 | Honeycomb monolith photocatalytic reactor | 0.23 s | TiO2 (NR) | MT/87% DMS/96% DMDS/91% 1-Butanol/95% AA/89% PA/98% BA/98% VA/99% | [126] |
| 40 °C/40% | Lab-scale | 2017 | UV-A (365 nm) | 0.061 | 12.2 | Glass plates | 200 s | TiO2 (10 μg/cm2) | DMDS/40.4% DEDS/81.0% DMTS/76.3% BA/86.9% Guaiacol/100% p-cresol/93.8% | [127] |
| 21.8–26.0 °C/46–84% | Pilot-scale | 2019 | UV-A (365 nm) | 0–0.04 | 0–1.89 | Rectangular tunnel photoreactor | 47.2 s | TiO2 (10 μg/cm2) | p-cresol/22.0% DMDS/23.6% a DMTS/41.1% a BA/6.8% a iso-VA/5.6% a Phenol/10.9% a Indole/47.5% a | [128] |
| 22–28 °C/55–63% | Pilot-scale | 2020 | UV-A-LED (365 nm) | 0–0.44 | 0–74.8 | Rectangular tunnel photoreactor | 170 s | TiO2 (10 μg/cm2) | DEDS/42% BA/62% p-cresol/49% Skatole/35% | [18] |
| NR/NR | Pilot-scale | 2021 | UV-A (365 nm) | 0.41 | 5.3 | Photocatalytic mobile laboratory (serpentine tunnel reactor) | 9.51 s | TiO2 (10 μg/cm2) | DMDS/62% iso-BA/44% BA/32% p-cresol/40% Indole/66% Skatole/49% | [17] |
| 28.5 ± 2.3 °C/66 ± 4.3% | Pilot-scale | 2021 | UV-A (367 nm) | 0.41 | 5.3 | Photocatalytic mobile laboratory (serpentine tunnel reactor) | 12.9 s | TiO2 (10 μg/cm2) | DMDS/62% IA/44% BA/32% p-cresol/40% Skatole/49% | [129] |
| UV-C (254 nm) | 3.7 × 10−4 | 1.6 × 10−3 | 4.32 s | iso-BA/10.3% a | ||||||
| UV-C (222 nm) | 5.9 × 10−4 | 2.55 × 10−3 | 4.32 s | iso-BA/11.8% a BA/1.6% a Indole/26.5% a | ||||||
| UV-C (185 + 254 nm) | 1 × 10−5 | 3 × 10−5 | 3 s | iso-BA/33.6% a p-cresol/49.6% a Skatole/16.5% a | ||||||
| 22 ± 5 °C/40% | Pilot-scale | 2020 | UV-A (365 nm) | 0.41 | 3.9 | Annular reactor | 9.51 s | TiO2 (10 μg/cm2) | AA/48.6% BA/52.6% p-cresol/66.5% Indole/32.3% | [122] |
| 16 ± 1 °C/40% | Pilot-scale | UV-C (185 + 254 nm) | 10 | 48 | 4.8 s | TiO2 (10 μg/cm2) | p-cresol/47.1% Indole/54.2% | |||
| 11 ± 3 °C/34 ± 6 °C | Pilot-scale | 2021 | UV-A-LED (367 nm) | 0.41 | 5.8 | Photocatalytic mobile laboratory (serpentine tunnel reactor) | 14.1 s | TiO2 (10 μg/cm2) | 1-Butanol/41% | [116] |
| 20 °C/60% | Lab-scale | 2023 | UV lamp (wavelength not reported) | 2.0 | <9.52 | Annular formed of two concentric Pyrex tubes | <4.76 s b | TiO2 Glass Fiber Tissue (13 g/m2) | PA/37% | [52] |
| 20 °C/60% | Lab-scale | 2015 | UV-A (360 nm) | 2.0 | NR | Annular reactor | NR | TiO2 Glass Fiber Tissue (6.5 g/m2) | TMA/19–68% | [130] |
| Ambient temperature/NR | Pilot-scale | 2017 | UV-A (wavelength not reported) | NR | NR | Rectangular tunnel photoreactor containing the pleated photocatalytic media | NR | TiO2 Glass Fiber Tissue (6.5 g/m2) | Isovaleraldehyde/~38% | [131] |
| 32.4 °C/56% | Industrial-scale | UV-A (wavelength not reported) | NR | NR | Two similar rectangular tunnel photoreactor connected in series | NR | TiO2 Glass Fiber Tissue (13 g/m2) | Isobutyraldehyde/~20% Isovaleraldehyde/~22% 2-methyl butyraldehyde/~23% | ||
| (NR/43%) | Pilot-scale | 2006 | UV-A (365 nm) | 1.28 | NR | Cubic photoreactor made from Pyrex glass with an effect volume of 33.4 L (inner surface was coated with Teflon film to avoid adsorption) | NR | NH4+-TiO2 (3.93 mg/cm2) | MT/86% | [132] |
| 25 °C/NR | Pilot-scale | 2008 | UV-C (185 + 254 nm) | 1.5 | 55.5 | Tubular photoreactor made from PTFE with quartz windows on its top for UV transmission | 37 s | P25 (NR) | MT/85.8% ET/76.6% DMS/77.4% BM/87.6% AA/58.8% PA/64.7% BA/61.2% iso-VA/55.4% p-cresol/73.4% | [133] |
| 33–35 °C/NR | Pilot-scale | 2020 | UV-A (340–400 nm, peak at 365 nm) | ~28 | ~50 | Quartz tubular photoreactor surrounded by UV lamps | 1.8 s | Anatase-TiO2 (NR) | DMDS/~50% DMTS/~99% Decane/~20–50% | [134] |
| ~28 | ~169.4 | 6.1 s | DMDS/80–100% DMTS/~99% p-cresol/~99% Decane/~90% | |||||||
| 4.8 | 26.4–30.24 | 5.5–6.3 s | DMDS/~90% DMTS/~90% Decane/2% | |||||||
| 10.8 | 59.4–68.04 | 5.5–6.3 s | DMDS/80–100% DMTS/~99% p-cresoll/~95% Decane/30–60% | |||||||
| 28.8 | 158.4–181.44 | 5.5–6.3 s | DMDS/80–100% DMTS/~99% p-cresol/~99% Decane/~80–95% |
| Wavelength (nm) | Fitting Range I (mW∙cm−2) | kK (mol m−2 s) | n |
|---|---|---|---|
| 357 | 0–1.0 | 3.51 × 10−8 | 0.78 |
| 1.0–2.5 | 3.47 × 10−8 | 0.16 | |
| 375 | 0–1.0 | 3.02 × 10−8 | 0.69 |
| 1.0–2.5 | 3.00 × 10−8 | 0.20 | |
| 385 | 0–2.5 | 1.16 × 10−8 | 0.75 |
| 402 | 0–2.5 | 4.12 × 10−8 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Sun, Q.; Yan, X.; Zhang, X.; Wang, X.; Wang, K. Review on Photocatalytic Applications for Deodorization in Livestock and Poultry Farms. Agriculture 2024, 14, 2216. https://doi.org/10.3390/agriculture14122216
Han D, Sun Q, Yan X, Zhang X, Wang X, Wang K. Review on Photocatalytic Applications for Deodorization in Livestock and Poultry Farms. Agriculture. 2024; 14(12):2216. https://doi.org/10.3390/agriculture14122216
Chicago/Turabian StyleHan, Dongxuan, Qinqin Sun, Xiaojie Yan, Ximing Zhang, Xiaoshuai Wang, and Kaiying Wang. 2024. "Review on Photocatalytic Applications for Deodorization in Livestock and Poultry Farms" Agriculture 14, no. 12: 2216. https://doi.org/10.3390/agriculture14122216
APA StyleHan, D., Sun, Q., Yan, X., Zhang, X., Wang, X., & Wang, K. (2024). Review on Photocatalytic Applications for Deodorization in Livestock and Poultry Farms. Agriculture, 14(12), 2216. https://doi.org/10.3390/agriculture14122216







