Application of a Portable Chlorophyll Meter to Assess the Nitrogen Sufficiency Index and Nitrogen Requirements in Sweet Potatoes
Abstract
1. Introduction
2. Materials and Methods
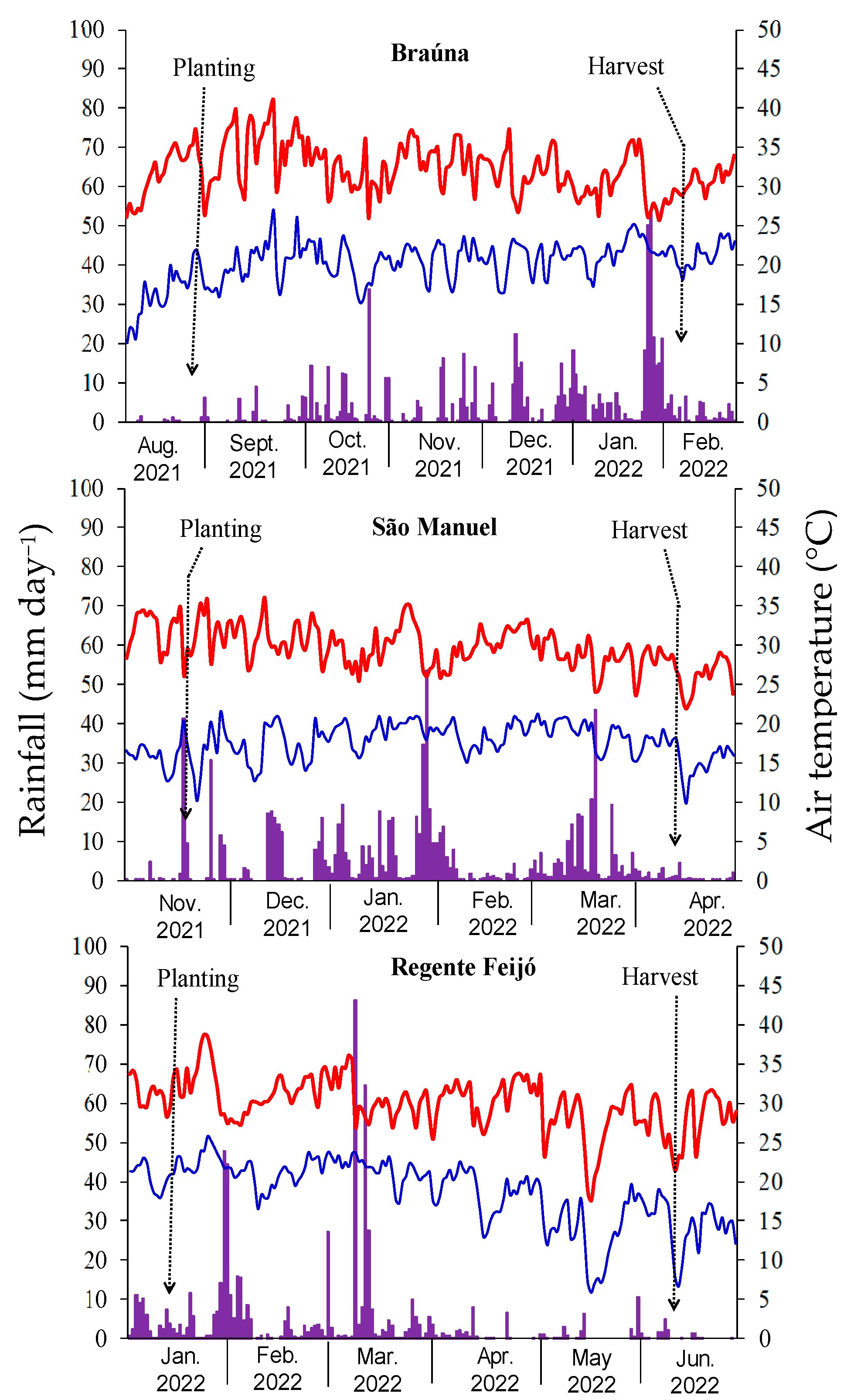
2.1. Site, Climate, and Soil
2.2. Experimental Design and Treatment
2.3. Sweet Potato Planting and Management
2.4. Plant Sampling and Analyses
2.5. Data Analyses
3. Results
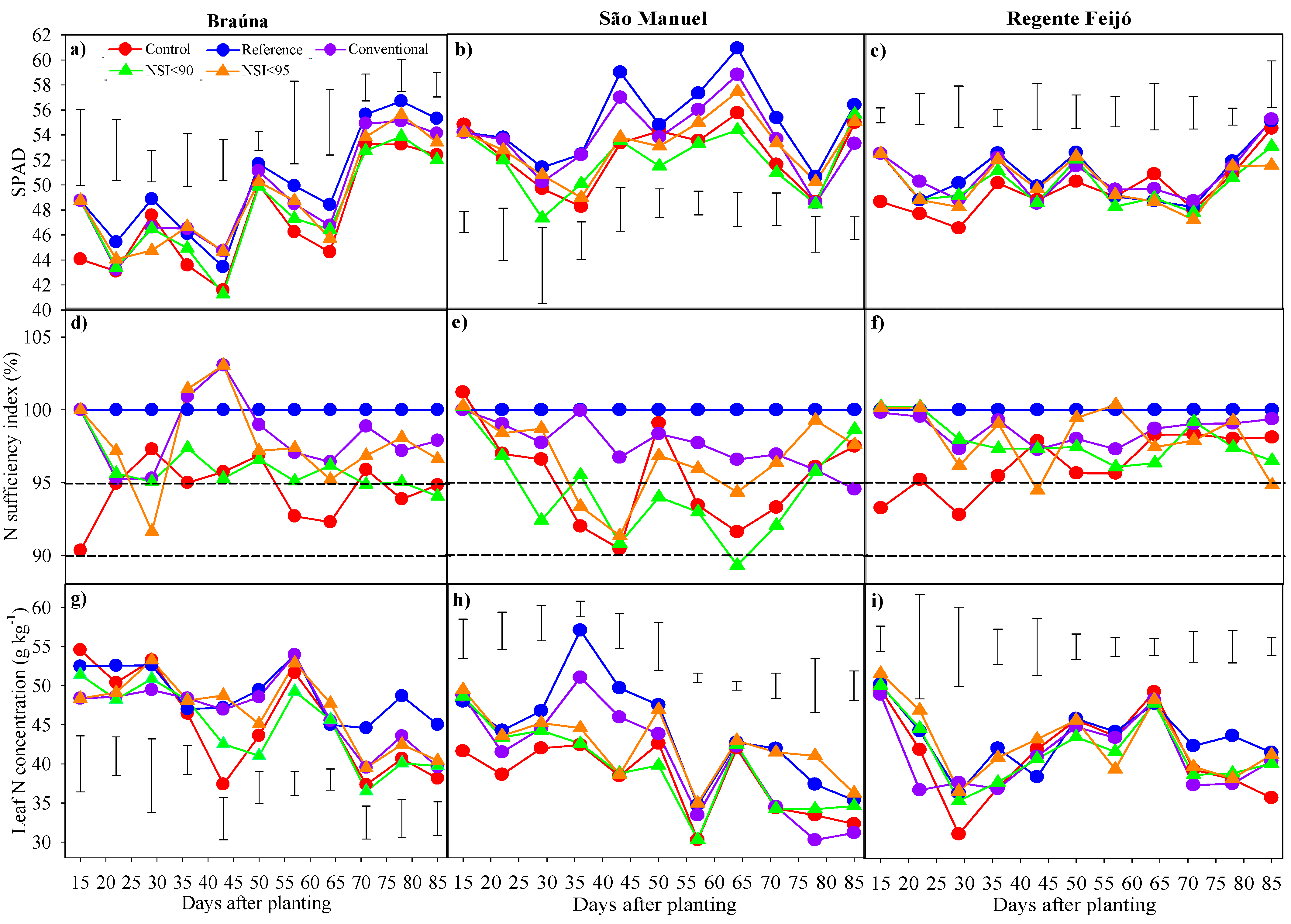
3.1. SPAD Index and N Status Monitoring in Leaves
3.2. Biomass Accumulation and N Uptake and Removal
3.3. Yield Components and Storage Root and Starch Yields
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, S.K.; Chattopadhyay, A.; Chakraborty, I.; Bhattacharya, I. Crops that feed the world 5. Sweetpotato. Sweetpotatoes for income and food security. Food Secur. 2011, 3, 283–305. [Google Scholar] [CrossRef]
- Adu-Kwarteng, E.; Baafi, E.; Amoa-Owusu, A.; Okyere, F.; Carey, E. Expanding industrial uses of sweetpotato for food security and poverty alleviation. Open Agric. 2021, 6, 382–391. [Google Scholar] [CrossRef]
- CIP, International Potato Center. Sweetpotato Processing and Uses. Available online: https://cipotato.org/sweetpotato/sweet-potato-processing-and-uses/ (accessed on 3 October 2024).
- FAOSTAT, Food and Agriculture Organization Corporate Statistical Database. Crops and Livestock Products: Sweet Potatoes. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 October 2024).
- IBGE—SIDRA, Instituto Brasileiro de Geografia e Estatística—Sistema de Recuperação Automática. Produção Agrícola Municipal. Available online: https://sidra.ibge.gov.br/tabela/5457 (accessed on 3 October 2024).
- Fernandes, A.M.; Ribeiro, N.P. Mineral nutrition and fertilization of sweet potato. Científica 2020, 48, 325–338. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Campos, L.G.; Senna, M.S.; Silva, C.L.; Assunção, N.S. Yield and nitrogen use efficiency of sweet potato in response to cover crop and nitrogen management. Agron. J. 2018, 110, 2004–2015. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Assunção, N.S.; Ribeiro, N.P.; Gazola, B.; Silva, R.M. Nutrient uptake and removal by sweet potato fertilized with green manure and nitrogen on sandy soil. Rev. Bras. Ciênc. Solo. 2020, 44, e0190127. [Google Scholar] [CrossRef]
- Ribeiro, N.P.; Fernandes, A.M.; Silva, R.M.; Pelvine, R.A.; Assunção, N.S. Growth and yield of sweet potato in response to the application of nitrogen rates and paclobutrazol. Bragantia 2021, 80, e3821. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 858. [Google Scholar]
- Echer, F.R.; Dominato, J.C.; Creste, J.E. Absorção de nutrientes e distribuição da massa fresca e seca entre órgãos de batata-doce. Hortic. Bras. 2009, 27, 176–182. [Google Scholar] [CrossRef]
- Hill, W.A. Nitrogen fertility and uses of sweet potato—Past, present, and future. In Proceedings of the Colloquium on Science in a Changing Agriculture, Urbana, IL, USA, 15 April 1982; University of Illinois: Urbana, IL, USA, 1982; pp. 89–112. [Google Scholar]
- Phillips, S.B.; Warren, J.G.; Mullins, G.L. Nitrogen rate and application timing affect ‘Beauregard’ sweetpotato yield and quality. HortScience 2005, 40, 2014–2017. [Google Scholar] [CrossRef]
- Yonebayashi, K.; Katsumi, N.; Nishi, T.; Okazaki, M. Activation of nitrogen-fixing endophytes is associated with the tuber growth of sweet potato. Mass Spectrom. 2014, 3, A0032. [Google Scholar] [CrossRef]
- Ueda, R.; Yano, K. Endophytic N2 fixation in sweet potato: Responses to N, P, and K inputs and visualization of 15N2 utilizing bacterial cells via Raman spectroscopy. Biol. Fertil. Soils 2023, 59, 275–283. [Google Scholar] [CrossRef]
- Okpara, D.A.; Okon, O.E.; Ekeleme, F. Optimizing nitrogen fertilization for production of white and orange-fleshed sweet potato in Southeast Nigeria. J. Plant Nutr. 2009, 32, 878–891. [Google Scholar] [CrossRef]
- Dong, H.T.; Li, Y.; Henderson, C.; Brown, P.; Xu, C.-Y. Optimum nitrogen application promotes sweetpotato storage root initiation. Horticulturae 2022, 8, 710. [Google Scholar] [CrossRef]
- Peng, S. Adjustment for specific leaf weight improves chlorophyll meter estimate of rice leaf nitrogen concentration. Agron. J. 1993, 85, 987–990. [Google Scholar] [CrossRef]
- Hussain, F.; Bronson, F.K.; Yadvinder, S.; Singh, B.; Peng, S. Use of chlorophyll meter sufficiency indices for nitrogen management of irrigated rice in Asia. Agron. J. 2000, 92, 875–879. [Google Scholar] [CrossRef]
- Argenta, G.; Silva, P.R.F.; Fosthofer, E.L.; Strieder, M.L.; Suhre, E.; Teichmann, L.L. Adubação nitrogenada em milho pelo monitoramento do nível de nitrogênio na planta por meio do clorofilômetro. Rev. Bras. Ciênc. Solo 2003, 27, 109–119. [Google Scholar] [CrossRef]
- Carvalho, M.A.C.; Furlani Junior, E.; Arf, O.; Sá, M.E.; Paulino, H.B.; Buzetti, S. Doses e épocas de aplicação de nitrogênio e teores foliares deste nutriente e de clorofila em feijoeiro. Rev. Bras. Ciênc. Solo 2003, 27, 445–450. [Google Scholar] [CrossRef]
- Furlani Junior, E.; Nakagawa, J.; Bulhoes, L.J.; Moreira, J.Á.A.; Grassi Filho, H. Correlação entre leituras de clorofila e níveis de nitrogênio aplicados em feijoeiro. Bragantia 1996, 55, 171–175. [Google Scholar] [CrossRef]
- Nunes, J.C.S.; Araújo, E.F.; Souza, C.M.; Bertini, L.A.; Ferreira, F.A. Efeito da palhada de sorgo localizada na superfície do solo em características de plantas de soja e milho. Rev. Ceres 2003, 50, 115–126. [Google Scholar]
- Schadchina, T.M.; Dmitrieva, V.V. Leaf chlorophyll content as a possible diagnostic mean for the evaluation of plant nitrogen uptake from the soil. J. Plant Nutr. 1995, 18, 1427–1437. [Google Scholar] [CrossRef]
- Piekielek, W.P.; Fox, R.H. Use of a chlorophyll meter to predict sidedress nitrogen requirements for maize. Agron. J. 1992, 84, 59–65. [Google Scholar] [CrossRef]
- Smeal, D.; Zhang, H. Chlorophyll meter evaluation for nitrogen management in corn. Commun. Soil Sci. Plant Anal. 1994, 25, 1495–1503. [Google Scholar] [CrossRef]
- Barbosa Filho, M.P.; Cobucci, T.; Fageria, N.K.; Mendes, P.N. Timing of nitrogen application on irrigated common bean measured by portable sensor. Ciênc. Agrotec. 2009, 33, 425–431. [Google Scholar] [CrossRef]
- Maia, S.C.M.; Soratto, R.P.; Nastaro, B.; Freitas, L.B. The Nitrogen sufficiency index underlying estimates of nitrogen fertilization requirements of common bean. Rev. Bras. Ciênc. Solo 2012, 36, 183–192. [Google Scholar] [CrossRef]
- Fernandes, F.M.; Soratto, R.P.; Fernandes, A.M.; Souza, E.F.C. Chlorophyll meter-based leaf nitrogen status to manage nitrogen in tropical potato production. Agron. J. 2021, 113, 1733–1746. [Google Scholar] [CrossRef]
- Silveira, P.M.; Gonzaga, A.C.O. Portable chlorophyll meter can estimate the nitrogen sufficiency index and levels of topdressing nitrogen in common bean. Pesqu. Agropec. Trop. 2017, 47, 1–6. [Google Scholar] [CrossRef]
- Varvel, G.E.; Schepers, J.S.; Francis, D.D. Ability for in-season correction of nitrogen deficiency in corn using chlorophyll meters. Soil Sci. Soc. Am. J. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- Koshy, A.M.; Joseph, V.; Ravi, V.; Byju, G. Rapid method for estimation of total chlorophyll, chlorophyll a and b and carotene content in leaves of cassava and sweet potato using SPAD meter. J. Root Crops. 2018, 44, 37–40. [Google Scholar]
- Sirabis, W.C.L.; Kantar, M.B.; Radovich, T.; Lincoln, N.K. Nitrogen dynamics and sweet potato production under indigenous soil moisture conservation practices in the leeward Kohala field system, Hawai’i Island. Soil Syst. 2022, 6, 16. [Google Scholar] [CrossRef]
- Darko, C.; Yeboah, S.; Amoah, A.; Opoku, A.; Berchie, J.N. Productivity of sweet potato (Ipomoea batatas (L) Lam) as influenced by fertilizer application in different agro-ecologies in Ghana. Sci. Afr. 2020, 10, e00560. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Jin, M.; Bai, J.; Shu, X.; Deng, L.; Wang, S.; Zhu, Y.; Liu, Y.; Lu, G.; et al. A new curve of critical leaf nitrogen concentration based on the maximum root dry matter for diagnosing nitrogen nutritional status of sweetpotato. Eur. J. Agron. 2024, 156, 127176. [Google Scholar] [CrossRef]
- Bohman, B.J.; Rosen, C.J.; Mulla, D.J. Evaluation of variable rate nitrogen and reduced irrigation management for potato production. Agron. J. 2019, 111, 2005–2017. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S. Use of a chlorophyll meter to monitor nitrogen status and schedule fertigation for corn. J. Prod. Agric. 1995, 8, 56–60. [Google Scholar] [CrossRef]
- Waskom, R.M.; Westfall, D.G.; Spellman, D.E.; Soltanpour, P.N. Monitoring nitrogen status of corn with a portable chlorophyll meter. Commun. Soil Sci. Plant Anal. 1996, 27, 545–560. [Google Scholar] [CrossRef]
- van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Lorenzi, J.O.; Monteiro, D.A.; Miranda Filho, H.S.; van Raij, B. Raízes e tubérculos. In Boletim 100: Recomendações de Adubação e Calagem para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 221–229. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1997. [Google Scholar]
- Nunes, J.G.S.; Leonel, M.; Fernandes, A.M.; Nunes, J.G.S.; Figueiredo, R.T.; Silva, J.A.; Menegucci, N.C. Yield and nutritional composition of sweet potatoes storage roots in response to cultivar, growing season and phosphate fertilization. Ciênc. Rural. 2024, 55, e20240046. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–390. [Google Scholar] [CrossRef]
- Cereda, M.P.; Vilpoux, O.; Takahashi, M. Balança hidrostática como forma de avaliação do teor de massa seca e amido. In Tecnologia, Usos e Potencialidade de Tuberosas Amiláceas Latino-Americanas; Cereda, M.P., Vilpoux, O., Eds.; Fundação Cargil: São Paulo, Brazil, 2003; pp. 30–47. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Cienc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Fontes, P.C.R.; Araújo, C. Adubações Nitrogenada de Hortaliças: Princípios e Práticas com o Tomateiro; Editora UFV: Viçosa, Brazil, 2007. [Google Scholar]
- Silva, M.C.C.; Fontes, P.C.R.; Miranda, G.V. Índice SPAD e produção de batata, em duas épocas de plantio, em função de doses de nitrogênio. Hortic. Bras. 2009, 27, 17–22. [Google Scholar] [CrossRef]
- Coelho, F.S.; Fontes, P.C.R.; Finger, F.L.; Cecon, P.R.; Silva, I.R. Valor e predição do nível crítico de índices para avaliar o estado nitrogenado da batateira. Rev. Ciênc. Agron. 2013, 44, 115–122. [Google Scholar] [CrossRef]
- Feltran, J.C.; Peresin, V.A.; Granja, N.P.; Silva Filho, H.M.; Lorenzi, J.O.; Fernandes, A.M.; Soratto, R.P.; Factor, T.L.; Rós, A.B.; Aguiar, E.B. Raízes e tubérculos. In Boletim 100: Recomendações de Adubação e Calagem Para o Estado de São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., van Raij, B., Eds.; Instituto Agronômico: Campinas, Brazil, 2022; pp. 314–338. [Google Scholar]
- Hartemink, A.E.; Johnston, M.; O’Sullivan, J.N.; Poloma, S. Nitrogen use efficiency of taro and sweet potato in the humid lowlands of Papua New Guinea. Agric. Ecosyst. Environ. 2000, 79, 271–280. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Moura, M.F.; Nogueira, D.H.; Chagas, N.G.; Braz, M.S.S.; Oliveira, M.R.T.; Barbosa, J.A. Yield of sweet potato roots in function of nitrogen levels applied in soil and foliating. Hortic. Bras. 2006, 24, 279–282. [Google Scholar] [CrossRef]
- Alves, A.U.; Oliveira, A.P.; Alves, E.U.; Oliveira, A.N.P.; Cardoso, E.A.; Matos, B.F. Manejo da adubação nitrogenada para a batata-doce: Fontes e parcelamento de aplicação. Ciênc. Agrotec. 2009, 33, 1554–1559. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Santos, J.F.; Cavalcante, L.F.; Pereira, W.E.; Santos, M.C.C.A.; Oliveira, A.N.P.; Silva, N.V. Yield of sweet potato fertilized with cattle manure and biofertilizer. Hortic. Bras. 2010, 28, 277–281. [Google Scholar] [CrossRef]
- Santos Neto, A.R.; Silva, T.O.; Blank, A.F.; Silva, J.O.; Araújo Filho, R.N. Yield of sweet potato clones in response to nitrogen doses. Hortic. Bras. 2017, 35, 445–452, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef][Green Version]
- Akoetey, W.; Britain, M.M.; Morawicki, R.O. Potential use of byproducts from cultivation and processing of sweet potatoes. Ciênc. Rural. 2017, 47, e20160610. [Google Scholar] [CrossRef]
- Zotarelli, L.; Scholberg, J.M.; Dukes, M.D.; Muñoz-Carpena, R. Monitoring of nitrate leaching in sandy soils: Comparison of three methods. J. Environ. Qual. 2007, 36, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecu. Tropl. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Souza, E.F.C.; Rosen, C.J.; Venterea, R.T. Co-application of DMPSA and NBPT with urea mitigates both nitrous oxide emissions and nitrate leaching during irrigated potato production. Environ. Pollut. 2021, 284, 117124. [Google Scholar] [CrossRef]

| Soil Properties 1 | Sites | ||
|---|---|---|---|
| Braúna | São Manuel | Regente Feijó | |
| pH (CaCl2) | 5.0 | 5.4 | 5.1 |
| SOM (g dm−3) | 6.3 | 11.0 | 12.0 |
| P resin (mg dm−3) | 7.2 | 9.0 | 9.0 |
| K (mmolc dm−3) | 1.2 | 1.8 | 1.5 |
| Ca (mmolc dm –3) | 18.4 | 21.0 | 12.0 |
| Mg (mmolc dm−3) | 8.1 | 9.0 | 4.0 |
| H + Al (mmolc dm−3) | 13.3 | 19.0 | 16.0 |
| CEC (mmolc dm−3) | 40.9 | 51.0 | 33.0 |
| Base saturation (%) | 67.6 | 62.0 | 52.0 |
| S (mg dm−3) | 4.5 | 2.0 | 9.0 |
| B (mg dm−3) | 0.4 | 0.3 | 0.1 |
| Cu (mg dm−3) | 0.5 | 1.3 | 0.6 |
| Fe (mg dm−3) | 9.0 | 7.0 | 15.0 |
| Mn (mg dm−3) | 4.7 | 5.3 | 6.2 |
| Zn (mg dm−3) | 0.2 | 1.2 | 0.3 |
| N Management | Treatments 1 | ||||
|---|---|---|---|---|---|
| Control | Reference | Conventional | NSI < 90 | NSI < 95 | |
| Braúna | |||||
| Application time | - | Planting, 15, 29, 57 DAP | Planting, 29 DAP | Planting | Planting, 29 DAP |
| N rate per each time | 0 | 17, 43, 45, 45 | 17, 33 | 17 | 17, 36 |
| Total N rate | 0 | 150 | 50 | 17 | 53 |
| São Manuel | |||||
| Application time | - | Planting, 15, 29, 57 DAP | Planting, 29 DAP | Planting, 64 DAP | Planting, 36, 43, 64 DAP |
| N rate per each time | 0 | 17, 43, 45, 45 | 17, 33 | 17, 11 | 17, 16, 44, 6 |
| Total N rate | 0 | 150 | 50 | 28 | 83 |
| Regente Feijó | |||||
| Application time | - | Planting, 15, 29, 57 DAP | Planting, 29 DAP | Planting | Planting, 42 DAP |
| N rate per each time | 0 | 17, 43, 45, 45 | 17, 33 | 17 | 17, 2 |
| Total N rate | 0 | 150 | 50 | 17 | 19 |
| Site | Control 1 | Reference | Conventional | NSI | F Probability | |
|---|---|---|---|---|---|---|
| <90 | <95 | |||||
| Shoot biomass (Mg ha−1) | ||||||
| Braúna | 4.0 ± 0.61 b 1 | 4.8 ± 0.76 a | 4.1 ± 0.72 b | 3.7 ± 0.39 b | 4.0 ± 0.48 b | 0.030 |
| São Manuel | 1.9 ± 0.62 a | 2.1 ± 0.27 a | 1.9 ± 0.45 a | 2.2 ± 0.72 a | 1.8 ± 0.47 a | NS 2 |
| Regente Feijó | 2.7 ± 0.66 a | 3.1 ± 0.65 a | 3.0 ± 0.40 a | 2.8 ± 0.50 a | 3.1 ± 0.73 a | NS |
| Storage root biomass (Mg ha−1) | ||||||
| Braúna | 8.5 ± 0.96 ab | 7.2 ± 1.24 b | 8.5 ± 0.98 ab | 9.2 ± 0.48 a | 9.4 ± 1.07 a | 0.050 |
| São Manuel | 7.2 ± 1.15 b | 8.2 ± 1.03 ab | 9.5 ± 1.25 a | 7.9 ± 0.74 ab | 8.3 ± 1.42 ab | 0.050 |
| Regente Feijó | 10.5 ± 0.96 ab | 9.6 ± 1.27 b | 10.2 ± 1.01 ab | 11.0 ± 0.83 a | 10.0 ± 0.76 ab | 0.050 |
| Whole plant biomass (Mg ha−1) | ||||||
| Braúna | 12.5 ± 1.02 a | 12.0 ± 1.15 a | 12.5 ± 1.16 a | 12.8 ± 0.42 a | 13.3 ± 1.14 a | NS |
| São Manuel | 9.1 ± 1.23 b | 10.3 ± 1.02 ab | 11.4 ± 1.32 a | 10.1 ± 0.72 ab | 10.1 ± 1.42 ab | 0.050 |
| Regente Feijó | 13.5 ± 0.81 a | 12.8 ± 1.16 a | 12.9 ± 1.0 a | 13.8 ± 0.79 a | 13.1 ± 0.55 a | NS |
| N uptake (kg ha−1) | ||||||
| Braúna | 92 ± 3.6 c | 151 ± 3.6 a | 129 ± 5.0 ab | 118 ± 2.0 bc | 148 ± 3.9 a | 0.002 |
| São Manuel | 56 ± 2.8 c | 98 ± 4.1 a | 77 ± 2.5 b | 64 ± 3.2 bc | 81 ± 3.4 b | 0.002 |
| Regente Feijó | 142 ± 4.0 a | 136 ± 3.7 a | 143 ± 4.4 a | 151 ± 3.5 a | 149 ± 5.8 a | NS |
| N removal (kg ha−1) | ||||||
| Braúna | 41 ± 2.5 a | 54 ± 3.0 a | 55 ± 3.7 a | 46 ± 1.8 a | 54 ± 2.7 a | NS |
| São Manuel | 29 ± 1.6 c | 68 ± 4.0 a | 53 ± 2.9 ab | 38 ± 2.7 bc | 53 ± 3.4 ab | 0.003 |
| Regente Feijó | 108 ± 4.6 a | 105 ± 4.3 a | 112 ± 4.2 a | 119 ± 3.3 a | 115 ± 5.3 a | NS |
| Site | Control 1 | Reference | Convential | NSI | F Probability | |
|---|---|---|---|---|---|---|
| <90 | <95 | |||||
| Total storage root number (no. pl−1) | ||||||
| Braúna | 7.4 ± 1.01 ab | 7.2 ± 0.80 b | 8.1 ± 0.60 ab | 8.3 ± 0.64 a | 8.3 ± 0.80 a | 0.050 |
| São Manuel | 6.3 ± 0.78 b | 6.6 ± 0.52 b | 7.5 ± 0.51 a | 6.7 ± 0.80 ab | 7.0 ± 0.73 ab | 0.046 |
| Regente Feijó | 11.1 ± 0.90 b | 11.9 ± 0.86 ab | 11.3 ± 0.92 ab | 12.5 ± 0.51 a | 11.5 ± 1.23 ab | 0.048 |
| Marketable storage root number (no. pl−1) | ||||||
| Braúna | 3.2 ± 0.86 b | 3.0 ± 0.82 b | 3.4 ± 0.77 ab | 3.5 ± 0.68 ab | 3.8 ± 0.76 a | 0.049 |
| São Manuel | 1.6 ± 0.50 b | 2.1 ± 0.60 a | 2.2 ± 0.44 a | 2.0 ± 0.76 ab | 1.8 ± 0.43 ab | 0.047 |
| Regente Feijó | 4.1 ± 0.88 ab | 3.5 ± 0.94 b | 4.2 ± 0.83 ab | 4.6 ± 0.53 a | 4.5 ± 1.09 ab | 0.045 |
| Storage root mean weight (g root−1) | ||||||
| Braúna | 252 ± 5.9 a | 236 ± 5.5 a | 251 ± 6.0 a | 246 ± 4.6 a | 243 ± 5.0 a | NS 2 |
| São Manuel | 204 ± 4.6 a | 215 ± 5.9 a | 220 ± 5.0 a | 212 ± 3.1 a | 204 ± 5.7 a | NS |
| Regente Feijó | 188 ± 4.5 a | 172 ± 4.3 a | 186 ± 4.5 a | 274 ± 2.5 a | 177 ± 4.7 a | NS |
| Total storage root yield (Mg ha−1) | ||||||
| Braúna | 38 ± 1.42 ab | 35 ± 2.19 b | 42 ± 2.65 a | 42 ± 1.50 ab | 42 ± 1.67 ab | 0.050 |
| São Manuel | 27 ± 2.26 b | 30 ± 2.11 b | 35 ± 2.10 a | 30 ± 1.31 b | 30 ± 1.92 b | 0.050 |
| Regente Feijó | 41.6 ± 1.84 a | 42.2 ± 2.46 a | 41.1 ± 1.41 a | 43.7 ± 1.54 a | 41.2 ± 1.62 a | NS |
| Marketable storage root yield (Mg ha−1) | ||||||
| Braúna | 22 ± 0.75 b | 23 ± 1.88 b | 27 ± 2.30 ab | 26 ± 2.51 ab | 28 ± 1.05 a | 0.047 |
| São Manuel | 13 ± 1.74 a | 15 ± 1.40 a | 16 ± 1.62 a | 15 ± 1.70 a | 12 ± 1.87 a | NS |
| Regente Feijó | 26 ± 1.67 a | 25 ± 2.12 a | 26 ± 1.14 a | 28 ± 1.39 a | 28 ± 1.88 a | NS |
| Storage root DM content (% fresh weight) | ||||||
| Braúna | 22.1 ± 1.29 ab | 20.5 ± 1.21 ab | 20.1 ± 1.34 b | 22.5 ± 0.66 a | 21.8 ± 1.25 ab | 0.050 |
| São Manuel | 26.2 ± 0.43 a | 27.1 ± 1.16 a | 27.4 ± 1.37 a | 26.8 ± 1.78 a | 27.4 ± 1.23 a | NS |
| Regente Feijó | 25.3 ± 0.62 a | 23.4 ± 1.47 a | 24.2 ± 1.32 a | 25.3 ± 0.76 a | 24.3 ± 1.10 a | NS |
| Storage root starch content (% fresh weight) | ||||||
| Braúna | 13.2 ± 1.21 a | 13.1 ± 1.22 a | 13.1 ± 1.18 a | 14.5 ± 1.01 a | 13.8 ± 1.59 a | NS |
| São Manuel | 19.6 ± 0.67 a | 19.3 ± 1.01 a | 20.7 ± 1.23 a | 20.0 ± 1.57 a | 19.6 ± 1.08 a | NS |
| Regente Feijó | 18.8 ± 1.19 a | 17.5 ± 1.64 a | 18.4 ± 0.93 a | 19.4 ± 1.16 a | 18.3 ± 1.52 a | NS |
| Starch yield (Mg ha−1) | ||||||
| Braúna | 5.1 ± 0.72 a | 4.6 ± 0.91 a | 5.6 ± 1.03 a | 6.1 ± 0.87 a | 5.8 ± 1.19 a | NS |
| São Manuel | 5.4 ± 0.97 b | 5.8 ± 0.96 b | 7.3 ± 0.79 a | 6.0 ± 0.73 b | 5.9 ± 0.92 b | 0.035 |
| Regente Feijó | 7.8 ± 0.62 a | 7.2 ± 1.10 a | 7.8 ± 0.7 a | 8.5 ± 0.90 a | 7.6 ± 1.00 a | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.E.; Fernandes, A.M.; Oliveira, A.V.; Vargas, P.F.; Souza, E.F.C.; Guedes, P.T.P.; Figueiredo, R.T.; Guimarães, Í.T. Application of a Portable Chlorophyll Meter to Assess the Nitrogen Sufficiency Index and Nitrogen Requirements in Sweet Potatoes. Agriculture 2024, 14, 2167. https://doi.org/10.3390/agriculture14122167
Rodrigues FE, Fernandes AM, Oliveira AV, Vargas PF, Souza EFC, Guedes PTP, Figueiredo RT, Guimarães ÍT. Application of a Portable Chlorophyll Meter to Assess the Nitrogen Sufficiency Index and Nitrogen Requirements in Sweet Potatoes. Agriculture. 2024; 14(12):2167. https://doi.org/10.3390/agriculture14122167
Chicago/Turabian StyleRodrigues, Fabrício E., Adalton M. Fernandes, Arthur V. Oliveira, Pablo F. Vargas, Emerson F. C. Souza, Politon T. P. Guedes, Ricardo T. Figueiredo, and Ítala T. Guimarães. 2024. "Application of a Portable Chlorophyll Meter to Assess the Nitrogen Sufficiency Index and Nitrogen Requirements in Sweet Potatoes" Agriculture 14, no. 12: 2167. https://doi.org/10.3390/agriculture14122167
APA StyleRodrigues, F. E., Fernandes, A. M., Oliveira, A. V., Vargas, P. F., Souza, E. F. C., Guedes, P. T. P., Figueiredo, R. T., & Guimarães, Í. T. (2024). Application of a Portable Chlorophyll Meter to Assess the Nitrogen Sufficiency Index and Nitrogen Requirements in Sweet Potatoes. Agriculture, 14(12), 2167. https://doi.org/10.3390/agriculture14122167






