Nitrogen Residual Effect of Winter Cover Crops on Maize in Uruguay: Conventional and Isotopic Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites Description
2.2. Treatments and Experimental Design
2.3. Soil and Plant Sampling
2.4. Sample Processing and Analytical Determinations
2.4.1. Soil Samples from Conventional and Isotopic Experiments
2.4.2. Plant Samples from Conventional and Isotopic Experiments
2.5. Statistical Analysis
3. Results
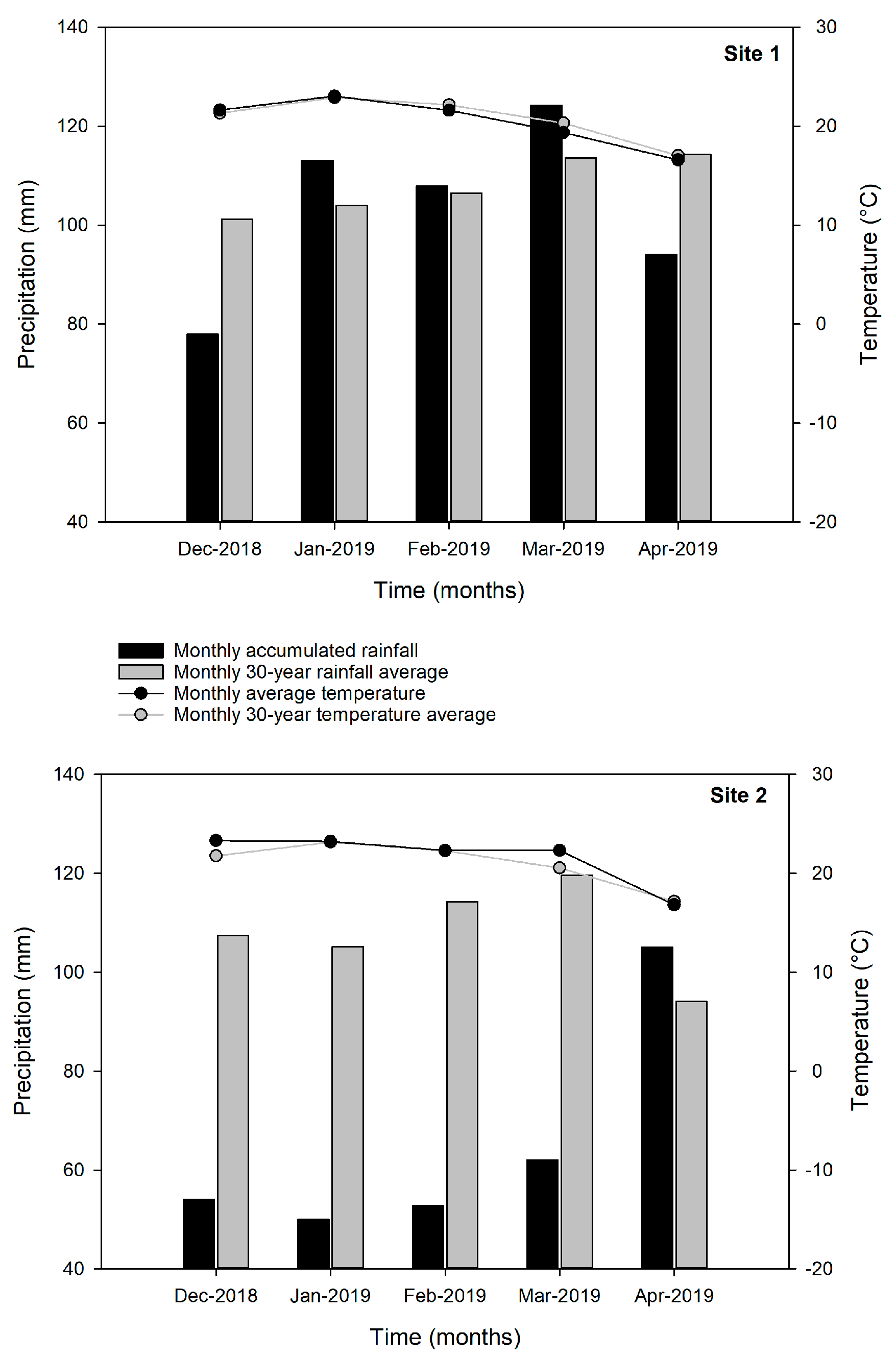
3.1. Weather Conditions During the Study Period
3.2. Phase 1: Aboveground Biomass Yield and Chemical and Biochemical Traits of CC
3.3. Phase 2: Conventional Experiment
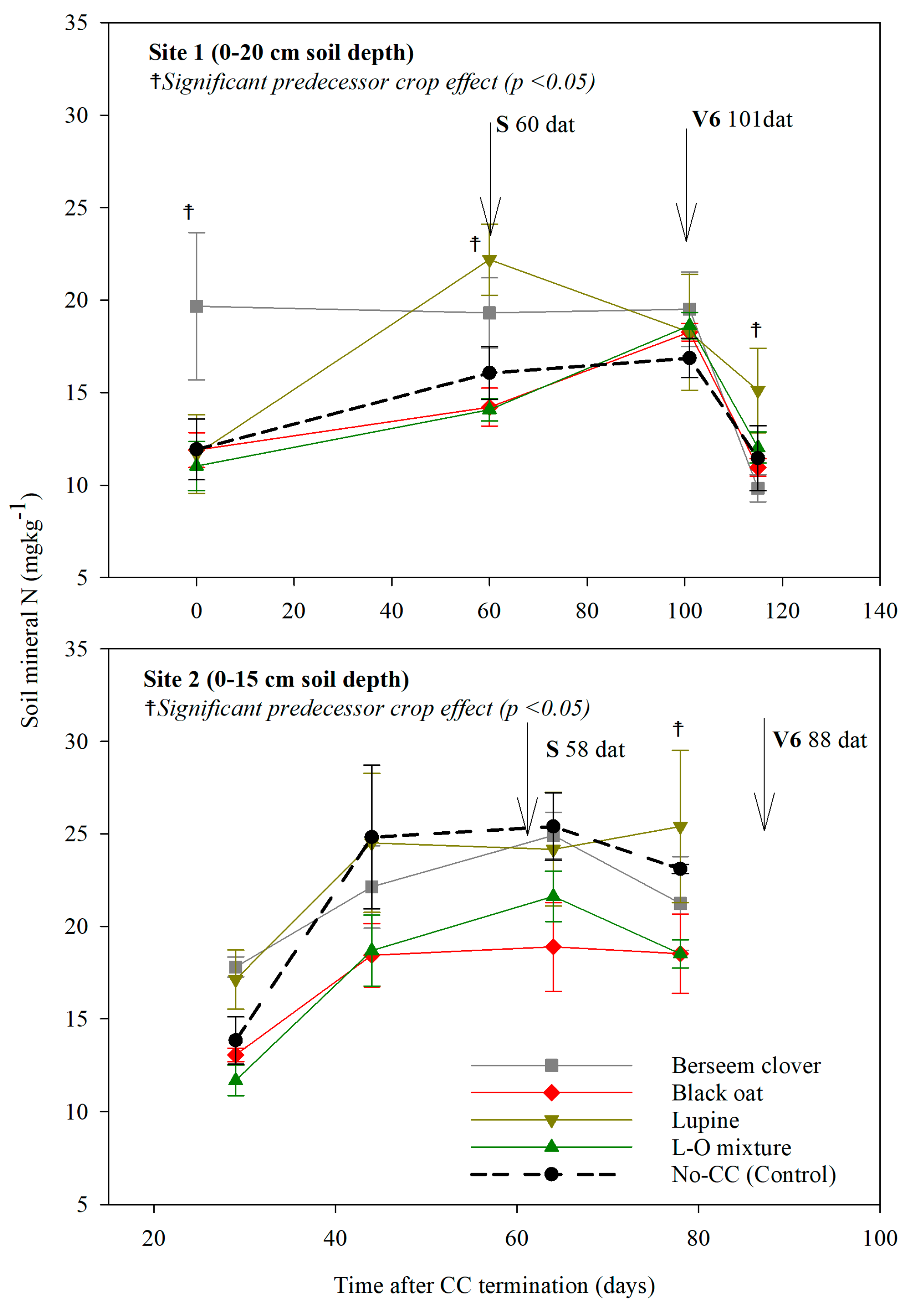
3.3.1. Soil Mineral N Dynamics After CC Termination
3.3.2. Grain Yield and Plant N Uptake by Maize Cropping
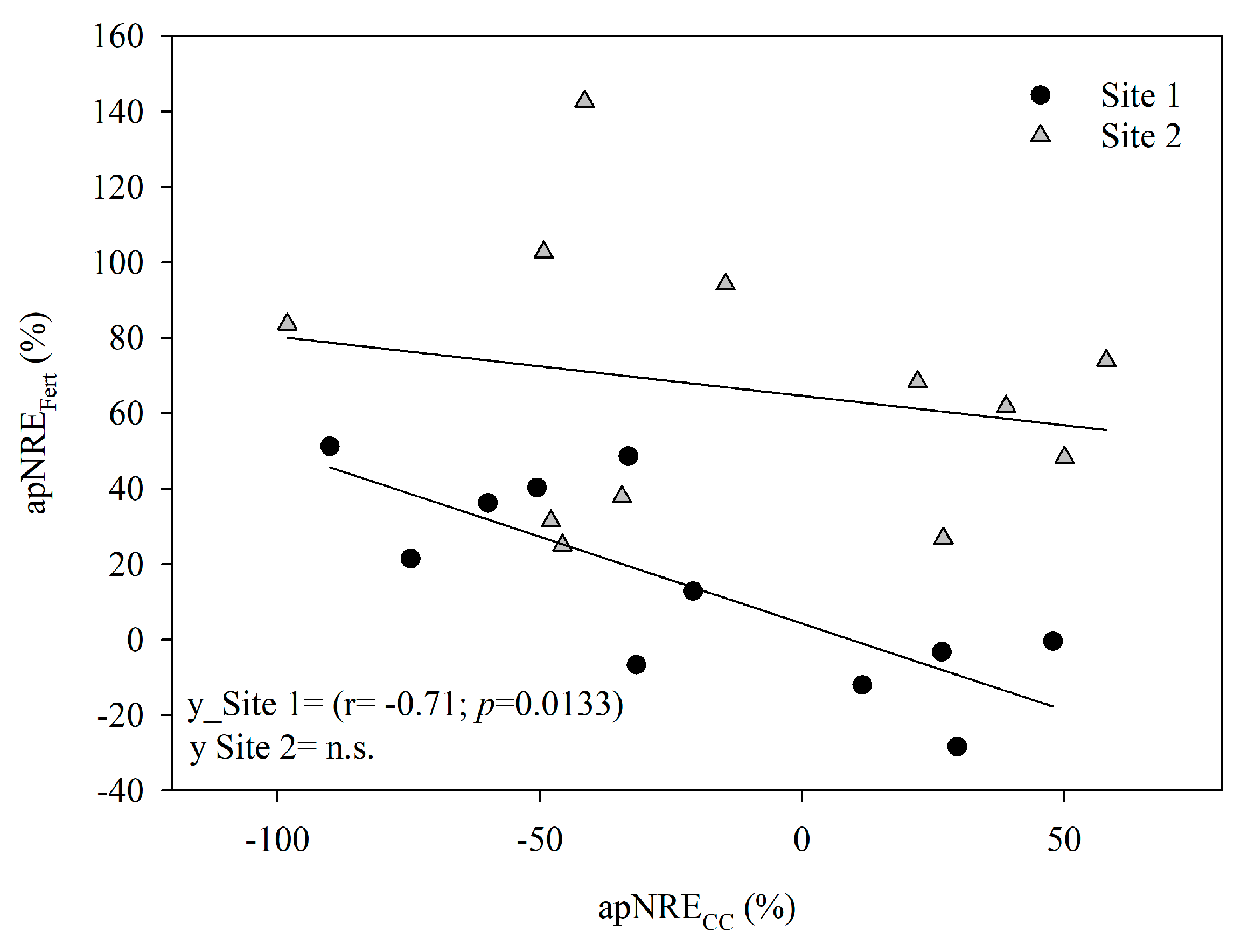
3.3.3. Apparent Nitrogen Recovery Efficiency from CCs and Fertilizer
3.4. Isotopic Experiment
3.4.1. Plant 15N Recovery in the Maize Cropping
3.4.2. Soil 15N Recovery After Corn Harvest
4. Discussion
4.1. Soil Mineral N Dynamic as Affected by Chemical and Biochemical Traits of CCs
4.2. Cover Crops Effects on Succeeding Maize Crop
4.3. Cover Crops Effects on Nitrogen Recovery Indices from Conventional and Isotopic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbeletche, P. Agribusiness in Uruguay: Evolution and Its Changeant Estrategies in the 20th Century. Rev. Iberoam. Vitic. Agroind. Rural. 2020, 7, 109–129. [Google Scholar] [CrossRef]
- Vassallo, M. Dinámica y Competencia Intrasectorial En La Agricultura Uruguaya: Los Cambios En La Última Década. Agrociencia Uruguay. 2013, 17, 170–179. [Google Scholar] [CrossRef]
- Ernst, O.R.; Dogliotti, S.; Cadenazzi, M.; Kemanian, A.R. Shifting Crop-Pasture Rotations to No-Till Annual Cropping Reduces Soil Quality and Wheat Yield. Field Crop. Res. 2018, 217, 180–187. [Google Scholar] [CrossRef]
- Rubio, V.; Quincke, A.; Ernst, O. Deep Tillage and Nitrogen Do Not Remediate Cumulative Soil Deterioration Effects of Continuous Cropping. Agron. J. 2021, 113, 5584–5596. [Google Scholar] [CrossRef]
- Pravia, M.V.; Kemanian, A.R.; Terra, J.A.; Shi, Y.; Macedo, I.; Goslee, S. Soil Carbon Saturation, Productivity, and Carbon and Nitrogen Cycling in Crop-Pasture Rotations. Agric. Syst. 2019, 171, 13–22. [Google Scholar] [CrossRef]
- Xie, H.; Huang, Y.; Chen, Q.; Zhang, Y.; Wu, Q. Prospects for Agricultural Sustainable Intensification: A Review of Research. Land 2019, 8, 157. [Google Scholar] [CrossRef]
- Rubio, V.; Núñez, A.; Berger, A.; van Es, H. Biomass Inputs Drive Agronomic Management Impacts on Soil Health. Agric. Ecosyst. Environ. 2024, 378, 109316. [Google Scholar] [CrossRef]
- Mazzilli, S.R.; Ernst, O.R. Rapeseed-to-Wheat Yield Ratio in Different Production Environments and Effects on Subsequent Summer Crops Yields. Agrosyst. Geosci. Environ. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Rossini, G.F.I. Soil and Subsoil Conservation from an Environmental Perspective. Rev. Derecho 2022, 21, 41–52. [Google Scholar] [CrossRef]
- Mazzilli, S.R.; Kemanian, A.R.; Ernst, O.R.; Jackson, R.B.; Piñeiro, G. Greater Humification of Belowground than Aboveground Biomass Carbon into Particulate Soil Organic Matter in No-Till Corn and Soybean Crops. Soil Biol. Biochem. 2015, 85, 22–30. [Google Scholar] [CrossRef]
- Ernst, O. Efecto De Una Leguminosa Invernal Como Cultivo de Cobertura Sobre Rendimiento En Grano y Respuesta a Nitrógeno DE Maíz Sembrado Sin Laboreo. Agrociencia Uruguay. 2006, 10, 25–35. [Google Scholar] [CrossRef]
- Constantin, J.; Mary, B.; Laurent, F.; Aubrion, G.; Fontaine, A.; Kerveillant, P.; Beaudoin, N. Effects of Catch Crops, No Till and Reduced Nitrogen Fertilization on Nitrogen Leaching and Balance in Three Long-Term Experiments. Agric. Ecosyst. Environ. 2010, 135, 268–278. [Google Scholar] [CrossRef]
- Prieto, G.S.; Ernst, O. Effect of Legume or Grass Cover Crops and Nitrogen Application Rate on Soil Properties and Corn Productivity. Agrociencia Uruguay. 2012, 16, 294–301. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using Cover Crops to Mitigate and Adapt to Climate Change. A Review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Iqbal, A.; Aslam, S.; Alavoine, G.; Benoit, P.; Garnier, P.; Recous, S. Rain Regime and Soil Type Affect the C and N Dynamics in Soil Columns that are Covered with Mixed-Species Mulches. Plant Soil 2015, 393, 319–334. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Randall, G.W. Estimating Nitrogen Budgets for Soil-Crop Systems. Manag. Nitrogen Groundw. Qual. Farm Profitab. 2015, 1, 85–124. [Google Scholar] [CrossRef]
- Moukanni, N.; Brewer, K.M.; Gaudin, A.C.M.; O’Geen, A.T. Optimizing Carbon Sequestration Through Cover Cropping in Mediterranean Agroecosystems: Synthesis of Mechanisms and Implications for Management. Front. Agron. 2022, 4, 844166. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A Critical Review of the Impacts of Cover Crops on Nitrogen Leaching, Net Greenhouse Gas Balance and Crop Productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Huang, W.; Chen, J.; Wu, F.; Jia, Y.; Han, Y.; Wang, G.; Feng, L.; Li, X.; et al. Cover Crops and N Fertilization Affect Soil Ammonia Volatilization and N2O Emission by Regulating the Soil Labile Carbon and Nitrogen Fractions. Agric. Ecosyst. Environ. 2022, 340, 108188. [Google Scholar] [CrossRef]
- Johnson, F.E.; Roth, R.T.; Ruffatti, M.D.; Armstrong, S.D. Cover Crop Impacts on Nitrogen Losses and Environmental Damage Cost. Agric. Ecosyst. Environ. 2024, 363, 108859. [Google Scholar] [CrossRef]
- Oberson, A.; Jarosch, K.A.; Frossard, E.; Hammelehle, A.; Fliessbach, A.; Mäder, P.; Mayer, J. Higher than Expected: Nitrogen Flows, Budgets, and Use Efficiencies Over 35 Years of Organic and Conventional Cropping. Agric. Ecosyst. Environ. 2024, 362, 108802. [Google Scholar] [CrossRef]
- Landriscini, M.R.; Galantini, J.A.; Martínez, J.M. Eficiencia de Recuperación de Nitrógeno Por Los Métodos de la Diferencia y de la Dilución Isotópica. Cienc. Suelo 2016, 34, 155–162. [Google Scholar]
- Ovalle, C.; del Pozo, A.; Peoples, M.B.; Lavín, A. Estimating the Contribution of Nitrogen From Legume Cover Crops to the Nitrogen Nutrition of Grapevines Using a 15N Dilution Technique. Plant Soil 2010, 334, 247–259. [Google Scholar] [CrossRef]
- Espinoza, S.; Ovalle, C.; Zagal, E.; Matus, I.; Tay, J.; Peoples, M.B.; Del Pozo, A. Contribution of Legumes to Wheat Productivity in Mediterranean Environments of Central Chile. Field Crop. Res. 2012, 133, 150–159. [Google Scholar] [CrossRef]
- Wang, J.; Sainju, U.M.; Zhang, S. A Mixture of Summer Legume and Nonlegume Cover Crops Enhances Winter Wheat Yield, Nitrogen Uptake, and Nitrogen Balance. Nitrogen 2024, 5, 871–890. [Google Scholar] [CrossRef]
- Sawchik, J.; Pérez-Bidegain, M.; García, C. Impact of Winter Cover Crops on Soil Properties Under Soybean Cropping Systems. Agrociencia Urug. 2012, 16, 288–293. [Google Scholar] [CrossRef]
- Sawchik, J.; Siri, G.; Ayala, W.; Barrios, E.; Bustamante, M.; Ceriani, M.; Gutierrez, F.; Mosqueira, J.; Otaño, C.; Perez, M.; et al. El Sistema Agrícola Bajo Amenaza: ¿Qué Aportanlos Cultivos de Cobertura y/o Laspasturas Cortas? IV Simp. Nac. Agric. 2015, 4, 149–168. [Google Scholar]
- Alvarez, S.; Ernst, O. Impact of Cropping Systems on Soil Quality. Eur. J. Agron. 2024, 158, 127197. [Google Scholar] [CrossRef]
- Durán, A.; Califra, A.; Molfino, J.H.; Lynn, W. Keys to Soil Taxonomy for Uruguay; USDA, Natural Resources Conservation Service (NRCS): Washington, DC, USA, 2006; p. 77.
- Durán, A.; Fernando, G.P. Suelos Del Uruguay, Origen, Clasificación, Manejo Y Conservación; Southern Hemisphere: Montevideo, Uruguay, 2007; Volume 2, ISBN 978-9974-674-02-8. [Google Scholar]
- Assefa, Y.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Smith, S.; Ciampitti, I.A. Analysis of Long Term Study Indicates Both Agronomic Optimal Plant Density and Increase Maize Yield per Plant Contributed to Yield Gain. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J. How a Corn Plant Develops; Iowa State University of Science and Technology: Ames, IA, USA, 1993; pp. 1–25. [Google Scholar]
- IAEA. Use of Nuclear Techniques in Studies of Soil-Plant Relationships. Training Course Series No 2; Hardarson, G., Ed.; International Atomic Energy Agency: Vienna, Austria, 1990; p. 223. [Google Scholar]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods; CSIRO Publishing: Clayton, Australia, 2011; ISBN 978-0-643-06768-4. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis; Page, A., Ed.; SSSA: Madison, WI, USA, 1983; pp. 159–165. [Google Scholar]
- Lajtha, K.; Driscoll, C.T.; Jarrell, W.M.; Elliott, E.T. Soil phosphorus: Characterization and total element analysis. In Standard Soil Methods for Long Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 115–143. ISBN 0-19-512083-3. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic Forms. In Methods of Soil Analysis, Part 3: Chemical Methods; Wiley: New Jersey, NJ, USA, 2018; pp. 1123–1184. ISBN 9780891188667. [Google Scholar]
- Rhine, E.D.; Mulvaney, R.L.; Pratt, E.J.; Sims, G.K. Improving the Berthelot Reaction for Determining Ammonium in Soil Extracts and Water. Soil Sci. Soc. Am. J. 1998, 62, 473–480. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis by Hydrometer: A Simplified Method for Routine Textural Analysis and a Sensitivity Test of Measurement Parameters. Soil Sci. Soc. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Van Cleemput, O.; Zapata, F.; Vanlauwe, B. Guidelines on Nitrogen Management in Agricultural Systems. In Guidelines on Nitrogen Management in Agricultural System; Soil Science Unit: Seibersdorf, Austria, 2008; pp. 19–125. [Google Scholar]
- Tamagno, S.; Eagle, A.J.; McLellan, E.L.; van Kessel, C.; Linquist, B.A.; Ladha, J.K.; Pittelkow, C.M. Quantifying N leaching losses as a function of N balance: A Path to Sustainable Food Supply Chains. Agric. Ecosyst. Environ. 2022, 324, 107714. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-Fixation in Field Settings: Estimations Based on Natural 15N Abundance. Aust. J. Plant Physiol. 1987, 13, 699–756. [Google Scholar] [CrossRef]
- Hardarson, G.; Danso, S.K.A. Methods for Measuring Biological Nitrogen Fixation in Grain Legumes. Plant Soil 1993, 152, 19–23. [Google Scholar] [CrossRef]
- Giménez, L. Producción de Maíz Con Estrés Hídrico Provocado En Diferentes Etapas de Desarrollo. Agrociencia 2012, 16, 92–102. [Google Scholar] [CrossRef]
- Soares, M.; Rousk, J. Microbial Growth and Carbon Use Efficiency in Soil: Links to Fungal-Bacterial Dominance, SOC-Quality and Stoichiometry. Soil Biol. Biochem. 2019, 131, 195–205. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen Mineralization from Organic Residues. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef]
- Chaves, B.; Redin, M.; Giacomini, S.J.; Schmatz, R.; Léonard, J.; Ferchaud, F.; Recous, S. The Combination of Residue Quality, Residue Placement AND Soil Mineral N Content Drives C and N Dynamics by Modifying N Availability to Microbial Decomposers. Soil Biol. Biochem. 2021, 163, 108434. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass Production and Carbon/Nitrogen Ratio Influence Ecosystem Services from Cover Crop Mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef]
- Sun, L.; Xia, Z.; Sang, C.; Wang, X.; Peng, B.; Wang, C.; Zhang, J.; Müller, C.; Bai, E. Soil Resource Status Affects the Responses of Nitrogen Processes to Changes in Temperature and Moisture. Biol. Fertil. Soils 2019, 55, 629–641. [Google Scholar] [CrossRef]
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crop. Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of High Nh+4 on k+ Uptake, Culm Mechanical Strength and Grain Filling IN Wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef] [PubMed]
- Dann, C.E.; Cabrera, M.L.; Thapa, R.; Mirsky, S.; Tully, K.; Reberg-Horton, C.; Hitchcock, R.; Morari, F. Modeling Water Potential of Cover Crop Residues on the Soil Surface. Ecol. Model. 2021, 459, 109708. [Google Scholar] [CrossRef]
- Guinet, M.; Nicolardot, B.; Revellin, C.; Durey, V.; Carlsson, G.; Voisin, A.S. Comparative Effect of Inorganic N on Plant Growth and N2 Fixation of Ten Legume Crops: Towards a Better Understanding of the Differential Response Among Species. Plant Soil 2018, 432, 207–227. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen Fixation in Annual and Perennial Legume-Grass Mixtures Across a Fertility Gradient. Plant Soil 2012, 357, 147–159. [Google Scholar] [CrossRef]
- Evans, J.; O’Connor, G.E.; Turner, G.L.; Bergersen, F.J. Influence of Mineral Nitrogen on Nitrogen Fixation by Lupin (Lupinus angustifolius) as Assessed by 15N Isotope Dilution Methods. Field Crop. Res. 1987, 17, 109–120. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Baddeley, J.A.; Watson, C.A. Models of Biological Nitrogen Fixation of Legumes. Sustain. Agric. 2009, 2, 883–905. [Google Scholar] [CrossRef]
- Williams, A.; Wells, M.S.; Dickey, D.A.; Hu, S.; Maul, J.; Raskin, D.T.; Reberg-Horton, S.C.; Mirsky, S.B. Establishing the Relationship of Soil Nitrogen Immobilization to Cereal Rye Residues in a Mulched System. Plant Soil 2018, 426, 95–107. [Google Scholar] [CrossRef]
- Koudahe, K.; Allen, S.C.; Djaman, K. Critical Review of the Impact of Cover Crops on Soil Properties. Int. Soil Water Conserv. Res. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Perdomo, C.; Cardellino, G. Respuesta de Maíz a Fertilizaciones Definidas con Diferentes Criterios de Recomendación. Agrociencia 2006, 10, 63–79. [Google Scholar] [CrossRef]
- De Oliveira, S.M.; De Almeida, R.E.M.; Ciampitti, I.A.; Junior, C.P.; Lago, B.C.; Trivelin, P.C.O.; Favarin, J.L. Understanding N Timing in Corn Yield and Fertilizer N Recovery: An Insight from an Isotopic Labeled-N Determination. PLoS ONE 2018, 13, e0192776. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Steinbach, H.S.; De Paepe, J.L. Cover Crop Effects on Soils and Subsequent Crops in the Pampas: A Meta-Analysis. Soil Tillage Res. 2017, 170, 53–65. [Google Scholar] [CrossRef]
- Fillery, I.R.P.; Recous, S. Use of Enriched 15N Sources to Study Soil N Transformations. Curr. Plant Sci. Biotechnol. Agric. 2001, 9, 167–194. [Google Scholar] [CrossRef]
- Schmatz, R.; Recous, S.; Aita, C.; Tahir, M.M.; Schu, A.L.; Chaves, B.; Giacomini, S.J. Crop Residue Quality and Soil Type Influence the Priming Effect but Not the Fate of Crop Residue C. Plant Soil 2017, 414, 229–245. [Google Scholar] [CrossRef]
- Liang, X.; Yuan, J.; Yang, E.; Meng, J. Responses of Soil Organic Carbon Decomposition and Microbial Community to the Addition of Plant Residues with Different C:N Ratio. Eur. J. Soil Biol. 2017, 82, 50–55. [Google Scholar] [CrossRef]
- Liang, Z.; Rasmussen, J.; Poeplau, C.; Elsgaard, L. Priming Effects Decrease with the Quantity of Cover Crop Residues-Potential Implications for Soil Carbon Sequestration. Soil Biol. Biochem. 2023, 184, 109110. [Google Scholar] [CrossRef]
- Baijukya, F.P.; De Ridder, N.; Giller, K.E. Nitrogen Release from Decomposing Residues of Leguminous Cover Crops and Their Effect on Maize Yield on Depleted Soils of Bukoba District, Tanzania. Plant Soil 2006, 279, 77–93. [Google Scholar] [CrossRef]
- Asagi, N.; Ueno, H. Nitrogen Dynamics in Paddy Soil Applied with Various 15N-labelled Green Manures. Plant Soil 2009, 322, 251–262. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Collins, F.; Meisinger, J.J.; Schomberg, H.H.; Liebig, M.A.; Kaspar, T.; Mitchell, J. Using Cover Crops and Cropping Systems for Nitrogen Management. In Advances in Nitrogen Management; Soil and Water Conservation Society: Ankeny, SW, USA, 2010; pp. 230–281. [Google Scholar]
- Chapagain, T.; Lee, E.A.; Raizada, M.N. The Potential of Multi-Species Mixtures to Diversify Cover Crop Benefits. Sustainability 2020, 12, 2058. [Google Scholar] [CrossRef]
- Moreno-Cadena, P.; Salmeron, M.; Canisares, L.P.; Poffenbarger, H.J. Productivity Benefits of Cereal-Legume Cover Crop Mixtures Under Variable Soil Nitrogen and Termination Times. Eur. J. Agron. 2024, 155, 127114. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Fox, R.H.; Rayner, J.H. Interactions Between Fertilizer Nitrogen and Soil Nitrogen—The so-Called ‘Priming’ Effect. J. Soil Sci. 1985, 36, 425–444. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of Mechanisms and Quantification of Priming Effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]

| Site | P Bray N°1 | Ca | Mg | K | Na | pH |
|---|---|---|---|---|---|---|
| mg kg−1 | cmolckg−1 | |||||
| 1 | 18.0 | 5.9 | 1.9 | 0.5 | 0.4 | 4.7 |
| 2 | 9.2 | 16.0 | 4.7 | 1.0 | 0.2 | 5.6 |
| Site | Soil Depth | TOC | TN | Sand | Clay | Silt | Texture |
|---|---|---|---|---|---|---|---|
| cm | % | ||||||
| 1 | 0–7 | 3.0 | 0.3 | 8.3 | 35.5 | 56.1 | Silty clay loam |
| 1 | 7–15 | 1.7 | 0.2 | 7.6 | 38.8 | 53.6 | Silty clay loam |
| 2 | 0–7 | 3.3 | 0.3 | 8.8 | 53.6 | 37.6 | Clayey |
| 2 | 7–15 | 2.2 | 0.2 | 8.0 | 55.0 | 37.0 | Clayey |
| Site | Predecessor Crop | AG Biomass | C | N | C:N | CARB | CELL | LIG | BNF | AG N Yield | AG N Yield Fixed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | % | % of Total C | % | kg ha−1 | |||||||
| 1 | Berseem clover | 2444.4 | 42.2 | 3.0 | 14.2 | 54.5 | 38.9 | 6.6 | 82.8 | 72.9 | 64.1 |
| Lupine | 4674.5 | 42.0 | 2.4 | 17.9 | 59.6 | 34.2 | 6.2 | 80.8 | 110.0 | 60.1 | |
| L-O mixtureL+O | 3253.0 | 44.3 | 1.1 | 40.2 | 49.8 | 45.1 | 5.1 | 80.4 | 35.8 | 1.4 | |
| Oat | 4849.9 | 40.4 | 1.0 | 40.6 | 31.4 | 61.7 | 6.9 | n.c | 48.3 | n.c | |
| 2 | Berseem clover | 2183.8 | 40.5 | 2.7 | 15.2 | 55.8 | 37.5 | 6.7 | 79.3 | 59.8 | 47.4 |
| Lupine | 1134.2 | 41.0 | 2.5 | 16.4 | 58.3 | 33.5 | 8.2 | 76.3 | 28.4 | 21.6 | |
| L-O mixtureL+O | 3619.7 | 41.2 | 1.8 | 28.2 | 44.4 | 48.9 | 6.7 | 93.8 | 65.1 | 13.5 | |
| Oat | 2621.4 | 41.9 | 1.0 | 40.2 | 35.8 | 56.4 | 7.8 | n.c | 27.4 | n.c | |
| Site | Predecessor Crop | NO3-N | NH4-N | Mineral N |
|---|---|---|---|---|
| mg kg−1 | ||||
| 1 | Berseem clover | 14.2 ± 1.5 a | 5.1 ± 0.4 | 19.3 ± 1.9 ab |
| Oat | 6.3 ± 1.0 b | 7.9 ± 0.3 | 14.2 ± 1.0 c | |
| Lupine | 14.8 ± 0.9 a | 7.4 ± 1.2 | 22.2 ± 1.9 a | |
| L-O mixture | 8.3 ± 0.1 b | 5.8 ± 0.6 | 14.1 ± 0.6 c | |
| No CC (Control) | 7.9 ± 0.6 b | 8.1 ± 0.9 | 16.1 ± 1.4 bc | |
| Significance of treatment effect | p-value | |||
| Predecessor crop | 0.0006 | n.s | 0.0187 | |
| 2 | Berseem clover | 8.5 ± 1.2 | 6.9 ± 0.1 | 15.4 ± 1.3 |
| Oat | 8.2 ± 0.9 | 7.4 ± 1.1 | 15.6 ± 1.8 | |
| Lupine | 9.4 ± 0.5 | 7.6 ± 0.5 | 17.0 ± 0.7 | |
| L-O mixture | 8.7 ± 0.8 | 8.9 ± 1.5 | 17.6 ± 2.3 | |
| No CC (Control) | 11.6 ± 0.2 | 7.4 ± 0.0 | 19.0 ± 0.2 | |
| Significance of treatment effect | p-value | |||
| Predecessor crop | n.s | n.s | n.s | |
| Site | N Rate | Predecessor Crop | NO3-N | NH4-N | Mineral N |
|---|---|---|---|---|---|
| mg kg−1 | |||||
| 1 | 0 | Berseem clover | 10.3 ± 0.7 | 9.1 ± 1.4 | 19.5 ± 2.0 |
| Oat | 8.6 ± 0.7 | 9.7 ± 0.7 | 18.3 ± 0.5 | ||
| Lupine | 8.5 ± 3.0 | 9.7 ± 0.7 | 18.3 ± 3.2 | ||
| L-O mixture | 9.0 ± 2.0 | 9.7 ± 2.3 | 18.6 ± 0.7 | ||
| No CC (control) | 7.3 ± 0.5 | 9.6 ± 1.4 | 16.9 ± 1.1 | ||
| 100 | Berseem clover | 12.6 ± 3.5 | 8.4 ± 0.6 | 20.9 ± 3.1 | |
| Oat | 11.9 ± 2.5 | 8.8 ± 0.6 | 20.8 ± 2.4 | ||
| Lupine | 12.6 ± 1.9 | 8.3 ± 0.6 | 20.9 ± 2.4 | ||
| L-O mixture | 7.9 ± 1.3 | 8.6 ± 0.1 | 16.5 ± 1.2 | ||
| No CC (control) | 10.2 ± 0.3 | 8.4 ± 0.3 | 18.6 ± 0.2 | ||
| Significance of treatment effect | p-value | ||||
| Nrate | n.s | n.s | n.s | ||
| Predecessor crop | n.s | n.s | n.s | ||
| Nrate× Predecessor crop | n.s | n.s | n.s | ||
| 2 | 0 | Berseem clover | 4.4 ± 0.9 abB | 8.8 ± 1.0 | 13.2 ± 1.6 B |
| Oat | 2.6 ± 0.6 b | 9.5 ± 1.0 | 12.1 ± 1.0 A | ||
| Lupine | 4.4 ± 1 abB | 9.5 ± 0.6 | 13.8 ± 1.5 B | ||
| L-O mixture | 3.2 ± 0.7 b | 8.1 ± 1.0 | 11.2 ± 0.6 | ||
| No CC (control) | 6.5 ± 2.0 a | 8.7 ± 1.0 | 15.3 ± 1.3 | ||
| 100 | Berseem clover | 11.6 ± 2.1 aA | 10.7 ± 1.5 | 22.3 ± 2.3 aA | |
| Oat | 1.9 ± 0.5 c | 7.5 ± 0.6 | 9.4 ± 0.6 cB | ||
| Lupine | 11.0 ± 0.7 aA | 10.3 ± 2.2 | 21.3 ± 1.8 aA | ||
| L-O mixture | 3.9 ± 0.7 bc | 7.6 ± 0.8 | 11.5 ± 0.1 bc | ||
| No CC (control) | 8.4 ± 1.8 b | 6.8 ± 0.2 | 15.2 ± 2.0 b | ||
| Significance of treatment effect | p-value | ||||
| Nrate | <0.0001 | n.s | 0.0002 | ||
| Predecessor crop | 0.0036 | n.s | 0.0113 | ||
| Nrate× Predecessor crop | 0.0014 | n.s | <0.0011 | ||
| Site | N Rate | Predecessor Crop | Grain Yield | Grain N Concentration | Grain N Content | Whole Plant N Content † | Relative N Response in Whole Plant N Content (Rs) |
|---|---|---|---|---|---|---|---|
| kg ha−1 | g kg−1 Dry Weight | kg ha−1 | |||||
| 1 | 0 | Berseem clover | 6439 ± 526 a | 10.3 ± 0.9 | 65.3 ± 1.1 a | 99.9 ± 8.2 a | |
| Oat | 2785 ± 232 cB | 9.9 ± 0.3 | 27.5 ± 2.3 cB | 53.9 ± 3.6 bB | |||
| Lupine | 2437 ± 846 cB | 9.4 ± 0.6 | 23.8 ± 9.5 cB | 62.2 ± 14.0 bB | |||
| Mixture | 3744 ± 135 bc | 9.4 ± 0.6 | 35.9 ± 2.1 bc | 66.9 ± 3.8 bB | |||
| No CC (control) | 5542 ± 546 ab | 9.4 ± 0.5 | 52.5 ± 7.4 ab | 82.4 ± 13.7 abB | |||
| 100 | Berseem clover | 5582 ±762 ab | 10.5 ± 0.2 | 58.3 ± 7.6 ab | 93.6 ± 11.4 | −0.08 ± 0.0 b | |
| Oat | 5870 ±558 abA | 10.4 ± 0.7 | 61.4 ± 8.9 abA | 96.9 ± 13.1 A | 0.43 ± 0.1 a | ||
| Lupine | 4882 ± 629 bA | 10.4 ± 1.2 | 49.9 ± 7.2 bA | 82.7 ± 8.5 A | 0.26 ± 0.1 a | ||
| Mixture | 5124 ± 374 ab | 9.6 ± 0.5 | 49.2 ± 5.0 b | 80.4 ± 10.3 A | 0.25 ± 0.0 a | ||
| No CC (control) | 7148 ± 1040 a | 10.2 ± 0.5 | 73.8 ± 13.7 a | 101.2 ± 14.0 A | 0.19 ± 0.0 a | ||
| Significance of treatment effect | p-value | ||||||
| Nrate | 0.0017 | n.s | 0.0028 | 0.0004 | |||
| Predecessor crop | 0.0313 | n.s | 0.0399 | n.s. | 0.0197 | ||
| Nrate× Predecessor crop | 0.0457 | n.s | n.s | 0.0197 | |||
| 2 | 0 | Berseem clover | 7267 ±676 | 11.3 ± 0.9 B | 81.3 ± 4.2 B | 149.3 ± 12.5 B | |
| Oat | 6574 ± 405 B | 10.3 ± 1.0 B | 68.0 ± 9.9 B | 124.6 ± 11.7 B | |||
| Lupine | 7866 ± 745 B | 10.2 ± 0.3 B | 80.1 ± 6.8 B | 131.1 ± 7.1 B | |||
| Mixture | 8056 ± 977 | 10.4 ± 0.2 B | 84.2 ± 11.2 B | 125.8 ± 16.2 B | |||
| No CC (control) | 7121 ± 570 | 11.7 ± 0.5 B | 82.7 ± 3.8 B | 136.7 ± 10.4 B | |||
| 100 | Berseem clover | 8707 ± 1275 | 16.3 ± 2.2 A | 136.9 ± 8.6 A | 214.3 ± 12.4 A | 0.30 ± 0.1 | |
| Oat | 8969 ± 815 A | 13.8 ± 0.4 A | 122.6 ± 8.2 A | 181.9 ± 18.0 A | 0.30 ± 0.1 | ||
| Lupine | 10,116 ± 952 A | 13.8 ± 1.3 A | 140.7 ± 24.4 A | 203.1 ± 29.7 A | 0.26 ± 0.1 | ||
| Mixture | 9583 ± 663 | 13.6 ± 0.3 A | 129.8 ± 7.1 A | 197.1 ± 11.0 A | 0.32 ± 0.1 | ||
| No CC (control) | 7999 ± 610 | 15.0 ± 1.0 A | 120.6 ± 10.0 A | 186.1 ± 6.4 A | 0.37 ± 0.1 | ||
| Significance of treatment effect | p-value | ||||||
| Nrate | 0.0022 | <0.0001 | <0.0001 | <0.0001 | |||
| Predecessor crop | n.s | n.s | n.s | n.s | n.s | ||
| Nrate× Predecessor crop | n.s | n.s | n.s | n.s | |||
| Site | 15N Application Timing | Predecessor Crop | 15NREcrop | Ndff Grain | Ndff Grain | Ndff Stubble † | Nddf Stubble |
|---|---|---|---|---|---|---|---|
| (%) | kg ha−1 | % | kg ha−1 | ||||
| 1 | Sowing | Berseem clover | 7.4 ± 2.6 B | 2.3 ± 0.4 B | 1.4 ± 0.5 B | 1.2 ± 0.2 B | 0.4 ± 0.1 B |
| Oat | 3.9 ± 1 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.1 B | 0.2 ± 0.1 B | ||
| Lupine | 7.2 ± 2.0 | 2.4 ± 0.5 B | 1.1 ± 0.3 B | 1.5 ± 0.3 | 0.6 ± 0.2 B | ||
| L-O mixture | 3.1 ± 0.1 B | 1.1 ± 0.1 B | 0.6 ± 0.1 B | 0.6 ± 0.0 B | 0.2 ± 0.0 B | ||
| No CC (control) | 7.3 ± 0.8 | 2.2 ± 0.2 | 1.5 ± 0.3 B | 0.9 ± 0.2 | 0.3 ± 0.0 | ||
| V6 stage | Berseem clover | 17.5 ± 2.0 aA | 12.3 ± 3.0 aA | 6.2 ± 0.7 aA | 6.7 ± 1.8 A | 2.0 ± 0.4 A | |
| Oat | 7.0 ± 2.1 c | 4.6 ± 1.5 c | 2.8 ± 1.0 b | 6.7 ± 1.8 A | 1.9 ± 0.2 A | ||
| Lupine | 11.4 ± 2.4 bc | 9.2 ± 2.5 abA | 4.2 ± 1.1 bA | 4.3 ± 1.1 | 1.5 ± 0.3 A | ||
| L-O mixture | 12.6 ± 2.2 abA | 9.9 ± 2.6 abA | 4.5 ± 0.8 abA | 5.0 ± 1.3 A | 1.5 ± 0.5 A | ||
| No CC (control) | 8.9 ± 1.2 c | 5.4 ± 1.2 bc | 4.0 ± 0.9 bA | 3.0 ± 0.6 | 0.9 ± 0.1 | ||
| Significance of treatment effect | p-value | ||||||
| 15N application timing | 0.0016 | 0.0001 | <0.0001 | 0.0001 | <0.0001 | ||
| Predecessor crop | 0.0201 | n.s | n.s | n.s | n.s | ||
| 15N appl. timing × Predecessor crop | n.s | n.s | n.s | n.s | n.s | ||
| 2 | Sowing | Berseem clover | 34.4 ± 1.7 a | 8.6 ± 0.6 B | 7.4 ± 1.3 aB | 8.8 ± 1.0 | 4.0 ± 0.8 |
| Oat | 19.6 ± 2.5 bB | 5.0 ± 0.2 B | 3.6 ± 0.5 bB | 8.5 ± 1.1 | 2.7 ± 0.4 | ||
| Lupine | 21.4 ± 0.9 bB | 5.1 ± 0.4 B | 4.8 ± 0.2 bB | 6.5 ± 0.1 B | 2.3 ± 0.1 B | ||
| L-O mixture | 22.8 ± 3.7 bB | 6.2 ± 0.7 B | 5.0 ± 0.9 bB | 7.9 ± 0.8 B | 2.5 ± 0.4 B | ||
| No CC (control) | 30.7 ± 4.4 aB | 7.6 ± 1.6 B | 6.0 ± 1.1 abB | 10.5 ± 0.6 | 4.1 ± 0.5 | ||
| V10 stage | Berseem clover | 32.4 ± 2.0 b | 19.2 ± 3.5 cA | 16.2 ± 1.3 bA | 11.6 ± 2.2 b | 5.2 ± 1.1 ab | |
| Oat | 30.4 ± 2.5 bA | 21.5 ± 4.1 bcA | 16.2 ± 1.8 bA | 11.7 ± 1.6 b | 3.9 ± 0.5 b | ||
| Lupine | 50.9 ± 3.0 aA | 25.6 ± 2.4 abA | 28.0 ± 2.1 aA | 14.5 ± 1.7 abA | 5.6 ± 0.7 abA | ||
| L-O mixture | 48.0 ± 2.3 aA | 30.9 ± 0.8 aA | 25.4 ± 0.8 aA | 18.3 ± 1.5 aA | 6.3 ± 0.9 aA | ||
| No CC (control) | 37.2 ± 1.4 bA | 24.3 ± 0.1 bcA | 19.4 ± 0.3 bA | 14.3 ± 1.3 ªb | 5.2 ± 0.9 ab | ||
| Significance of treatment effect | p-value | ||||||
| 15N application timing | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Predecessor crop | 0.0444 | n.s | 0.0009 | n.s | n.s | ||
| 15N appl. timing × Predecessor crop | <0.0001 | 0.0284 | 0.0002 | 0.0091 | 0.0214 | ||
| Site | 15N Application Timing | Predecessor Crop | Ndff-0–30 cm Soil Depth | Ndff-0–5 cm Soil Depth | Unaccounted-for N |
|---|---|---|---|---|---|
| kg ha−1 | |||||
| 1 | Sowing | Berseem clover | 2.9 ± 0.3 B | 1.2 ± 0.1 | 27.6 ± 1.0 B |
| Oat | 3.0 ± 0.3 | 1.4 ± 0.2 | 28.7 ± 0.4 B | ||
| Lupine | 4.2 ± 1.1 | 2.5 ± 0.9 | 26.4 ± 0.4 B | ||
| L-O mixture | 3.2 ± 0.2 B | 1.5 ± 0.2 B | 28.8 ± 0.2 B | ||
| No CC (control) | 2.9 ± 0.2 B | 1.5 ± 0.2 | 27.7 ± 0.1 B | ||
| V6 stage | Berseem clover | 9.7 ± 2.8 A | 5.3 ± 1.8 | 44.8 ± 4.1 A | |
| Oat | 6.2 ± 0.5 | 2.4 ± 0.2 | 55.2 ± 1.7 A | ||
| Lupine | 8.2 ± 1.2 | 5.3 ± 1.4 | 50.3 ± 1.7 A | ||
| L-O mixture | 11.1 ± 4.4 A | 6.6 ± 3.7 A | 46.6 ± 5.7 A | ||
| No CC (control) | 9.7 ± 3.1 A | 5.8 ± 3.0 | 50.4 ± 2.9 A | ||
| Significance of treatment effect | p-value | ||||
| 15N application timing | 0.0008 | 0.0055 | <0.0001 | ||
| Predecessor crop | n.s | n.s | n.s | ||
| 15N appl. timing × Predecessor crop | n.s | n.s | n.s | ||
| 2 | Sowing | Berseem clover | 12.0 ± 2.5 B | 5.6 ± 1.8 | 9.7 ± 3.0 |
| Oat | 9.7 ± 0.9 B | 4.9 ± 1.1 | 16.8 ± 1.7 | ||
| Lupine | 11.4 ± 1.1 B | 5.2 ± 1.2 | 14.5 ± 1.4 | ||
| L-O mixture | 12.0 ± 3.2 B | 6.8 ± 2.5 | 13.5 ± 4.4 | ||
| No CC (control) | 8.5 ± 1.1 B | 3.6 ± 0.9 B | 14.4 ± 1.9 | ||
| V10 stage | Berseem clover | 25.2 ± 4.7 A | 8.7 ± 0.7 | 19.5 ± 5.2 ab | |
| Oat | 22.7 ± 2.2 A | 9.7 ± 2.0 | 23.2 ± 3.1 a | ||
| Lupine | 25.7 ± 3.6 A | 8.7 ± 1.1 | 6.7 ± 5.0 b | ||
| L-O mixture | 23.7 ± 1.8 A | 9.3 ± 1.1 | 10.7 ± 2.9 ab | ||
| No CC (control) | 19.8 ± 3.4 A | 8.9 ± 2.2 A | 21.7 ± 3.1 ab | ||
| Significance of treatment effect | p-value | ||||
| 15N application timing | <0.0001 | 0.0043 | n.s | ||
| Predecessor crop | n.s | n.s | n.s | ||
| 15N appl. timing × Predecessor crop | n.s | n.s | n.s | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori Alvez, C.; Perdomo Varela, C.; del Pino Machado, A. Nitrogen Residual Effect of Winter Cover Crops on Maize in Uruguay: Conventional and Isotopic Evaluation. Agriculture 2024, 14, 2123. https://doi.org/10.3390/agriculture14122123
Mori Alvez C, Perdomo Varela C, del Pino Machado A. Nitrogen Residual Effect of Winter Cover Crops on Maize in Uruguay: Conventional and Isotopic Evaluation. Agriculture. 2024; 14(12):2123. https://doi.org/10.3390/agriculture14122123
Chicago/Turabian StyleMori Alvez, Cristina, Carlos Perdomo Varela, and Amabelia del Pino Machado. 2024. "Nitrogen Residual Effect of Winter Cover Crops on Maize in Uruguay: Conventional and Isotopic Evaluation" Agriculture 14, no. 12: 2123. https://doi.org/10.3390/agriculture14122123
APA StyleMori Alvez, C., Perdomo Varela, C., & del Pino Machado, A. (2024). Nitrogen Residual Effect of Winter Cover Crops on Maize in Uruguay: Conventional and Isotopic Evaluation. Agriculture, 14(12), 2123. https://doi.org/10.3390/agriculture14122123






