Abstract
The synergistic application of far-red (FR) and ultraviolet A (UVA) irradiation presents a promising approach for enhancing growth and the enrichment of secondary metabolites in plants. However, prolonged exposure to these combined light qualities imposes significant stress on plants, hindering their development. Therefore, an initial period of FR irradiation to promote plant growth, followed by a subsequent period of UVA irradiation to enhance the accumulation of plant quality, constitutes a viable strategy. Our study, focusing on purple lettuce, aims to elucidate the response mechanisms of the lettuce leaf under standard white light in commercial production, with the addition of different durations of FR and UVA irradiation, and to explore the complex dynamic changes at the multi-omics level. The results indicate that the duration of FR exposure is crucial in determining biomass-related phenotypes such as fresh weight, while the duration of UVA exposure significantly influences the accumulation of phenotypic markers like anthocyanins. At the transcriptional level, the most extensive transcriptional regulation was observed when FR was applied throughout the entire growth period, and UVA was applied eight days before harvest, significantly impacting pathways such as MAPK signaling cascades, plant hormone signal transduction, photosynthetic processes, and the biosynthesis of secondary metabolites. Metabolomic analysis corroborated the transcriptomic findings, with particular emphasis on antioxidant activity, photoprotection, and defense mechanisms. Our comprehensive analysis suggests that short-term UVA irradiation prior to harvest, based on full growth period FR irradiation, is feasible. The combined application of FR and UVA irradiation fine-tunes plant growth, developmental trajectories, and stress responses by modulating light signals, hormonal signals, and secondary metabolic pathways. These findings not only reveal the adaptive mechanisms of plants to fluctuating light environments but also provide a scientific basis for optimizing light management strategies in controlled plant production systems and precision agriculture.
1. Introduction
The growth and development of plants are significantly influenced by the light environment, with light quality, light intensity, and photoperiod being key parameters that regulate plant growth. The physiologically effective radiation spectrum for plants has specific intensity and periodicity, which play a decisive role in plant physiological activities and growth patterns [1,2,3]. In the field of plant photobiology, visible light has previously been the focus of research, while the study of far-red light and other non-visible light is relatively new, with many potential effects and mechanisms not yet fully understood [4,5]. With innovations in light source technology, the impact of non-visible light environments on crop growth is increasingly receiving attention.
Within plant communities, interspecific interactions substantially alter the ambient light environment. Under conditions of reduced light, plants manifest symptoms indicative of Shade Avoidance Syndrome (SAS), which is marked by a series of adaptive physiological reactions triggered by the perception of alterations in far-red light [6,7]. These adaptations encompass elongation of stems and leaves, modulation of leaf angles, suppression of lateral branch growth, and modifications in reproductive development. Through these adaptive changes, plants optimize their photosynthetic efficiency within the competitive light environment, thereby ensuring their survival and reproductive success [8,9]. In the context of SAS, plants modulate the synthesis and equilibrium of auxins (IAA), gibberellins (GA), and ethylene to adapt to environmental conditions [10,11]. The intricate interplay among these hormones constitutes a complex regulatory network that is pivotal for plants to adapt to fluctuations in light availability and to augment their competitive edge. Research evidence indicates that the application of FR irradiation is associated with an accelerated increase in the fresh weight of lettuce [12], concurrent with a potential decrease in the concentrations of certain secondary metabolites, including anthocyanins [13]. FR irradiation could also promote the growth of Viola [14].
UVA radiation exerts multifaceted influences on plants, altering morphological characteristics such as leaf size and impacting physiological and biochemical processes through the modulation of photosynthesis, stomatal conductance, and interactions with phytochromes and other pigments [15,16,17]. It induces changes in phenolic compounds within the plant and responds to UVA exposure by regulating gene expression at multiple levels [18,19]. Research has indicated that UVA radiation significantly influences the accumulation of anthocyanins and phenolic compounds in purple leaf lettuce [20]. UVA exposure enhances the biosynthesis of these protective pigments and antioxidants, vital for plant defense against environmental stresses. However, excessive UVA irradiation can stress plants, negatively impacting their growth and development [21]. Furthermore, UVA signaling could interact with UVB signaling pathways to collectively modulate secondary metabolism in plants [22]. Although the effects of UVA on plants are complex and diverse, research indicates that UVA plays a significant role in plant growth and metabolism, with specific mechanisms requiring further investigation across various environmental conditions [23].
FR and UVA composite light quality can achieve a balance between higher yield and better quality in artificial photosynthetic plant factories [24]. Studies have shown that the combination of FR and UVA light irradiation significantly promotes the biomass of Chinese broccoli (Brassica alboglabra Bailey), with significant increases in petiole length, stem thickness, main stem length, and leaf area [25]. However, the underlying mechanisms of how the timing of FR and UVA composite light irradiation affect crop growth and antioxidant capacity remain unclear. Lettuce (Lactuca sativa L.), widely cultivated globally and available throughout the year, is a leafy vegetable appreciated for its antioxidant content and favored by consumers worldwide. Research indicates that red- or purple-leaf lettuce varieties possess significantly higher levels of antioxidants and free radical scavenging rates compared to green-leaf lettuce [24,26]. Therefore, this study explored the regulatory mechanisms of lettuce at the leaf omics level under different timing sequences of FR and UVA composite light irradiation.
2. Materials and Methods
2.1. Cultivation Environment Setting
Phenotypic data collection experiments were conducted from October to December 2023 at the artificial light plant factory laboratory of the Zibo Institute for Digital Agriculture and Rural Research (Zibo, China). Lettuce seeds (Alaine, Rijk Zwann, Delft, The Netherlands) were initially sown in moistened sponges (24 mm × 24 mm) and cultivated under white LED lights with a photosynthetic photon flux density of 200 μmol·m−2·s−1 until the emergence of the second leaf at the center, after which they were transplanted into cultivation channels (1200 mm × 600 mm × 60 mm). Following a two-day acclimation period for seedlings, the experiment commenced. The light source panels were provided by Guangzhou Smart Core Agricultural Technology Co., Ltd., (Guangzhou, China). Photosynthetic photon flux density within the plant canopy was measured using an Avantes spectrometer (Avaspec-2048CL, Avantes, Apeldoorn, The Netherlands) and adjusted weekly in accordance with plant growth. A nutrient solution specifically designed for the growth of lettuce, provided by Shanghai Yongtong Chemical Co., Ltd. (Shanghai, China), was used for hydroponic cultivation. The specific parameters of nutritional elements are as follows: Ca ≥ 10.0%; Hg ≤ 5 mg/kg; As ≤ 10 mg/kg; Cd ≤ 10 mg/kg; Pb ≤ 50 mg/kg; Cr ≤ 50 mg/kg; S ≤ 0.1%; Cl ≤ 0.1%; Na ≤ 1%, etc. Indoor environmental conditions were maintained at a daytime temperature of (24 ± 1) °C and a nighttime temperature of (21 ± 1) °C, with relative humidity at (65 ± 5)%, and carbon dioxide concentration kept at ambient levels. The photoperiod was set to a 16-h light and 8-h dark cycle, which is an effective light environment configuration for enhancing both the yield and quality of lettuce. To investigate the effect of non-visible composite light timing on lettuce, as shown in Figure 1, white light (W, 200 μmol·m−2·s−1) was set as the control group, and the light durations of FR (728 nm, 120 μmol·m−2·s−1) and UVA (378 nm, 20 μmol·m−2·s−1) were set separately.
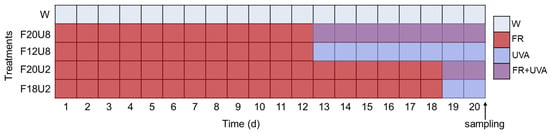
Figure 1.
Details of experimental setup.
2.2. Multi-Omics Data Determination
- (a)
- SPAD Measurement
Chlorophyll content was assessed using a SPAD-502 chlorophyll meter, conducted two hours prior to the end of the light period.
- (b)
- Fresh and Dry Weight Measurement
Fresh weight was measured two hours before the conclusion of the light period. Initially, canopy phenotyping was performed using a phenotyping platform. Subsequently, the plants were separated at the stem base, and the fresh weight of the aerial parts was recorded using an analytical balance. The leaves were then removed for total leaf area assessment. Finally, the leaves were placed in an oven and dried at a constant temperature for 48 h until reaching a stable weight for weighing.
- (c)
- Measurement of Leaf Biochemical Components
The total anthocyanin content was determined according to the instructions provided for the reagent kit (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). The levels of malondialdehyde and hydrogen peroxide were measured following the guidelines outlined in the reagent kit (Suzhou Grace Biotechnology Co., Ltd., Suzhou, China).
- (d)
- Image phenotype extraction
Canopy RGB images were extracted using the existing phenotype platform (Figure S1), and referring to previous research results, the phenotype indicators (Table S1) suitable for this experiment were summarized, and corresponding algorithms for extraction were constructed.
- (e)
- RNA Extraction and Transcriptome Sequencing
At specific time points (1 h after morning light exposure), leaf samples of lettuce (excluding petioles, retaining only leaf tissue) were collected after different light quality treatments. Total RNA from each sample was extracted using the TRIzol method (Invitrogen, Carlsbad, CA, USA). To ensure the integrity and uniformity of the extracted RNA, total RNA was obtained by pooling leaf tissues from multiple lettuce plants, including three groups of independent biological replicates, with each group containing at least 8 individual plants. RNA purity was assessed using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA), and RNA integrity was evaluated using an Agilent 2100 (Agilent Technologies, Santa Clara, CA, USA). All RNA samples that passed quality control were sent to Beijing Allwegene Technology Co., Ltd., (Beijing, China). for library construction and sequencing. Library construction was performed using Illumina’s (Illumina, San Diego, CA, USA) compatible kits.
The sequencing data were processed using Trimomatic software (version 0.33) to remove adapter sequences, reads with N content exceeding 10%, and reads with low-quality bases (Q ≤ 20) exceeding 50%, resulting in clean reads. Subsequently, they were compared with the reference genome using STAR software (v2.5.2b). The gene expression levels of each sample were analyzed using HTSeq software (v0.5.4. p3). FPKM values of 0.1 or 1 were set as the criteria for determining gene expression. In subsequent analysis, only genes with FPKM values greater than 1 were considered for in-depth analysis.
- (f)
- Metabolome acquisition
After freeze-drying the samples, 20 mg of the sample was weighed and added to 1000 μL of extraction solvent (methanol: acetonitrile: water = 2:2:1 (V/V/V), containing an isotope-labeled internal standard mixture). The mixture was vortexed for 30 s, ground at 35 Hz for 4 min, and then sonicated for 5 min in an ice water bath. Steps 2 and 3 were repeated three times. The samples were then allowed to stand at −40 °C for 1 h. Subsequently, the samples were centrifuged at 4 °C and 12,000 rpm (13,800× g, radius 8.6 cm) for 15 min. The supernatant was collected into sample vials for analysis. Additionally, equal amounts of supernatant from all samples were combined to create a QC sample for machine detection and analysis.
The analysis was conducted using a UHPLC system (Vanquish, Thermo Fisher Scientific, Waltham, MA, USA) interfaced with an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific). UHPLC separation was achieved on an Acquity UHPLC platform (Waters) equipped with a Waters UPLC BEH Amide column (1.8 μm, 2.1 × 100 mm). The mobile phases comprised A: a solution of 25 mM ammonium acetate and 25 mM ammonium hydroxide in water, and B: pure ACN. The flow rate was maintained at 0.5 mL/min, with an injection volume of 2 μL, and samples were kept at 4 °C in the autosampler.
The Orbitrap Exploris 120 mass spectrometer was selected for its capability to generate MS/MS spectra under information-dependent acquisition (IDA) mode, managed by Xcalibur software (v4.4). This mode allows for continuous assessment of the full-scan MS spectrum by the acquisition software. The electrospray ionization (ESI) source parameters were optimized as follows: sheath gas flow at 50 Arb, auxiliary gas flow at 15 Arb, capillary temperature at 320 °C, full MS resolution at 60,000, MS/MS resolution at 15,000, and collision energy set to SNCE values of 20/30/40. The spray voltage was adjusted to 3.8 kV in positive mode and −3.4 kV in negative mode.
- (1)
- Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) is a statistical method for supervised pattern recognition. A model established through this analysis method can highlight the metabolic differences between different sample groups and screen metabolic markers related to classification from the data. OPLS-DA models were constructed between every two sample groups, where high values of model parameters (R2 and Q2) indicate strong reliability of the model. In addition, the stability of model parameters R2 and Q2 was verified through 200 permutation tests.
- (2)
- Screening of differential metabolites
We used multivariate statistical analysis to process the characteristics of LC-OE-MS metabolomics data. The screening criteria we set included: Student’s t-test p-value less than 0.05, Fold Change value either greater than 1.5 or less than 0.67, and Variable Importance in the Projection (VIP) of the first principal component in the OPLS-DA model exceeding 1.
- (3)
- Differential metabolite enrichment analysis
Firstly, we compared the differential metabolites with authoritative databases such as KEGG and PubChem to determine the metabolic pathways associated with these metabolites. By comprehensively analyzing the pathways of these differential metabolites, including enrichment analysis and topological analysis, we could further screen for the main pathways most relevant to metabolic differences.
3. Results
3.1. The Effect of Non-Visible-Light Irradiation Duration on the Phenomics of Purple Lettuce
The phenotypic effects of non-visible composite light irradiation sequences on lettuce exhibit distinct differences (Figure 2). Unsupervised clustering analysis suggests that these effects can be broadly categorized into two groups for discussion. In terms of certain morphological structures, gray-level co-occurrence matrix, and color phenotypes (homogeneity_0, homogeneity_90, uniformity, energy_0, energy_90, ASM_0, ASM_90, small_gradient, skew_r, skew_g, lab_mean_a), the phenotypes under non-visible light irradiation were lower than those under control conditions. Conversely, another set of phenotypic indicators shows an opposite trend. Under the same FR treatment, the phenotypic group changed consistently, indicating that in this experiment, the duration of UVA irradiation had essentially no impact on the phenotypic groups of lettuce. Furthermore, under the same UVA irradiation duration before harvest, the phenotypic group indices of F18U2 were higher than those of F20U2, a trend also observed with F12U8 and F20U8. This suggests that FR irradiation influences the phenotype. In summary, the phenotypic effects of non-visible composite light irradiation sequences on lettuce exhibit a certain degree of regularity. These findings provide significant data support for further research into the regulatory effects of light on plant growth and development.
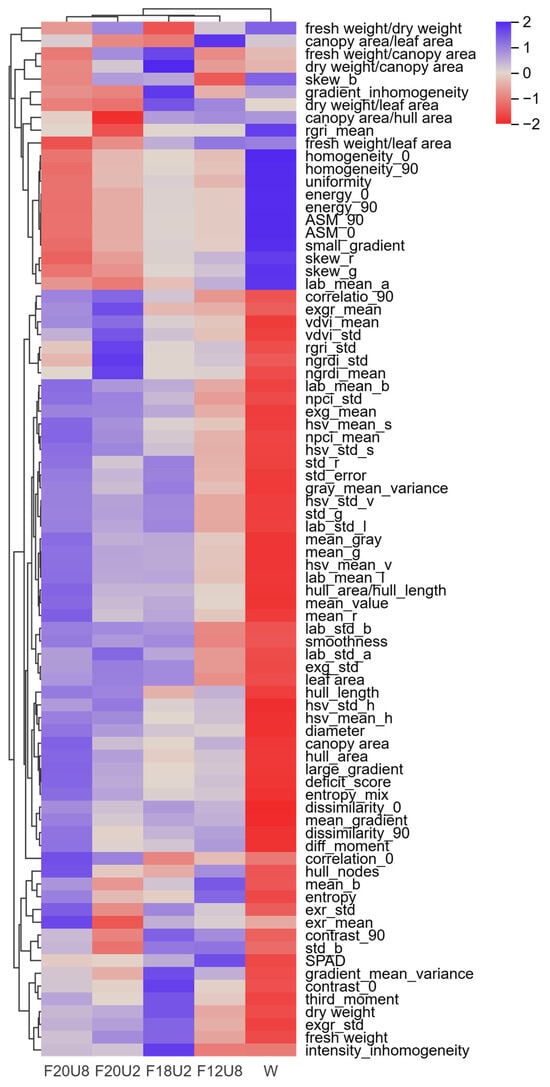
Figure 2.
The effects of FR and UVA irradiation time on the phenomics of purple lettuce.
Quantitative assessments of anthocyanin, malondialdehyde (MDA), and hydrogen peroxide (H2O2) levels along with catalase (CAT) activity were performed at the culmination of the growth period (20 d) to ascertain the effects of various treatments on the antioxidant capacity and oxidative stress in plants (Figure 3). Notably, the F20U8 treatment elicited the highest anthocyanin levels at 501.67 μg/g·FW, which were comparable to those under the F12U8 treatment, indicating that extended UVA exposure enhances anthocyanin biosynthesis in lettuce, independent of FR irradiation duration. The highest MDA levels, reaching 24.56 nmol/g·FW, were observed under the F18U2 treatment, with no significant divergence from the F20U8 treatment, suggesting that both UVA and FR irradiation contribute to increased MDA accumulation. H2O2 levels were also maximized under the F20U8 treatment, showing no significant difference from the F12U8 treatment. CAT activity remained consistent across all treatments. These results collectively suggest that the imposition of FR and UVA irradiation significantly alters the antioxidant profile and the oxidative stress response in plants.
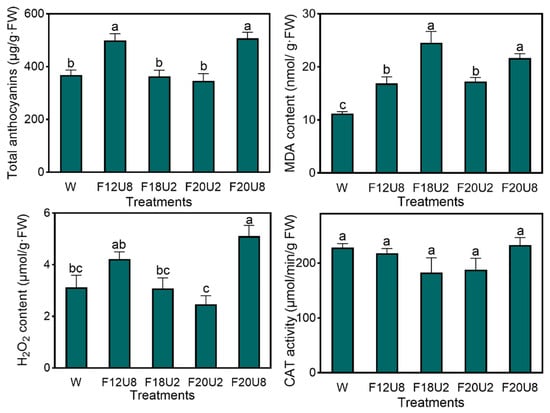
Figure 3.
The effects of FR and UVA irradiation time on the stress resistance of purple lettuce. The anthocyanin content, MDA content, H2O2 content, and CAT activity were analyzed separately. Different letters indicate significant differences between data (p < 0.05).
3.2. The Effect of FR and UVA Irradiation Time on the Transcriptome of Purple Lettuce
To investigate the effects of different durations of non-visible composite light exposure on the mRNA transcriptome of lettuce leaves, we conducted RNA sequencing analysis on lettuce leaves subjected to various light treatments prior to harvest (Figure 4). The results indicated that different durations of composite light exposure have varying regulatory effects on RNA expression in lettuce leaves. Specifically, the F20U8 treatment triggered the most extensive transcriptional regulation, identifying 10,420 differentially expressed genes (DEGs). The F20U2 treatment induced a broad transcriptional response, affecting 8268 DEGs. The F18U2 treatment resulted in fewer transcriptional changes, impacting 7993 DEGs. The F12U8 treatment elicited the least transcriptional regulation, identifying 6391 DEGs. Notably, 2853 genes exhibited changes in transcriptional expression across all four treatments. These findings elucidate the complexity of gene expression regulation in lettuce leaves in response to varying durations of non-visible light exposure, providing valuable insights into how such exposure durations affect plant growth and development.
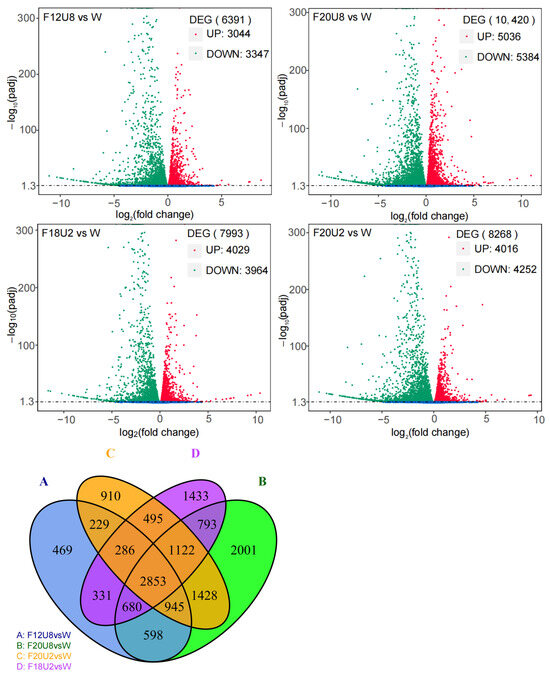
Figure 4.
Analysis of differential gene expressions caused by non-visible light irradiation duration.
Under various light treatments, significant differences in gene expression were observed, which are further categorized into eight distinct clusters (Figure 5). Gene Ontology (GO) enrichment analysis indicates that differentially expressed genes (DEGs) associated with biological processes were significantly enriched in multiple biological functions related to plant metabolic regulation and signal transduction. Specifically, the expression of genes involved in Carbon Fixation and Gene Expression Regulation (C1), Signal Transduction and Membrane Transport (C2), Cell Cycle Regulation and Plant Hormone Response (C7), Cell Recognition and Ribosome Biogenesis (C6), Hormone Response and Metabolic Regulation (C8), Protein Modification and Secretion (C4), Glycoprotein Metabolism and Biosynthesis (C5), and Microtubule Movement and Lysine Metabolism (C3) were notably affected. DEGs related to cellular components were significantly enriched in various biological functions associated with organelle and membrane structures, as well as protein modification and genetic information processing (Figure S2). Notably, the expression of genes associated with Proton-Transporting ATPase Complexes (C1), Golgi Apparatus and Associated Structures (C2), Intermediate Filaments and Ubiquitination Complexes (C7), Ribonucleoprotein Complexes and Pre-Ribosomal Particles (C6), Ubiquitin Ligase Complexes (C8), Chromatin and Protein-DNA Complexes (C4), and Proteasome Complexes (C3) were significantly influenced. Molecular function-related DEGs were significantly enriched in biological functions related to plant enzyme activity and molecular modification, as well as DNA interaction and protein synthesis (Figure S3). The expression of genes involved in Photosynthesis and Energy Conversion (C1), Oxidoreductase Activity and Cytoskeleton Binding (C2), DNA Binding and Methylation Modification (C7), Translation Regulation and Oxidoreductase Activity (C8), Calcium Ion Binding and Oxidoreductase Activity (C4), Protein Folding and Molecular Chaperone Activity (C5), and Proteolysis and Microtubule Motor Activity (C3) were all significantly impacted. Compared to growth under visible light (W), a combination of far-red (FR) and ultraviolet A (UVA) light inhibited the expression of genes in C4 and C5, while non-visible light combinations had no significant effect on gene expression in C4. Additionally, specific light combinations, such as F20U8, promoted the expression of genes in C1, while both F20U8 and F20U2 enhanced gene expression in C2 and suppressed it in C6. F12U8 promotes gene expression in C7, F18U2 in C8, and F20U2 in C3. These findings reveal the nuanced regulatory effects of light conditions on plant gene expression and suggest that different light treatments could modulate plant growth and development by influencing specific biochemical pathways and molecular mechanisms.
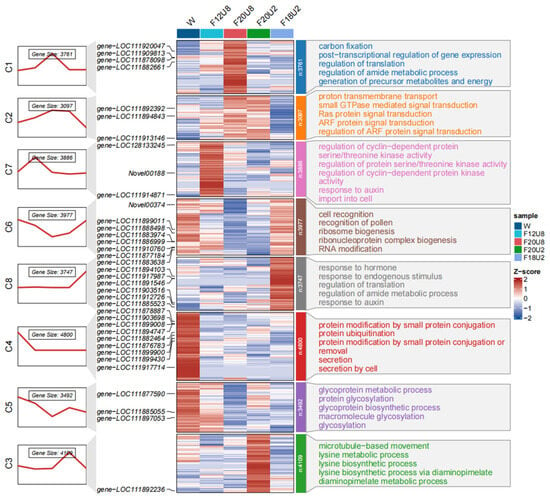
Figure 5.
GO analysis of differentially expressed genes related to biological processes induced by FR and UVA irradiation time.
KEGG pathway enrichment analysis of gene expression under varying durations of non-visible light exposure revealed significant alterations in several pathways (Figure 6, Figures S4–S6). Specifically, the F12U8 treatment had a pronounced effect on plant hormone signal transduction, plant MAPK signaling pathway, ribosome, carbon metabolism, amino acid biosynthesis, nucleotide sugar metabolism, and plant-pathogen interactions. Similarly, the F18U2 treatment significantly influenced plant hormone signal transduction, plant MAPK signaling pathway, ribosome, carbon metabolism, and amino acid biosynthesis. The F20U2 treatment notably impacted pathways related to phagosome, plant hormone signal transduction, ribosome, carbon metabolism, secondary metabolite biosynthesis, amino acid biosynthesis, and nucleotide sugar metabolism. The F20U8 treatment significantly affected the ribosome, pyruvate metabolism, carbon metabolism, and amino acid biosynthesis pathways. These results indicate that different durations of non-visible light exposure significantly affect gene expression in lettuce, particularly in key metabolic pathways such as plant hormone signal transduction, ribosome biogenesis, carbon metabolism, and amino acid biosynthesis. This suggests that non-visible light exposure not only influences the fundamental metabolic processes in plants but could also regulate their adaptive responses to environmental changes, including energy metabolism and plant defense mechanisms.
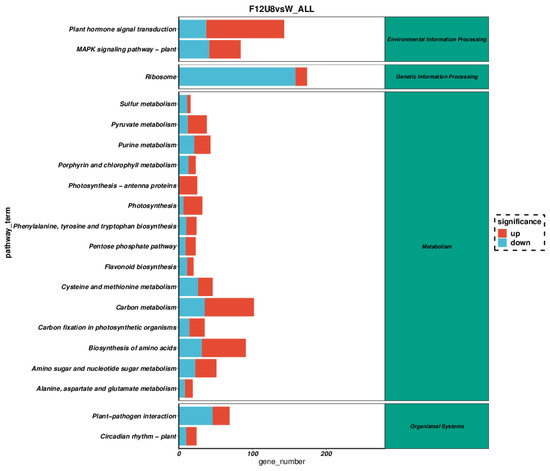
Figure 6.
Path enrichment analysis under non-visible composite light treatment.
We further discovered that the MAPK signaling pathway, plant hormone signal transduction, photosynthesis, and the biosynthesis of secondary metabolites are significantly affected in plants (Table S2). Under the F20U2 treatment, the changes in gene expression of plants revealed their sensitivity and adaptability to specific light conditions. These changes primarily involved key biological processes such as light signal transduction, stress response, and plant hormone signaling. For example, the change in the calcium-binding protein CML45 (LOC111877740) could play a crucial role in regulating light signal transduction and stress response in plants. The downregulation of members of the WRKY transcription factor family and 1-aminocyclopropane-1-carboxylic acid synthase (LOC111886468) could indicate reduced sensitivity of the plant to stress under these light conditions. Under the F20U8 treatment, the downregulation of genes related to protein phosphatase 2C 24 (LOC111880373) could reveal the inhibitory effect of this light condition on light signal transduction and stress response. In the F18U2 treatment, the number of upregulated genes decreased, while stress response-related genes such as PP2C24 (LOC111880373), BAK1 (LOC111899008), and ERF1A (LOC111905113) were downregulated, indicating the plant’s adaptive regulation to different light intensities and durations. Additionally, transcription factors such as MYC2 (LOC111884132) and PIF3 (LOC111890722), as well as signaling proteins like BIRK1 (LOC111899011), could play key roles in regulating the plant’s response to light. Under the F12U8 treatment, plants exhibited high sensitivity to light signals, with the highest number of upregulated genes primarily involved in light signal transduction and stress response, including protein phosphatase 2C 6 (LOC111883708) and EBF2 (LOC111893352). This suggests that plants could enhance their light signal transduction and stress adaptation capabilities by regulating these genes. Long-term exposure to FR combined with UVA significantly upregulated genes related to auxin synthesis and signaling, such as IAA16 (LOC111881233) and AUX1 (LOC111887891). The upregulation of these genes reflects an increased demand for auxin in plants under specific light conditions to adapt to their growth processes. In summary, changes in light conditions influence multiple signaling pathways that collectively affect the physiological functions of plants, revealing the complex mechanisms of growth regulation under different environmental conditions.
We conducted a comprehensive analysis of mRNA transcriptional level variations in lettuce under different durations of non-visible light exposure to elucidate the responses of specific transcription factor families to these changes (Figure 7, Figures S7–S9). Notably, in the F12U8 treatment, transcription factors from the WRKY, ERF, C2H2, and bHLH families also displayed significant expression changes. The WRKY family had 24 members downregulated and one upregulated, the ERF family had all 24 members downregulated, the C2H2 family had 12 members downregulated and one upregulated, and the bHLH family had nine members downregulated and four upregulated. Transcription factors OpuncBB_FGP21054, Lsa011539, and Thhalv10022273m frequently interacted within the regulatory network, highlighting their crucial regulatory roles in the F12U8 treatment’s network. Under F18U2 treatment, transcription factors from the ERF, bHLH, and C2H2 families showed significant expression changes as well, with 26 members of the ERF family downregulated and five upregulated, 14 members of the bHLH family downregulated and four upregulated, and 15 members of the C2H2 family downregulated with one upregulated. Transcription factors Thecc1EG014762t1, Lsa016760, and Han008863 frequently interacted within the regulatory network, indicating their significant centrality in the F18U2 treatment’s network. Under F20U2 treatment, transcription factors from the ERF, WRKY, and bHLH families exhibited significant expression alterations. Within the ERF family, 30 members were downregulated while one was upregulated. The WRKY family showed uniform downregulation across its 29 members. In contrast, the bHLH family had 21 members downregulated and two upregulated. Transcription factors such as Potri.006G000800.1, Pbr030871.1, and MA_11351g0010 demonstrated frequent interactions within the regulatory network, suggesting their central roles in the F20U2 treatment’s network, potentially linking to various biological processes including plant growth and development, stress response, signal transduction, and metabolic regulation. Under F20U8 treatment, transcription factors from the WRKY, bHLH, and ERF families again showed significant expression changes, with 31 members of the WRKY family downregulated, 23 members of the bHLH family downregulated and two upregulated, and 22 members of the ERF family downregulated with one upregulated. Transcription factors Potri.T074200.1, MA_11351g0010, and PH01000668G0290 frequently interacted within the regulatory network, indicating their significant centrality in the F20U8 treatment’s network. Collectively, our findings unravel the distinct impacts of varying durations of non-visible light exposure on the lettuce transcriptome regulatory network, particularly concerning the transcriptional reprogramming associated with environmental stress responses.
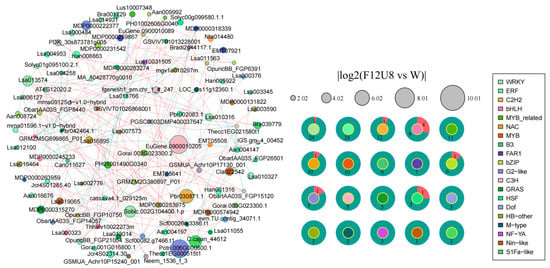
Figure 7.
Analysis of transcription factor interaction network.
3.3. The Effect of FR and UVA Irradiation Time on the Metabolome of Purple Lettuce
We conducted an untargeted metabolomic analysis on lettuce leaves following various durations of non-visible light exposure to investigate the impact on the metabolic profile (Figure 8). Our findings revealed that different exposure durations exert varying degrees of regulatory effects on the metabolism of lettuce leaves. The treatment designated F20U8 elicited the most extensive metabolic modulation, with 675 differentially expressed genes (DEMs). The F20U2 treatment induced a broader metabolic modulation, with 458 DEMs, while the F18U2 treatment resulted in a lower degree of metabolic modulation, with 450 DEMs. The F12U8 treatment showed the least metabolic modulation, with 434 DEMs. Moreover, the number of shared metabolic modulations among different treatments varied; specifically, there were 205 DEMs common to all four treatments. These findings underscore the complexity of the regulatory effects of non-visible light exposure duration on the number of metabolites in lettuce leaves, providing valuable insights into how such exposure durations could influence plant growth and development.
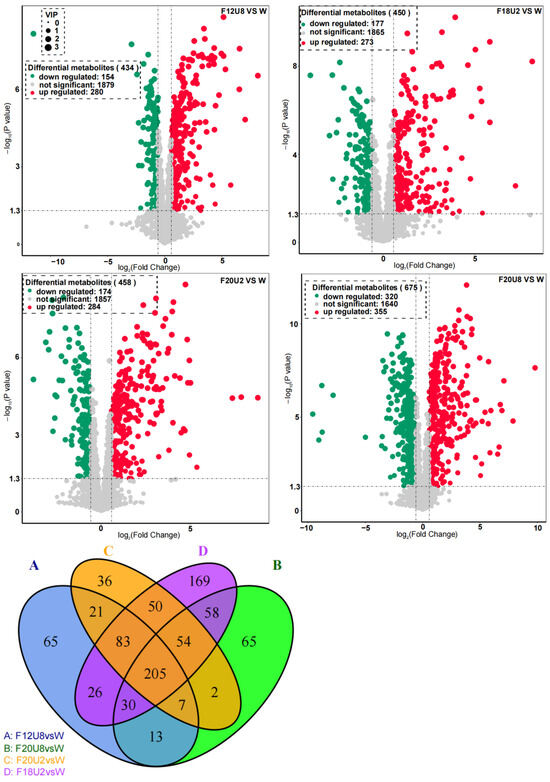
Figure 8.
Differential expression metabolite analysis.
The changes in metabolic pathways under different treatments were analyzed (Figure 9, Figures S10–S12). In comparison with the control group W, the treatment with F20U2 predominantly influenced multiple metabolic pathways, including plant hormone signal transduction, starch and sucrose metabolism, photosynthesis, galactose metabolism, and ABC transporters. Notably, the ABC transporter pathway was enriched with five metabolites, the highest among all pathways assessed. The F20U8 treatment primarily elicited changes in starch and sucrose metabolism, ABC transporters, galactose metabolism, and carbon fixation in photosynthetic organisms, with the starch and sucrose metabolism and ABC transporters pathways accumulating eight metabolites, highlighting their central role in the metabolic network and potential significant impact on pathway regulation and functionality. Furthermore, the F18U2 treatment revealed alterations in photosynthesis, plant hormone signal transduction, oxidative phosphorylation, and nucleotide metabolism pathways. The nucleotide metabolism pathway was particularly enriched with five metabolites, marking it as the most enriched among the assessed pathways. Lastly, the F12U8 treatment predominantly affected plant hormone signal transduction and the one-carbon pool by folate, photosynthesis, phenylpropanoid biosynthesis, and biosynthesis of cofactors. Among these, the biosynthesis of cofactors pathway was the most enriched, with nine metabolites identified. In summary, under various treatment conditions, specific metabolic pathways exhibited significant enrichment of metabolites, indicating their pivotal role in plant metabolic regulation. Pathways such as starch and sucrose metabolism, plant hormone signal transduction, and ABC transporters demonstrated pronounced metabolite enrichment, suggesting their substantial influence on the plant’s metabolic network and physiological functions.
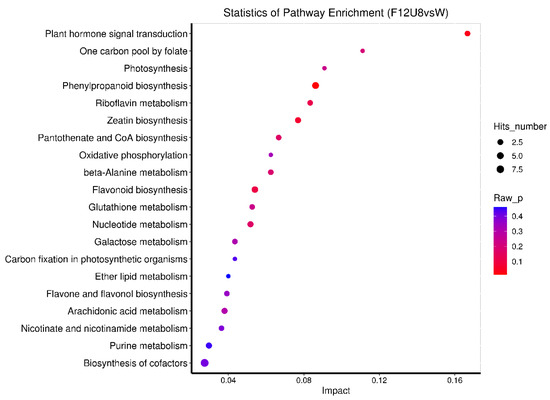
Figure 9.
The metabolic KEGG pathway of TOP20.
In the comprehensive metabolomic analysis using the KEGG metabolic network (Figure 10, Figures S13–S15), it was observed that under the F12U8 treatment, there was a significant upregulation in the expression of metabolic pathways such as the biosynthesis of secondary metabolites and biosynthesis of cofactors. Particularly within the lipid metabolism category, the linoleic acid metabolism and the biosynthesis of unsaturated fatty acids pathways were notably upregulated in response to F12U8 treatment. Furthermore, within the biosynthesis of other secondary metabolites category, the phenylpropanoid biosynthesis pathway was upregulated, while the glucosinolate biosynthesis and flavone and flavonol biosynthesis pathways were downregulated. In carbohydrate metabolism, the galactose metabolism pathway was upregulated, and in the metabolism of terpenoids and polyketides category, the sesquiterpenoid and triterpenoid biosynthesis pathway was also upregulated. Additionally, in the metabolism of cofactors and vitamins, the pantothenate and CoA biosynthesis pathway was upregulated. Similarly, the F18U2 treatment led to an upregulation in the expression of metabolic pathways and the biosynthesis of secondary metabolites. In the biosynthesis of other secondary metabolites, the flavonoid biosynthesis pathway was upregulated, along with galactose metabolism in carbohydrate metabolism, oxidative phosphorylation in energy metabolism, the biosynthesis of unsaturated fatty acids in lipid metabolism, and porphyrin metabolism in the metabolism of cofactors and vitamins. The F20U8 treatment resulted in an upregulation of the biosynthesis of secondary metabolites, while downregulating the expression of metabolic pathways. Within energy metabolism, the oxidative phosphorylation pathway was upregulated, as was linoleic acid metabolism in lipid metabolism and sesquiterpenoid and triterpenoid biosynthesis in the metabolism of terpenoids and polyketides. Lastly, the F20U2 treatment predominantly upregulated the biosynthesis of secondary metabolites. In the biosynthesis of other secondary metabolites, the flavonoid biosynthesis pathway was upregulated, the galactose metabolism in carbohydrate metabolism was upregulated, and the biosynthesis of unsaturated fatty acids in Lipid metabolism was also upregulated. These findings indicate that specific metabolic pathways are significantly influenced by the respective treatments, with certain pathways such as the biosynthesis of secondary metabolites, linoleic acid metabolism, and galactose metabolism being consistently upregulated across different conditions.
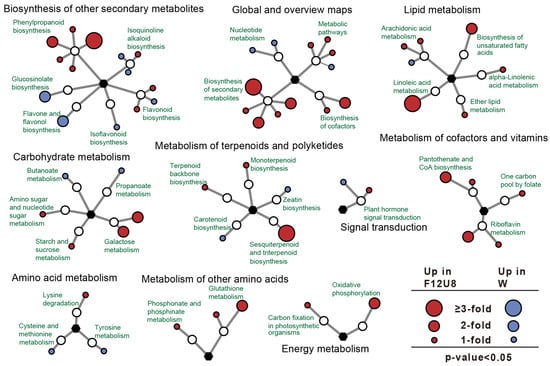
Figure 10.
KEGG network analysis of differential metabolites.
3.4. Joint Analysis of Transcriptomics and Metabolomics
Transcriptomic and metabolomic association analysis of lettuce leaf tissues reveals significant pathway regulation in response to varying durations of non-visible light exposure (Figure 11). The F12U8 treatment induced transcriptional regulation in 126 differentially expressed genes (DEGs) and metabolic regulation in 54 DEGs, with 46 DEGs overlapping between the induced transcriptional and metabolic pathways. Notably, extensive modulation occurred in pathways such as plant hormone signal transduction, biosynthesis of secondary metabolites, and flavonoid biosynthesis. Integrating O2PLS association analysis with KEGG annotation, it is evident that a suite of genes and their encoded proteins play crucial roles in sustaining metabolic activities within plant cells. For instance, the photosystem I reaction center subunit psaK (LOC111880682) is involved in light-dependent photosynthesis reactions, while TIC 20-II (LOC111884260) is implicated in chloroplast protein targeting. The 60S ribosomal protein L27a-3 (LOC111904754) is a central component in protein synthesis, and the V-type proton ATPase catalytic subunit A (LOC111910396) is involved in regulating intracellular and extracellular pH balance and ion transport. Glyceraldehyde-3-phosphate dehydrogenase B (LOC111921246) is a key enzyme in the glycolytic pathway. These genes and their products are pivotal in multiple metabolic pathways, including photosynthesis, protein synthesis, and energy metabolism, significantly impacting the growth, development, and environmental adaptability of plants. Furthermore, in plant metabolic pathways, specific metabolites are closely associated with particular biosynthetic routes. For example, 17phenyl-trinor-PGE2 participates in plant lipid metabolism and signal transduction. Phellamurin, a flavonoid compound, is involved in antioxidant activity, photoprotection, and defense responses, with its biosynthesis likely mediated by key enzymes such as chalcone synthase (CHS) and flavanone 3-hydroxylase (F3H). 13,14-Dihydro-15-keto-PGE2, a derivative of prostaglandin compounds, could also be related to lipid metabolism and signal transduction. Oxododecadienoylcarnitine, a type of acylcarnitine, is associated with the β-oxidation process of fatty acids, involving the transport and metabolism of fatty acids. Glu-Asp, referring to glutamic acid and aspartic acid, play multiple roles in plants, including protein synthesis, nitrogen metabolism, and acting as signaling molecules in plant growth, development, and defense responses. Glutamic acid is also related to the synthesis of glutathione, while aspartic acid is involved in the aspartate metabolic pathway. The specific roles of these metabolites within plants could be influenced by various factors, including plant species, growth conditions, and environmental stimuli.
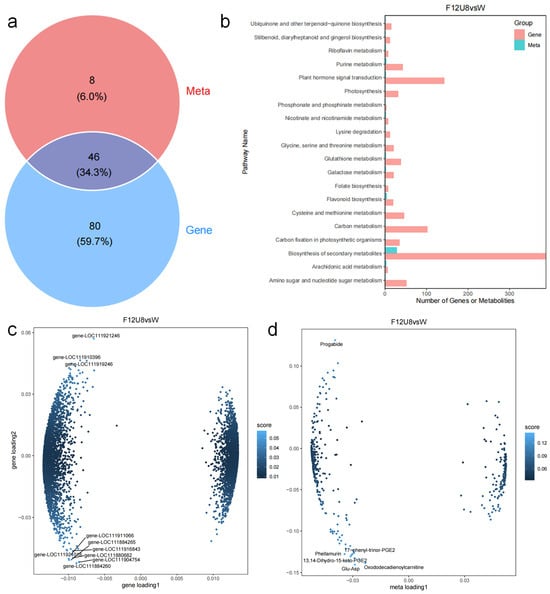
Figure 11.
Joint analysis of transcriptome and metabolome. (a) Venn analysis of transcriptomics and metabolomics; (b) KEGG enrichment analysis of transcriptomics and metabolomics; (c) Loading of genes based on O2PLS analysis; (d) Loading of metabolites based on O2PLS analysis.
Treatment with F18U2 induced transcriptional regulation in 129 differentially expressed genes (DEGs) and metabolic regulation in 57 DEGs, with 48 DEGs overlapping between transcriptional and metabolic regulation (Figure S16). Extensive modulation was observed, primarily in pathways such as biosynthesis of secondary metabolites and carbon metabolism. Through orthogonal partial least squares (O2PLS) correlation analysis combined with KEGG annotation, we were able to elucidate the roles of key genes and their encoded proteins in metabolic activities within plant cells. For instance, gene LOC111884904 encodes a photosystem I reaction center subunit, which is a crucial component in chloroplasts for capturing light energy and participating in photosynthesis. LOC111888547 encodes a YlmG-like protein 2; while its specific function remains to be further investigated, its localization in chloroplasts suggests a potential role in photosynthesis-related metabolic processes. The product of gene LOC111890942, carbamoyl-phosphate synthase, is essential for amino acid biosynthesis and is located in the mitochondria. LOC111900690 encodes a triose phosphate/phosphate translocator that regulates the transport of triose phosphate and inorganic phosphate in the chloroplast during energy metabolism. Additionally, gene LOC111909172 encodes the CURVATURE THYLAKOID 1C protein, which significantly impacts the shaping of thylakoid membrane structure and the efficiency of photosynthesis. These genes and their encoded proteins play central roles in multiple metabolic pathways, including photosynthesis, protein synthesis, and energy metabolism, exerting a substantial influence on plant growth, development, and environmental adaptability. Furthermore, the metabolome primarily includes compounds involved in nucleotide metabolism, enhancement of stress resistance, promotion of antioxidant activity, regulation of nitrogen metabolism, and synthesis of secondary metabolites. These compounds play vital roles in plant defense mechanisms, signal transduction, and responses to environmental stress. Further research is essential to more deeply understand the specific mechanisms of these compounds and their potential applications in plant biology.
The F20U2 treatment induced the transcriptional regulation of 127 differentially expressed genes (DEGs) and metabolic regulation of 59 DEGs, with 51 DEGs overlapping in both transcriptional and metabolic pathways (Figure S17). Notably, extensive regulation occurred in pathways such as biosynthesis of secondary metabolites and carbon metabolism. By integrating Orthogonal Partial Least Squares (O2PLS) correlation analysis with Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation, we could elucidate the roles of key genes and their encoded proteins in the metabolic activities of plant cells. For instance, gene LOC111891591 encodes FRIGIDA-like protein 3, which is involved in the regulation of plant growth. Another gene, LOC111893052, encodes a tyrosine aminomutase enzyme that plays a crucial role in phenylalanine metabolism, contributing to the synthesis of plant hormones and secondary metabolites. Additionally, gene LOC111902280 encodes a SPIRAL1-like 2 protein that is essential for maintaining cellular morphology and structure. Gene LOC111903099 encodes a photosynthetic NDH subunit localized in chloroplasts, a vital component of electron transport during photosynthesis. The product of gene LOC111910454, S-adenosylmethionine decarboxylase precursor, participates in various biosynthetic pathways, including the synthesis of plant hormones. Lastly, gene LOC111919698 encodes a heavy chain class I KRAS protein that is involved in endocytosis and cellular transport, critical for the intracellular trafficking of substances. Collectively, these genes and their products contribute to plant metabolism and physiological functions. In terms of metabolomic analysis, the compounds primarily include phospholipids, fatty acids, flavonoids, amides, and iridoid glycosides. These metabolites play pivotal roles in various biological processes such as cellular structure, signal transduction, energy storage, antioxidant defense, and immune response in plants. For example, phospholipids are integral components of the cell membrane, while flavonoids and iridoid glycosides are involved in plant defense mechanisms against environmental stresses. Fatty acids like stearic acid function in energy metabolism and cellular signaling. The diversity and complexity of these compounds reflect the richness of plant metabolism.
The F20U8 treatment induced transcriptional regulation in 129 differentially expressed genes (DEGs) and metabolic regulation in 77 DEGs (Figure S18). Notably, there was an overlap of 68 DEGs in the transcription–metabolism pathways that were induced. Extensive modulations were observed particularly in pathways such as plant hormone signal transduction and amino sugar and nucleotide sugar metabolism. By employing O2PLS correlation analysis combined with KEGG annotation, we could elucidate the roles of key genes and their encoded proteins in metabolic activities within plant cells. For instance, gene LOC111876122 encodes serine/threonine protein kinase STN8, which is an important regulator in chloroplasts and participates in signal transduction related to photosynthesis. Gene LOC111892670 encodes 30S ribosomal protein S5, which is responsible for protein synthesis in chloroplasts, thereby supporting cellular metabolism. Additionally, gene LOC111910223 encodes a subunit of cytochrome c oxidase, which is crucial for cellular respiration, while gene LOC111914553 encodes photosystem I type a/b-binding protein 3-1, a key component in capturing light energy during photosynthesis. Metabolomic analysis has characterized the multifaceted roles of these compounds within the plant, including acting as signaling molecules or defensive compounds, or being involved in energy storage, highlighting the complexity of plant adaptation to the environment and maintenance of physiological functions.
3.5. Joint Analysis of Transcriptomics and Phenomics
Through WGCNA analysis of the expression patterns of 20,903 DEGs obtained from transcriptome sequencing, these genes were classified into 16 distinct modules based on their expression similarities (Figure 12). Each module’s characteristic genes represent the overall gene expression profile, resulting in 16 different expression patterns, each indicated by a unique color. Gene expression ultimately drives phenotypic changes; therefore, a correlation analysis was conducted between 17 phenotypic traits and the 16 transcriptome modules. The results indicate that the navajowhite2 module exhibited the highest correlation with various phenotypic metrics, with a correlation coefficient of −0.97 for leaf area. Subsequently, a correlation network analysis was performed on the top five phenotypic traits most closely associated with the navajowhite2 module genes. This analysis identified 65 representative genes significantly correlated with key phenotypic indicators. Based on KEGG annotations, several findings emerged: ACCS (LOC111886468) and BAK1 (LOC111899008) are involved in hormone signaling and growth regulation, while PR1 (LOC111886999) is linked to plant disease resistance. CAT (LOC111878432) serves as a critical gene for antioxidant stress response, protecting plants from oxidative damage. GAPDH (LOC111881660) is a key enzyme in photosynthesis, and DGK5 (LOC111878102) is associated with energy metabolism, influencing cellular energy supply and signaling pathways. Additionally, Glycine cleavage system H protein 2 (LOC111883680) and inosine-5′-monophosphate dehydrogenase 2 (LOC111877491) are involved in amino acid and nucleotide synthesis, supporting plant growth and development. Lipoxygenase 1 (LOC111882177) and peroxidase N1 (LOC111896403) play crucial roles in lipid metabolism and antioxidant responses, enhancing the plant’s ability to cope with stress. Thylakoid lumenal 29 kDa protein (LOC111888025) and ATP-sulfurylase 3 (LOC111890798) are involved in photosynthesis and sulfur metabolism, promoting physiological functions in plants. Other genes, such as calmodulin-7 (LOC111883491) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (LOC111903696), also contribute to signaling pathways and steroid synthesis. The selection of these genes in the transcriptome correlation network analysis underscores their significant roles in plant growth, development, and stress responses.
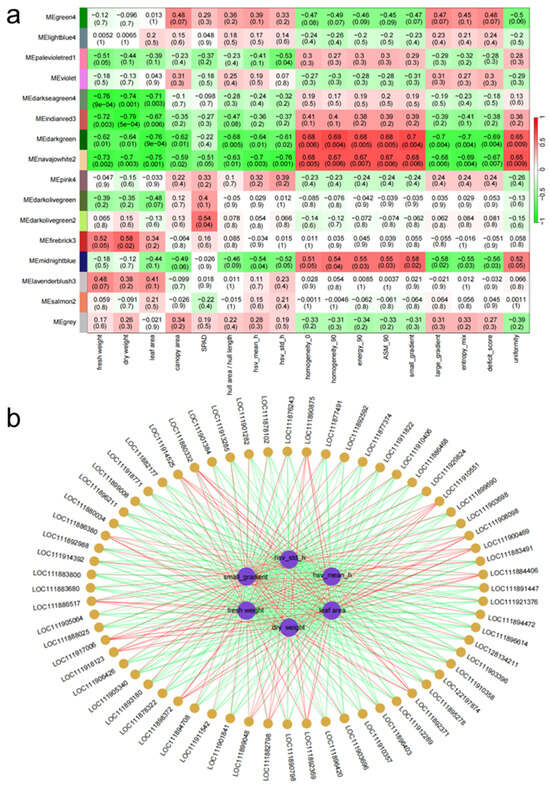
Figure 12.
Joint analysis of transcriptome and phenotype based on WGCNA. (a) Correlation network diagram between gene modules and phenotypes. (b) The correlation network analysis of key gene modules and representative phenotypic traits in WGCNA.
4. Discussion
4.1. The Effect of Combined Irradiation Time of FR and UVA on the Phenomics of Purple Lettuce
Light is the energy source for photosynthesis in plants, while the light spectrum serves as an important signal that influences plant growth, development, and metabolic processes. Supplementing with non-visible light has advantages in improving plant growth and crop quality in controlled environments, as well as promoting the accumulation of primary and secondary metabolites [27,28,29]. It has been reported that FR positively affects plant internode length, height, and yield [30]. He et al. [24] found that supplemental UVA significantly increased anthocyanin content in lettuce, while FR supplementation promoted lettuce growth, especially under UVA irradiation. This aligns with the results of our study, which further revealed that phenotypic changes exhibited a strong time effect with FR and UVA exposure; specifically, the longer the exposure time, the greater the changes in biomass and physicochemical phenotypes. The antioxidant capacity of crops refers to their ability to resist oxidative stress and free radical damage, primarily achieved through antioxidant enzymes (such as peroxidase) and non-enzymatic antioxidants (such as vitamin C and anthocyanins) [31,32]. The strength of antioxidant capacity is closely related to a crop’s tolerance to environmental stress, with stronger antioxidant capacity contributing to improved growth, yield, and nutritional quality of the crops. We found that anthocyanin content was also higher under the treatment with higher hydrogen peroxide content. In terms of phenotypic traits, compared to the W treatment, non-visible light treatments such as F20U8 resulted in lower indicators like ASM and energy, while indicators like intensity inhomogeneity and entropy were higher, indicating disruption of canopy image information in lettuce under non-visible light treatment. This serves as a visual manifestation of stress immune dysregulation within the lettuce leaves. These results reveal the comprehensive regulatory effects of non-visible light irradiation on plant metabolism, signaling regulation, and stress resistance mechanisms, providing an important molecular basis for understanding plant adaptability to environmental changes.
4.2. The Effect of Combined Irradiation Time of FR and UVA on the Transcriptomics of Purple Lettuce
During the growth and development of lettuce, non-visible light treatment has a significant impact on its molecular metabolic levels. FR treatment notably affects pathways related to the MAPK signaling pathway, plant hormone signal transduction, carbon metabolism, and ribosomes. Specifically, genes such as JAR6 (LOC111876787), IAA16 (LOC111881233), and AUX1 (LOC111887891) are upregulated under FR irradiation, promoting plant growth [33,34]. When faced with environmental threats, plants can respond rapidly through metabolic shifts and gene reprogramming [35,36]. These changes primarily involve key biological processes such as light signal transduction, stress responses, and plant hormone signaling. For example, the upregulation of the calcium-binding protein CML45 (GH3.17, LOC111877740) could play a crucial role in regulating plant light signal transduction and stress responses. Additionally, plants changed genes related to the calcium-binding protein CP1, such as LOC111911900, LOC111914172 and LOC111914173, which could reflect enhanced sensitivity to light signals and stress adaptation under this treatment. The concentrations of Ca2+ in the cytoplasm and reactive oxygen species (ROS) signals are among the early responses of plants to stressors such as damage, pathogen attack, and abiotic stress, and there is an interaction between them [37]. Ca2+ signaling has been identified in various signaling pathways in plants and established as a secondary messenger capable of responding to a wide range of environmental and developmental stimuli, triggering appropriate physiological responses in plants [38]. These signaling processes involve specific metabolic and transcriptional reprogramming, enabling plants to adapt to specific stress conditions [39]. In summary, the expression changes in auxin-related genes and Ca2+ signaling play a crucial role in plant responses to environmental stress. These processes not only affect plant growth and development but also determine the plant’s adaptability and resistance to adversity.
4.3. The Effect of Combined Irradiation Time of FR and UVA on the Metabolomics of Purple Lettuce
In the early stages of stress adversity, plants respond to short-term environmental changes by rapidly adjusting their metabolic pathways [40,41]. As the duration of UVA irradiation before harvest increases, gene expression and metabolic regulation in plants exhibit complex dynamic changes, reflecting the plants’ adaptability to environmental pressures. This response not only helps to cope with oxidative stress but also promotes plant growth, demonstrating that plants can quickly activate defense mechanisms in the short term. This aligns with findings from metabolomics studies, which indicate that changes in metabolites are a direct reflection of an organism’s physiological phenotype and biochemical levels [42,43]. Non-visible light irradiation also activates secondary metabolic pathways such as flavonoid and phenylpropanoid biosynthesis, which are often closely related to plant stress resistance, indicating that plants tend to enhance their defensive capabilities when facing short-term environmental pressures. Under prolonged UVA irradiation (8 days), the expression of antioxidant genes remains higher than that of the control, suggesting that plants maintain strong antioxidant capacity when subjected to continuous light stress. Additionally, the expression of abscisic acid receptors and ethylene signaling pathway genes further supports the inhibitory effects of prolonged light exposure on these signaling pathways [44,45]. In summary, the effects of non-visible light irradiation on plants not only exhibit a clear time dependency in gene expression but also show different emphases in the regulation of metabolic pathways. These findings provide important guidance for plant production management. By optimizing the duration of non-visible composite light exposure, it is possible to promote plant growth, enhance the accumulation of secondary metabolites, and improve the nutritional value and market competitiveness of plants, thereby supporting sustainable agricultural development.
4.4. Mechanistic Insights into the Growth of and Gene Expression Changes in Purple Leaf Lettuce Mediated by FR and UVA Combined Irradiation Time
The influence of FR and UVA radiation on plants is multifaceted, and over the years, researchers have explored the roles and mechanisms of FR and UVA in regulating plant growth, with a particular focus on phenotypic traits and molecular responses [12,23]. To gain a comprehensive understanding of lettuce’s multi-omics cascade response to FR and UVA, we employed an analysis integrating transcriptomics, metabolomics, and phenomics. Our findings indicate that prolonged exposure to FR radiation elicits more pronounced changes in phenotypic indicators (Figure 2), which can be attributed to the shade avoidance response triggered by FR, promoting plant growth and counteracting the stress responses induced by extended UVA exposure, leading to significant alterations in morphological appearance [24,46]. At a deeper molecular level, chronic FR irradiation induces the upregulation of a broad spectrum of auxin-related genes. RNA sequencing data reveal that numerous genes encoding AUX/IAA and SAUR are significantly upregulated (Supplementary Table S2). Plant hormone signaling, including auxins and cytokinins, plays a pivotal role in various developmental processes of plant growth, such as macromolecular biosynthesis, photosynthesis, and environmental stress responses [47,48]. Studies have shown that FR influences plant growth and development by affecting the synthesis and distribution of auxins. Upon perceiving FR light, auxins migrate towards the shaded area, promoting cell elongation in that region, thereby facilitating phototropism. This process not only enhances photosynthetic efficiency but also impacts the overall morphological adaptation of the plant. Furthermore, FR stimulates the intricate interplay of various plant hormones, bolstering the plant’s adaptive capabilities under diverse environmental conditions [49]. Under the influence of FR light, photomorphogenesis, shade avoidance responses, and anthocyanin biosynthesis are intricately interwoven, facilitating the maintenance of a balance between growth and reproduction in plants. The ratio of red to far-red (R/FR) light, sensed by phytochrome (phy) photoreceptors [50], triggers a cascade of developmental responses in plants upon light exposure, including the biosynthesis of anthocyanins. In conditions of reduced R/FR light ratios, the expression of CmbHLH16 is suppressed, while the anthocyanin repressor CmMYB4 is upregulated, leading to the formation of the anthocyanin inhibitory complex CmMYB4-CmTPL [51].
The modulation of secondary metabolite production and accumulation is a key strategy employed by plants to endure environmental stress. Our multi-omics analysis elucidates how UVA radiation selectively influences the generation of secondary metabolites in lettuce. The study reveals that UVA exerts the most effective positive impact on phenylpropanoids, inducing the majority, albeit not all, of phenylpropanoid compounds. Furthermore, the stimulatory effect of UVA on flavonoids results in broad enhancement at both transcriptional and metabolic levels (Supplementary Table S3). The primary absorption range for most flavonoids lies within the UVA spectrum (315–400 nm) rather than the UVB range (280–315 nm) [52]. This observation provides compelling indirect evidence that UVA radiation specifically triggers the production of flavonoids. Flavonoids are principal secondary metabolites involved in plant defense against abiotic stresses [53]. In Arabidopsis, flavonoid compounds are considered determinants of tolerance to abiotic stress [54]. Metabolomic analysis in Brassica oleracea revealed a significant increase in flavonol metabolites in seedlings following cold stress [55]. In Solanum lycopersicum, flavonols reduce ROS accumulation and modulate ABA-dependent ROS bursts in guard cells, facilitating stomatal opening to regulate leaf gas exchange [56].
4.5. Prospects of Research
The majority of UVA-induced secondary metabolites are nitrogen-containing compounds or derived from amino acids, such as phenylpropanoids, suggesting a heightened demand for carbon and nitrogen sources under UVA exposure. Transcriptional data also indicate that UVA enhances carbon assimilation efficiency through various mechanisms. The impact of UVA on plants varies with the duration of exposure. Prolonged UVA irradiation typically leads to negative effects such as decreased photosynthesis, cellular damage, oxidative stress, and growth inhibition, while potentially promoting the synthesis of antioxidants and secondary metabolites to counteract the damage. In contrast, brief UVA exposure stimulates adaptive responses in plants, enhances antioxidant capacity, and even stimulates photosynthesis and growth in certain cases. Therefore, moderate UVA exposure may be beneficial to plants, but excessive exposure can cause harm. In general, short-wavelength light increases the antioxidant content and capacity within plants, while long-wavelength light decreases them [57]. Anthocyanin synthesis is primarily regulated by enzymes encoded by various structural genes and transcriptional regulators, with plant hormones also participating in the regulation of anthocyanin biosynthesis. Ethylene, abscisic acid, and jasmonic acid have been identified as positive regulators of anthocyanin synthesis, while auxins and gibberellins inhibit anthocyanin accumulation. Previous studies have reported that overexpression of MdIAA26 in apple callus tissue and Arabidopsis promotes anthocyanin accumulation, with auxins inhibiting anthocyanin by degrading MdIAA26 protein [58]. Exogenous ethylene treatment increases anthocyanin accumulation in grape skins and induces the expression of genes related to anthocyanin biosynthesis [59]. FR and UVA light stimulate the production of plant hormones through the expression of hormone signal transduction genes, thereby affecting the biosynthetic pathways of anthocyanins.
The synergistic effects of FR and UVA irradiation on plants are complementary. FR primarily influences plant growth by modulating morphological and developmental processes, whereas UVA contributes by enhancing the synthesis of secondary metabolites and bolstering stress tolerance. The equilibrium between these two types of radiation is crucial for plant health and development; excessive UVA can lead to detrimental effects, while FR plays a significant role in adaptive growth of plants. Further investigation into the interplay of these two types of light and their impact on plant physiology will aid in optimizing conditions for plant growth. Consequently, in practical applications, it is imperative to judiciously regulate the intensity and duration of these light sources to achieve optimal plant growth outcomes. Optimizing the spectral composition of light to enhance plant growth and development, as well as to improve energy utilization efficiency, is a feasible approach [30]. Although the cost of high-intensity non-visible LED light sources is relatively high, our research has demonstrated that by precisely adjusting the photoperiod of FR light in conjunction with low-intensity UVA light, there is significant enhancement in crop yield and quality. It is imperative to note that, for the best outcomes in practical large-scale industrial applications, the irradiation parameters must be meticulously analyzed and adjusted based on specific growth environments and plant species. Furthermore, studies have demonstrated that the introduction of low doses of UVA in the growth spectrum can significantly enhance the antioxidant capacity of lettuce, thereby promoting its storage capability during postharvest storage [60]. Additionally, exposure to FR radiation during the cultivation period also influences the content of primary metabolic substances and antioxidant components in lettuce [12]. Consequently, the impact of combined FR and UVA irradiation on the postharvest storage period and quality of lettuce necessitates further investigation.
5. Conclusions
The duration of invisible composite light exposure has a significant impact on the growth and physiological metabolism of lettuce. Specifically, the duration of FR light exposure directly determines the biomass of lettuce, while the duration of UVA exposure affects the anthocyanin content. Additionally, UVA exposure enhances the stress response of lettuce leaves. At the transcriptional level, sustained application of FR followed by UVA exposure for the last 8 days before harvest resulted in significant transcriptional regulation effects. The exposure to non-visible light significantly influenced multiple signaling pathways, including MAPK signaling, plant hormone signaling, photosynthesis, and the synthesis of secondary metabolites. Metabolomic analysis further confirmed the impact of different light exposure durations on the gene expression of lettuce, particularly regarding antioxidant activity, photoprotection, and defense responses. Overall, non-visible light exposure finely regulates plant growth, development, and stress responses by influencing light signal transduction, hormone signaling, and secondary metabolism pathways. Therefore, long-term FR treatment combined with short-term UVA irradiation before harvesting can promote lettuce growth while increasing nutrient content.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14112019/s1.
Author Contributions
Y.Z. designed the research study, performed the experiments, analyzed the data, and wrote the manuscript. Z.L. assisted in writing the manuscript and improving the work. N.Z. and X.C. supervised the project and designed the research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by Beijing Smart Agriculture Innovation Consortium Project (BAIC10-2024); the Central Public-interest Scientific Institution Basal Research Fund [No. Y2022QC17]; Innovation Program of Chinese Academy of Agricultural Sciences [No. CAAS-CAE-202302, No. CAAS-ASTIP-2024-AII]; the National Natural Science Foundation of Sichuan Province (2022NSFSC1645), Key R&D Program Project of Xinjiang Province (2023B02020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data and code will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leister, D. Enhancing the Light Reactions of Photosynthesis: Strategies, Controversies, and Perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant Cell Environ. 2020, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Hughes, J.; Winkler, A. New Insight Into Phytochromes: Connecting Structure to Function. Annu. Rev. Plant Biol. 2024, 75, 153–183. [Google Scholar] [CrossRef]
- Fankhauser, C.; Chen, M. Transposing Phytochrome into the Nucleus. Trends Plant Sci. 2008, 13, 596–601. [Google Scholar] [CrossRef]
- Smith, H. Physiological and Ecological Function within the Phytochrome Family. CABI Databases 1995, 46, 289–315. [Google Scholar] [CrossRef]
- Pierik, R.; de Wit, M. Shade Avoidance: Phytochrome Signalling and Other Aboveground Neighbour Detection Cues. J. Exp. Bot. 2014, 65, 2815–2824. [Google Scholar] [CrossRef]
- Fankhauser, C.; Batschauer, A. Shadow on the Plant: A Strategy to Exit. Cell 2016, 164, 15–17. [Google Scholar] [CrossRef]
- Keuskamp, D.H.; Pollmann, S.; Voesenek, L.A.C.J.; Peeters, A.J.M.; Pierik, R. Auxin Transport through PIN-FORMED 3 (PIN3) Controls Shade Avoidance and Fitness during Competition. Proc. Natl. Acad. Sci. USA 2010, 107, 22740–22744. [Google Scholar] [CrossRef]
- Hisamatsu, T.; King, R.W.; Helliwell, C.A.; Koshioka, M. The Involvement of Gibberellin 20-Oxidase Genes in Phytochrome-Regulated Petiole Elongation of Arabidopsis. Plant Physiol. 2005, 138, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of Growth, Flowering, and Chlorophyll Fluorescence Responses of Viola cornuta cv. Penny Red Wing According to Spectral Power Distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, J.E.; Oh, M.-M. Growth and Phenolic Compounds of Lactuca sativa L. Grown in a Closed-Type Plant Production System with UV-A, -B, or -C Lamp. J. Sci. Food Agric. 2013, 94, 197–204. [Google Scholar] [CrossRef]
- Tezuka, T.; Yamaguchi, F.; Ando, Y. Physiological Activation in Radish Plants by UV-A Radiation. J. Photochem. Photobiol. B Biol. 1994, 24, 33–40. [Google Scholar] [CrossRef]
- Štroch, M.; Materová, Z.; Vrábl, D.; Karlický, V.; Šigut, L.; Nezval, J.; Špunda, V. Protective Effect of UV-A Radiation during Acclimation of the Photosynthetic Apparatus to UV-B Treatment. Plant Physiol. Biochem. 2015, 96, 90–96. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of Selenium and UVA Radiation Affects Growth and Phytochemicals of Broccoli Microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV Irradiance as a Major Influence on Growth, Development and Secondary Products of Commercial Importance in Lollo Rosso Lettuce ‘Revolution’ Grown under Polyethylene Films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 492746. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB Radiation as Innovative Tools to Biofortify Horticultural Crops with Nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Li, Y.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Supplemental UV-A Affects Growth and Antioxidants of Chinese Kale Baby-Leaves in Artificial Light Plant Factory. Horticulturae 2021, 7, 294. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Yavaş, İ.; Ünay, A.; Ali, S.; Abbas, Z. UV-B Radiations and Secondary Metabolites. Turk. JAF Sci. Technol. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Kotiranta, S.; Pihlava, J.-M.; Kotilainen, T.; Palonen, P. The Morphology, Inflorescence Yield, and Secondary Metabolite Accumulation in Hemp Type Cannabis sativa Can Be Influenced by the R:FR Ratio or the Amount of Short Wavelength Radiation in a Spectrum. Ind. Crops Prod. 2024, 208, 117772. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Breusegem, F.V. Improving Oxidative Stress Resilience in Plants. Plant J. 2021, 109, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.R.; Glick, B.R. Indole-3-Acetic Acid Biosynthesis and Its Regulation in Plant-Associated Bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2019, 62, 25–54. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium Signaling and Transport Machinery: Potential for Development of Stress Tolerance in Plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Fichman, Y.; Xiong, H.; Sengupta, S.; Morrow, J.; Loog, H.; Azad, R.K.; Hibberd, J.M.; Liscum, E.; Mittler, R. Phytochrome B Regulates Reactive Oxygen Signaling during Abiotic and Biotic Stress in Plants. New Phytol. 2022, 237, 1711–1727. [Google Scholar] [CrossRef]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant Adaptation and Tolerance to Environmental Stresses: Mechanisms and Perspectives. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 117–145. ISBN 9789811521560. [Google Scholar]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Baker, S.A.; Rutter, J. Metabolites as Signalling Molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Sulpice, R.; Keurentjes, J. Metabolic Networks: How to Identify Key Components in the Regulation of Metabolism and Growth. Plant Physiol. 2010, 152, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.F.; Rodriguez-Concepcion, M. Molecular Mechanisms of Shade Tolerance in Plants. New Phytol. 2023, 239, 1190–1202. [Google Scholar] [CrossRef]
- Wong, C.; Alabadí, D.; Blázquez, M.A. Spatial Regulation of Plant Hormone Action. J. Exp. Bot. 2023, 74, 6089–6103. [Google Scholar] [CrossRef]
- Hudeček, M.; Nožková, V.; Plíhalová, L.; Plíhal, O. Plant Hormone Cytokinin at the Crossroads of Stress Priming and Control of Photosynthesis. Front. Plant Sci. 2023, 13, 1103088. [Google Scholar] [CrossRef]
- Jaillais, Y.; Chory, J. Unraveling the Paradoxes of Plant Hormone Signaling Integration. Nat. Struct. Mol. Biol. 2010, 17, 642–645. [Google Scholar] [CrossRef]
- Holalu, S.V.; Finlayson, S.A. The Ratio of Red Light to Far Red Light Alters Arabidopsis Axillary Bud Growth and Abscisic Acid Signalling before Stem Auxin Changes. J. Exp. Bot. 2017, 68, 943–952. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Wang, Y.; Wang, Y.; Song, A.; Jiang, J.; Chen, S.; Ding, B.; Guan, Z.; Chen, F. Transcription Factor CmbHLH16 Regulates Petal Anthocyanin Homeostasis under Different Lights in Chrysanthemum. Plant Physiol. 2022, 190, 1134–1152. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The Use of Chlorophyll Fluorescence Excitation Spectra for the Non-Destructive in Situ Assessment of UV-Absorbing Compounds in Leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Zha, L.; Wei, S.; Huang, D.; Zhang, J. Multi-Omics Analyses of Lettuce (Lactuca sativa) Reveals Primary Metabolism Reorganization Supporting Distinct Features of Secondary Metabolism Induced by Supplementing UV-A Radiation. J. Agric. Food Chem. 2024, 72, 15498–15511. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids Are Determinants of Freezing Tolerance and Cold Acclimation in Arabidopsis Thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Xie, L.; Wang, Y.; Cao, Y.; Wan, M.; Lv, D.; Li, J.; Lu, K.; Xu, X.; Liu, L. Characterization of Cold Stress Responses in Different Rapeseed Ecotypes Based on Metabolomics and Transcriptomics Analyses. PeerJ 2020, 8, e8704. [Google Scholar] [CrossRef]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.; Han, X.; Pan, H.; Dai, J.; Wei, Y.; Zhuo, R.; Liu, J. Integrated Transcriptomic and Metabolomic Analysis Reveal the Underlying Mechanism of Anthocyanin Biosynthesis in Toona Sinensis Leaves. Int. J. Mol. Sci. 2023, 24, 15459. [Google Scholar] [CrossRef]
- Wang, C.-K.; Han, P.-L.; Zhao, Y.-W.; Ji, X.-L.; Yu, J.-Q.; You, C.-X.; Hu, D.-G.; Hao, Y.-J. Auxin Regulates Anthocyanin Biosynthesis through the Auxin Repressor Protein MdIAA26. Biochem. Biophys. Res. Commun. 2020, 533, 717–722. [Google Scholar] [CrossRef]
- Wang, P.; Ge, M.; Yu, A.; Song, W.; Fang, J.; Leng, X. Effects of Ethylene on Berry Ripening and Anthocyanin Accumulation of ‘Fujiminori’ Grape in Protected Cultivation. J. Sci. Food Agric. 2022, 102, 1124–1136. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA Intensity during Cultivation Improves the Lettuce Shelf-Life, an Effect That Is Not Sustained at Higher Intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).