Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Material Preparation
2.3. Experimental Device
2.4. Sample Analysis
2.4.1. Plants
2.4.2. Soil
- 1.
- Analysis of soil chemical properties and heavy metal content
- 2.
- Aggregate particle size fraction of soil
- 3.
- Analysis of surface morphology and mineral composition of soil aggregates
2.5. Methodology for Calculating the Indicators
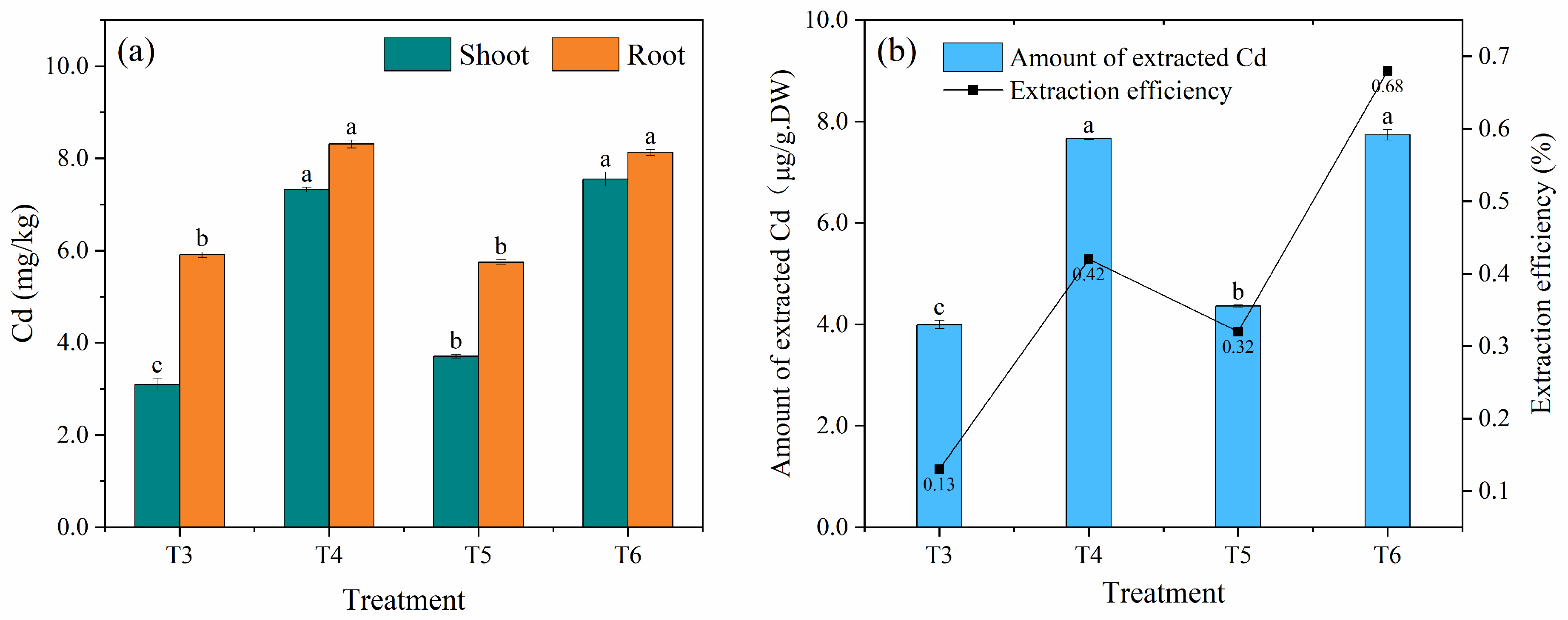
2.5.1. Biological Concentration Factor
2.5.2. Translocation Factor
2.5.3. Extraction Efficiency
2.5.4. Mean Weighted Diameter
2.5.5. Distribution Factor
2.5.6. Grain-Sized Fraction Metals Loading
3. Results
3.1. Growth of Maize Under Different Treatments in the Soil Column Experiment
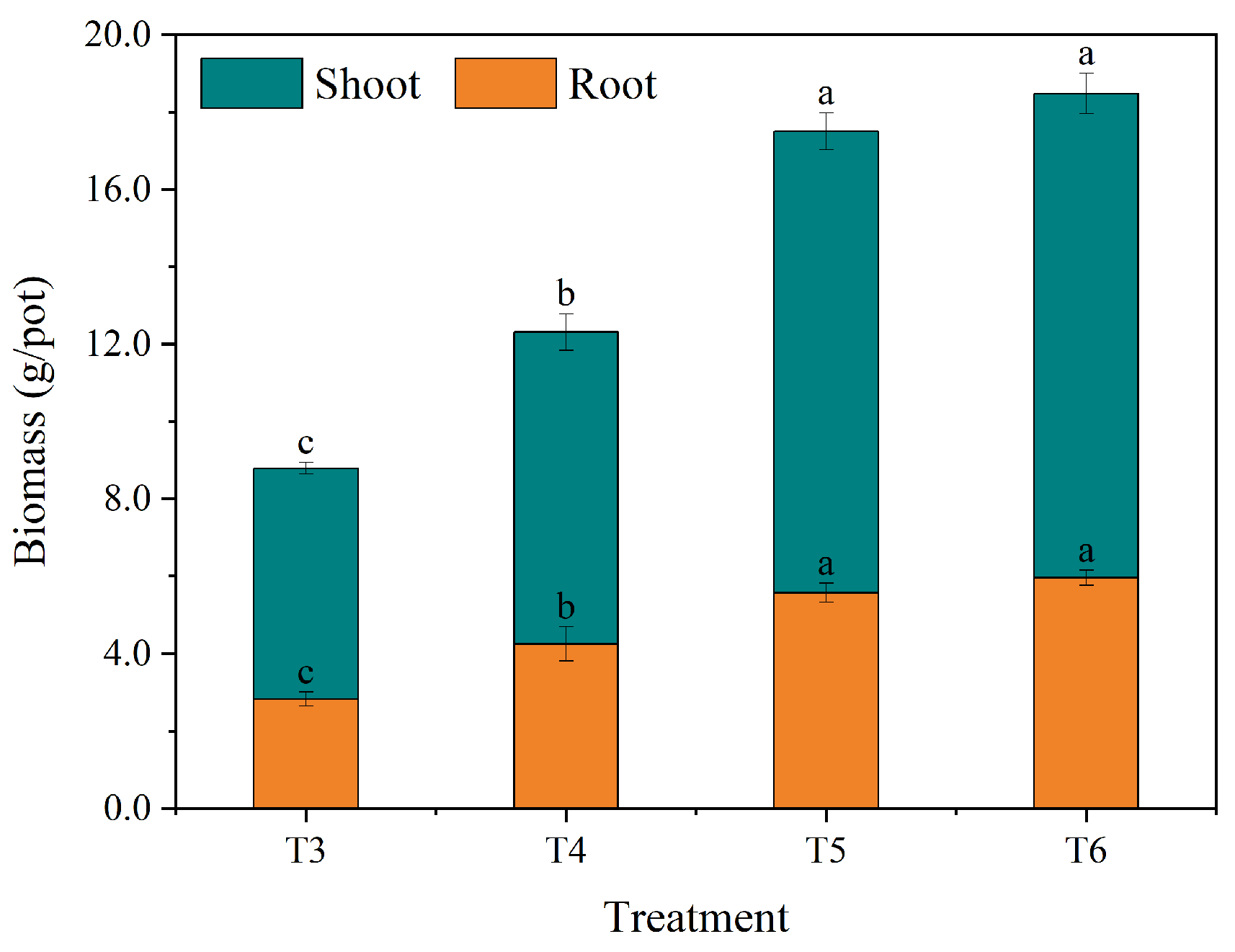
3.1.1. Maize Biomass
3.1.2. Physiological and Biochemical Activities of Maize
3.1.3. Remediation Effect of Cd-Contaminated Soil under Different Treatments in Soil Column Experiments
3.2. Composition of Aggregates and Distribution of Cd in Each Soil Layer under Different Treatments in the Soil Column Experiment
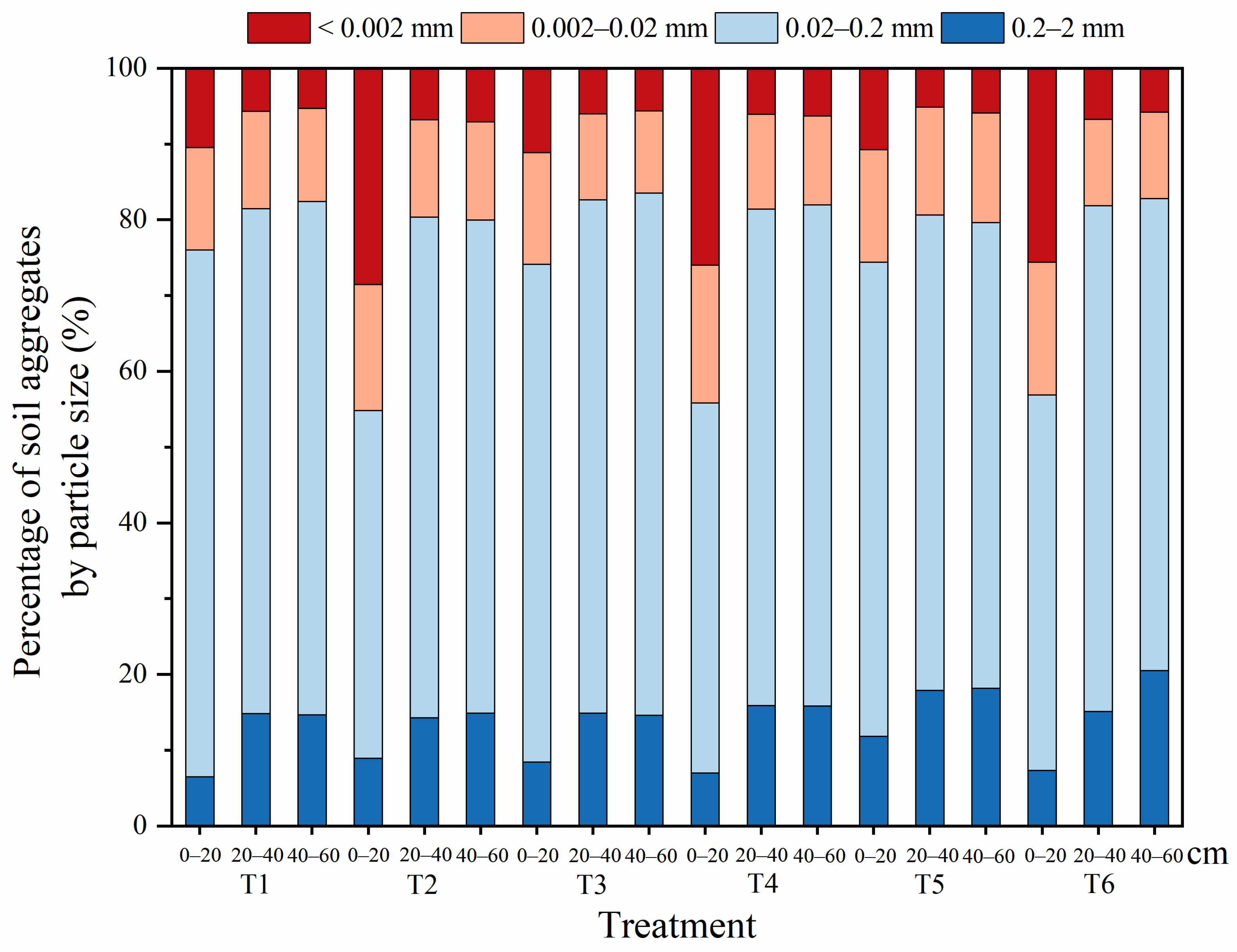
3.2.1. Composition of Aggregates in Each Soil Layer under Different Treatments in the Soil Column Experiment
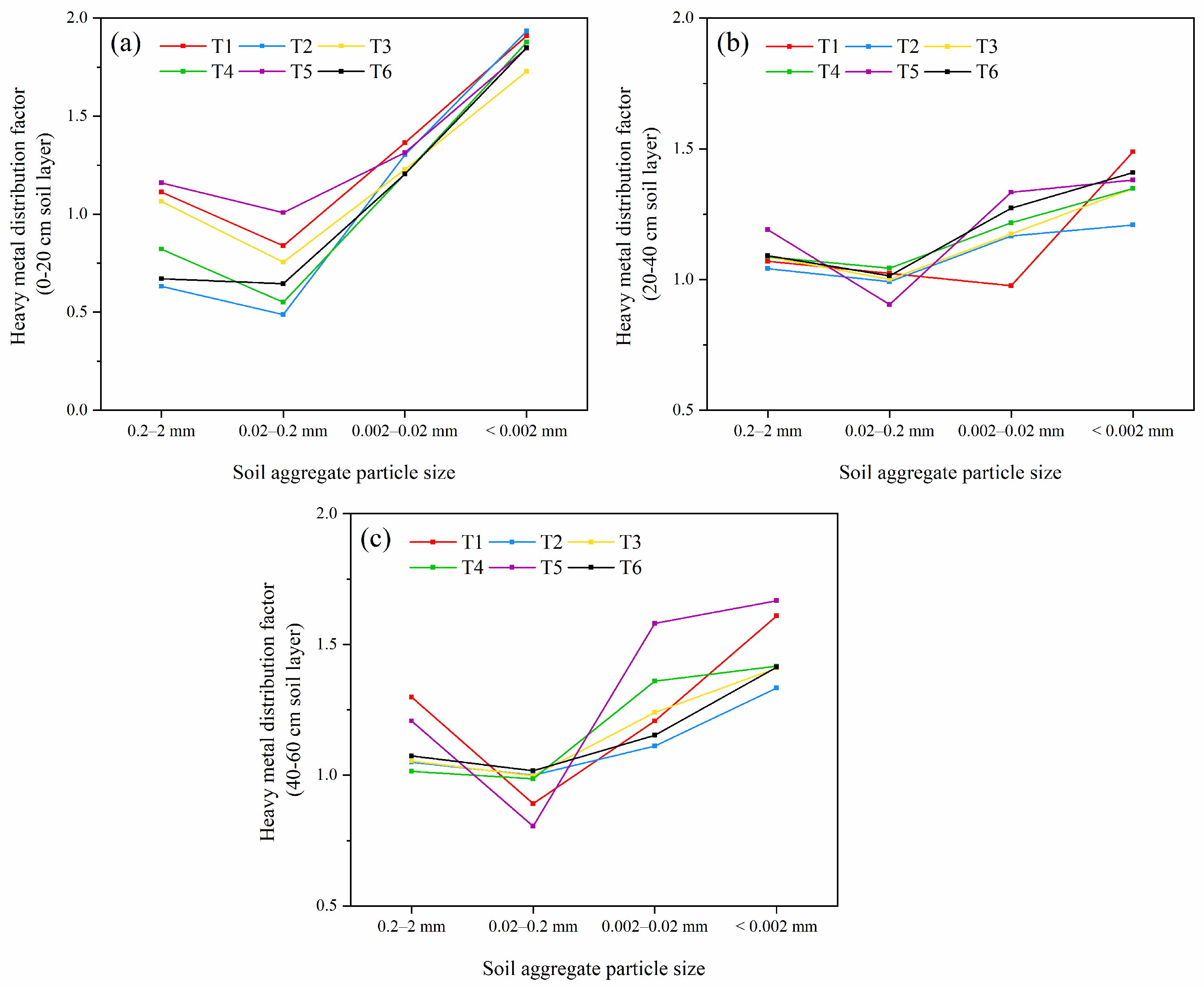
3.2.2. Distribution of Cd in Soil Aggregates of Different Particle Sizes
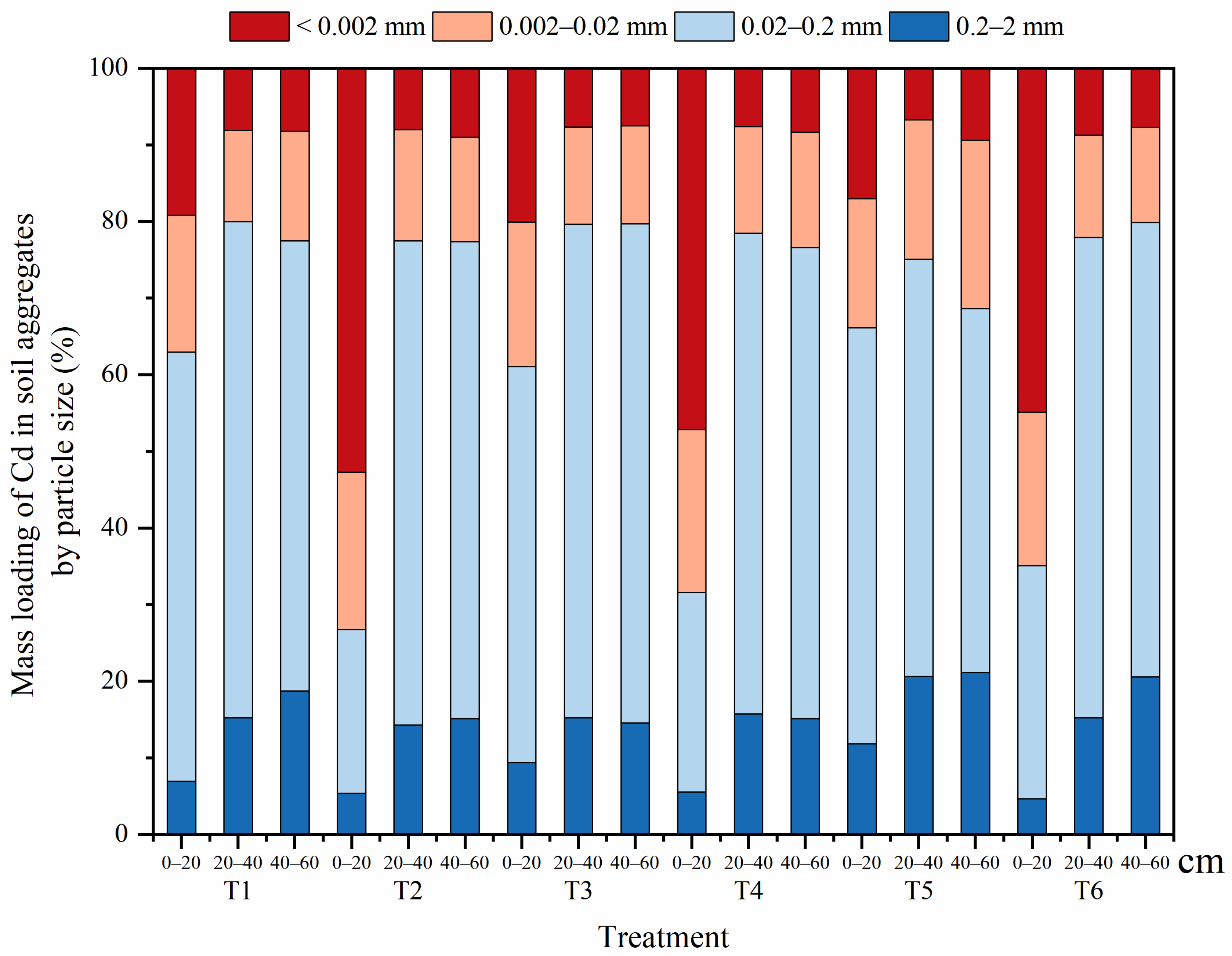
3.2.3. Mass Loading of Cd in Soil Aggregates of Different Grain Sizes
3.3. Morphology and Mineral Composition of Surface Soil Aggregates under Different Treatments in the Soil Column Experiment
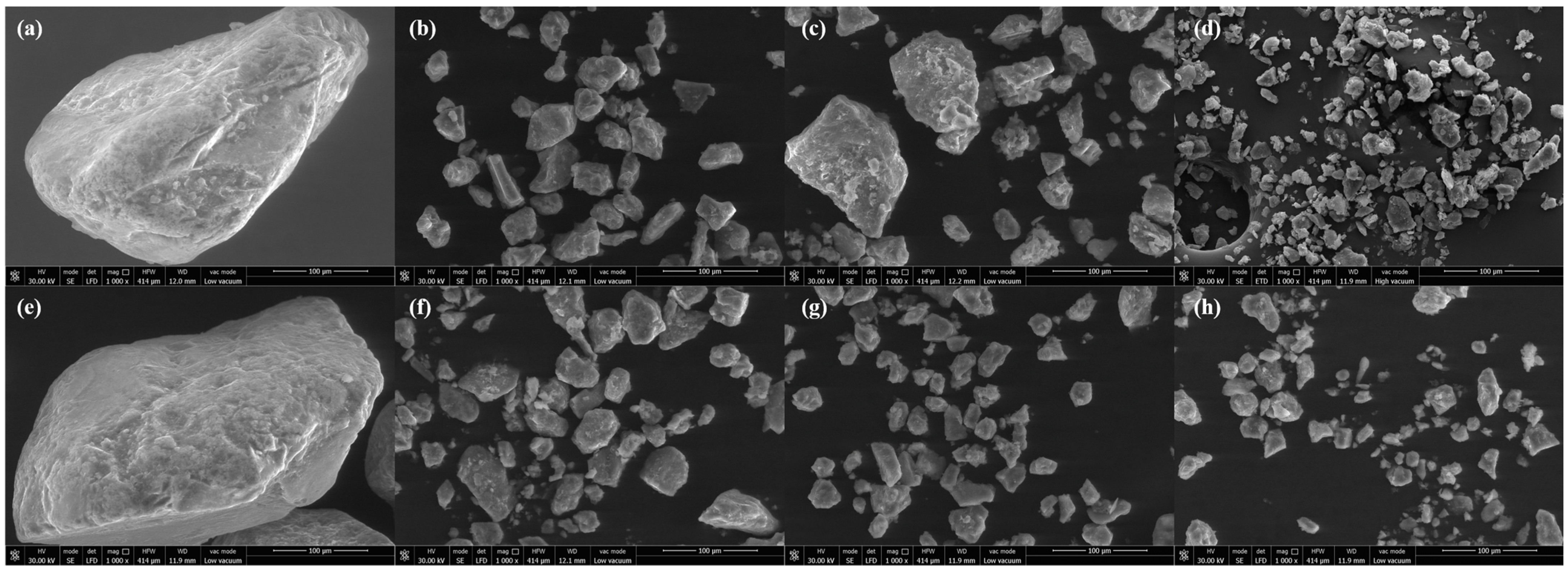
3.3.1. Surface Morphology of Soil Aggregates
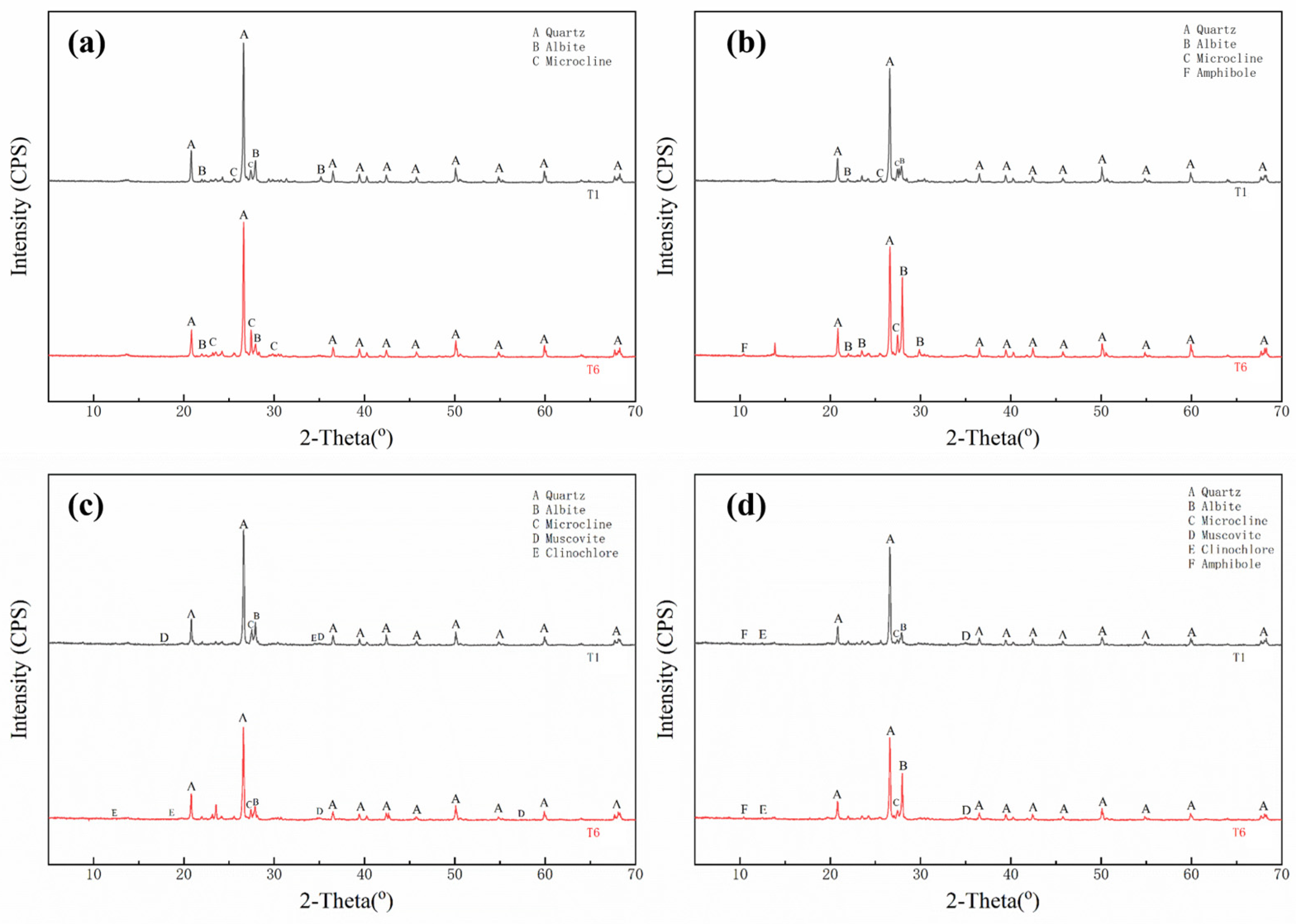
3.3.2. Mineral Composition of Soil Aggregates
4. Discussion
4.1. Remediation Techniques Change the Distribution of Cd in Soil Aggregates
4.2. Effects of Remediation Techniques on Changes in Soil Aggregate Stability
4.3. Structural Changes in Soil Aggregates and Remediation Mechanisms of Cd
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, S.; Zhong, Q.; Xu, X.; Li, T.; Jia, Y.; Zhang, Y.; Peijnenburg, W.J.G.M.; Vijver, M.G. Effect of soil washing with biodegradable chelators on the toxicity of residual metals and soil biological properties. Sci. Total Environ. 2018, 625, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, X.; Zheng, L.; Zhang, Y.; Sun, L.; Feng, Y.; Du, J.; Lu, X.; Wang, G. Comprehensive assessment of health and ecological risk of cadmium in agricultural soils across China: A tiered framework. J. Hazard. Mater. 2024, 465, 133111. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Wu, Y.; Xu, B.; Zhang, S.; Deng, X.; Zhang, Y.; Siddique, K.H.; Chen, Y. Nitrogen supply improved plant growth and Cd translocation in maize at the silking and physiological maturity under moderate Cd stress. Ecotoxicol. Environ. Saf. 2022, 230, 113137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, C.; Yu, H.; Zhao, Z.; Bai, Z. The addition of degradable chelating agents enhances maize phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2020, 269, 129373. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, H.; Yang, C.; Zhao, Z.; Ju, Z.; Wang, J.; Bai, Z. Enhanced phytoremediation of cadmium contaminated soil using chelating agents and plant growth regulators: Effect and mechanism. R. Soc. Open Sci. 2024, 11, 240672. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.; Ishaque, W.; Kamran, M.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Mojiri, A. The Potential of Corn (Zea mays) for Phytoremediation of Soil Contaminated with Cadmium and Lead. J. Biol. Environ. Sci. 2011, 5, 17–22. [Google Scholar]
- Kosnar, Z.; Mercl, F.; Tlustos, P. Ability of natural attenuation and phytoremediation using maize (Zea mays L.) to decrease soil contents of polycyclic aromatic hydrocarbons (PAHs) derived from biomass fly ash in comparison with PAHs-spiked soil. Ecotoxicol. Environ. Saf. 2018, 153, 16–22. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, D.; Lai, A.; Liu, G.; Liu, L.; Long, J.; Zhang, H.; Chen, Y. Leaching characteristics of EDTA-enhanced phytoextraction of Cd and Pb by Zea mays L. in different particle-size fractions of soil aggregates exposed to artificial rain. Environ. Sci. Pollut. Res. 2017, 24, 1845–1853. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Liang, X.; Sun, Y.; Huang, Q.; Qin, X.; Zhao, L. Effects of mercapto-palygorskite on Cd distribution in soil aggregates and Cd accumulation by wheat in Cd contaminated alkaline soil. Chemosphere 2021, 271, 129590. [Google Scholar] [CrossRef]
- Zhao, Z.; Mao, Y.; Gao, S.; Lu, C.; Pan, C.; Li, X. Organic carbon accumulation and aggregate formation in soils under organic and inorganic fertilizer management practices in a rice-wheat cropping system. Sci. Rep. 2023, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, X.; Perfect, E.; Xiao, T.; Peng, G. Effects of organic and inorganic fertilization on soil aggregation in an Ultisol as characterized by synchrotron based X-ray micro-computed tomography. Geoderma 2013, 195–196, 23–30. [Google Scholar] [CrossRef]
- Lv, C.; Bi, R.; Guo, X.; Chen, D.; Guo, Y.; Xu, Z. Erosion characteristics of different reclaimed substrates on iron tailings slopes under simulated rainfall. Sci. Rep. 2020, 10, 4275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; He, W.; Liu, Y.; Chen, Z.; Dong, Z.; Zhu, C.; Chen, Y.; Xiong, Y. Characteristics of soil erodibility in the Yinna mountainous area, eastern Guangdong Province, China. Int. J. Environ. Res. Public Health 2022, 19, 15703. [Google Scholar] [CrossRef] [PubMed]
- Shentu, J.; Li, X.; Han, R.; Chen, Q.; Shen, D.; Qi, S. Effect of site hydrological conditions and soil aggregate sizes on the stabilization of heavy metals (Cu, Ni, Pb, Zn) by biochar. Sci. Total Environ. 2022, 802, 149949. [Google Scholar] [CrossRef] [PubMed]
- Junyang, L.; Zhengzhao, Z.; Xuemeng, S. A review of the mechanism of plant root action on soil aggregate formation. J. Soil Water Conserv. 2020, 34, 8. [Google Scholar]
- Li, Y.; Zong, L.; Huang, Y. Application of chelators to phytoremediation of heavy metal polluted soils. Soil Environ. 2002, 11, 303–306. [Google Scholar] [CrossRef]
- Xu, W. Study on the Mechanism of Cadmium Tolerance and Enhanced Enrichment in Super Sweet 38 Maize. Master’s Thesis, South China University of Technology, Guangzhou, China, 2014. [Google Scholar]
- GB 15618-2018; Soil Environmental Quality Agricultural Land Soil Pollution Risk Control Standard. China Environment Publishing Group: Beijing, China, 2018.
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Z.; Bai, Z.; Hou, H.; Yuan, Y.; Guo, A.; Li, Y. Effects of mycorrhizae and water conditions on perennial ryegrass growth in rare earth tailings. RSC Adv. 2019, 9, 10881–10888. [Google Scholar] [CrossRef]
- Lu, R. Soil Agrochemical Analysis Method; China Agricultural Science Technology Press: Beijing, China, 2000. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- Zhu, T.; Fu, D.; Yang, F. Effect of saponin on the phytoextraction of Pb, Cd and Zn from soil using Italian Ryegrass. Bull. Environ. Contam. Toxicol. 2015, 94, 129–133. [Google Scholar] [CrossRef]
- Widdup, E.E.; Chatfield-Reed, K.; Henry, D.; Chua, G.; Samuel, M.A.; Muench, D.G. Identification of detoxification pathways in plants that are regulated in response to treatment with organic compounds isolated from oil sands process-affected water. Chemosphere 2015, 139, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, A.M.A.E.; Awad, E.-S.A.M.; Ibrahim, A.H.; Merwad, A.-R.M.A.; Desoky, E.-S.M. Integrative application of heavy metal–resistant bacteria, moringa extracts, and nano-silicon improves spinach yield and declines its contaminant contents on a heavy metal–contaminated soil. Front. Plant Sci. 2022, 13, 1019014. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, W.; Zhuang, L.; Wang, S.; Tsang, D.C.W.; Qiu, R. Two-stage multi-fraction first-order kinetic modeling for soil Cd extraction by EDTA. Chemosphere 2018, 211, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Liao, S. Effect of Chelating Agent EDDS and Growth Regulators on Cadmium Enrichment in Cowslip Chrysanthemum (Galinsoga parviflora). Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2016. [Google Scholar]
- Cheng, J. Effect of Chemical Drenching on the Structure of Soil Aggregates and the Desorption Characteristics of Heavy Metals. Master’s Thesis, Chongqing University, Chongqing, China, 2020. [Google Scholar]
- Asensio, V.; Covelo, E.F. Physical distribution of Ni, Pb and Zn in reclaimed mine soils observed by FE-SEM with an EDS detector. Environ. Sci. Pollut. Res. 2015, 22, 13372–13381. [Google Scholar] [CrossRef]
- Wei, M.; Chen, J.; Wang, Q. Remediation of sandy soil contaminated by heavy metals with Na2EDTA washing enhanced with organic reducing agents: Element distribution and spectroscopic analysis. Eur. J. Soil Sci. 2018, 69, 719–731. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Huang, J.; Guo, L.; Nie, X.; Wang, Y.; Zhang, Y.; Zeng, G. Adsorption characteristics of Cu and Zn onto various size fractions of aggregates from red paddy soil. J. Hazard. Mater. 2014, 264, 176–183. [Google Scholar] [CrossRef]
- Kim, H.; Cho, K.; Purev, O.; Choi, N.; Lee, J. Remediation of toxic heavy metal contaminated soil by combining a washing ejector based on hydrodynamic cavitation and soil washing process. Int. J. Environ. Res. Public Health 2022, 19, 786. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Li, D.; Yuan, Z.; Chen, Z.; Huang, J. Distribution characteristics of heavy metal(loid)s in aggregates of different size fractions along contaminated paddy soil profile. Environ. Sci. Pollut. Res. 2017, 24, 23939–23952. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Zhao, Z.; Li, X.; Han, Y.; Chen, S. Alleviation of cadmium phytotoxicity to wheat is associated with Cd re-distribution in soil aggregates as affected by amendments. RSC Adv. 2018, 8, 17426–17434. [Google Scholar] [CrossRef]
- Gong, C.; Ma, L.; Cheng, H.; Liu, Y.; Xu, D.; Li, B.; Liu, F.; Ren, Y.; Liu, Z.; Zhao, C.; et al. Characterization of the particle size fraction associated heavy metals in tropical arable soils from Hainan Island, China. J. Geochem. Explor. 2014, 139, 109–114. [Google Scholar] [CrossRef]
- Hou, R.; Li, T.; Fu, Q.; Liu, D.; Li, M.; Zhou, Z.; Li, Q.; Zhao, H.; Yu, P.; Yan, J. The effect on soil nitrogen mineralization resulting from biochar and straw regulation in seasonally frozen agricultural ecosystem. J. Clean. Prod. 2020, 255, 120302. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Chen, X.; Li, Y.; Yan, C.; Wang, C.; Wang, Y.; Xu, H. Enhanced Cd activation by coprinus comatus endophyte bacillus thuringiensis and the molecular mechanism. Environ. Pollut. 2024, 342, 123052. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, B.; Chen, H.; Li, J.; Xu, J.; Li, X. Gamma-aminobutyric acid enhances cadmium phytoextraction by coreopsis grandiflora by remodeling the rhizospheric environment. Plants 2023, 12, 1484. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tóth, T.; Yang, F.; Wang, Z. Effects of different types of vegetation cover on soil microorganisms and humus characteristics of soda-saline land in the Songnen Plain. Front. Microbiol. 2023, 14, 1163444. [Google Scholar] [CrossRef] [PubMed]
- Alemu, E.; Selassie, Y.G.; Yitaferu, B. Effect of lime on selected soil chemical properties, maize (Zea mays L.) yield and determination of rate and method of its application in Northwestern Ethiopia. Heliyon 2022, 8, e08657. [Google Scholar] [CrossRef]
- Liu, C.; Lin, H.; Li, B.; Dong, Y.; Yin, T.; Chen, X. Endophyte inoculation redistributed bioavailable Cd and nutrient in soil aggregates and enhanced Cd accumulation in Phytolacca acinosa. J. Hazard. Mater. 2021, 416, 125952. [Google Scholar] [CrossRef]
- Mondal, S.; Mishra, J.S.; Poonia, S.P.; Kumar, R.; Dubey, R.; Kumar, S.; Verma, M.; Rao, K.K.; Ahmed, A.; Dwivedi, S.; et al. Can yield, soil C and aggregation be improved under long-term conservation agriculture in the eastern Indo-Gangetic plain of India? Eur. J. Soil Sci. 2021, 72, 1742–1761. [Google Scholar] [CrossRef]
- Nie, X.; Wang, D.; Ren, L.; Ma, K.; Chen, Y.; Yang, L.; Du, Y.; Zhou, G. Distribution characteristics and controlling factors of soil total nitrogen: Phosphorus ratio across the Northeast Tibetan Plateau Shrublands. Front. Plant Sci. 2022, 13, 825817. [Google Scholar] [CrossRef]
- Soinne, H.; Hyväluoma, J.; Ketoja, E.; Turtola, E. Relative importance of organic carbon, land use and moisture conditions for the aggregate stability of post-glacial clay soils. Soil Tillage Res. 2016, 158, 1–9. [Google Scholar] [CrossRef]
- Fliessbach, A.; Martens, R.; Reber, H.H. Soil microbial biomass and microbial activity in soils treated with heavy metal contaminated sewage sludge. Soil Biol. Biochem. 1994, 26, 1201–1205. [Google Scholar] [CrossRef]
- Bernasconi, D.; Caviglia, C.; Destefanis, E.; Agostino, A.; Boero, R.; Marinoni, N.; Bonadiman, C.; Pavese, A. Influence of speciation distribution and particle size on heavy metal leaching from MSWI fly ash. Waste Manag. 2022, 138, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Zhang, X.; He, F.; Liu, X.; Bian, R.; Cheng, K.; Li, L.; Pan, G. Effects of different levels of heavy metal pollution on the composition of rice soil aggregates. SOIL 2017, 49, 337–344. [Google Scholar]
- Yu, H.; Li, C.; Yan, J.; Ma, Y.; Zhou, X.; Yu, W.; Kan, H.; Meng, Q.; Xie, R.; Dong, P. A review on adsorption characteristics and influencing mechanism of heavy metals in farmland soil. RSC Adv. 2023, 13, 3505–3519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, Z.D.; Liu, Y.; Jiang, J.; Xu, R.K. Relative abundance of chemical forms of Cu(II) and Cd(II) on soybean roots as influenced by pH, cations and organic acids. Sci. Rep. 2016, 6, 36373. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Cai, C.; Yuan, Z.; Li, D.; Liao, Y.; Deng, Y. Quantifying the contribution of phyllosilicate mineralogy to aggregate stability in the East Asian monsoon region. Geoderma 2021, 393, 115036. [Google Scholar] [CrossRef]
- Walia, S.S.; Dhaliwal, S.S.; Gill, R.S.; Kaur, T.; Kaur, K.; Randhawa, M.K.; Obročník, O.; Bárek, V.; Brestic, M.; Gaber, A.; et al. Improvement of soil health and nutrient transformations under balanced fertilization with integrated nutrient management in a rice-wheat system in Indo-Gangetic Plains—A 34-year Research outcomes. Heliyon 2024, 10, e25113. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]

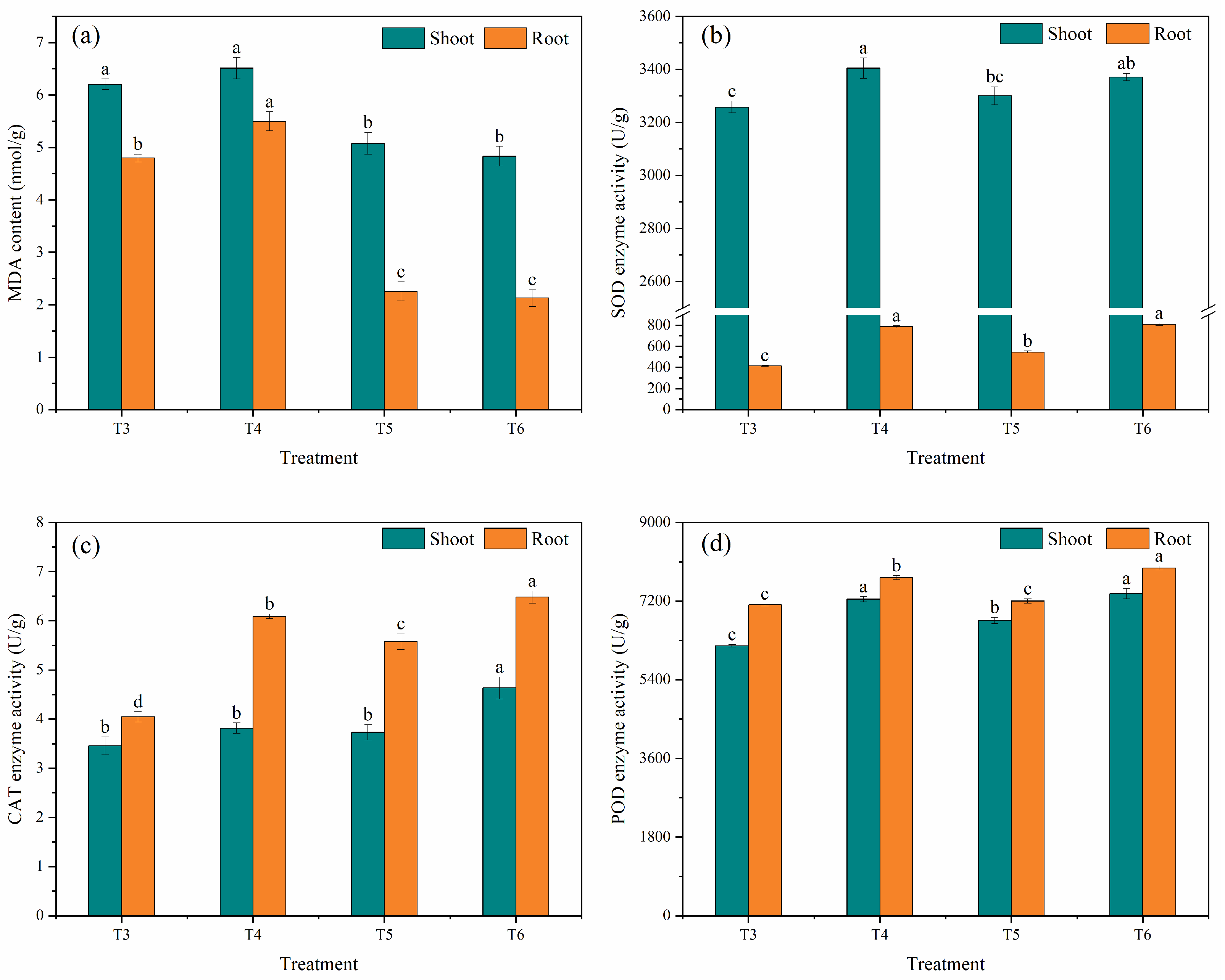
| Treatment | Plant | Chelating Agent | GA3 |
|---|---|---|---|
| T1 | – | – | – |
| T2 | – | 6 mmol/kg AES | – |
| T3 | Maize | – | – |
| T4 | Maize | 6 mmol/kg AES | – |
| T5 | Maize | – | 10−6 mol/L GA3 |
| T6 | Maize | 6 mmol/kg AES | 10−6 mol/L GA3 |
| Ratio | T3 | T4 | T5 | T6 |
|---|---|---|---|---|
| BCF | 0.47 ± 0.02 c | 1.12 ± 0.01 a | 0.57 ± 0.01 b | 1.16 ± 0.02 a |
| TF | 0.52 ± 0.02 c | 0.88 ± 0.01 a | 0.65 ± 0.01 b | 0.93 ± 0.02 a |
| Treatment | MWD (mm) | ||
|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | |
| T1 | 0.15 ± 0.001 c | 0.24 ± 0.008 b | 0.24 ± 0.001 c |
| T2 | 0.15 ± 0.003 c | 0.23 ± 0.003 b | 0.24 ± 0.003 c |
| T3 | 0.17 ± 0.001 b | 0.24 ± 0.003 b | 0.24 ± 0.005 c |
| T4 | 0.13 ± 0.003 d | 0.25 ± 0.008 b | 0.25 ± 0.006 c |
| T5 | 0.20 ± 0.003 a | 0.27 ± 0.005 a | 0.27 ± 0.005 b |
| T6 | 0.14 ± 0.002 d | 0.24 ± 0.007 b | 0.30 ± 0.007 a |
| Treatment | Soil Depth /cm | Total Cd (mg/kg) | |||
|---|---|---|---|---|---|
| 0.2–2 mm | 0.02–0.2 mm | 0.002–0.02 mm | <0.002 mm | ||
| T1 | 0–20 | 6.12 ± 0.23 | 4.61 ± 0.20 | 7.50 ± 0.24 | 10.50 ± 0.22 |
| 20–40 | 0.23 ± 0.01 | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.32 ± 0.03 | |
| 40–60 | 0.23 ± 0.02 | 0.16 ± 0.01 | 0.21 ± 0.04 | 0.28 ± 0.02 | |
| T2 | 0–20 | 3.95 ± 0.19 | 3.05 ± 0.16 | 8.14 ± 0.21 | 12.09 ± 0.30 |
| 20–40 | 0.25 ± 0.02 | 0.24 ± 0.02 | 0.28 ± 0.02 | 0.29 ± 0.02 | |
| 40–60 | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.20 ± 0.04 | 0.24 ± 0.04 | |
| T3 | 0–20 | 6.02 ± 0.29 | 4.27 ± 0.32 | 6.94 ± 0.18 | 9.77 ± 0.28 |
| 20–40 | 0.25 ± 0.04 | 0.23 ± 0.01 | 0.27 ± 0.01 | 0.31 ± 0.01 | |
| 40–60 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.03 | 0.25 ± 0.02 | |
| T4 | 0–20 | 4.90 ± 0.07 | 3.29 ± 0.11 | 7.19 ± 0.09 | 11.20 ± 0.13 |
| 20–40 | 0.25 ± 0.02 | 0.24 ± 0.01 | 0.28 ± 0.01 | 0.31 ± 0.02 | |
| 40–60 | 0.18 ± 0.01 | 0.17 ± 0.004 | 0.24 ± 0.01 | 0.25 ± 0.02 | |
| T5 | 0–20 | 6.17 ± 0.19 | 5.36 ± 0.10 | 6.99 ± 0.10 | 9.82 ± 0.20 |
| 20–40 | 0.25 ± 0.01 | 0.19 ± 0.01 | 0.28 ± 0.01 | 0.29 ± 0.02 | |
| 40–60 | 0.21 ± 0.01 | 0.14 ± 0.01 | 0.28 ± 0.03 | 0.29 ± 0.01 | |
| T6 | 0–20 | 3.95 ± 0.09 | 3.80 ± 0.05 | 7.09 ± 0.10 | 10.89 ± 0.08 |
| 20–40 | 0.24 ± 0.01 | 0.22 ± 0.01 | 0.28 ± 0.04 | 0.31 ± 0.02 | |
| 40–60 | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.20 ± 0.02 | 0.25 ± 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Yu, H.; Zhao, Z.; Ju, Z. Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure. Agriculture 2024, 14, 1882. https://doi.org/10.3390/agriculture14111882
Yang Q, Yu H, Zhao Z, Ju Z. Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure. Agriculture. 2024; 14(11):1882. https://doi.org/10.3390/agriculture14111882
Chicago/Turabian StyleYang, Qiao, Hao Yu, Zhongqiu Zhao, and Zhengshan Ju. 2024. "Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure" Agriculture 14, no. 11: 1882. https://doi.org/10.3390/agriculture14111882
APA StyleYang, Q., Yu, H., Zhao, Z., & Ju, Z. (2024). Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure. Agriculture, 14(11), 1882. https://doi.org/10.3390/agriculture14111882





