Effects of Cover Measures on Soil Organic Nitrogen Fractions and Total Soluble Nitrogen Pools in Citrus Orchards of the Red Soil Hilly Region of Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Soil Sampling and Measurement Methods
2.4. Statistical Analysis
3. Results and Analysis
3.1. Basic Soil Physicochemical Properties
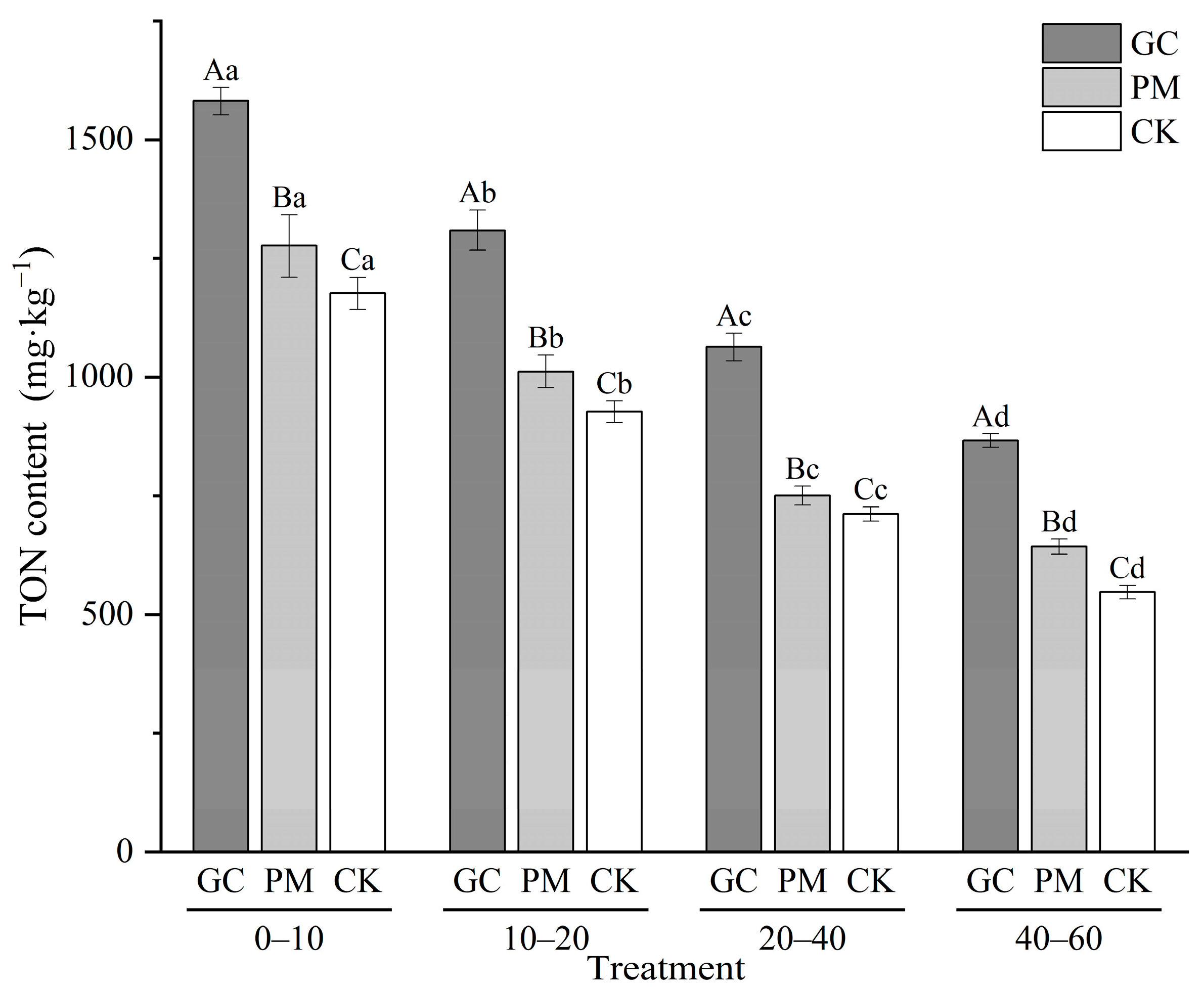
3.2. TON in Soil
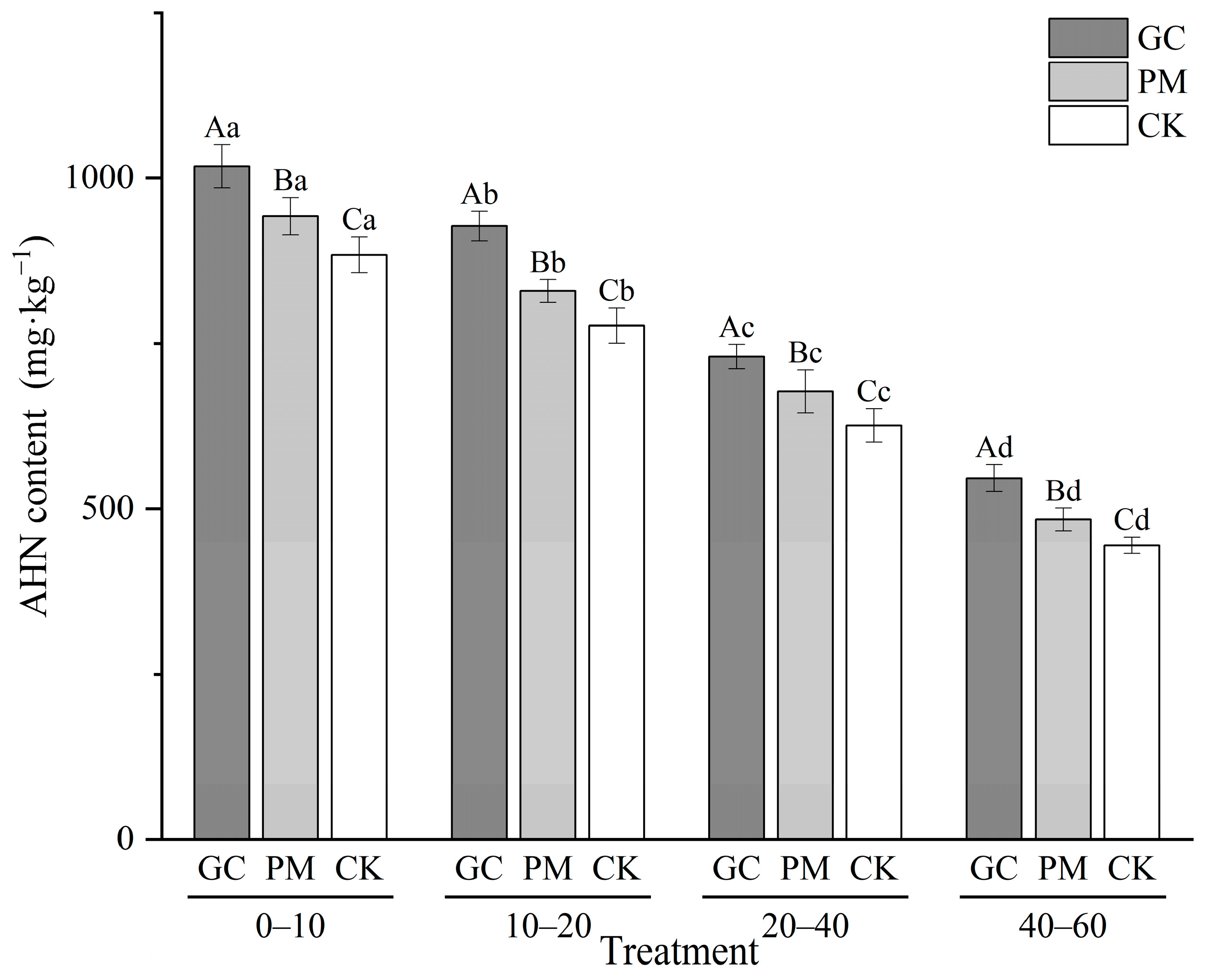
3.3. Acid-Hydrolyzable TN
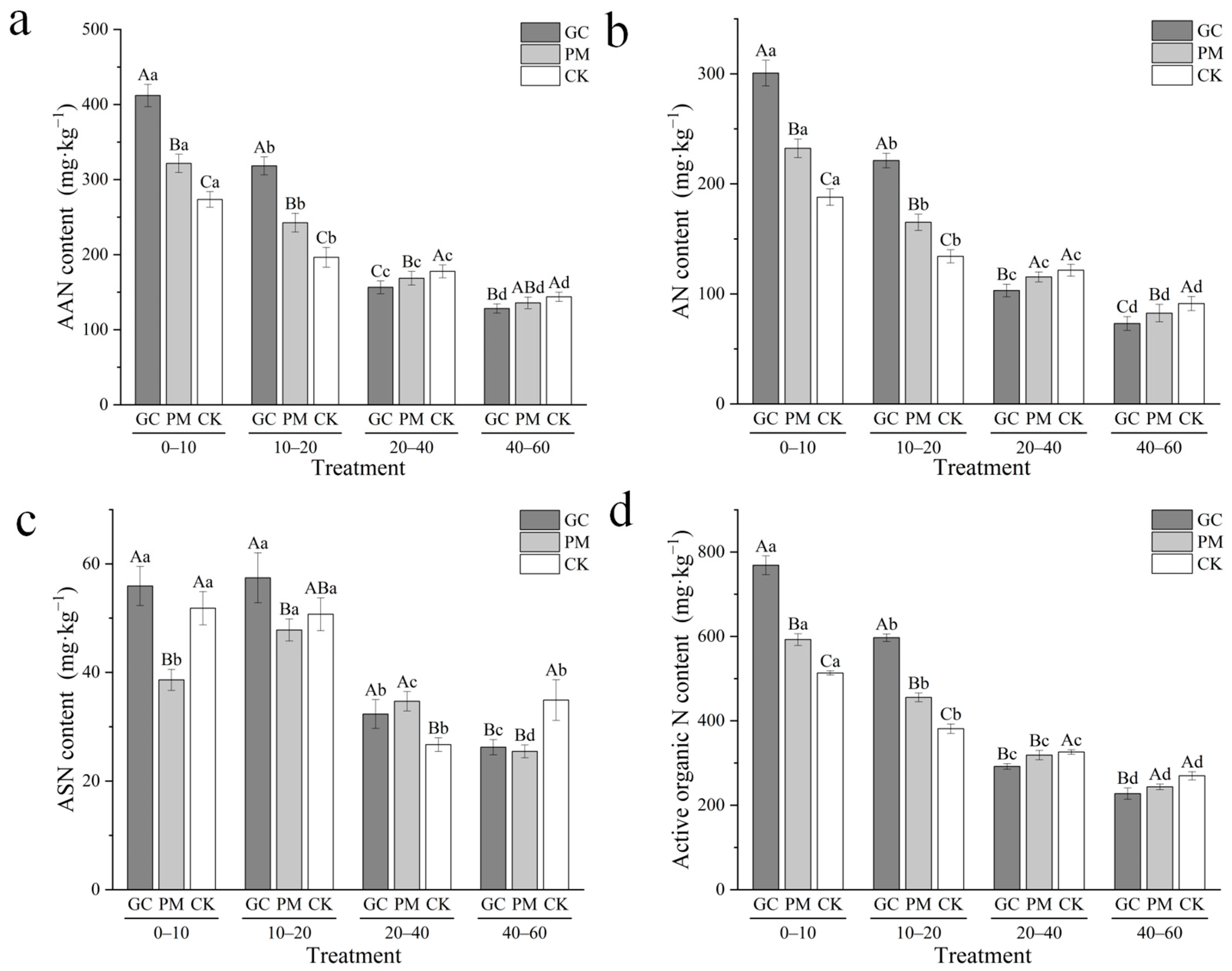
3.4. Active Organic N
3.5. Stable Organic N
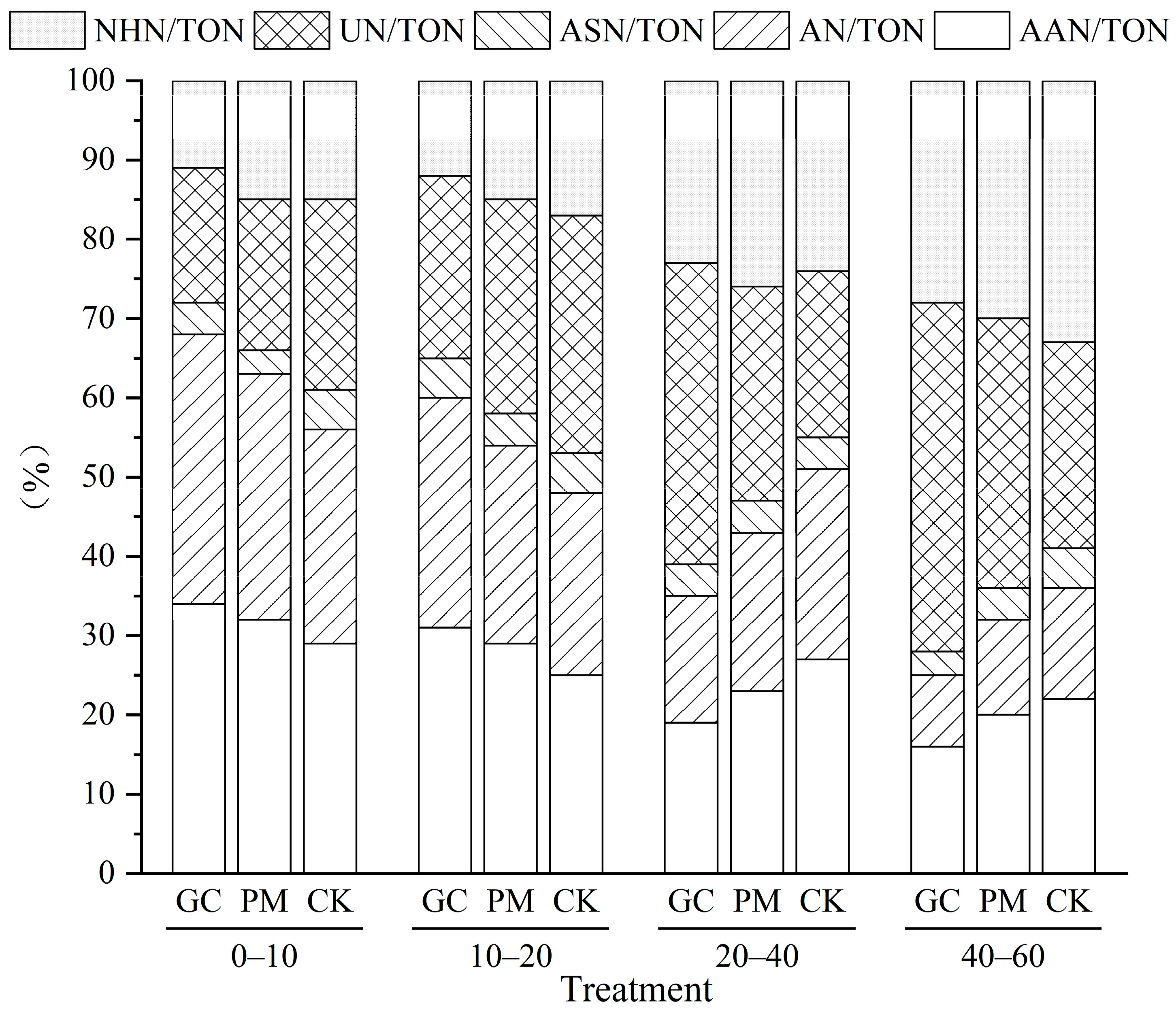
3.6. Proportion of Organic N Fractions
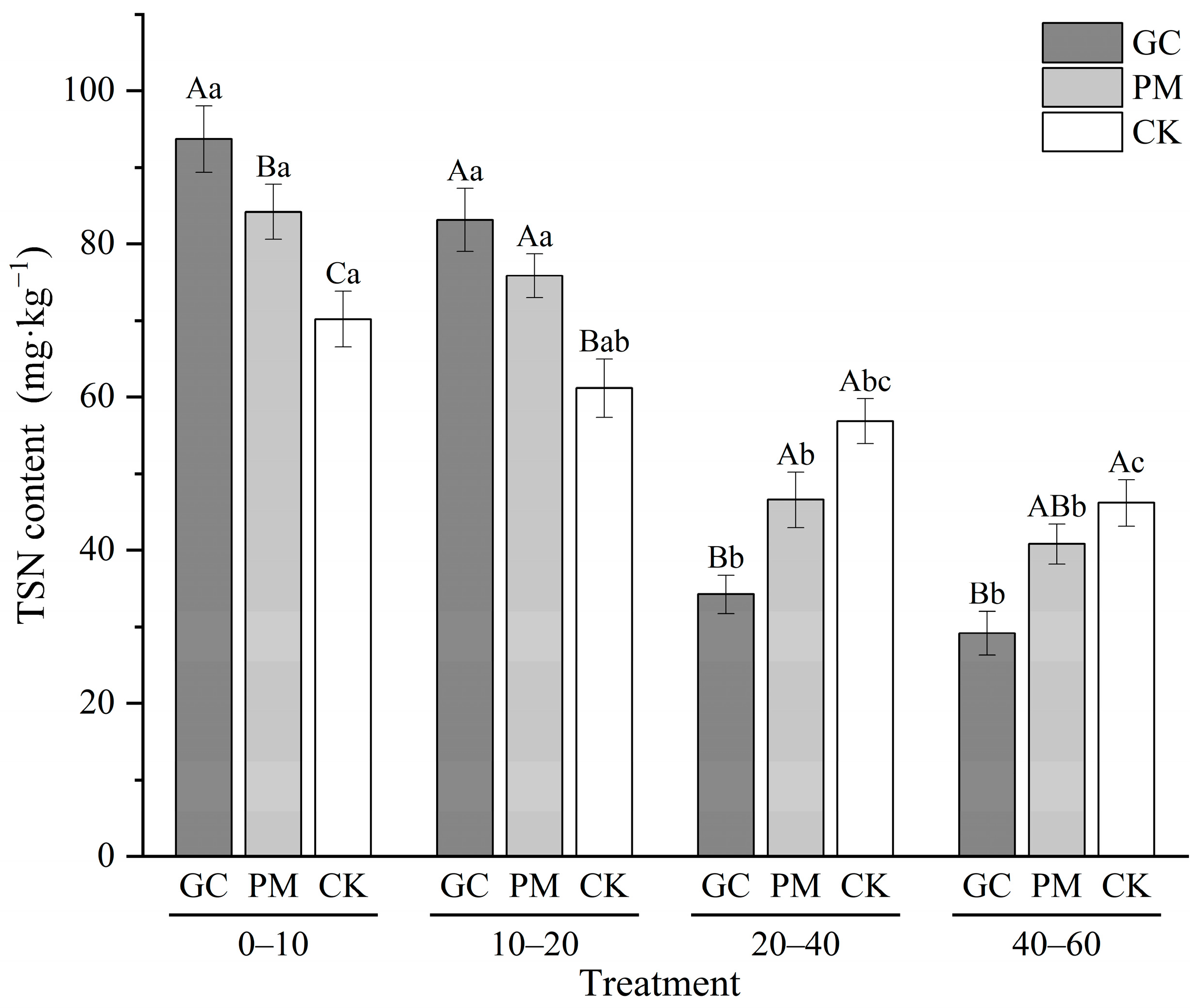
3.7. Total Soluble N
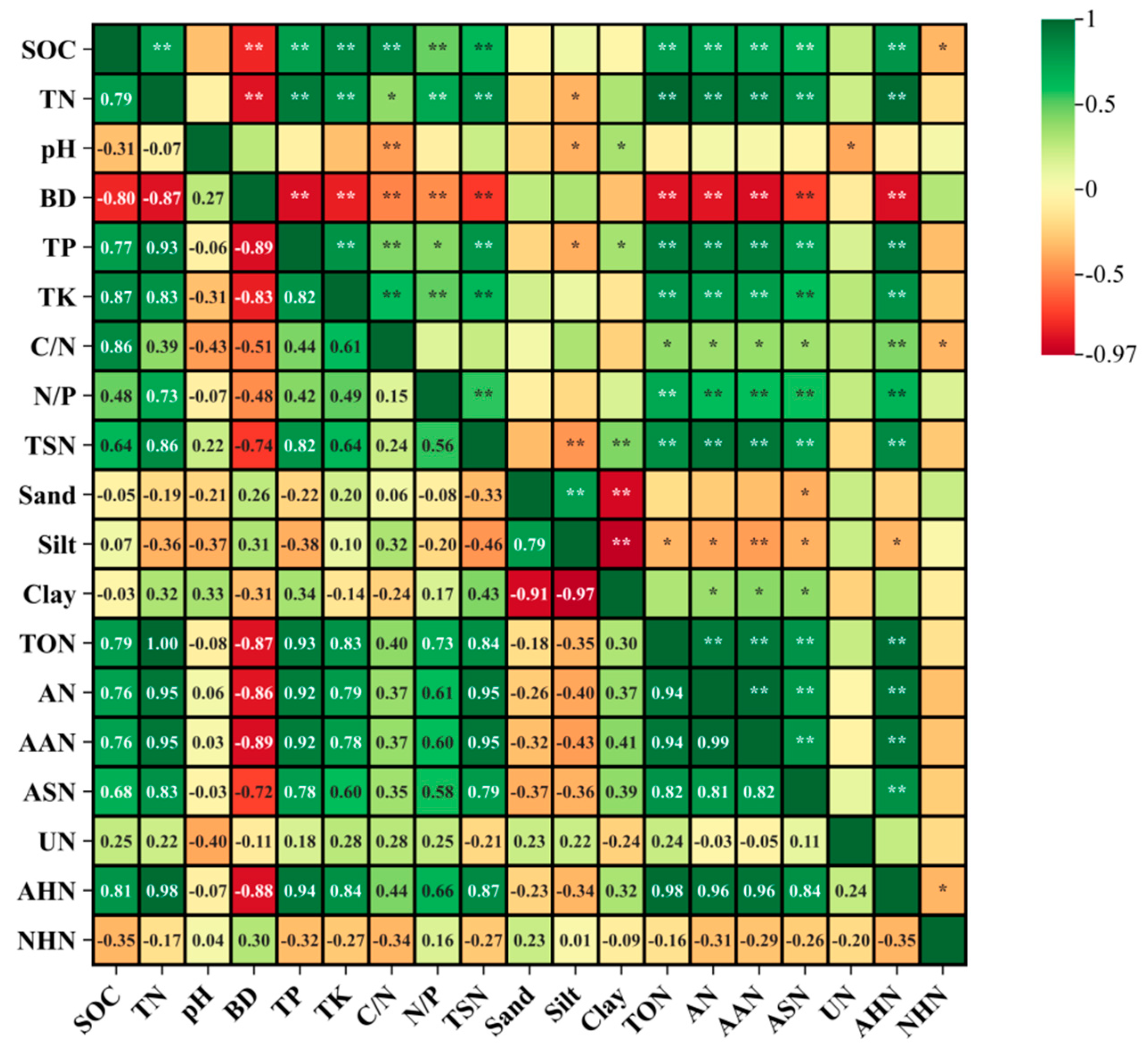
3.8. Relationships between Soil Physicochemical Properties, Soil Organic N Fractions, and Total Soluble N Pools
4. Discussion
4.1. Effect of Cover Measures on the Soil Physicochemical Properties in Orchards
4.2. Effect of Cover Measures on Soil Organic N Fractions in Orchards
4.3. Effect of Cover Measures on the Total Soluble N Pools in Orchard Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Yang, J.-L.; Zhao, X.-R.; Yang, S.-H.; Mulder, J.; Dörsch, P.; Zhang, G.-L. Seasonal dynamics of soil pH and N transformation as affected by N fertilization in subtropical China: An in situ 15N labeling study. Sci. Total Environ. 2022, 816, 151596. [Google Scholar] [CrossRef] [PubMed]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J.; Xu, G. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.; Yuan, H.; Chen, B.; Zhuang, S. Climatic effects on soil organic nitrogen fractions and amino acid chirality in paddy soils. Pedosphere 2023, 33, 579–588. [Google Scholar] [CrossRef]
- Miller, A.E.; Bowman, W.D.; Suding, K.N. Plant uptake of inorganic and organic nitrogen: Neighbor identity matters. Ecology 2007, 88, 1832–1840. [Google Scholar] [CrossRef]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 2020, 150, 103452. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Farzadfar, S.; Knight, J.D.; Congreves, K.A. Soil organic nitrogen: An overlooked but potentially significant contribution to crop nutrition. Plant Soil 2021, 462, 7–23. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, D. Nitrogen fertilizer use in China–Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Bremner, J.M. Organic Forms of Soil Nitrogen. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1238–1255. [Google Scholar]
- Su, H.; Zhang, Y.; Wu, G.; Chen, Z.; Jiang, N.; Qiu, W.; Chen, L. Effects of different maize residue managements on soil organic nitrogen cycling in different soil layers in northeast China. GCB Bioenergy 2024, 16, e13123. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Li, Z.; Li, D.; Wang, Q.; Zhou, Y.; Guo, W.; Ma, D.; Zhang, J.; Zhao, B. Long-term nitrogen fertilization-induced enhancements of acid hydrolyzable nitrogen are mainly regulated by the most vital microbial taxa of keystone species and enzyme activities. Sci. Total Environ. 2023, 874, 162463. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol. Biochem. 2013, 66, 119–129. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Noll, L.; Zhang, S.; Wanek, W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zhou, Y.; Zhang, J.; Nadeem, M.Y.; Miao, C.; You, J.; Li, W.; Jiang, Y.; Ding, Y.; et al. Long-term straw returning improved soil nitrogen sequestration by accelerating the accumulation of amino acid nitrogen. Agric. Ecosyst. Environ. 2024, 362, 108846. [Google Scholar] [CrossRef]
- Stevenson, F.J. Organic Forms of Soil Nitrogen. Nitrogen Agric. Soils 1982, 22, 67–122. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Murphy, D.V.; Farrar, J. Role of dissolved organic nitrogen (DON) in soil N cycling in grassland soils. Soil Biol. Biochem. 2004, 36, 749–756. [Google Scholar] [CrossRef]
- Murphy, D.V.; Macdonald, A.J.; Stockdale, E.A.; Goulding, K.W.T.; Fortune, S.; Gaunt, J.L.; Poulton, P.R.; Wakefield, J.A.; Webster, C.P.; Wilmer, W.S. Soluble organic nitrogen in agricultural soils. Biol. Fertil. Soils 2000, 30, 374–387. [Google Scholar] [CrossRef]
- Christou, M.; Avramides, E.J.; Jones, D.L. Dissolved organic nitrogen dynamics in a Mediterranean vineyard soil. Soil Biol. Biochem. 2006, 38, 2265–2277. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Zhang, Y.; Yu, N.; Zou, H.; Li, Y.; Liu, S.; Tong, Y. Soil nitrogen mineralisation dynamics under long-term different irrigation methods in greenhouses. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2014, 63, 117–126. [Google Scholar] [CrossRef]
- Ros, G.H.; Hoffland, E.; van Kessel, C.; Temminghoff, E.J.M. Extractable and dissolved soil organic nitrogen—A quantitative assessment. Soil Biol. Biochem. 2009, 41, 1029–1039. [Google Scholar] [CrossRef]
- VanZomeren, C.M.; Bhomia, R.K.; Tfaily, M.M.; Inglett, K.S.; Cooper, W.T.; White, J.R.; Reddy, K.R. Influence of vegetation on soil organic nitrogen composition and mineralization in a subtropical wetland. Ecol. Eng. 2024, 200, 107186. [Google Scholar] [CrossRef]
- Yang, J.; Guo, W.; Wang, F.; Wang, F.; Zhang, L.; Zhou, B.; Xing, S.; Yang, W. Dynamics and influencing factors of soluble organic nitrogen in paddy soil under different long-term fertilization treatments. Soil Tillage Res. 2021, 212, 105077. [Google Scholar] [CrossRef]
- Han, Y.; Yi, D.; Ye, Y.; Guo, X.; Liu, S. Response of spatiotemporal variability in soil pH and associated influencing factors to land use change in a red soil hilly region in southern China. CATENA 2022, 212, 106074. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Xu, Z.H.; Zhang, S.L.; Keay, P. Soluble Organic Nitrogen Pools in Forest soils of Subtropical Australia. Plant Soil 2005, 277, 285–297. [Google Scholar] [CrossRef]
- Yang, S.; Dong, Y.; Wu, H.; Song, X.; Zhao, X.; Yang, J.; Zhang, G.-L. Deep accumulation of soluble organic nitrogen after land-use conversion from woodlands to orchards in a subtropical hilly region. Sci. Total Environ. 2023, 863, 160931. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Aslam, M.U.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 75. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; Korthals, G.W.; Brussaard, L.; Mainardi, G.; De Deyn, G.B. Litter quality drives nitrogen release, and agricultural management (organic vs. conventional) drives carbon loss during litter decomposition in agro-ecosystems. Soil Biol. Biochem. 2021, 153, 108115. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhao, J.S.; Shi, Z.H.; Wang, L. Soil aggregates are key factors that regulate erosion-related carbon loss in citrus orchards of southern China: Bare land vs. grass-covered land. Agric. Ecosyst. Environ. 2021, 309, 107254. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014 (Update2015). International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Yang, J.; Duan, Y.; Zhang, R.; Liu, C.; Wang, Y.; Li, M.; Ding, Y.; Awasthi, M.K.; Li, H. Connecting soil dissolved organic matter to soil bacterial community structure in a long-term grass-mulching apple orchard. Ind. Crops Prod. 2020, 149, 112344. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a Sustainable Water and Soil Saving Practice in Agriculture: A Review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Dong, Q.; Dang, T.; Guo, S.; Hao, M. Effect of different mulching measures on nitrate nitrogen leaching in spring maize planting system in south of Loess Plateau. Agric. Water Manag. 2019, 213, 654–658. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Han, C.-L.; Kong, M.; Shi, X.-Y.; Zdruli, P.; Li, F.-M. Plastic film mulch promotes high alfalfa production with phosphorus-saving and low risk of soil nitrogen loss. Field Crops Res. 2018, 229, 44–54. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Hu, W.; Qin, J.; Zheng, Y.; Wang, J.; Wang, Q.; Xu, Y.; Guo, G.; Hu, S.; et al. Effects of plastic mulch film residues on soil-microbe-plant systems under different soil pH conditions. Chemosphere 2021, 267, 128901. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S.; Zhu, Z. Soil organic nitrogen composition and mineralization of paddy soils in a cultivation chronosequence in China. J. Soils Sediments 2017, 17, 1588–1598. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, W.; Goodwin, P.H.; Wang, Y.; Zheng, S.-J.; Li, X. Effect of cover crop on soil fertility and bacterial diversity in a banana plantation in southwestern China. Soil Tillage Res. 2024, 240, 106092. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Ritz, K.; Cantarella, H.; Galdos, M.V.; Hawkesford, M.J.; Whalley, W.R.; Mooney, S.J. Enhanced Plant Rooting and Crop System Management for Improved N Use Efficiency. Adv. Agron. 2017, 146, 205–239. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Pan, H.-j.; Zhang, K.-y.; Sun, W.; Wang, X.-j.; Gao, H. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Nevins, C.J.; Lacey, C.; Armstrong, S. The synchrony of cover crop decomposition, enzyme activity, and nitrogen availability in a corn agroecosystem in the Midwest United States. Soil Tillage Res. 2020, 197, 104518. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, L.; Fang, Q.; Zi, R.; Fang, F.; Fan, C.; Ding, G. Effects of biochar on runoff generation, soil and nutrient loss at the surface and underground on the soil-mantled karst slopes. Sci. Total Environ. 2023, 889, 164081. [Google Scholar] [CrossRef]
- Brangarí, A.C.; Lyonnard, B.; Rousk, J. Soil depth and tillage can characterize the soil microbial responses to drying-rewetting. Soil Biol. Biochem. 2022, 173, 108806. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Song, Y.; Sun, L.; Chen, X.; Wu, J.; Song, C.; Zhao, Y. Slowed down nitrogen mineralization under bacterial community-driven conditions by adding inhibitors during rice straw composting. Bioresour. Technol. 2022, 362, 127778. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, Z.; Yang, X.; Shen, Y. The effects of cocksfoot cover crop on soil water balance, evapotranspiration partitioning, and system production in an apple orchard on the Loess Plateau of China. Soil Tillage Res. 2021, 205, 104788. [Google Scholar] [CrossRef]
- Tu, A.; Xie, S.; Zheng, H.; Li, H.; Li, Y.; Mo, M. Long-term effects of living grass mulching on soil and water conservation and fruit yield of citrus orchard in south China. Agric. Water Manag. 2021, 252, 106897. [Google Scholar] [CrossRef]
- Tian, M.; Jiang, N.; Yu, C.; Zhang, Y.; Qiu, W.; Chen, Z.; Wang, J.; Chen, L. Sensitivity of active and stable organic nitrogen to nitrogen and carbon additions: Insights from enzymatic hydrolyses in a semi-arid steppe. Plant Soil 2024, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Peng, Y.; Zhang, S.; Lv, S.; Li, J.; Abdo, A.I.; Zhou, C.; Wang, L. Film mulching, residue retention and N fertilization affect ammonia volatilization through soil labile N and C pools. Agric. Ecosyst. Environ. 2021, 308, 107272. [Google Scholar] [CrossRef]
- Rocha, K.F.; de Souza, M.; Almeida, D.S.; Chadwick, D.R.; Jones, D.L.; Mooney, S.J.; Rosolem, C.A. Cover crops affect the partial nitrogen balance in a maize-forage cropping system. Geoderma 2020, 360, 114000. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co-incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Tillage Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhang, J.; Li, D.; Yu, J.; Gao, H.; Li, H.; Zhao, Z. Soil phytoremediation reveals alteration in soil microbial metabolic activities along time gradient of cover crop mulching. Environ. Res. 2022, 209, 112884. [Google Scholar] [CrossRef]
- Sun, X.; Tao, R.; Xu, D.; Qu, M.; Zheng, M.; Zhang, M.; Mei, Y. Role of polyamide microplastic in altering microbial consortium and carbon and nitrogen cycles in a simulated agricultural soil microcosm. Chemosphere 2023, 312, 137155. [Google Scholar] [CrossRef]
- Wu, H.; Du, S.; Zhang, Y.; An, J.; Zou, H.; Zhang, Y.; Yu, N. Effects of irrigation and nitrogen fertilization on greenhouse soil organic nitrogen fractions and soil-soluble nitrogen pools. Agric. Water Manag. 2019, 216, 415–424. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Hou, H.; Yu, X.; Ma, Y.; Fang, Y.; Lei, K. Did plastic mulching constantly increase crop yield but decrease soil water in a semiarid rain-fed area? Agric. Water Manag. 2020, 241, 106380. [Google Scholar] [CrossRef]
- Ridgeway, J.R.; Morrissey, E.M.; Brzostek, E.R. Plant litter traits control microbial decomposition and drive soil carbon stabilization. Soil Biol. Biochem. 2022, 175, 108857. [Google Scholar] [CrossRef]
- Mengel, K.; Schneider, B.; Kosegarten, H. Nitrogen compounds extracted by electroultrafiltration (EUF) or CaCl2 solution and their relationships to nitrogen mineralization in soils. J. Plant Nutr. Soil Sci. 1999, 162, 139–148. [Google Scholar] [CrossRef]



| Depth /(cm) | Treatment | BD /(kg·m−3) | Soil Texture/% | ||
|---|---|---|---|---|---|
| Sand 0.05–2 mm | Silt 0.05–0.002 mm | Clay <0.002 mm | |||
| 0–10 | GC | 1050 ± 22 Cc | 21.15 ± 0.87 Aa | 67.75 ± 0.19 Ab | 11.1 ± 0.87 Ab |
| PM | 1120 ± 25 Bc | 23.99 ± 0.86 Ba | 65.07 ± 0.61 Bc | 10.95 ± 0.28 Ab | |
| CK | 1170 ± 47 Ac | 27.51 ± 0.59 Ca | 63.34 ± 0.71 Cd | 9.16 ± 0.74 Bb | |
| 10–20 | GC | 1090 ± 3 Cc | 20.09 ± 1.02 Aa | 68.71 ± 1.09 Aab | 11.21 ± 0.07 Ab |
| PM | 1180 ± 4 Bb | 22.87 ± 0.20 Bab | 66.04 ± 0.57 Bbc | 11.09 ± 0.37 Aab | |
| CK | 1240 ± 3 Ab | 25.79 ± 0.68 Cb | 64.98 ± 0.60 Bc | 9.23 ± 0.19 Bb | |
| 20–40 | GC | 1180 ± 10 Cb | 19.59 ± 0.72 Aa | 69.04 ± 0.57 Aab | 11.37 ± 0.19 Ab |
| PM | 1220 ± 20 Bb | 21.77 ± 0.58 Bb | 67.02 ± 0.47 Bb | 11.21 ± 0.13 Aab | |
| CK | 1290 ± 30 Ab | 24.44 ± 0.4 Cc | 66.07 ± 0.21 Bb | 9.49 ± 0.19 Bb | |
| 40–60 | GC | 1400 ± 40 Aa | 16.59 ± 0.86 Ab | 70.47 ± 1.07 Aa | 12.94 ± 0.21 Aa |
| PM | 1410 ± 14 Aa | 18.99 ± 1.39 Bc | 68.96 ± 0.80 ABa | 12.04 ± 0.61 Ba | |
| CK | 1420 ± 10 Aa | 21.97 ± 0.11 Cd | 67.26 ± 0.02 Ba | 10.77 ± 0.09 Ca | |
| Depth /(cm) | Treatment | SOC /(g·kg−1) | TN /(g·kg−1) | TP /(g·kg−1) | TK /(g·kg−1) | pH | C/N | N/P |
|---|---|---|---|---|---|---|---|---|
| 0–10 | GC | 26.62 ± 3.87 Aa | 1.64 ± 0.037 Aa | 1.75 ± 0.60 Aa | 27.98 ± 0.98 Aa | 5.31 ± 0.024 Ba | 16.28 ± 2.41 Aa | 1 ± 0.31 Aa |
| PM | 16.88 ± 1.43 Ba | 1.33 ± 0.10 Ba | 1.69 ± 0.07 Ba | 21.54 ± 0.50 Ba | 5.49 ± 0.037 Ab | 12.73 ± 0.42 Bb | 0.79 ± 0.091 Ba | |
| CK | 15.69 ± 0.19 Ba | 1.22 ± 0.038 Ba | 1.47 ± 0.097 Ca | 17.23 ± 1.23 Ca | 5.19 ± 0.041 Cc | 12.91 ± 0.54 Ba | 0.83 ± 0.030 Ba | |
| 10–20 | GC | 23.39 ± 0.43 Ab | 1.36 ± 0.17 Ab | 1.54 ± 0.20 Ab | 23.6 ± 0.57 Ab | 4.98 ± 0.004 Cb | 17.24 ± 0.51 Aa | 0.89 ± 0.11 Ab |
| PM | 15.84 ± 1.11 Bb | 1.06 ± 0.018 Bb | 1.49 ± 0.13 Bb | 18.6 ± 0.57 Bb | 5.38 ± 0.002 Bb | 15.01 ± 1.23 Ba | 0.71 ± 0.056 Ba | |
| CK | 13.15 ± 0.43 Cb | 0.96 ± 0.023 Cb | 1.26 ± 0.002 Cb | 14.23 ± 0.62 Cb | 5.48 ± 0.002 Aa | 13.70 ± 0.64 Ca | 0.76 ± 0.032 Bb | |
| 20–40 | GC | 19.96 ± 3.77 Ac | 1.09 ± 0.015 Ac | 1.22 ± 0.092 Ac | 19.78 ± 1.11 Ac | 4.94 ± 0.02 Cb | 18.32 ± 3.27 Aa | 0.89 ± 0.054 Ab |
| PM | 10.35 ± 1.35 Bc | 0.79 ± 0.014 Bc | 1.19 ± 0.072 Bc | 12.96 ± 0.17 Bc | 5.07 ± 0.020 Bd | 13.12 ± 1.49 Bb | 0.67 ± 0.029 Bb | |
| CK | 9.44 ± 0.66 Cc | 0.75 ± 0.016 Cc | 1.05 ± 0.011 Cc | 11.38 ± 0.39 Cc | 5.26 ± 0.021 Ab | 12.54 ± 0.85 Ba | 0.71 ± 0.0083 Bb | |
| 40–60 | GC | 10.47 ± 6.67 Ad | 0.89 ± 0.14 Ad | 1.02 ± 0.018 Bd | 16.47 ± 1.13 Ad | 5 ± 0.030 Cc | 11.72 ± 7.29 Ab | 0.87 ± 0.022 Ab |
| PM | 7.91 ± 2.33 Bd | 0.67 ± 0.011 Bd | 1.07 ± 0.010 Ad | 12.96 ± 0.98 Bd | 5.61 ± 0.010 Aa | 11.78 ± 3.65 Ac | 0.63 ± 0.064 Bb | |
| CK | 7.3 ± 0.62 Cd | 0.58 ± 0.013 Cd | 0.88 ± 0.014 Cd | 10.76 ± 1.15 Cd | 5.47 ± 0.020 Ba | 12.57 ± 1.33 Aa | 0.66 ± 0.02 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhou, B.; Zhuo, Z.; Wang, L.; Wang, Z.; Xie, C.; Jiang, F.; Lin, J.; Huang, Y.; Zhang, Y. Effects of Cover Measures on Soil Organic Nitrogen Fractions and Total Soluble Nitrogen Pools in Citrus Orchards of the Red Soil Hilly Region of Southern China. Agriculture 2024, 14, 1879. https://doi.org/10.3390/agriculture14111879
Li H, Zhou B, Zhuo Z, Wang L, Wang Z, Xie C, Jiang F, Lin J, Huang Y, Zhang Y. Effects of Cover Measures on Soil Organic Nitrogen Fractions and Total Soluble Nitrogen Pools in Citrus Orchards of the Red Soil Hilly Region of Southern China. Agriculture. 2024; 14(11):1879. https://doi.org/10.3390/agriculture14111879
Chicago/Turabian StyleLi, Heming, Bangning Zhou, Zuopin Zhuo, Lei Wang, Zumei Wang, Chuanjin Xie, Fangshi Jiang, Jinshi Lin, Yanhe Huang, and Yue Zhang. 2024. "Effects of Cover Measures on Soil Organic Nitrogen Fractions and Total Soluble Nitrogen Pools in Citrus Orchards of the Red Soil Hilly Region of Southern China" Agriculture 14, no. 11: 1879. https://doi.org/10.3390/agriculture14111879
APA StyleLi, H., Zhou, B., Zhuo, Z., Wang, L., Wang, Z., Xie, C., Jiang, F., Lin, J., Huang, Y., & Zhang, Y. (2024). Effects of Cover Measures on Soil Organic Nitrogen Fractions and Total Soluble Nitrogen Pools in Citrus Orchards of the Red Soil Hilly Region of Southern China. Agriculture, 14(11), 1879. https://doi.org/10.3390/agriculture14111879






