Characterization of Changes in Ripening Process of Volatile Apple Compounds Based on HS-SPME-GC-MS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
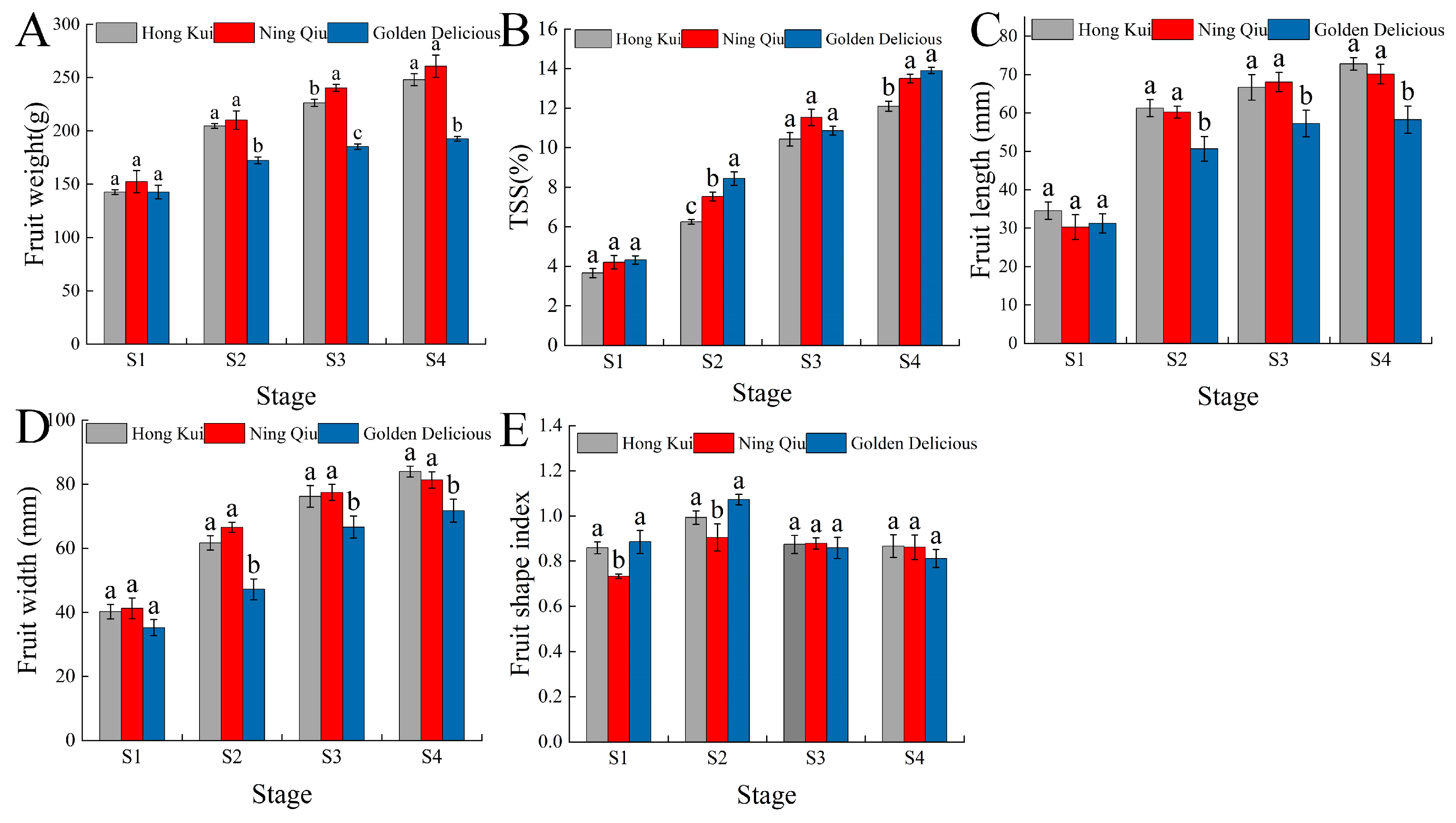
2.2. Fruit Quality
2.3. HS-SPME
2.4. GC-MS
2.5. Qualitative and Quantitative Analysis of Aromatic Compounds
2.6. Calculation of rOAVs
2.7. Data Analysis
3. Results
3.1. Fruit Quality
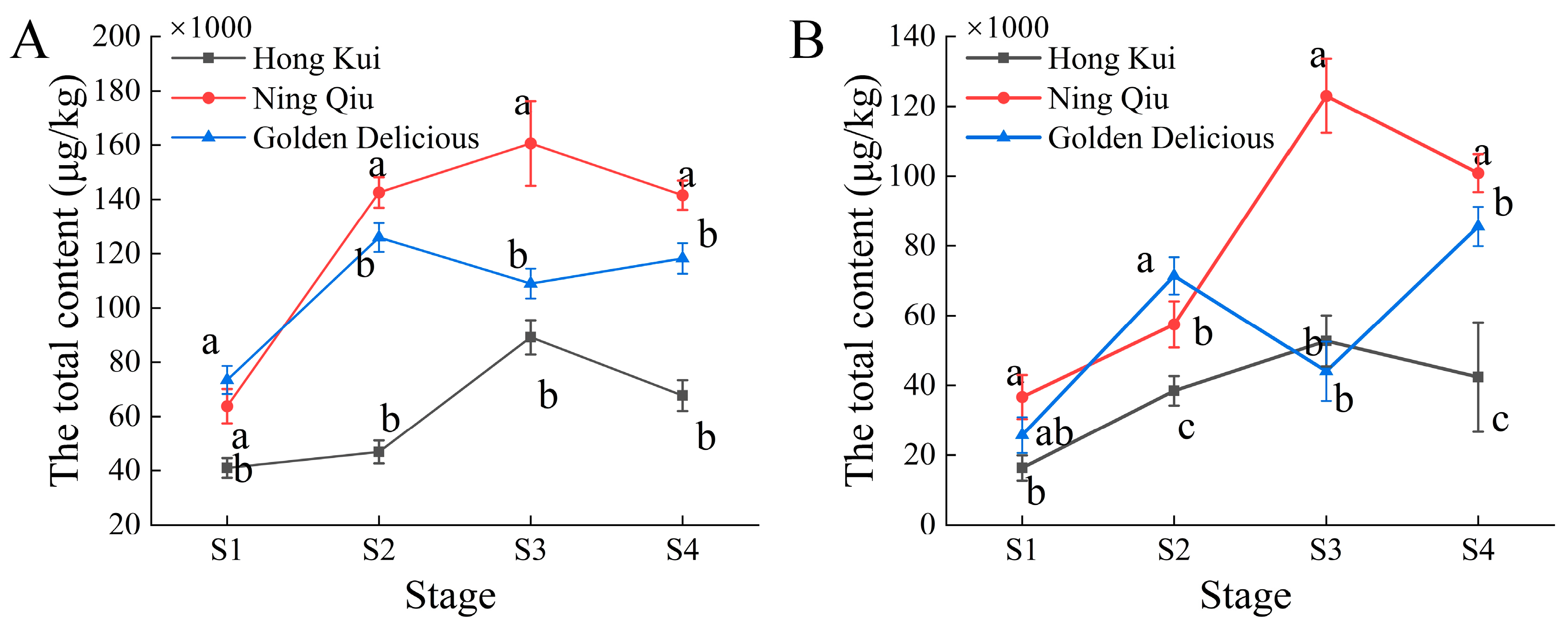
3.2. Volatile Compound Content of Apple Variety
3.3. Percentage Content of Aroma Types in Apple Variety
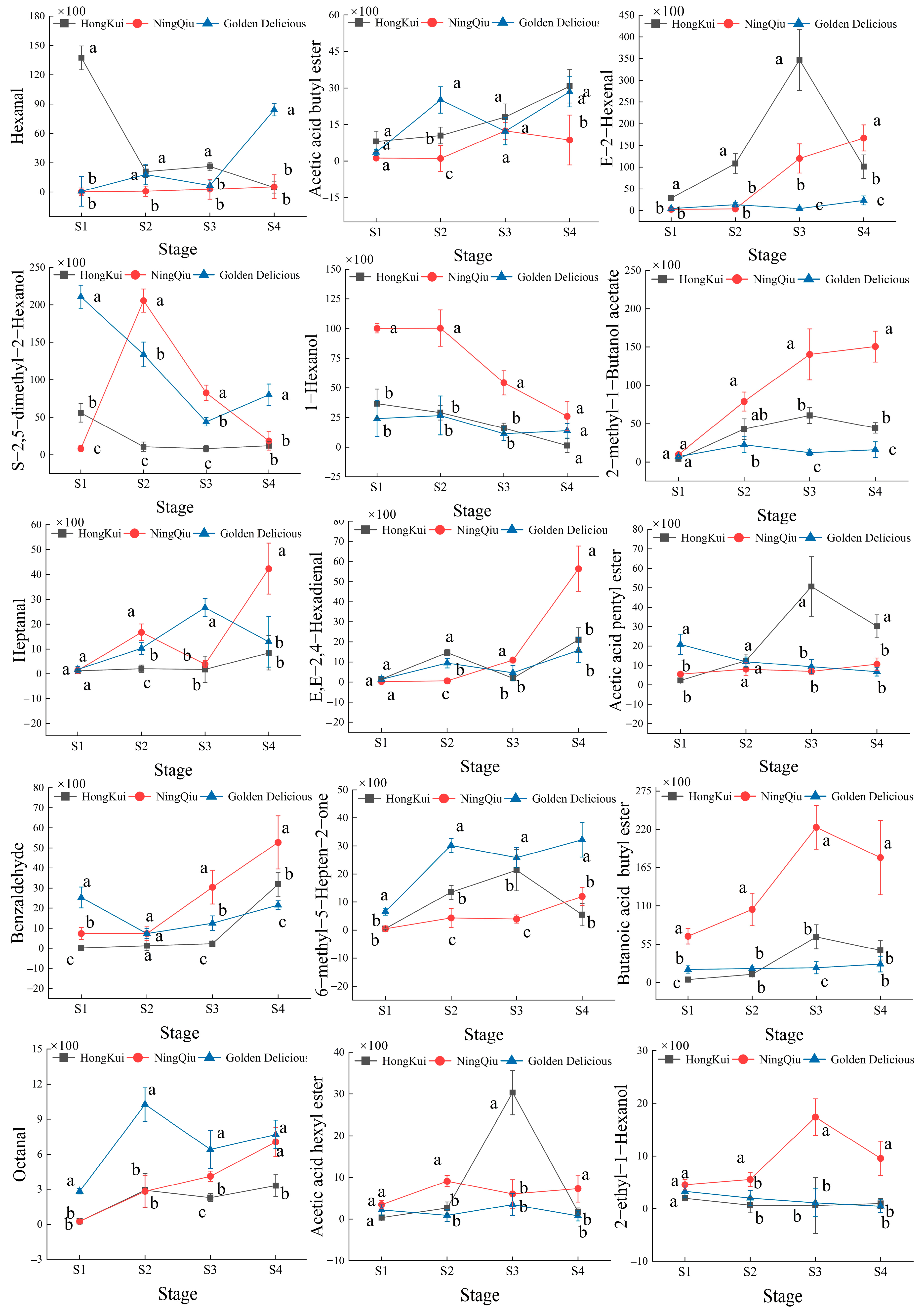
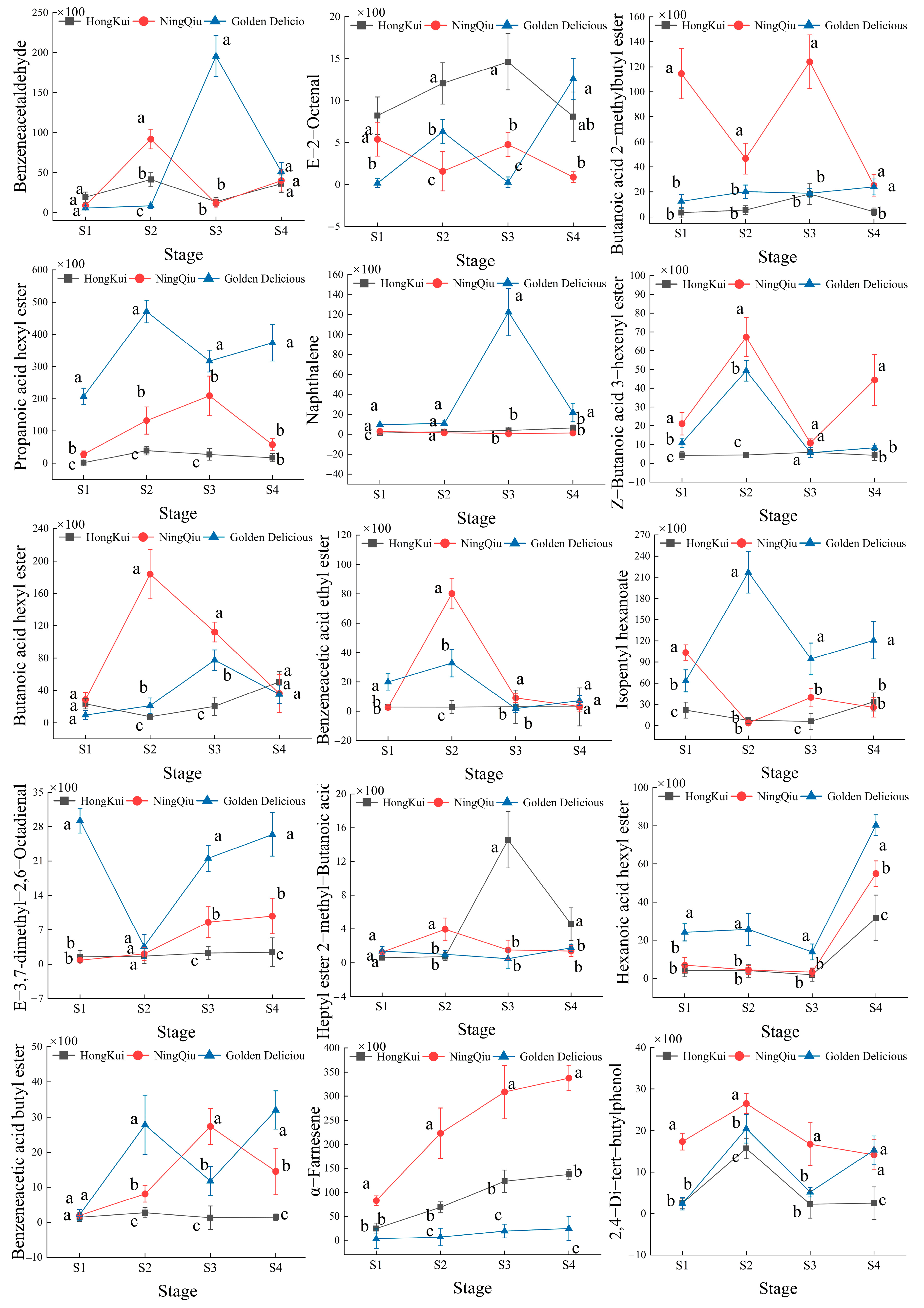
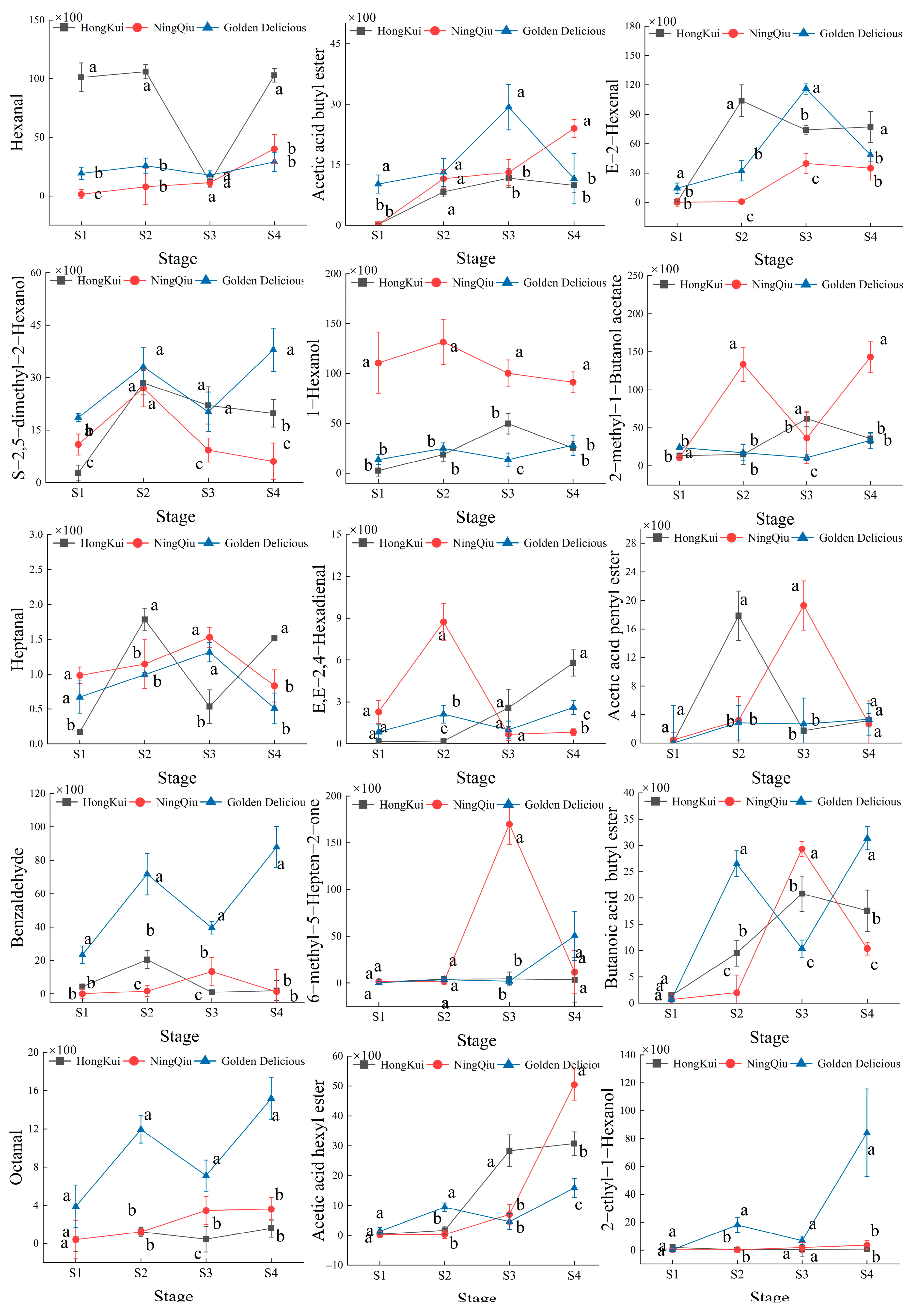
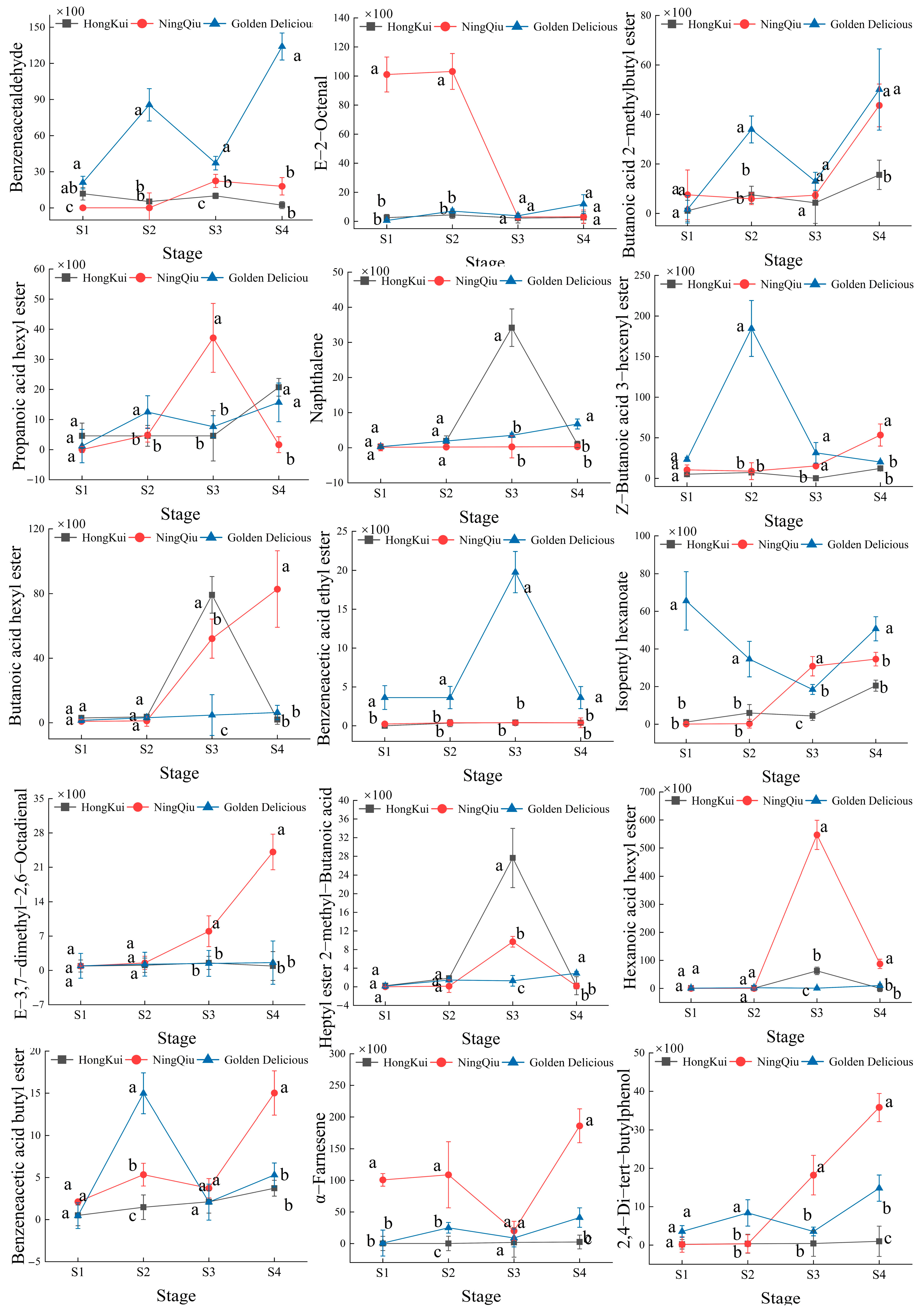
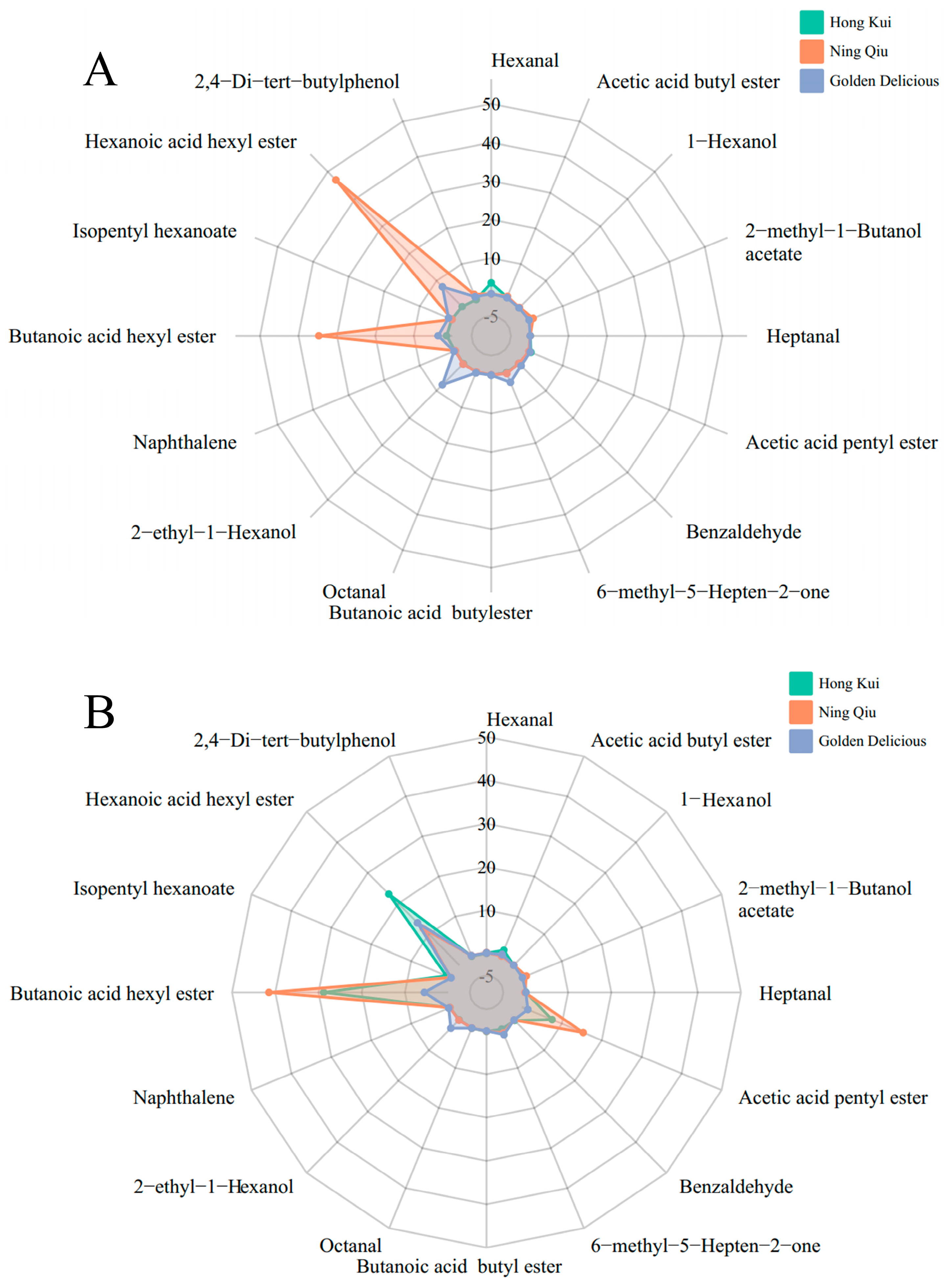
3.4. Changes in Content of Volatile Compounds in Apple Variety
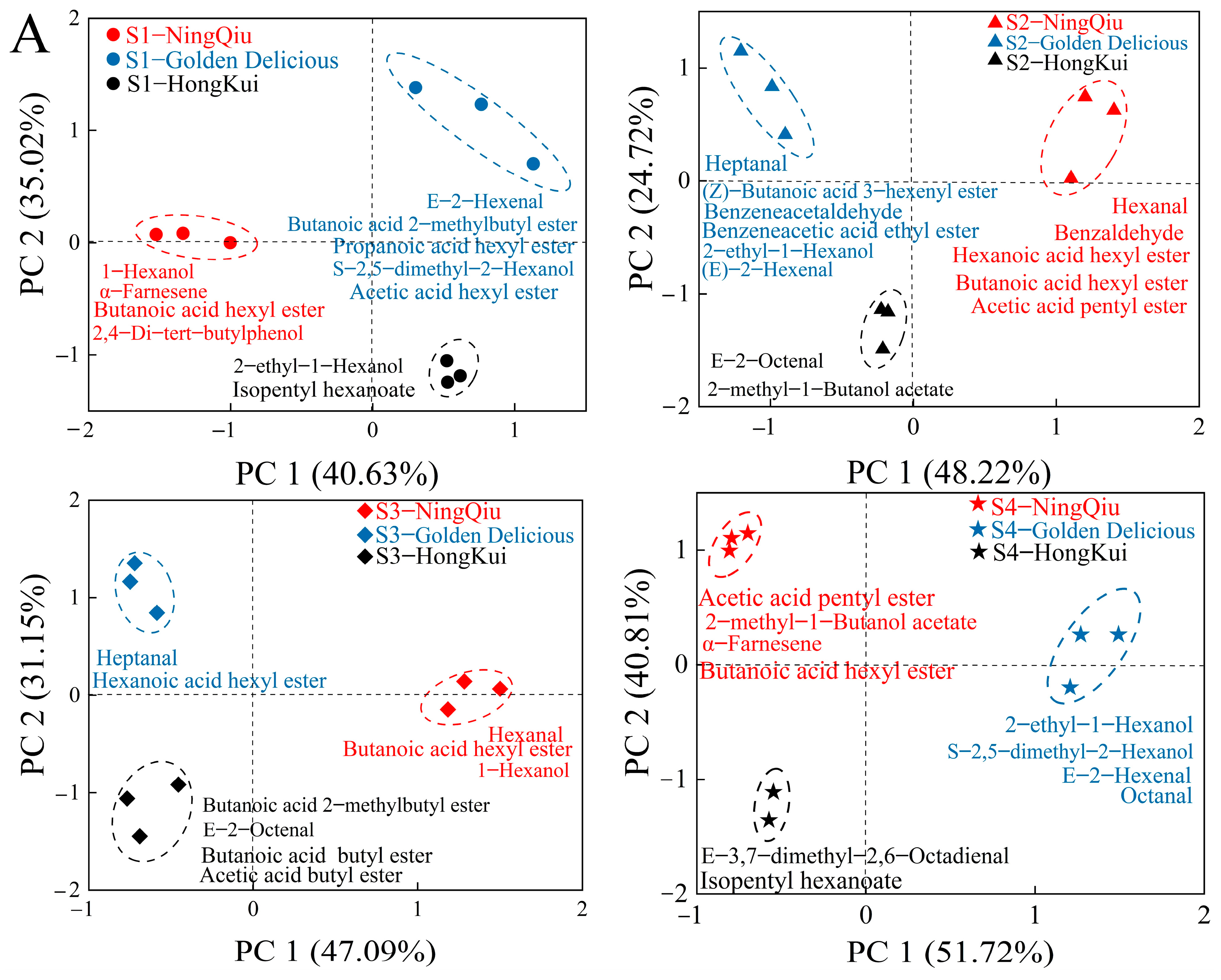
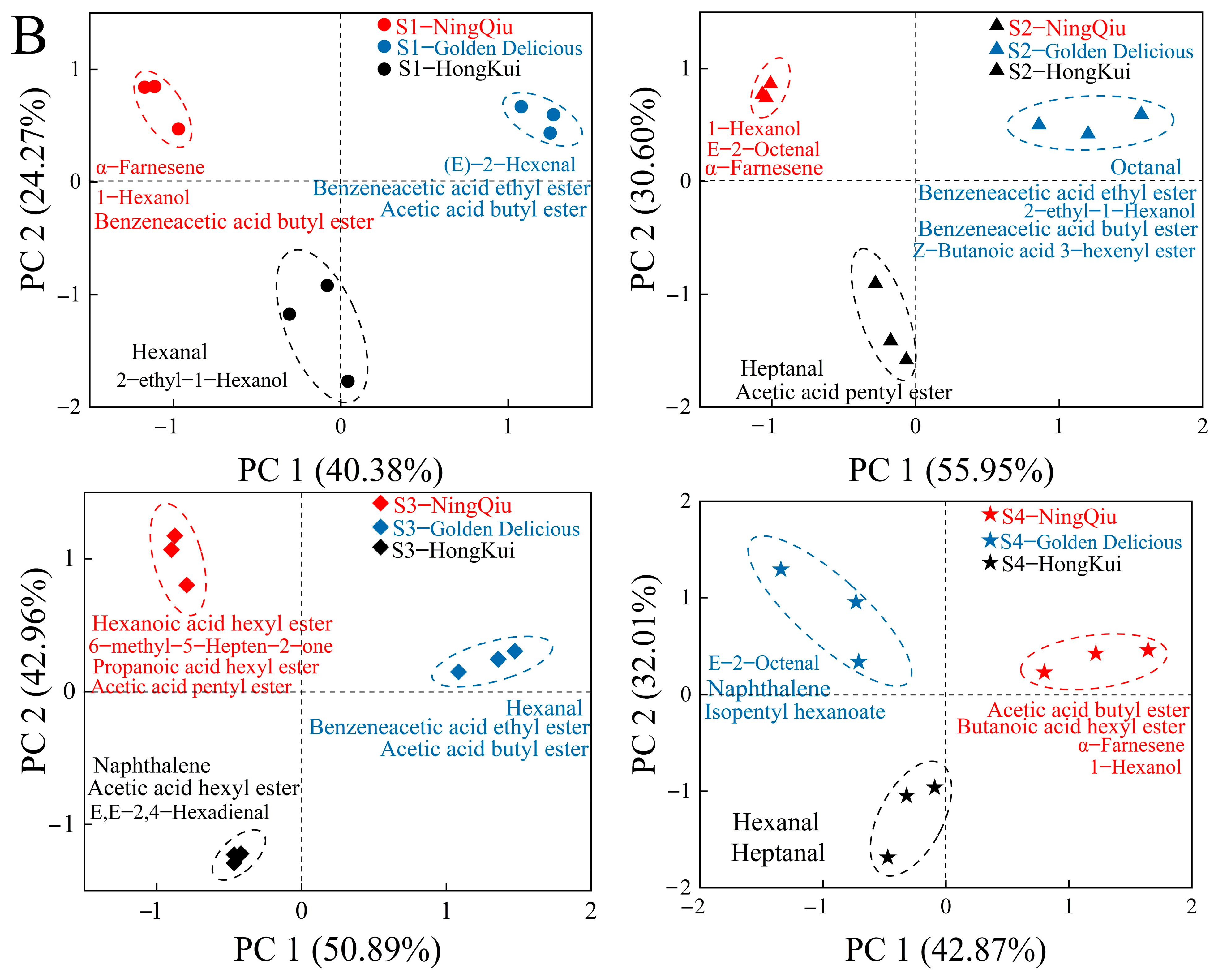
3.5. Principal Component Analysis (PCA) of Aromas of Three Varieties
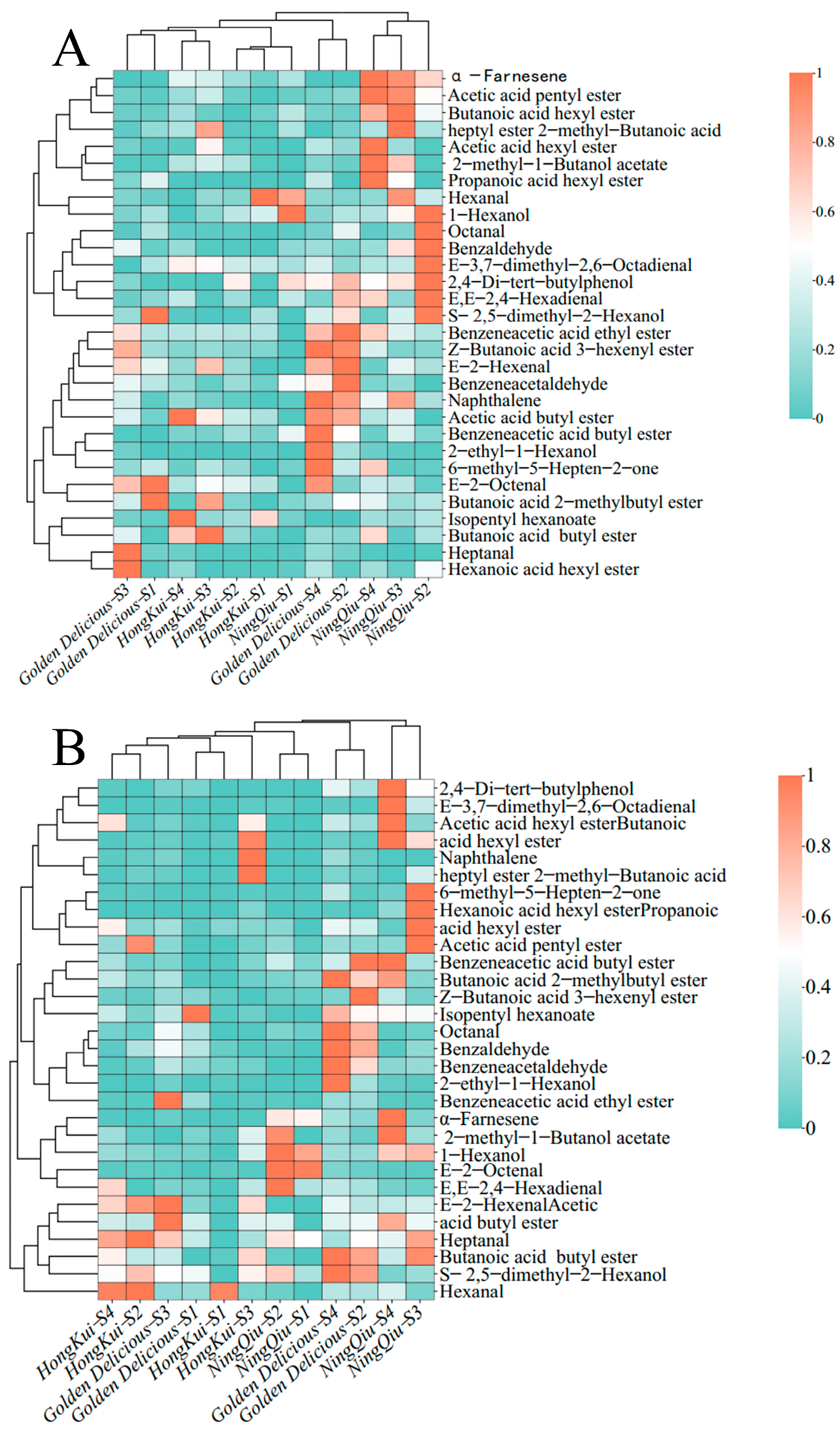
3.6. Cluster Analysis of Aromatic Components of Different Varieties and Maturation Processes
3.7. rOAV Analysis of Key Volatile Compounds in Fruits of Different Varieties at Maturity Stage
4. Discussion
4.1. Aroma Characterization
4.2. Changes in Volatile Compounds during Apple Ripening and Choice of Harvest Time
4.3. Evaluation of Aromatic Compounds’ rOAVs in Different Apple Varieties at Harvest
4.4. Research Value of Aroma of “Ning Qiu”
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Zealand J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Kim, K.; Chun, I.J.; Suh, J.H.; Sung, J. Relationships between sensory properties and metabolomic profiles of different apple cultivars. Food Chem. X 2023, 18, 100641. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Rune, C.J.; Thybo, A.K.; Clausen, M.P.; Orlien, V.; Giacalone, D. Sensory quality and consumer perception of high pressure processed orange juice and apple juice. LWT 2023, 173, 114303. [Google Scholar] [CrossRef]
- Pontesegger, N.; Rühmer, T.; Siegmund, B. Physicochemical attributes, volatile profile and sensory quality of organic Crimson Crisp apples during on-tree Maturation. Foods 2023, 12, 1425. [Google Scholar] [CrossRef]
- Jiaxin, L.I.; Xinye, W.U.; Jinfeng, B.I.; Min, G.O. Characterization of key aroma compounds of apple slices dried by hot-air at different temperatures by GC-MS and electronic nose. AGRIS—Int. Syst. Agric. Sci. Technol. 2022, 43, 272–282. [Google Scholar]
- George, J.; Nguyen, T.; Williams, D.; Hardner, C.; Sanewski, G.; Smyth, H.E. Review of the aroma chemistry of pineapple (Ananas comosus). J. Agric. Food Chem. 2023, 71, 4069–4082. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Gašić, U.; Tosti, T.; Natić, M.; Meland, M. Analysis of apple fruit (Malus × domestica Borkh.) quality attributes obtained from organic and integrated production systems. Sustainability 2022, 14, 5300. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Bi, J.; Wu, X.; Zhang, B. Cultivar classification of cloudy apple juices from substandard fruits in China based on aroma profile analyzed by HS-SPME/GC-MS. LWT 2019, 102, 304–309. [Google Scholar] [CrossRef]
- Sedov, E.N.; Yanchuk, T.V.; Korneeva, S.A.; Makarkina, M.A. Russian Adaptive Apple (Malus × domestica Borkh.) Varieties of Vniispk—Continuity of Goals and Developed Technologies. Agric. Biol. 2022, 57, 897–910. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Glycosidically-bound aroma volatile compounds in the peel and pulp of ‘Kensington Pride’mango fruit at different stages of maturity. Postharvest Biol. Technol. 2003, 29, 205–218. [Google Scholar] [CrossRef]
- Fellman, J.K.; Miller, T.W.; Mattinson, D.S.; Mattheis, J.P. Factors that influence biosynthesis of volatile flavor compounds in apple fruits. HortScience 2000, 35, 1026–1033. [Google Scholar] [CrossRef]
- Börjesson, J.; Karlsson HO, E.; Trägårdh, G. Pervaporation of a model apple juice aroma solution: Comparison of membrane performance. J. Membr. Sci. 1996, 119, 229–239. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Yang, L.; Li, J.; Zhu, X. Enhancement of the aroma in low-alcohol apple-blended pear fermented beverages mixed fermented with Saccharomyces cerevisiae and non-Saccharomyces yeasts. LWT 2022, 155, 112994. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Silsby, K.J. Predicting Harvest Date Windows for Apples; Cornell Cooperative Extension: New York, NY, USA, 1992. [Google Scholar]
- Fang, X.; Liu, Y.; Xiao, J.; Ma, C.; Huang, Y. GC–MS and LC-MS/MS metabolomics revealed dynamic changes of volatile and non-volatile compounds during withering process of black tea. Food Chem. 2023, 410, 135396. [Google Scholar] [CrossRef]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food aroma compounds. In Nutraceutical and Functional Food Components; Academic Press: Cambridge, MA, USA, 2022; pp. 363–409. [Google Scholar]
- Xu, Y.; Wang, Y.; Li, R.; Sun, P.; Chen, D.; Shen, J.; Feng, T. Characteristic aroma analysis of finger citron in four different regions based on GC–MS-HS-SPME and ROAV. J. Food Process. Preserv. 2022, 46, e17191. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Li, J.; Zhang, J.; Wei, Y.; Yan, Y.; Wang, H.; Pan, Y.; Xiong, Z.; Wang, R.; et al. Aroma formation mechanism by the drying step during Congou black tea processing: Analyses by HP-SPME and SAFE with GC-MS. LWT 2024, 198, 116019. [Google Scholar] [CrossRef]
- Wu, W.; Wu, R.; Tao, N.P. Identification of volatile compounds in cooked pulp through of farmed obscure puffer (Takifugu obscurus) using SDE and HS-SPME combined with GC-MS. Adv. Mater. Res. 2013, 706, 399–402. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Yang, H.; Zhao, Z. Genetic analysis and QTL mapping of aroma volatile compounds in the apple progeny ‘Fuji’בCripps Pink’. Front. Plant Sci. 2023, 14, 1048846. [Google Scholar] [CrossRef]
- Červenčík, K.; Dimitrov, F.; Tobolkova, B.; Žemlička, L.; Bírošová, L.; Hrouzkova, S.; Sadecka, J. Principal aroma-active compounds of Red Moon RM-1 apple fruit cultivar as determined by a combined technique of gas chromatography-olfactometry. J. Food Nutr. Res. 2022, 61, 251. [Google Scholar]
- Liang, Z.; Zhang, P.; Fang, Z. Modern technologies for extraction of aroma compounds from fruit peels: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1284–1307. [Google Scholar] [CrossRef] [PubMed]
- Masi, E.; Taiti, C.; Vignolini, P.; Petrucci, A.W.; Giordani, E.; Heimler, D.; Romani, A.; Mancuso, S. Polyphenols and aromatic volatile compounds in biodynamic and conventional ‘Golden Delicious’ apples (Malus domestica Bork.). Eur. Food Res. Technol. 2017, 243, 1519–1531. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘Orin’apples (Malus domestica) at different ripening stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Aroma volatiles: Biosynthesis and mechanisms of modulation during fruit ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar]
- Wu, X.; Bi, J.; Fauconnier, M.L. Characteristic volatiles and cultivar classification in 35 apple varieties: A case study of two harvest years. Foods 2022, 11, 690. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Turnbaugh, J.G.; Benson, M. Odor thresholds of various branched esters. LWT-Food Sci. Technol. 1995, 28, 153–156. [Google Scholar] [CrossRef]
- Oliveira, I.; de Pinho, P.G.; Malheiro, R.; Baptista, P.; Pereira, J.A. Volatile profile of Arbutus unedo L. fruits through ripening stage. Food Chem. 2011, 128, 667–673. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, X.; Liu, S.Q. Aroma modulation of vegetable oils—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1538–1551. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Grechkin, A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res. 1998, 37, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, X.; Zhang, C.; Kong, X.; Chen, Y.; Hua, Y. Formation mechanism of hexanal and (E)-2-hexenal during soybean [Glycine max (L.) Merr] processing based on the subcellular and molecular levels. J. Agric. Food Chem. 2021, 70, 289–300. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Fernandez-Cruz, T.; Sieiro-Sampedro, T.; Gonzalez-Barreiro, C. Dissipation of fungicide residues during fermented beveragesmaking and their effects on fermentation and the volatile composition of fermented beveragess. J. Agric. Food Chem. 2016, 64, 1344–1354. [Google Scholar] [CrossRef]
- Ferrandino, A.; Duverney, C.; Di Stefano, R. The evolution of apple volatiles during storage as influenced by fruit maturity. Ital. J. Food Sci./Riv. Ital. Di Sci. Degli Aliment. 2007, 19. [Google Scholar]
- Hijaz, F.; Gmitter Jr, F.G.; Bai, J.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar] [CrossRef]
- Mai, J.; Li, W.; Ledesma-Amaro, R.; Ji, X.-J. Engineering plant sesquiterpene synthesis into yeasts: A review. J. Agric. Food Chem. 2021, 69, 9498–9510. [Google Scholar] [CrossRef]
- Esparza, X.; Moyano, E.; Cosialls, J.; Galceran, M. Determination of naphthalene-derived compounds in apples by ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2013, 782, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Sharma, S.; Kumar, V. Cider: The production technology. In Fermented Beveragesmaking; CRC Press: Boca Raton, FL, USA, 2021; pp. 581–607. [Google Scholar]
- Singh, J.; Mirza, A.; Singh, S. Study on Physiochemical Properties of Fruits as Influenced by Naphthalene Acetic Acid: A Review. Int. J. Eng. Res. Technol. 2017, 6, 941–945. [Google Scholar]
- Najar-Rodriguez, A.; Orschel, B.; Dorn, S. Season-long volatile emissions from peach and pear trees in situ, overlapping profiles, and olfactory attraction of an oligophagous fruit moth in the laboratory. J. Chem. Ecol. 2013, 39, 418–429. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Irigoyen, A.; Ortigosa, M.; Juansaras, I.; Oneca, M.; Torre, P. Influence of an adjunct culture of Lactobacillus on the free amino acids and volatile compounds in a Roncal-type ewe’s-milk cheese. Food Chem. 2007, 100, 71–80. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, Z.; Li, P.; Xu, Y.; Zhang, G.; Ma, W.; Ren, Z. Drivers of Net Primary Productivity Spatio-Temporal Variation in Ningxia, China. Forests 2023, 14, 1170. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and pulp through tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Zellner, B.D.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Chen, X.; Chen, D.; Deng, S. Use of relative odor activity value (ROAV) to link aroma profiles to volatile compounds: Application to fresh and dried eel (Muraenesox cinereus). Int. J. Food Prop. 2020, 23, 2257–2270. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Tang, Z.; Xia, Q.; Wang, X.; Lianliang, L.; Qi, P.; Wang, Z.; Di, S.; Liu, Z. Flavor characteristics of key aroma compounds in bayberry juice, fruit, and thinning fruit using HS-SPME coupled with GC/Q-TOF-MS. J. Food Compos. Anal. 2024, 128, 106032. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
| Structure Type | Compound Name | Ta (mg/kg) A | rOAV | Odor Description B | |||||
|---|---|---|---|---|---|---|---|---|---|
| Peel | Pulp | ||||||||
| Hong Kui | Ning Qiu | Golden Delicious | Hong Kui | Ning Qiu | Golden Delicious | ||||
| C6 | Hexanal | 0.23 | 0.125 | 0.268 | 0.231 | 3.594 | 0.774 | 0.778 | Herbal, green, grass, fruity |
| C6 | Acetic acid butyl ester | 0.46 | 1.611 | 0.184 | 0.547 | 0.693 | 0.926 | 0.619 | Fruity, fruity, banana |
| C6 | 1-Hexanol | 0.034 | <0.1 | <0.1 | <0.1 | 0.129 | 0.260 | 0.111 | Green; herbaceous; woody; sweet |
| C7 | 2-methyl-1-Butanol acetate | 0.14 | 0.709 | 1.084 | 0.136 | 0.768 | 1.681 | 0.547 | Estery, fruity, banana, pear, sweet |
| C7 | Heptanal | 0.26 | 0.250 | <0.1 | 0.237 | <0.1 | <0.1 | <0.1 | Aromatic, nutty, almond flavor |
| C7 | Acetic acid pentyl ester | 2.2 | 7.555 | 15.338 | 1.476 | 1.061 | 0.497 | 0.856 | Ethereal, fresh–fruity, reminiscent of pear, banana, and apple |
| C7 | Benzaldehyde | 0.085 | 0.309 | 0.143 | 0.125 | <0.1 | <0.1 | 0.871 | Aromatic, nutty, almond flavor |
| C8 | 6-methyl-5-Hepten-2-one | 0.5 | 0.312 | 1.271 | 1.679 | 0.259 | 0.479 | 2.946 | Phenolic aroma, moldy aroma, ketone aroma |
| C8 | Butanoic acid butyl ester | 0.028 | 0.147 | <0.1 | <0.1 | <0.1 | <0.1 | 0.102 | Fruity, banana, pineapple |
| C8 | Octanal | 0.17 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.301 | Fruity, green, herbal, diffused |
| C8 | 2-ethyl-1-Hexanol | 0.8 | <0.1 | 0.205 | 2.817 | <0.1 | 0.237 | 7.855 | Citrus, fresh, floral, oily, sweet |
| C10 | Naphthalene | 0.45 | 0.322 | 0.302 | 0.602 | 0.065 | <0.1 | 0.355 | Pungent, dry, tarry |
| C10 | Butanoic acid hexyl ester | 5 | 28.817 | 41.480 | 5.527 | 1.509 | 34.717 | 3.715 | Fruity, pineapple, strawberry |
| C11 | Isopentyl hexanoate | 0.32 | 1.220 | 0.108 | <0.1 | 0.996 | 0.928 | 1.893 | Fruity, sweet, pineapple, a slightly pungent sour cheesy note |
| C12 | Hexanoic acid hexyl ester | 6.4 | 23.116 | 11.872 | 13.729 | 0.562 | 47.005 | 7.842 | Fatty wax flavor, soybean flavor, fruit flavor |
| C14 | 2,4-Di-tert-butylphenol | 0.5 | 0.144 | 0.329 | 0.320 | <0.1 | 1.501 | 0.865 | Pungent, dry resin scent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Li, X.; Chu, Y.; Yue, H.; Xu, Z.; Li, B.; Wu, X.; Gan, J.; Jia, Y. Characterization of Changes in Ripening Process of Volatile Apple Compounds Based on HS-SPME-GC-MS Analysis. Agriculture 2024, 14, 1787. https://doi.org/10.3390/agriculture14101787
Ma J, Li X, Chu Y, Yue H, Xu Z, Li B, Wu X, Gan J, Jia Y. Characterization of Changes in Ripening Process of Volatile Apple Compounds Based on HS-SPME-GC-MS Analysis. Agriculture. 2024; 14(10):1787. https://doi.org/10.3390/agriculture14101787
Chicago/Turabian StyleMa, Jun, Xiaolong Li, Yannan Chu, Haiying Yue, Zehua Xu, Baiyun Li, Xianyi Wu, Jun Gan, and Yonghua Jia. 2024. "Characterization of Changes in Ripening Process of Volatile Apple Compounds Based on HS-SPME-GC-MS Analysis" Agriculture 14, no. 10: 1787. https://doi.org/10.3390/agriculture14101787
APA StyleMa, J., Li, X., Chu, Y., Yue, H., Xu, Z., Li, B., Wu, X., Gan, J., & Jia, Y. (2024). Characterization of Changes in Ripening Process of Volatile Apple Compounds Based on HS-SPME-GC-MS Analysis. Agriculture, 14(10), 1787. https://doi.org/10.3390/agriculture14101787




