The Suitability of Algae Solution in Pea Microgreens Cultivation under Different Light Intensities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Light Intensity
2.2. Algae Cultivation Conditions
2.3. Treatment of Pea Microgreens
2.4. Harvest, Biometrical and Chlorophyll Fluorescence Measurements
2.5. FT-Raman Spectroscopy Measurements
2.6. Experiment Design and Statistical Analysis
3. Results
3.1. Growth and Morphology of Pea Microgreens
3.2. Fluorescence Parameters
3.3. Chemical Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.; Wang, T.T.Y. The Science Behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Frąszczak, B.; Kula-Maximenko, M. The Biometric Parameters of Microgreen Crops Grown under Various Light Conditions. Agriculture 2022, 12, 576. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; Szymczak, K.; Bonikowski, R. LED Light Quality Affected Bioactive Compounds, Antioxidant Potential, and Nutritional Value of Red and White Cabbage Microgreens. Appl. Sci. 2023, 13, 5435. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In Vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Despommier, D. Farming up the city: The rise of urban vertical farms. Trends Biotechnol. 2013, 31, 388–389. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in “Florida Broadleaf” mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential Effects of Low Light Intensity on Broccoli Microgreens Growth and Phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsinia, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2021, 102, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Samuoliené, G.; Brazaityte, A.; Jankauskiene, J.; Virsile, A.; Sirtautas, R.; Novickovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef]
- Ilieva, I.; Ivanova, T.; Naydenov, Y.; Dandolov, I.; Stefanov, D. Plant experiments with light-emitting diode module in Svet space greenhouse. Adv. Space Res. 2010, 46, 840–845. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Metting, B.; Pyne, J.W. Biologically active compounds from microalgae. Enzym. Microb. Technol. 1986, 8, 386–394. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Utln. 2012, 3, 89–100. [Google Scholar]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2015, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Németh, A.; Horváth, N.; Katona, S.; Molnár, Z. Review of the Use of Biostimulant Microalgae to Influence the Growth and Development of Ornamental Plants. Acta Agron. Óvár. 2024, 65, 36–55. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Stevanato, P.; Fascella, G.; Baglieri, A. Morpho-biometric and biochemical responses in lettuce seedlings treated by different application methods of Chlorella vulgaris extract: Foliar spray or root drench? J. Appl. Phycol. 2022, 34, 889–901. [Google Scholar] [CrossRef]
- Force, L.; Critchley, C.; Van Rensen, J.J.S. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17–33. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M.H. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B 2016, 157, 22–31. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Schulz, H.; Baranski, R.; Nothnagel, T.; Christensen, L.P. In Situ simultaneous analysis of polyacetylenes, carotenoids and polysaccharides in carrot roots. J. Agric. Food Chem. 2005, 53, 6565–6571. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Andreev, G.; Schrader, B.; Schulz, H.; Fuchs, R.; Popov, S.; Handjieva, N. Non-destructive NIR-FT-Raman analyses in practice. Part 1. Analyses of plants and historic textiles. Fresenius J. Anal. Chem. 2001, 371, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Ayora-Canada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- De Souza, M.H.B.; Calijuri, M.L.; Assemany, P.P.; Castro, J.d.S.; de Oliveira, A.C.M. Soil application of microalgae for nitrogen recovery: A life-cycle approach. J. Clean. Prod. 2019, 211, 342–349. [Google Scholar] [CrossRef]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as Biofertilizer in Modern Agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. [Google Scholar]
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar] [CrossRef]
- Karbarz, M.; Piziak, M.; Żuczek, J.; Duda, M. Influence of Microalgae Planktochlorella nurekis Clones on Seed Germination. Agronomy 2023, 13, 9. [Google Scholar] [CrossRef]
- Asif, R.; Yasmin, R.; Mustafa, M.; Ambreen, A.; Mazhar, M.; Rehman, A.; Umbreen, S.; Ahmad, M.; Asif, R.; Yasmin, R.; et al. Chapter 7: Phytohormones as Plant Growth Regulators and Safe Protectors against Biotic and Abiotic Stress. In Plant Hormones: Recent Advances, New Perspectives and Applications; Hano, C., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and Effects on Growth and Metabolites of Microalgae: A Review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Adasme, C.; Palma-Dias, R.; Escalona, V.H. The Effect of Light Intensity and Photoperiod on the Yield and Antioxidant Activity of Beet Microgreens Produced in an Indoor System. Horticulturae 2023, 9, 493. [Google Scholar] [CrossRef]
- Youssef, R.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Q. The combined effects of light intensity, temperature, and water potential on wall deposition in regulating hypocotyl elongation of Brassica rapa. Peer J. 2020, 8, e9106. [Google Scholar] [CrossRef]
- Vetchinnikov, A.; Filatov, D.A.; Olonina, S.I.; Kazakov, A.V.; Olonin, I.Y. Influence of the radiation intensity of LED light sources of the red-blue spectrum on the yield and energy consumption of microgreens. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 32–46. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B. The scaling of leaf area and mass: The cost of light interception increases with leaf size. Proc. R. Soc. B 2007, 274, 2109–2115. [Google Scholar] [CrossRef]
- Cowden, R.J.; Markussen, B.; Ghaley, B.B.; Henriksen, C.B. The Effects of Light Spectrum and Intensity, Seeding Density, and Fertilization on Biomass, Morphology, and Resource Use Efficiency in Three Species of Brassicaceae Microgreens. Plants 2024, 13, 124. [Google Scholar] [CrossRef]
- Walters, R.G.; Shephard, F.; Rogers, J.J.M.; Rolfe, S.A.; Horton, P. Identification of mutants of Arabidopsis defective in acclimation of photosynthesis to the light environment. Plant Physiol. 2003, 131, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, P.; Wu, Y.; Tang, J. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hort. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef]
- Janeeshma, E.; Johnson, R.; Amritha, M.S.; Noble, L.; Aswathi, K.P.R.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in chlorophyll a fluorescence-based on intensity and spectral variations of light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Imranul, C.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 25, 97–101. [Google Scholar] [CrossRef]
- Choi, H.G. Correlation among phenotypic parameters related to the growth and photosynthesis of strawberry (Fragaria × ananassa Duch.) grown under various light intensity conditions. Front. Plant Sci. 2021, 12, 647585. [Google Scholar] [CrossRef]
- Flores, M.; Hernández-Adasme, C.; Guevara, M.J.; Escalona, V.H. Effect of different light intensities on agronomic characteristics and antioxidant compounds of Brassicaceae microgreens in a vertical farm system. Front. Sustain. Food Syst. 2024, 8, 1349423. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, J.-E.; Truong, T.Q.; Koo, S.Y.; Choi, J.-H.; Kim, S.M. Effect of Chlorella vulgaris on the Growth and Phytochemical Contents of “Red Russian” Kale (Brassica napus var. Pabularia). Agronomy 2022, 12, 2138. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid impacts tomato carotenoids, soluble sugars, and organic acids. HortScience. 2016, 51, 370–376. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.S.; Hafez, M.M.; Habib, A.M. Seaweed extract improves growth, yield and quality of different watermelon hybrids. Res. J. Agric. Biol. Sci. 2010, 6, 161–168. [Google Scholar]
- Djuric, M.; Mladenovic, J.; Pavlovic, R.; Zdravkovic, J. Application of bio-stimulator biocomplex 900 in producing tomato (Lycopersicon Essculentum Mill.) seedling. Acta Agric. Serb. 2014, 19, 97–103. [Google Scholar] [CrossRef]
- Hajnal-Jafari, T.; Seman, V.; Stamenov, D.; Đurić, S. Effect of Chlorella vulgaris on Growth and Photosynthetic Pigment Content in Swiss Chard (Beta vulgaris L. subsp. cicla). Pol. J. Microbiol. 2020, 69, 235–238. [Google Scholar] [CrossRef] [PubMed]
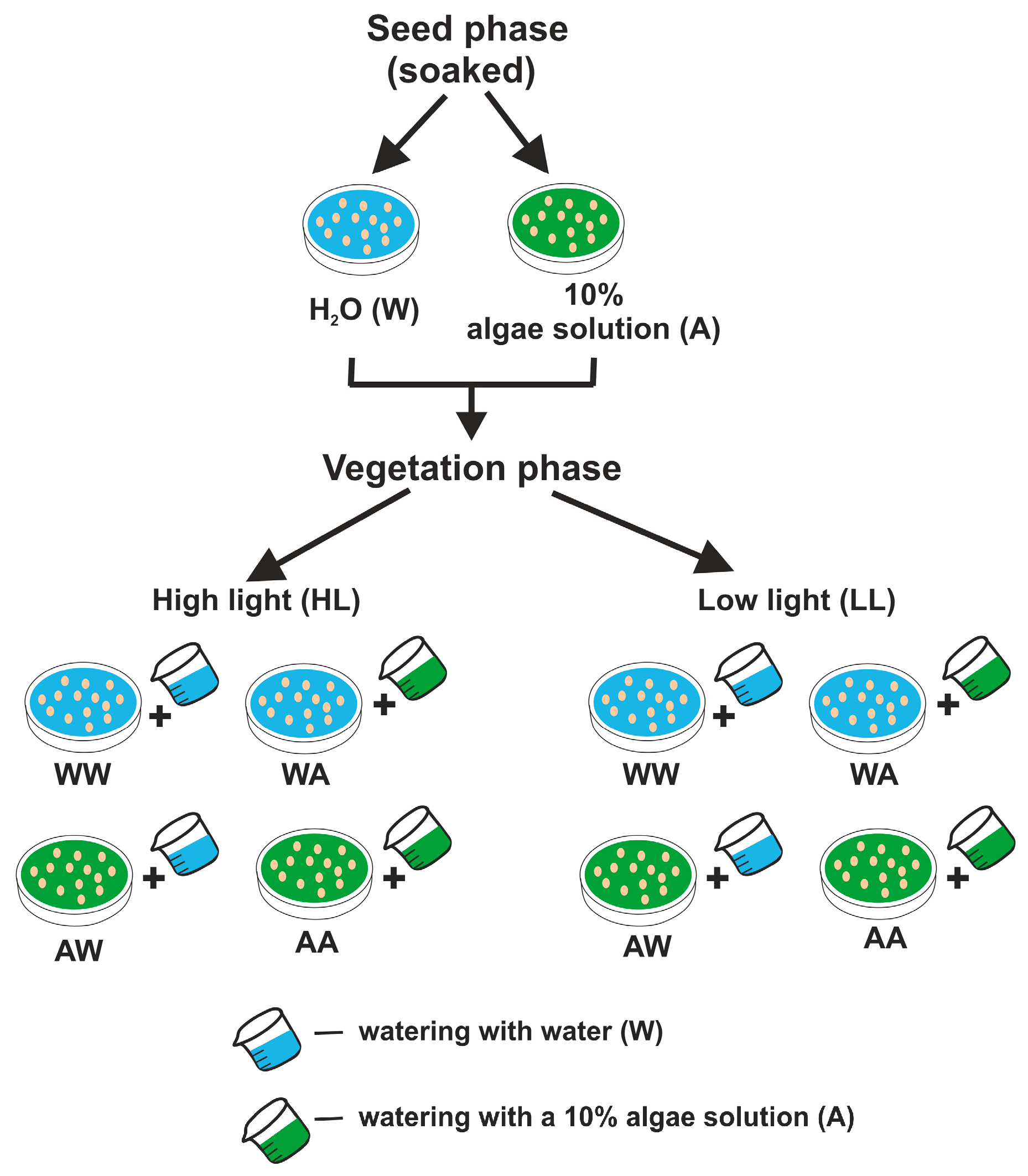
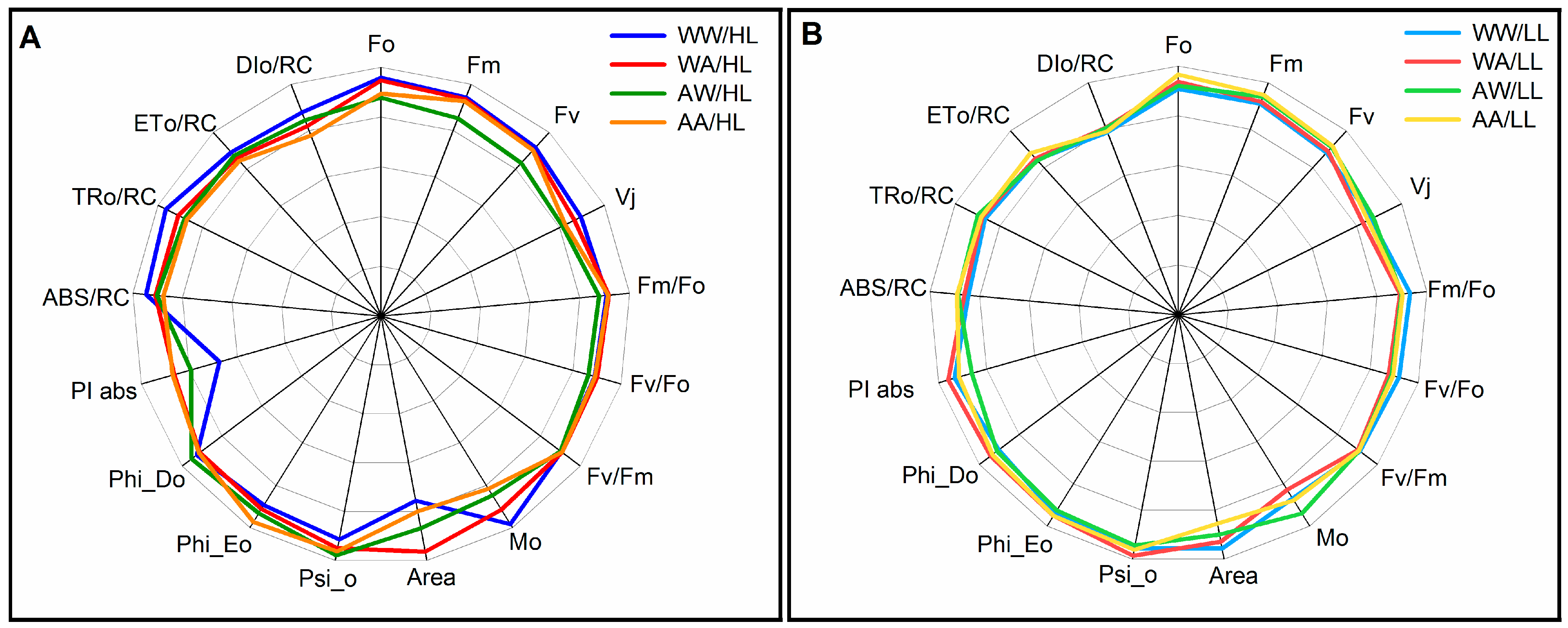
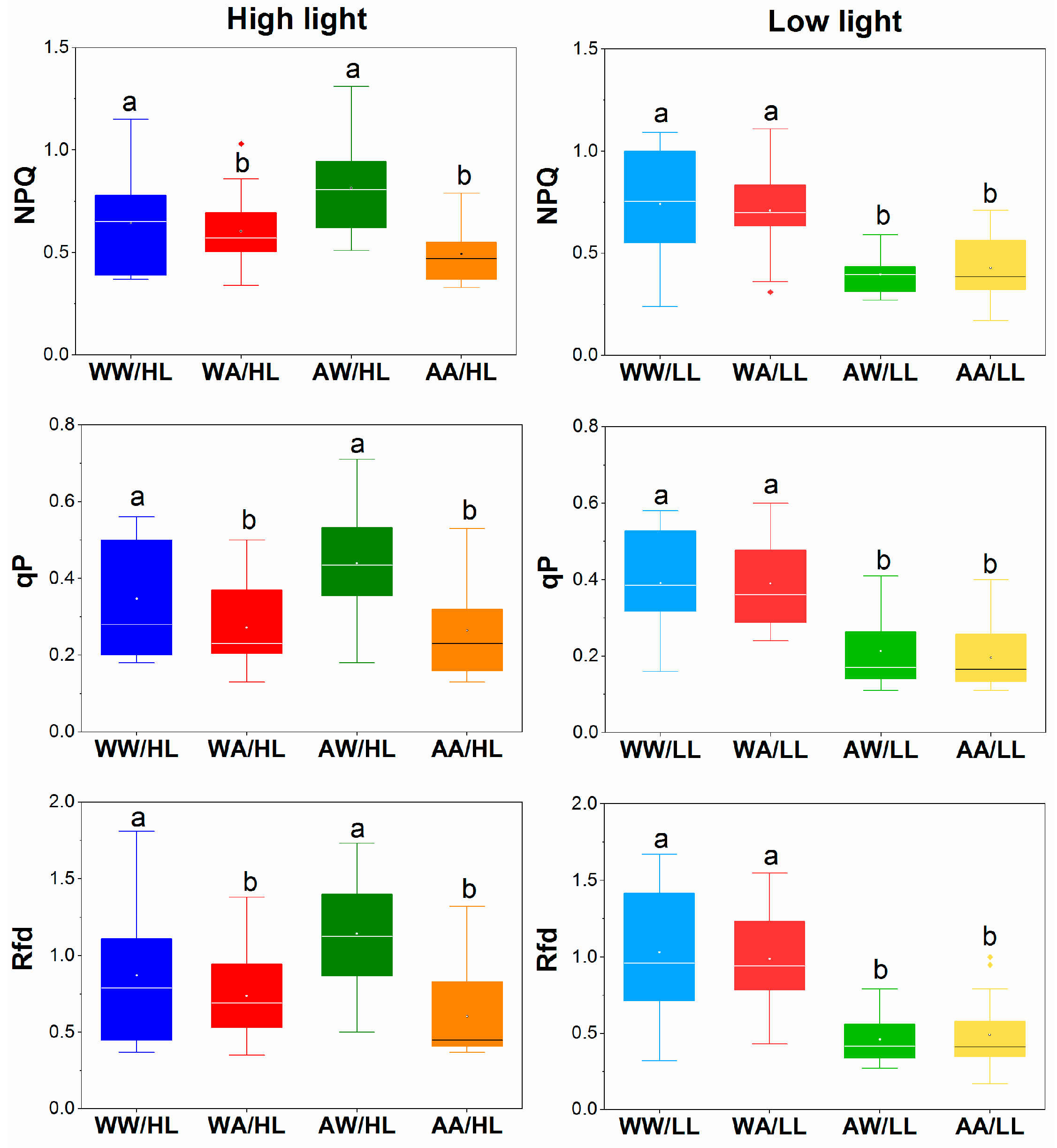
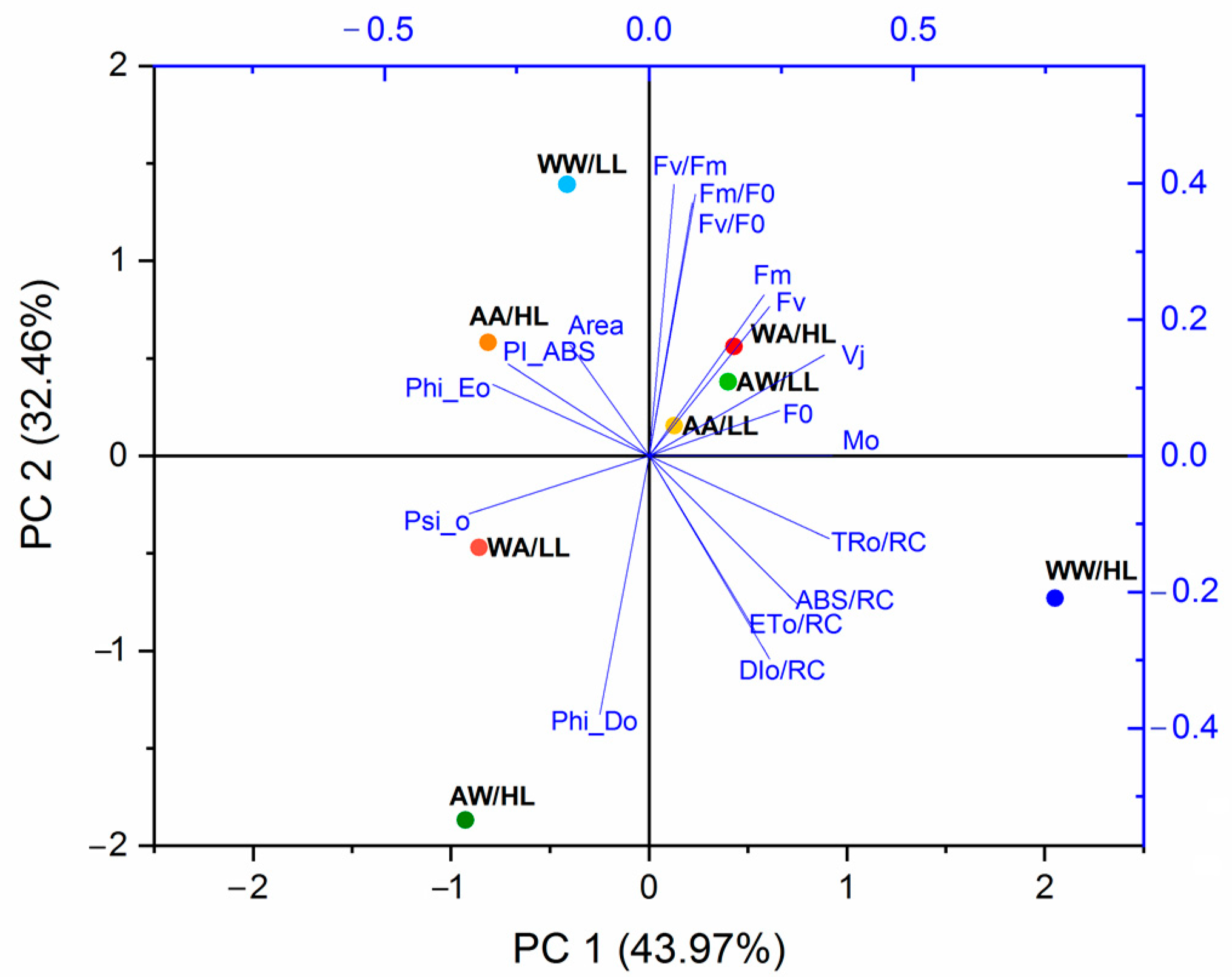

| Parameters | Description |
|---|---|
| Energy flow rate | |
| Fo | Minimal fluorescence, when all PSII reaction centers (RCs) are open. |
| Fm | Maximal fluorescence, when all PSII RCs are closed. |
| Fv | Maximal variable fluorescence, which is measured after dark adaptation. The extent of Fv is related to the maximum quantum yield of PSII. |
| Vj | information about the number of closed RCs in relation to the total number of RCs that can be closed. |
| Fm/Fo | The ratio of maximum fluorescence to zero-time fluorescence. The low ratio Fm/Fo could indicate the destruction of PSII. |
| Fv/Fo | Ratio of rate constants for photochemical reaction and nonphotochemical deactivation of PSII excitations. |
| Fv/Fm | Maximum photochemical quantum PSII after dark adaptation. |
| Mo | approximated initial slope (in ms−1) of the fluorescence transient normalized on the maximal variable fluorescence Fv. |
| Area | The area above the fluorescence induction curve is proportional to the pool size of electron acceptors in PSII. |
| Quantum yields and efficiencies | |
| Psi_o | The probability of electron transport beyond QA-, i.e., the efficiency with which the exciton trapped using an RC drives the electron along ETC beyond QA-. |
| Phi_Eo | The quantum efficiency of electron transfer from QA- to electron transport chain beyond QA-. |
| Phi_Do | The quantum efficiency of energy dissipation. |
| Performance indexes | |
| PI abs | Performance index (potential) for energy conservation from excitation to the reduction of intersystem electron acceptors. |
| Specific energy fluxes per reaction center | |
| ABS/RC | The light energy absorbed using the PSII antenna photon flux per active reaction center. |
| TRo/RC | Total energy used to reduce QA by the unit reaction center of PSII per energy captured/trapped using a single active RC. |
| ETo/RC | The rate of electron transport through a single RC |
| DIo/RC | Total energy dissipated per reaction center (RC) as heat, fluorescence, and energy transfer to PSI. |
| Parameters of nonphotochemical and photochemical fluorescence quenching | |
| NPQ | Nonphotochemical quenching per reaction center of PSII. This parameter is associated with heat losses. For the majority of healthy plants, NPQ values are 0.5–3.5, although they differ in various species or plants cultivated at different growth conditions. |
| qP | Photochemical quenching. This is the fraction of light energy consumed by the open centers for photosynthetic reactions concerning the total amount of energy absorbed by PSII. |
| Rfd | Vitality index of PSII. This parameter is indicative of interactions between the light-stage reactions activated by PAR absorption and the dark reactions of photosynthesis. This parameter is diminished when the balance between photochemical reactions in thylakoids and the rates of enzymatic reactions in the chloroplast stroma is disturbed. |
| Treatment | Seeds | Mean for Light | |||
|---|---|---|---|---|---|
| Water (W) | Algae (A) | ||||
| Watering/Spraying | |||||
| Water (WW) | Algae (WA) | Water (AW) | Algae (AA) | ||
| Fresh mass [g plant−1] | |||||
| Low light intensity (LL) | 0.94 abc * | 0.91 bc | 0.85 c | 0.83 c | 0.88 b |
| High light intensity (HL) | 1.07 a | 0.97 abc | 0.98 abc | 1.05 ab | 1.02 a |
| Mean for water/algae | 1.01 a | 0.94 a | 0.92 a | 0.94 a | |
| Dry mass content [%] | |||||
| Low light intensity (LL) | 7.92 ab | 7.96 ab | 7.75 b | 7.72 b | 7.84 a |
| High light intensity (HL) | 7.93 ab | 7.82 b | 8.39 a | 7.64 b | 7.95 a |
| Mean for water/algae | 7.93 ab | 7.89 ab | 8.07 a | 7.68 b | |
| Height [cm] | |||||
| Low light intensity (LL) | 12.13 bc | 11.99 bc | 12.36 abc | 11.55 c | 12.01 b |
| High light intensity (HL) | 12.94 ab | 12.53 abc | 12.93 ab | 13.59 a | 13.00 a |
| Mean for water/algae | 12.53 a | 12.26 a | 12.64 a | 12.57 a | |
| The leaf length [cm] | |||||
| Low light intensity (LL) | 1.71 b | 1.74 b | 1.65 b | 1.64 b | 1.69 b |
| High light intensity (HL) | 1.95 a | 1.81 ab | 1.72 b | 1.84 ab | 1.83 a |
| Mean for water/algae | 1.83 a | 1.77 a | 1.69 a | 1.74 a | |
| The leaf width [cm] | |||||
| Low light intensity (LL) | 2.13 bc | 1.81 d | 1.85 d | 1.84 d | 1.91 b |
| High light intensity (HL) | 2.40 a | 2.30 ab | 2.04 cd | 2.17 abc | 2.23 a |
| Mean for water/algae | 2.27 a | 2.05 b | 1.94 b | 2.00 b | |
| Plant area [cm2] | |||||
| Low light intensity (LL) | 40.58 ab | 35.07 bc | 32.87 c | 31.95 c | 35.12 b |
| High light intensity (HL) | 44.94 a | 39.68 ab | 37.29 bc | 39.66 ab | 40.39 a |
| Mean for water/algae | 42.76 a | 37.37 b | 35.08 b | 35.80 b | |
| Specific Leaf Area [SLA, m2 kg−1] | |||||
| Low light intensity (LL) | 20.43 ab | 17.63 cd | 17.06 d | 16.44 d | 17.89 b |
| High light intensity (HL) | 22.84 a | 20.57 ab | 17.63 cd | 19.97 bc | 20.25 a |
| Mean for water/algae | 21.64 a | 19.1 b | 17.35 b | 18.21 b | |
| Chlorophyll Content Index (CCI) | |||||
| Low light intensity (LL) | 17.83 d | 20.20 c | 19.95 c | 23.45 a | 20.36 a |
| High light intensity (HL) | 15.70 e | 21.75 b | 17.25 d | 23.20 a | 19.48 b |
| Mean for water/algae | 16.76 d | 20.98 b | 18.60 c | 23.33 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąszczak, B.; Kula-Maximenko, M.; Li, C. The Suitability of Algae Solution in Pea Microgreens Cultivation under Different Light Intensities. Agriculture 2024, 14, 1665. https://doi.org/10.3390/agriculture14101665
Frąszczak B, Kula-Maximenko M, Li C. The Suitability of Algae Solution in Pea Microgreens Cultivation under Different Light Intensities. Agriculture. 2024; 14(10):1665. https://doi.org/10.3390/agriculture14101665
Chicago/Turabian StyleFrąszczak, Barbara, Monika Kula-Maximenko, and Caihua Li. 2024. "The Suitability of Algae Solution in Pea Microgreens Cultivation under Different Light Intensities" Agriculture 14, no. 10: 1665. https://doi.org/10.3390/agriculture14101665
APA StyleFrąszczak, B., Kula-Maximenko, M., & Li, C. (2024). The Suitability of Algae Solution in Pea Microgreens Cultivation under Different Light Intensities. Agriculture, 14(10), 1665. https://doi.org/10.3390/agriculture14101665







