Abstract
Oil crops are the second most cultivated economic crop in the world after food crops, and they are an important source of both edible and industrial oil. The growth of oil crops is limited by biotic and abiotic stresses, which hinder their yield and quality. Among all the agronomic measures, plant growth promoting bacteria (PGPB) play a crucial role in improving the yield, quality, and adaptability of oil crops. In this review, we considered the recent research on the sources of beneficial bacteria and their interactions with and influences on host plants, with a focus on summarizing the important roles and molecular mechanisms of PGPB in promoting growth and resisting biotic and abiotic stresses in oil crops. Finally, we outlined the current opportunities and challenges of microbial strategies for the improvement of the yield, quality, and adaptability of oil crops, providing a theoretical basis for the future use of microbial inoculants in these crops.
1. Introduction
Oil crops, including soybeans, rapeseed, peanuts, palms, sunflowers, cottonseed, and sesame, are the second most cultivated crops after food crops in terms of yield and area [1]. They are not only a major source of edible oil, vegetable protein, and animal feed, but are also an important source of biodiesel [2]. Animal-derived edible oil is primarily composed of saturated fat, while plant-derived edible oil is mainly composed of unsaturated fat, which provides greater benefits to the human body [3]. Soybeans are rich in proteins, amino acids, and unsaturated fatty acids, and they also contain numerous phytochemicals and bioactive components that confer significant benefits on human health and enhance disease resistance [4,5]. Rapeseed is rich in antioxidants, vitamin C, and phenols. Therefore, it has a powerful protection against oxidative stress [6]. Sunflowers not only have value in the production of biodiesel and biogas but are also widely used for the phytoremediation of heavy metals (HMs) and organic pollutant-contaminated soil [7]. The increasing significance of oil crops has resulted in a progressively rising demand for them.
According to the United Nations forecast, the global population will reach 9.7 billion by 2050 [8]. The rapid growth of the population will lead to an increase in the demand for food and energy. A report issued by the Food and Agriculture Organization (FAO) emphasized that in order to meet the demands of a growing population, global food production must increase by 70% before 2050 [9]. The researchers project a 95% increase in palm production, a 23% increase in soybean production, and a 24% increase in rapeseed production from 2019 to 2050 to meet the demands of the growing population [10]. Oil crops are also a potential source of biodiesel. The production of biodiesel in 2021 amounted to 1.5 joules, representing a twofold increase compared to the production in 2010. It is expected that the global demand for biodiesel will reach 10.5 joules by 2050 [2]. Based on the above, with rapid global population growth, advancements in living standards, expansion of the livestock and poultry breeding industries, as well as the increasing production of biodiesel, have led to a surge in the demand for oil crops, as shown in Figure 1.
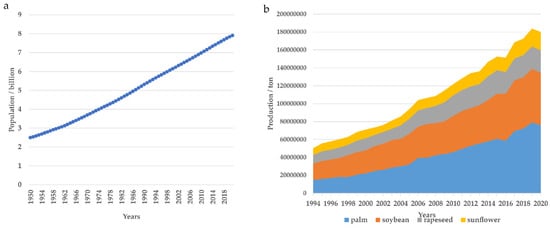
Figure 1.
The growth of the global population and the expansion of palm, soybean, rapeseed, and sunflower production. (a). The global population has experienced a rapid growth from 1950 to 2021. Data from the FAO (https://www.fao.org/faostat/zh/#data/OA/visualize, accessed on 10 December 2023). (b). The global production of palms, soybeans, rapeseed, and sunflowers exhibited a consistent upward trajectory from 1994 to 2021. Data from the FAO (https://www.fao.org/faostat/zh/#data/QCL/visualize, accessed on 10 December 2023).
There are numerous factors that constrain the enhancement of both the yield and quality of oil crops. Global climate change is a major factor affecting crop yields [11]. The growth and development process of oil crops may be subject to unforeseen challenges, such as salinization, drought, diseases, and pests, which can result in a reduction in both the yield and quality [12,13]. Some soil-borne fungal diseases, such as Sclerotinia stem rot, root rot, and blackleg, result in significant global economic losses annually [14]. The occurrence of root and stem rot in soybeans can lead to a significant decrease in the yield and potentially result in crop failure [14,15]. Adverse environmental conditions usually lead to adverse changes in the fatty acid composition of seed oil, and a decrease in seed oil accumulation [16]. Rapeseed is the second largest oil crop after soybeans, and drought and salt stresses seriously impact its growth and yield [17]. The daytime temperature was adjusted to 29 °C from the 38th day of rapeseed sowing until harvest, inducing high temperature stress (compared to the normal growth temperature of 23 °C). Ultimately, it was observed that heat stress led to a significant reduction of 85.3% in the rapeseed yield and a decrease of 52% in the oil content [18]. In summary, the yield and quality of oil crops are significantly influenced by both biotic and abiotic stresses, necessitating the implementation of efficient strategies to mitigate their impact on oil crop growth.
In the past few decades, scientists have used breeding plans and agronomic practices to increase the yield and resistance of oil crops. However, these strategies often entail significant costs and time investments. The application of molecular breeding is constrained by the availability of high-quality reference gene sequences and entails a protracted timeline from development to production [19,20,21,22,23,24]. Due to the large and complex genome of palms and the low efficiency of genetic transformation, the application of gene editing technology in palms is difficult [25]. The efficacy of traditional methods, such as crop rotation, in combating fungal diseases is limited due to the production of dormant sclerotia by fungi, which can live in the soil for up to 10 years [14]. The prolonged use of fungicides for disease management has led to a range of concerns, including the contamination of surface water, soil pollution, and damage to soil ecosystems. More seriously, the presence of these fungicides on crops poses a significant threat to human health if ingested [26]. Increasing evidence has indicated that plant growth promoting bacteria (PGPB) have tremendous potential for biological control and growth promotion in oil crops [27,28]. PGPB can promote plant growth by facilitating nitrogen fixation, phosphorus solubilization, potassium hydrolysis, and the synthesis of hormones and 1-Aminocyclopropane-1-carboxylate (ACC) deaminases. Additionally, they possess the ability to induce systemic resistance in host plants, thereby aiding in their defense against biotic and abiotic stresses [29,30,31,32,33,34].
This review aimed to elucidate the important roles and underlying molecular mechanisms of plant growth promoting bacteria (PGPB) for promoting growth and resisting biotic and abiotic stresses, including drought, salinity, and heavy metals, in oil crops. In this review, we emphasized harnessing PGPB as bioinoculants to improve the yield, quality, and adaptability of oil crops.
2. Source and Influence of Beneficial Bacteria
2.1. Source and Function of PGPB
Bacteria play an important role in all stages of plant growth and development, including seed germination, root and shoot growth, flowering, and fruiting [35]. There are two sources of bacteria in plants: vertical transmission from the mother plant and the external environment such as the soil and air [36]. Beneficial endophytic bacteria are a type of microorganisms that live within plant tissue without causing any disease symptoms [37]. These endophytic bacteria are mostly transmitted between consecutive plant generations through vertical transmission, which weakens the pathogenic intensity of microorganisms to support plant growth and development [36,38]. The rhizosphere is the stronghold of bacteria, and the composition and structure of the rhizosphere bacteria community are crucial for plant health [39,40,41]. The rhizosphere refers to the narrow area on the surface of plant roots, where numerous beneficial bacteria are recruited due to root-secreted amino acids, sugars, phenols, terpenoids, and lipids [42]. Rhizosphere bacteria play a significant role in promoting growth as well as stress and disease resistance. Therefore, they are widely used when reducing fertilizer use and in green sustainable production [43]. Moreover, the interactions between rhizosphere bacteria and plants are extensively used for the bioremediation of heavy metals and organic pollutants in contaminated soil [44].
The phyllosphere refers to the aerial part of the plant, including the stems, leaves, flowers, and fruits [45,46]. Due to the large biomass and surface area of the phyllosphere, it is a good habitat for bacteria [46]. The complexity of the soil environment results in significant variations in the rhizosphere bacteria community structure. However, the phyllosphere offers a relatively simplified environment with a high community similarity across different plants [47]. Phyllosphere microbiota originates from diverse sources, including the soil, seeds, air, and insects. Soil serves as the primary reservoir of phyllosphere bacteria that can be transmitted during seed germination or transported by roots to the shoots during the subsequent growth stages [48]. Consequently, there is a functional overlap between rhizosphere microbiota and phyllosphere microbiota [49]. Additionally, pollen acts as a niche for bacteria colonization while insects like bees facilitate transportation of both beneficial and pathogenic bacteria during the pollination process [50,51]. Phyllosphere bacteria play a pivotal role in the carbon and nitrogen cycling of ecosystems, growth promotion, stress resistance, and pathogens control [52,53,54,55]. The volatile compounds emitted by plants, such as methanol and methane, have a significant impact on global climate change. Phyllosphere bacteria can metabolize these volatile compounds to alleviate adverse effects on the climate [56]. Additionally, phyllosphere bacteria exhibit beneficial functions, including stimulating plant growth, absorbing nutrients such as nitrogen and phosphorus, and producing hormones such as auxin and cytokinin [57]. Moreover, they alleviate adverse environmental stresses for host plants, such as salt stress and drought stress by producing ACC deaminases [58,59]. Furthermore, phyllosphere bacteria synthesize a large number of secondary metabolites involved in the communication between beneficial and pathogenic bacteria [60].
2.2. Interaction and Influence between Bacteria and Plants
In nature, plants and bacteria interact and co-evolve with each other [61,62]. Microorganisms, known as the second genome of plants, establish functional symbiotic relationships with plants during evolution, exerting a significant impact on the nutrient absorption, growth promotion, and resistance against biotic and abiotic stresses [61]. Plants recruit beneficial bacteria by secreting various metabolites [63,64]. The influence of root exudates on the microbial community is defined as the rhizosphere effect [42,65,66]. Previous studies have demonstrated that the GsMYB10 gene can regulate the composition of rhizosphere microbiota to reduce aluminum toxicity in soybeans. The GsMYB10 gene regulates the properties of the root cell wall and influences the levels of proline and malondialdehyde, as well as impacts the phosphorus-related metabolic pathways. Consequently, this alters the soybean root exudates and the rhizosphere environment, leading to the recruitment of beneficial microorganisms in trans-GsMYB10 plants. These recruited microorganisms have important functions in reducing Al toxicity in plants through various mechanisms, such as nitrogen fixation, phosphorus dissolution, and the production of hormones [67]. Compared with the wild-type plants, triple-transgenic soybean plants with insect-resistant, glyphosate-tolerant, and drought-resistant varieties exhibit a different α diversity and β diversity in their root microbiota [68]. Notably, significant variations exist not only between plant species but also within different varieties of a single species regarding rhizosphere bacterial communities. Research has found that there are differences in the root microbial communities recruited by low cadmium accumulation variety L351 and high cadmium accumulation variety L338 in rapeseed. This provides evidence for studying the cadmium resistance mechanisms in rapeseed varieties [69]. In summary, the composition of root microbial communities is regulated by factors such as the rhizosphere effect, plant genotype, and host response to stress, which in turn affects the growth and development of the host plants.
Plant species influence the community structure of root microorganisms. Conversely, the root microbiota impacts the plant nutrient uptake and the expression of genes related to biotic and abiotic stresses [70]. It has been proven that the expression of genes related to nutrient absorption and stress resistance are influenced by changes in the structure and function of rhizosphere microbiota. Plant rhizosphere secretions reshape rhizosphere microorganisms, providing feedback on plant growth and flowering by regulating soil nitrogen cycling and secreting hormones such as indole-3-acetic acid (IAA) [71]. The synthetic community is a community that has been artificially constructed [72]. The application of synthetic communities has been shown to induce the gene expression related to the soybean phosphate starvation response, phosphate transport, and metabolism, indicating that synthetic communities not only facilitate phosphorus solubilization but also activate the plant phosphate starvation signal [73]. Overall, rhizosphere microorganisms can regulate the plant growth-related gene expression by mobilizing nutrients, altering plant nutrient utilization efficiency, and producing hormones such as IAA and volatile compounds.
To optimize the beneficial effects between bacteria and plants, it is essential to identify advantageous bacteria and construct a tailored synthetic community for specific crops, considering the interaction and influence between them. However, it should be noted that while beneficial bacteria may exhibit positive impacts on one crop, their inoculation on another crop might not always yield the desired effect due to variations in the environmental conditions and genotypes.
3. Mechanisms of Action of PGPB
3.1. Plant Growth Promotion
The quality and oil content of oil crops, such as rapeseed and soybeans, are closely related to the composition and activity of rhizosphere microorganisms [74]. PGPB promote the absorption and acquisition of nutrients by oil crops through direct mechanisms, such as nitrogen fixation, phosphate solubilization, and potassium hydrolysis [75,76]. They can also promote the growth of oil crops through indirect mechanisms, such as the synthesis of 1-Aminocyclopropane-1-carboxylate (ACC) deaminases and siderophores [77,78]. The promotion of oil crop growth by plant growth promoting bacteria (PGPB) may be attributed to the synergistic effects of multiple mechanisms. The mechanisms of action of PGPB are shown in Table 1.
3.1.1. Promotion of Nutrient Acquisition
Peanuts and soybeans can utilize rhizobia for symbiotic nitrogen fixation. Nitrogen-fixing bacteria convert atmospheric nitrogen into ammonia and nitrate by nitrogenase. Nodule endophyte Bacillus megaterium LNL6 and Methylobacterium oryzae CBMB20 symbiosis was shown to result in a measurable increase in the nodule activity, number, and total nitrogen content [79]. In addition to the symbiotic nitrogen fixation between leguminous crops and rhizobia, non-leguminous crops can also harbor PGPB that are capable of atmospheric nitrogen fixation. Paenibacillus polymyxa P2b-2R was isolated from the internal tissues of lodgepole pine trees. P2b-2R fixed 22% nitrogen from the atmosphere and significantly enhanced the canola height by 25% and the biomass by 30% [75]. The crust contains abundant phosphorus and potassium elements, but the majority of them exist in insoluble forms that cannot be directly absorbed and used by plants. Some bacteria present in the soil possess the ability to solubilize phosphorus and potassium from insoluble minerals for plant utilization through mechanisms such as the production of inorganic and organic acids, pH reduction, acidolysis, chelation, exchange reactions, and complexation [80,81]. The inoculation of phosphorus solubilizing bacteria in the field significantly enhanced rapeseed yields by 20~41%, reaching a level comparable to that achieved with chemical fertilization, thus reducing the use of synthetic fertilizers [76].
Additionally, PGPB also promote plant nutrient absorption and transportation by regulating the expression of the genes related to nutrient transport. Serratia marcescens PLR significantly upregulated the expression of the nitrate transporter genes NRT1.1 and NRT2.3, inorganic phosphate transporter gene PHO1; H1, and high-affinity K+ transporter gene HAK5 to increase the nutrient uptake by the host plants [82]. NRT2.5 and NRT2.6 participated in root growth, mediated by Phyllobacterium brassicaceae STM196 [83]. Inoculation with STM196 significantly upregulated the expression of the nitrate transport-related genes NRT2.5 and NRT2.6. Inoculation with STM196 did not result in an increase in biomass of the plant mutant nrt2.5 and nrt2.6, indicating that NRT2.5 and NRT2.6 are essential for strain STM196 to promote plant growth [83,84]. In summary, the promotion of the gene expression related to nutrient absorption and transport in host plants by PGPB facilitates an enhanced uptake of nutrients, such as N, P, and K, thereby promoting growth.
Therefore, PGPB contribute to plant nutrient acquisition, whether they dissolve nutrients into forms that plants can directly absorb and utilize or promote the expression of genes related to nutrient absorption.
3.1.2. Synthesis of Hormones and the Induced Expression of Plant Hormone-Related Genes
PGPB can produce hormones, such as indole-3-acetic acid (IAA), cytokinin (CTK), and gibberellin (GA), to promote the growth and development of host plants [33,85]. The rhizobia produce the penultimate intermediate GA9 for soybeans, then the soybean nodules express functional GA 3-oxidases that convert GA9 into bioactive GA4, thereby significantly increasing the nodule size [86]. The seed-endophytic bacterium Paenibacillus glycanilyticus YMR3 increased the levels of the hexanoate, succinate, and jasmonic acid (JA) content, as well as peanut biomass and the number of nodules compared to non-inoculation YMR3 [87]. In addition to promoting plant growth through hormone production, PGPB can also facilitate the expression of genes related to hormone synthesis and response in plants. Researchers typically use the model plant Arabidopsis thaliana to study the mechanisms at the molecular level [88]. Serratia marcescens PLR can enhance the expression of genes involved in the auxin synthesis pathway of Arabidopsis, particularly the IPA pathway, thereby promoting plant auxin biosynthesis [82]. Pseudomonas sp. CM11 regulated root branching by activating the PLT3/5/7-induced lateral root formation, thereby promoting plant growth and development [89]. The colonization of Achromobacter sp. 5B1 altered the root growth and branching patterns by altering the response and transport of root auxin [90].
All in all, phytohormones regulate the growth and development of plants, and PGPB promote plant growth through direct hormone production or the promotion of hormone synthesis and response-related genes expression. This is the most common and important mechanism by which PGPB promotes plant growth.
3.1.3. Production of ACC Deaminases
Gaseous phytohormone ethylene (ET) plays a pivotal role in promoting fruit ripening and flower senescence, while excessive levels of ET hinder plant growth and development [91]. Adverse environmental conditions, such as drought, waterlogging, salinization, and heavy metals stress, induce the synthesis of ET in plants [92,93,94]. The synthesis of ET in plants is precisely regulated. Methionine is converted into S-adenosylmethionine (SAM) under the catalysis of S-adenosylmethionine synthase (SAMS), then SAM is used as the substrate by 1-aminocyclopropane-1-carboxylic acid (ACC) synthase to generate ACC, and ACC converts into ET under the catalysis of ACC oxidase (ACO) [95]. ACC is the precursor of ET, and the conversion of ACC into ET is regarded as a rate-limiting step in ET biosynthesis [96]. Some PGPB can produce ACC deaminases that decompose ACC into non-toxic α-ketobutyrate and ammonia, thereby alleviating the adverse effects of excessive ET on plant growth [97,98,99]. The acds gene has been identified in PGPB, which can effectively reduce ethylene production under various pressure conditions [100]. Streptomyces sp. CLV45 significantly increased the leguminous crop shoot weight by 36.63% and dry weight by 17.97% due to its high production of IAA and ACC deaminases [101]. In summary, PGPB produce ACC deaminases, which are typically used to eliminate excess ET produced by the host plants under stress conditions.
3.1.4. Production of Siderophores
Siderophores are low molecular weight natural chelating agents produced by microorganisms or plants [102]. The siderophores produced by bacteria, such as azotochelin, entobactin, metanobactin, pyochelin, and rhizoferrin, play an essential role in promoting host plant growth and alleviating heavy metal and salt stresses [102,103,104]. Although the Earth’s crust is rich in iron (Fe), its bioavailability remains relatively low. The strains Micrococcus yunnanensis YIM 65004 and Stenotrophomonas chelatiphaga LPM-5 were isolated from the rhizosphere of canola and showed a strong capacity for siderophore production. These strains observably increased the canola weight and the Fe content of the roots and shoots [78]. The endophytic bacteria Pseudomonas brassicacearum CDVBN10, found in rapeseed plants, exhibits the ability to produce siderophores, solubilize phosphate, synthesize cellulose, and increase the rapeseed height [105]. The growth promoting bacteria that produce siderophores improve the utilization of iron in the rhizosphere, thereby facilitating the absorption of iron by host plants.
Promoting the growth of oil crops by PGPB is the result of multiple mechanisms working together, not just one mechanism at play. These mechanisms are similar to the mechanisms by which PGPB promotes the growth of general crops, lacking new mechanisms specifically targeting oil crops. For example, how PGPB promote oil synthesis and metabolism in oil crops remains elusive. Future research should focus on exploring the new mechanisms by which PGPB promotes plant growth, rather than being limited to the already discovered mechanisms. Additionally, most experiments were conducted in environmentally controlled greenhouses, lacking an exploration of the effects and mechanisms under field conditions. Future research should focus more on the practical application effects and molecular mechanisms in the field.

Table 1.
The effects and mechanisms of PGPB in promoting growth and enhancing the resistance of disease stress, salt stress, drought stress, and heavy mental stress in oil crops.
Table 1.
The effects and mechanisms of PGPB in promoting growth and enhancing the resistance of disease stress, salt stress, drought stress, and heavy mental stress in oil crops.
| Characteristics | Bacteria | Host Plant | Effect | Action Mechanism | References |
|---|---|---|---|---|---|
| Growth Promotion | Bacillus sp. 1L6 | Rapeseed | Inoculation with 1L6 increased the total biomass weight of rapeseed by 87.5%. | Phosphate solubilization (457.5 ± 6.37 mg/L), IAA (58.0 ± 0.77 mg/L), and HCN (221.8 ± 1.55 mg/L) production. | [106] |
| Paenibacillus polymyxa P2b-2R | Canola | After 60 days of inoculation with P2B-2R, the height and biomass of canola seedlings increased by 25% and 30%, respectively. | Fixed nitrogen (fixed 19–22% nitrogen in the atmosphere). | [75] | |
| Pseudomonas brassicacearum CDVBN10 | Rapeseed | In the field, inoculation with CDVBN10 increased the number of pods, the dry weight of the pods, and the dry weight of the shoot by 216%, 174%, and 198%, respectively. | Produced IAA (8.18 μg/mL), siderophores, solubilized phosphate, and synthesized cellulose. | [105] | |
| Bacillus cereus T4S | Sunflower | Inoculation with T4S in sunflowers in a greenhouse increased the shoot dry weight, root dry weight, and head dry weight by 19%, 36%, and 11%, respectively. | Produced IAA (11.29 ± 0.01 μg/mL), siderophore (87.30 ± 0.38%), and solubilized phosphate (30.43 ± 0.18 μg/mL). | [107] | |
| Disease stress | Pseudomonas fluorescens BRZ63 | Rapeseed | The inhibitory rates of BRZ63 on the pathogenic fungi Colletotrichum dematium K, Sclerotinia sclerotiorum K2291, Fusarium avenaceum, and Rhizoctonia solani W70 were 61.8%, 39.9%, 40.3%, and 37%, respectively. | Produced biosurfactants, siderophores, IAA (59.62 ± 1.11 μg/mL), ACC deaminase, ammonia, and exopolysaccharide; phosphate solubilization. | [108] |
| Bacillus velezensis LDO2 | Peanut | The inhibitory rates of Fusarium oxysporum and Fusarium moniliforme, which caused peanut Fusarium wilt and root rot, were 81.03% and 81.28%, respectively. | Produced antibacterial metabolites, such as fengycin, surfactin, bacilysin, butirosin, bacillaene, difficidin, and macrolactin; phosphate solubilization; siderophore production. | [109] | |
| Bacillus velezensis GA1 | Peanut | Inoculation with GA1 reduced the incidence of peanut stem rot caused by Athelia rolfsi by 60%. | Produced the lipopeptides surfactin, iturin, and fengycin. | [110] | |
| Bacillus altitudinis JSCX-1 | Soybean | The inhibitory rate of JSCX-1 on Phytophthora sojae was 63.94%, and the lesion length of soybean leaves was reduced by 61.11% after inoculation with JSCX-1. | Upregulated the expression of the salicylate-responsive gene GmPR1a; induced the systemic resistance of soybeans. | [111] | |
| Corallococcus sp. EGB | Soybean | The control efficiency of EGB on Phytophthora sojae wild-type P6497 reached 50.34%. | Scavenged thiamine in the soybean rhizosphere via outer membrane vesicle-secreted thiaminase I. | [112] | |
| Salt stress | Bacillus amyloliquefaciens H-2-5 | Soybean | Inoculation with H-2-5 increased the plant height of soybeans by 9.8% under salt stress. | Production of gibberellin (2.1 ng/100 mL) and solubilized phosphate. | [113] |
| Enterobacter cloacae HSNJ4 | Canola | Under 100 mM NaCl stress, inoculation with HSNJ4 increased the length of the root and shoot and the chlorophyll content of canola by 35.7%, 15.6%, and 25.4%, respectively. | Produced IAA (112.62 mg/L) and ACC deaminase; decreased the malondialdehyde content (19.6%) and increased the proline content (47.2%) and antioxidant enzyme activity. | [114] | |
| Curtobacterium sp. SAK1 | Soybean | Under 300 mM NaCl stress, the plant height and shoot fresh weight of soybeans increased by 23.7% and 39.3%, respectively, when inoculated with SAK1. | Produced ACC deaminase (330 nmol α-ketobutyrate mg−1 h−1); decreased endogenous JA and the ABA content in soybeans under salt stress. | [115] | |
| Drought stress | Pseudomonas putida GAP-P45 | Sunflower | Under drought stress, inoculation with GAP-P45 increased the total dry weight of sunflower seedlings by 64.6%. | Produced exopolysaccharides (304%) and increased the percentage of stable soil aggregates (149%). | [116] |
| Heavy metal stress | Pseudomonas lurida EOO26 | Sunflower | Under the stress of 100 mg/L copper, the growth of sunflower seedlings was inhibited, and the shoot fresh weight and root fresh weight of sunflower increased by 1.4 times and 9.4 times, respectively, when inoculation with EOO26. | Produced ACC deaminase, siderophore, indole-3-acetic acid, and ammonia under copper stress; phosphate solubilization. | [117] |
| Acinetobacter bouvetii P1 | Sunflower | Inoculation with P1 enabled sunflower to bear 1200 μg/mL of Cr6+ without affecting its growth. | Transformed Cr6+ into a stable and less toxic Cr3+ form; strengthened the host antioxidant system; produced flavonoids, phenolics, proline, and glutathione. | [118] | |
| Brevundimonas diminuta MYS6 | Sunflower | Inoculation with MYS6 in copper-contaminated soil increased the fresh weight and dry weight of sunflower by 9.9 times and 15.8 times, respectively. | Produced EPS; increased leaf chlorophyll under copper stress. | [119] | |
| Staphylococcus arlettae MT4 | Sunflower | Compared with no chromate stress and no inoculation, 100 ppm chromate stress reduced the growth rate of sunflower by five times, while the growth rate of sunflower under 100 ppm chromate stress increased by about eight times when inoculated with MT4. | Secreted phytohormones and secondary metabolites; strengthened the host’s antioxidant system and suppressed chromate intake by the host. | [120] |
3.2. Disease Stress
Oil crops are susceptible to diseases caused by soil-borne pathogens, including stem rot, root-knot nematode disease, blackleg disease, and soybean Phytophthora root rot. These diseases significantly reducing the yield and quality of oil crops [14,23,121]. Soybean root rot is a widely prevalent soil-borne disease, mainly caused by Fusarium spp., Pithium spp., Phytophthora spp., and Rhizoctonia spp. [122,123,124,125]. Currently, those soil-borne diseases are mainly managed through methods such as seed coating, pesticide spraying, tolerant variety screening, and biological control [22,24]. However, these strategies often give rise to issues like soil pollution, pesticide residue accumulation, and pathogen resistance development [26]. In recent years, the pursuit of sustainable development methods for controlling soil-borne diseases has become a persistent scientific focus. Some beneficial microorganisms play a very vital role in maintaining plant health. They not only effectively restrict invasion and induce plant resistance, but also help hosts cope with environmental pressures [126,127]. Therefore, microbial biocontrol can be an effective alternative strategy for managing plant diseases [128,129].
Some bacteria, such as Bacillus spp., Pseudomonas spp., and Streptomyces spp., have significant potential for suppressing phytopathogens [130,131,132]. A novel endophyte of rapeseed, Pseudomonas fluorescens BRZ63, demonstrated biocontrol potential against fungal pathogens [108]. Blackleg disease caused by Leptosphaeria maculans can drastically decrease rapeseed yields. Bacillus pumilus 1L6 has the highest phosphate solubilization capacity, as well as IAA and HCN production. Moreover, 1L6 significantly decreased the leaf area damaged by L. maculans [106]. Two endophytic strains Pseudomonas protegens Sneb1997 and Serratia plymuthica Sneb2001 have a high biological efficacy for controlling root-knot nematode disease [133]. Fusarium oxysporum is a common pathogen responsible for various plant diseases, including vascular wilt, root rot, and damping-off. By isolating and screening bacteria from sunflowers leaf tissues, three endophytic bacterial strains showed extensive antifungal activity against Fusarium oxysporum in sunflowers [134]. Among the isolated bacterial endophytes from peanut seeds, Pseudomonas sp. EGN1 and Acinetobacter sp. EGN4 showed positive results for IAA and siderophore production as well as phosphate solubilization, and they also displayed a 100% inhibition rate against Sclerotium rolfsii Sacc [135].
3.2.1. Production of Secondary Metabolites
PGPB can produce antimicrobial compounds and metabolites that can reduce plant pathogenicity [136,137,138,139]. Pseudomonas fluorescens is capable of producing a series of secondary metabolites, including hydrogen cyanide, phloroglucinol, pyrrolidine, and cyclic lipopeptide, to control plant pathogens [130]. Bacillus amyloliquefaciens BZ6-1, which exhibited the highest antimicrobial activity among those isolated from healthy peanut plants, was found to produce surfactin and fengycin A homologues identified using a liquid chromatograph mass spectrometer (LC-MS) [140]. Additionally, it was observed that Bacillus velezensis FZB42 effectively resisted Phytophthora sojae. However, a mutant lacking in bacilysin biosynthesis lost its ability to resist Phytophthora sojae. This suggests that the production of bacilysin by Bacillus velezensis FZB42 plays an important role in its antagonism against pathogens [15]. Furthermore, some PGPB have been found to produce hydrolytic substances with an antibacterial activity, such as chitinase, protease, cellulase, and pectinase. These substances not only degrade the cell wall of pathogenic bacteria but also improve plant defense mechanisms. For instance, Pseudomonas chlororaphis IRHB3 isolated from the soybean rhizosphere has been shown to produce proteases, caseinases, and cellulases, effectively preventing soybean root rot [141]. The secondary metabolites produced by PGPB have been developed into biostimulant products for disease control in oil crops.
3.2.2. Competition for Nutrients and Niches
There are thousands of microorganisms coexisting in nature, sharing resources secreted by other microorganisms or plants in public spaces. Beneficial microorganisms can compete with pathogens for nutrients and ecological niches, thereby eliminating the pathogens [142]. Phytophthora spp., an important class of pathogenic fungi, poses a threat to the soybean industry by causing soybean root rot, particularly Phytophthora sojae [143]. Phytophthora sojae P6497 relies on external sources for its growth and infection due to its deficiency in thiamine. Myxobacteria EGB produces a novel thiamine enzyme called CcThi1, which is transported outside the cell via outer membrane vesicles and decomposes common thiamine in the environment. This process effectively blocks the pathway for Phytophthora sojae P6497 to obtain thiamine and inhibits its growth [112]. Fe is an essential trace element for most organisms, as it serves as a cofactor for enzymes involved in fundamental cellular processes such as respiration and DNA synthesis. Rhizosphere microorganisms compete for limited Fe3+ resources [144]. PGPB with a strong siderophore production ability can outcompete pathogenic bacteria for scarce Fe resources in the rhizosphere, thus reducing the harm caused by pathogenic bacteria to host plants [104]. The competition between PGPB and pathogens for nutrients and ecological niches depends on their respective abilities.
3.2.3. Induction of Systemic Resistance
PGPB help host plants resist pathogens by increasing their systemic resistance [127,145]. Sclerotinia stem rot, caused by the fungal pathogen Sclerotinia sclerotiorum, poses a major threat to rapeseed crops and causes severe yield losses. Previous studies have shown that rapeseed bio-priming with the hypovirulent strain DT-8 of S. sclerotiorum induced systemic resistance and reduced the severity of sclerotinia stem rot disease, thereby increasing rapeseed yields [146,147]. Pathogenic bacteria in soybeans promote infection by regulating host plant hormone signals [148]. Salicylic acid is a plant hormone related to plant immunity [149]. Bacillus altitudinis JSCX-1, isolated from the rhizosphere of healthy soybeans, was found to stimulate the expression of GmPR1a (the soybean salicylic acid-responsive gene), significantly reducing infection by Phytophthora sojae [111]. Bacillus velezensis CB13 effectively controlled peanut stem rot through upregulation of plant defense-related enzymes, including phenylalamine ammonia lyase (PAL), polyphenol oxidase (PPO), catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), chalcone isomerase (CHI), and staphylococcus protease (GLU) [150]. PGPB enhance plant system resistance by promoting the production of antioxidant enzymes that support the immune system of plants in combating pathogenic bacteria, as shown in Figure 2.
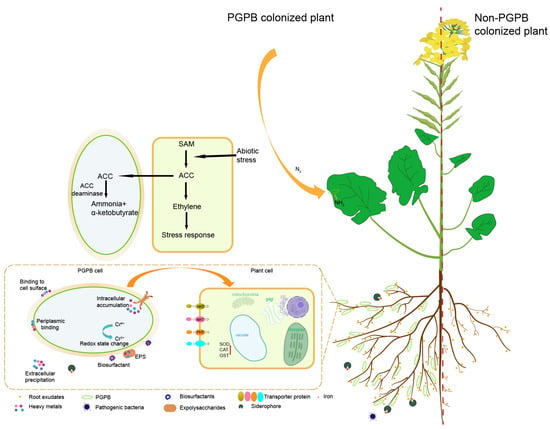
Figure 2.
Molecular mechanisms for promoting growth and enhancing the stress resistance of oil crops by growth promoting bacteria.
3.3. Drought Stress
Drought is a global natural disaster that adversely affects agricultural productivity. The widespread and persistent occurrence of drought poses a serious threat to global food production [17]. Drought limits the mobility of nutrients and reduces the diversity and abundance of microorganisms in the soil. In order to cope with drought stress, plants have developed various adaptation mechanisms through long-term evolution, encompassing morphology, physiology, biochemistry, and other factors [151]. Drought triggers various adaptive responses in plants, including leaf curling, stomata closure to reduce the transpiration rate, increased root depth and diameter for enhanced water absorption and utilization efficiency, the modulation of plant hormone signals such as abscisic acid to promote flowering and shorten the life cycle, and the regulation of the osmotic balance and antioxidant system to strengthen the defense mechanisms [152]. The exposure of rapeseed to drought stress triggers the molecular mechanisms that are activated to cope with the stress [17]. The BnaHsf gene is reactive to stress caused by high temperatures, drought, and carbon dioxide [153]. Further research found that the overexpression of the BnaHsf gene enhanced the drought tolerance of transgenic rapeseed by modifying the quantity, composition, and structure of keratin wax [154]. In recent years, plant growth promoting bacteria (PGPB) have been widely used to alleviate drought stress in crops [43,155]. PGPB facilitates the growth of oil crops under drought conditions by synthesizing hormones to modulate the root architecture, enhance the antioxidant enzyme activity, and produce extracellular polymers to improve soil water retention.
3.3.1. Production of Hormones
The promotion of nutrient absorption under drought conditions represents an important mechanism, through which PGPB can improve the plant drought tolerance [43,155]. Drought stress leads to soil structure damage, seriously hindering the migration and diffusion of nutrients within the soil [43]. When inoculated with PGPB, the root system architecture is enhanced; for example, more and longer lateral roots are formed. The change in the root system architecture facilitates an enhanced absorption of nutrients and water under drought conditions [156]. The changes in the root system architecture are often attributed to hormones produced by PGPB. The senescence induced by abiotic stress results in crop yield and significant economic losses for farmers, which is mainly due to the crosstalk between plant hormones under stress, especially cytokinin and abscisic acid [157,158,159,160,161,162]. Drought stress leads to the accumulation of abscisic acid, and excessive abscisic acid reduces the cytokinin content via the MYB2-dependent repression of IPT genes [159]. The PGPB Bacillus amyloliquefaciens GB03 produced diacetyl, which alleviated ABA-mediated senescence under various abiotic stress conditions [163].
PGPB alters the root system architecture of plants through the production of hormones, which stimulate an increased absorption of water and nutrients during drought conditions. Additionally, PGPB regulates hormone crosstalk in plants to alleviate senescence.
3.3.2. Enhancement of Antioxidant Enzyme Activity
Under normal environmental conditions, the production and clearance of reactive oxygen species (ROS) in plant cells are normally maintained in a dynamic balance. However, this balance is disrupted when plants experience drought stress, resulting in disturbances in ROS production and metabolism within plants [164]. Plants produce a range of enzymes to neutralize these ROS [13]. PGPB can induce specific signaling pathways in plants, thereby enhancing the plant’s ability to synthesize antioxidant enzymes [62]. The application of Paraburkholderia megapolitana MGT9 was found to increase the activity of plant stress markers, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and glutathione reductase (GR). Therefore, it exhibited a positive impact on the growth of soybean plants under drought conditions [165].
The application of PGPB enhances drought tolerance by stimulating the synthesis of antioxidant enzymes to clear excess ROS and enhancing the plant’s antioxidant defense system.
3.3.3. Production of Extracellular Polymers to Increase Water Retention
Exopolysaccharides are secondary metabolites secreted by PGPB outside the cell wall during their growth and metabolism [166]. The exopolysaccharides secreted by PGPB improve plants’ stress resistance, facilitate the nutrient absorption and water retention capacity, and improve soil aggregate stability and porosity, thereby promoting plant growth under drought stress conditions [167,168,169]. The strain Lysobacter sp. CJ11T, isolated from the roots of soybeans, produced a novel exopolysaccharide with a potential antioxidant capacity [170]. Pseudomonas spp. has been shown to improve plant tolerance against abiotic stresses [171]. The EPS produced by the Pseudomonas strain GAP-P45 alleviated the effects of drought stress on sunflowers by improving the soil structure and producing plant growth promoting substances [116]. The PGPB Bacillus amyloliquefaciens FZB42 enhanced plants resistance to stress. Plants inoculated with exopolysaccharide-deficient mutant epsC exhibited a reduced ability to resist drought stress compared to the wild-type strain, indicating the important role of exopolysaccharides for enhancing plant drought resistance [172]. The trehalose metabolism of PGPB plays a crucial role in improving the stress resistance of host plants. Soybeans inoculated with a mutant strain of Rhizobium etli CE3 that overexpressed the trehalose-6-phosphate synthase gene exhibited increased nodule formation and biomass compared to those inoculated with the wide-type strain under drought stress. When soybeans were inoculated with a mutant strain that had a deficiency in trehalose synthesis, they showed reduced nodules and biomass compared to those inoculated with the wild-type strain under drought conditions [173]. The co-inoculation of Pseudomonas putida and SA resulted in an increased production of osmolytes, phenols, and flavonoids while reducing damage caused by drought stress [174].
The extracellular polymers produced by PGPB play a pivotal role in enhancing plant tolerance to drought, saline, and heavy metals stress. However, their mechanisms of action exhibit distinct variations. Under drought stress, the extracellular polymers mainly enhance the soil’s capacity to retain water and improve its aggregate structure, thereby facilitating plants to cope with drought stress. Therefore, modern industrial techniques can be used to synthesize polysaccharides, trehalose, and other substances similar to the extracellular polymers produced by PGPB, which in turn can be used to promote oil crops growth in arid soils.
3.4. Salt Stress
Soil salinization is a widespread global problem, affecting approximately 7% of the Earth’s land surface [175]. Globally, 2.5 billion acres of soil are severely affected by salinization, with Asia having the highest continuous scale of salinization. Salinization is also one of the main causes of desertification in the European Union [176]. Salinization causes an accumulation of Na+ and CI− in plants, thereby inhibiting the synthesis of proteins and enzymes [177]. The presence of a high salt content in the soil impacts the osmotic pressure of solutions, leading to a scarcity of water for crops [177]. Salinization also diminishes the photosynthetic rate of crops, causing stomatal closure, which in turn affects cell elongation and division. Ultimately, this leads to impaired leaf development and a decrease in plant height, thereby significantly reducing crop yield and quality [178]. Salinization causes an excessive production of reactive oxygen species (ROS) in plants, which can damage plant tissues, alter DNA, damage cell membranes, and degrade lipids, proteins, and other biological molecules [175].
The gene regulatory network of plant salt tolerance helps to reveal the mechanisms of salt tolerance. The GmSIN1 gene is highly induced by salt stress, and it not only promotes root growth but also enhances plant tolerance to salt stress. Research has found that it promotes the expression of the ABA and ROS synthesis genes, leading to the accumulation of ABA and ROS. Consequently, excessive levels of ABA and ROS further promote the GmSIN1 expression. This positive feedback loop facilitated by GmSIN1 leads to the rapid transformation and amplification of early salt stress signals into ABA and ROS signals [179]. The overexpression of the GmTGA13 gene is beneficial for the absorption of K+ and Ca2+ into cells, thereby regulating ion homeostasis balance under salt stress [180]. The use of plant growth promoting bacteria (PGPB) is an important strategy for alleviating salt stress in plants [181,182,183]. Halotolerant PGPB are usually isolated from the rhizosphere or endosphere of plants growing in salt-stressed environments, as only the bacteria capable of withstanding salt stress can effectively enhance plant growth in saline conditions [184]. Halotolerant PGPB alleviate salt stress in host plants through the production of hormones, ACC deaminases, and extracellular polymers. They also maintain ion homeostasis, induce gene expression, and enhance systemic resistance [183,184,185].
3.4.1. Production of Hormones and ACC Deaminases
The modulation of plant hormones by bacteria plays a pivotal role in improving plants’ resistance to salt stress [186]. Hormones such as auxin, produced by bacteria, regulate the root system architecture of host plants under salt stress, thereby enhancing their resistance. The strain Acinetobacter pittii YNA40 showed a significantly higher concentration of indole-3-acetic acid (IAA) at 4% NaCl and enhanced the chlorophyll content and antioxidant activity of soybean plants during and after salt stress [187]. Abscisic acid (ABA) is one of the most important stress phytohormones. Salt stress forces plants to produce a large amount of ABA, and an excessive accumulation of ABA exerts detrimental effects on plant growth [161]. Inoculation with Bacillus aryahattai ALT29 and Arthrobacter woluwensis ALT43 significantly reduced the endogenous ABA levels of soybeans under salt stress [188]. The strain Bacillus amyloliquefaciens H-2-5 has the ability to secrete gibberellin. Inoculation with H-2-5 significantly increased the gibberellin content in soybean plants, resulting in a threefold increase compared to non-inoculated soybeans. This ultimately led to an improved growth of soybean plants under salt stress [113]. When canola plants were subjected to salt stress and inoculated with wild-type Pseudomonas putida UW4 or the ACC deaminase-deficient mutant, it was observed that the wild-type strains significantly improved plant growth, while the mutant strains failed to promote plant growth. This suggests that ACC deaminases confer salt tolerance to plants by reducing ethylene synthesis induced by salt stress [189].
Therefore, under salt stress conditions, PGPB modulate the root system architecture to enhance nutrient uptake through the production of indole-3-acetic acid (IAA) and gibberellins. Additionally, PGPB mitigate the detrimental effects of excessive ABA and ethylene by reducing ABA accumulation and synthesizing ACC deaminase.
3.4.2. Production of Extracellular Polymeric Substances
Bacterial exopolysaccharides (EPS) have been found to possess functions such as sodium ion flow restriction and the enhancement of plant osmotic stress tolerance, thus exhibiting extraordinary potential for improving plant salt tolerance [185]. The production of exopolysaccharides in bacteria varies in response to environmental fluctuations. By exposing Pseudomonas putida GAP-P45 to stressors such as drought, extreme temperatures, and salinity in order to assess its capacity for exopolysaccharide production and tolerance, it was found that the inoculation with Pseudomonas putida GAP-P45 improved the soil aggregates and stability under diverse stress conditions [190]. Pseudomonas aeruginosa PF23 exhibited a salinity tolerance of up to a 2000 mM NaCl concentration, and the mutant lacking synthetic EPS was unable to promote growth and resist pathogenic bacteria under salt stress. This indicated the importance of exopolysaccharides for promoting growth and biocontrol of strain PF23 under salt stress [191]. The EPS produced by Pseudomonas sp. AK-1 facilitated the sequestration of free sodium ions in the soil, thereby inhibiting their uptake by soybean plants [192]. The negatively charged nature of EPS makes it more prone to aggregate with positively charged sodium ions rather than negatively charged chloride ions.
The extracellular polymers produced by PGPR serve distinct functions under drought stress and salt stress. Specifically, under salt stress conditions, the primary role of these extracellular polymers is to impede the movement of sodium ions and hinder their interaction with the root system.
3.4.3. Maintenance of Ion Homeostasis
Salt stress forces plants to absorb a large amount of Na+ and CI−, and excessive accumulation of Na+ and CI− not only has a negative impact on metabolic processes and photosynthesis but also impairs the ability of plants to absorb water [177]. Inoculation with PGPB maintains ion homeostasis and improves malnutrition in plants. Compared with non-inoculated soybean seedlings under salt stress, the relative expression levels of nitrate and ammonium transporter-related genes were significantly upregulated when they were inoculated with Bradyrhizobium japonicum under salt stress [193]. This indicated that inoculation with PGPB exerts an influence on the absorption and transport of anions and cations, thereby regulating ion homeostasis in plants [193]. A high concentration of Na+ in the soil can disrupt intracellular ion homeostasis and inhibit K+ absorption in the roots. The high-affinity K+ transporter protein HKT is a crucial family of cationic transporter, extensively involved in the absorption and transportation of K+ and Na+ [194]. PGPB enhanced the salt tolerance of the host plants by regulating the HKT genes [195]. Bacillus subtilis GB03 exhibited an ability to downregulate the expression of HKT1 in the Arabidopsis roots and upregulate the expression of HKT1 in the shoots, thereby limiting the influx of Na+ into the roots and facilitating its transport from the roots to the shoots [195]. Under salt stress, soybean plants inoculated with Bacillus sp. SJ-5 and Pseudomonas sp. AK-1 exhibited an increase in the proline content, which facilitated the osmotic balance and maintained positive water potential for efficient water uptake by the roots [196].
In brief, salt stress disrupts the homeostasis of ions in plants, while PGPB can regulate the absorption and transport proteins to maintain ion homeostasis. Additionally, PGPB can regulate plant substances like proline to maintain the water potential balance and enhance root water absorption in the presence of salt stress.
3.4.4. Induction of Systemic Resistance
The volatile compounds produced by Bacillus amyloliquefaciens FZB42 activated the antioxidant system in plants under salt stress. Compared to plants without volatile compound treatment, higher levels of peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) activities were detected in the plants treated with volatile compounds. Further investigation revealed that the volatile compounds primarily activated the plant’s antioxidant system through the jasmonic acid pathway [197]. Bacillus licheniformis AP6 and Pseudomonas plecoglossicida PB5 were both capable of producing IAA and biofilm. The inoculation of sunflowers with AP6 and PB5 resulted in a significantly higher activity of CAT, SOD, and GPOD. Furthermore, AP6 and PB5 also significantly increased the photosynthetic pigments and nutrient uptake in sunflowers under salt stress [198]. Bacillus firmus SW5 enhanced the salt tolerance in soybeans by upregulating the expression of the genes involved in antioxidant enzyme synthesis and salt tolerance [199]. Inoculation with endophytic bacteria Bacillus firmus J22N, Bacillus tequilensis SEN15N, and Bacillus sp. REN51N increased the activities of the oxidative stress enzymes involved in scavenging ROS under salinity. They also enhanced the accumulation of osmoprotectant proline [200].
The impact of PGPB on the systemic resistance of plants under salt stress is analogous to its effect under other stresses, both of which stimulate the production of antioxidant enzymes for the elimination of excessive reactive oxygen species.
3.5. Heavy Metal Stress
Soil heavy metals (HMs) pollution is a major environmental issue globally. HMs, such as arsenic, mercury, nickel, chromium, lead, and copper, have many direct and indirect effects on plant growth. For example, they can deactivate and denature enzymes, alter protein structures, damage membrane integrity, and affect plant photosynthesis, respiration, and transpiration, ultimately leading to slow plant growth, yellowing of leaves, and stunted root development [201,202,203]. Unlike other stresses, heavy metals can easily enter the food chain through agricultural products, and this poses a threat to human health [204]. Plants have evolved various strategies to cope with HM toxicity, focusing primarily on limiting HMs absorption by the roots to prevent their entry into the plants. For example, some plants secrete organic acids from their roots to chelate HMs and exhibit enhanced antioxidant enzyme activities [205,206]. Additionally, the detoxification and defense antioxidant mechanisms of plants are activated when HMs enter plant tissues [207]. However, in soil contaminated with high concentrations of HMs, the majority of plants display poor growth and yields.
Various physical and chemical technologies, such as vapor extraction, incineration, thermal desorption, and ion exchange, have been used to mitigate soil heavy metal pollution. However, these technologies are expensive, laborious, time-consuming, and even may cause secondary environmental pollution [208,209]. Bioremediation is an ecologically neutral technology that utilizes green plants and microorganisms to restore contaminated sites to their original state [210]. Oil crops like rapeseed and sunflowers are commonly used for phytoremediation of HM-contaminated soil due to their well-developed roots, large shoot biomass, and strong stress resistance [211,212,213]. Plants remediate HM-contaminated soil through the mechanisms encompassing accumulation, stability, volatilization, and degradation [214,215]. Nevertheless, when the concentration of HMs in the soil exceeds a certain threshold, it can severely impede plant growth [169]. The integration of PGPB with plants has shown promise in improving the plant remediation efficiency while bolstering plant resistance against heavy metal stress [34,216,217,218]. PGPB change the soil pH and metals bioavailability by producing and secreting various organic acids, polymer compounds, chelating agents, and hormones, while the polymer compounds help stabilize the metals by reducing metals mobility [219,220].
3.5.1. Production of Hormones and ACC Deaminases
PGPB can promote plant growth and enhance plant tolerance to HM stress through the production of hormones, siderophores, ACC deaminases, nitrogen fixation, and phosphorus solubilization [169,221]. The exogenous application of plant hormones is also an effective strategy for mitigating HM toxicity [222]. Plant hormones alleviate HM stress by activating the expression of the genes involved in antioxidant biosynthesis [222,223,224]. PGPB that produce ACC deaminases assist plants in tolerating heavy metal stress by regulating the root system architecture and ethylene levels [225,226]. The ACC deaminases production strain Pseudomonas sp. TR15a and siderophores production strain Bacillus aerophilus TR15c had a synergistic effect on the resistance of sunflower plants to copper. Compared to the non-inoculated control, co-inoculation with TR15a and TR15c significantly increased the seed germination rate and total dry biomass [227]. Pseudomonas lurida EOO26 produced IAA and solubilized phosphate under a wide range of Cu contents. Sunflowers inoculated with EOO26 exhibited a significantly increased Cu uptake compared to non-inoculated plants, indicating its potential for Cu phytoremediation [117]. Pseudomonas libanensis TR1 and Pseudomonas reactans Ph3R3 were capable of producing IAA, siderophores, and ACC deaminases. They demonstrated a high resistance against various heavy metals, antibiotics, salts, and extreme temperatures. Inoculation with TR1 and Ph3R3 significantly improved Brassica oxyrrhina growth as well as the mineral element contents under drought and heavy metal stresses [228].
In heavy metal-contaminated soil, PGPB produced hormones and ACC deaminases, which changed the root system architecture and promoted the absorption of nutrients. Consequently, oil crops grown in PGPB-inoculated HM-contaminated soil exhibited superior growth compared to those without inoculation. However, further investigation is required to determine whether PGPB inoculation has any detrimental effects on the grain quality and oil composition of oil crops in HM-contaminated soil.
3.5.2. Decrease in the Bioavailability of Heavy Metals
PGPB have the ability to produce proteins, extracellular polysaccharides, and surfactants that can chelate, complex, and precipitate HMs, thereby reducing their bioavailability in soil [217]. The cellular structure of PGPB enables it to capture heavy metal ions and absorb them at binding sites on the cell wall. Negatively charged sulfhydryl, carboxyl, hydroxyl, sulfonic, amino, and amide groups on the cell surface bind to positively charged HMs, fixing them onto the cell surface [229]. Some PGPB also produce biosurfactants, such as lipopeptide, subtilisin, and rhamnolipid, which form complexes with HMs [230]. These biosurfactants have a high emulsification and surface activity that improve metal desorption by reducing the interfacial tension [231]. Exopolysaccharides are high molecular weight biopolymerics secreted by PGPB. Research has shown that exopolysaccharides have good adsorption properties for HMs, such as Cu, Cd, and Pb, due to their charge interactions, which can effectively alleviate the accumulation of HMs in agricultural crops [232,233]. Exposure to arsenate in sunflowers can lead to growth damage. However, the arsenate stress was alleviated when they were inoculated with Pantoaa conspicua due to its ability to produce exopolysaccharides and lignify the roots. The exopolysaccharide produced by Pantoaa conspicua had a filtering capacity of 75.1% for arsenate in the growth medium [234]. Serratia liquefaciens CL-1 and Bacillus thuringiensis X30 fixed cadmium through polyamine production, pH increase, extracellular adsorption, and intracellular accumulation mechanisms. Moreover, they also reduced the transfer of cadmium from rapeseed roots to shoots [235]. The nitrogen-fixing bacterium Burkholderia sp. GN6 exhibited a high tolerance to Cd and its inoculation increased the pectin methylesterase content in rapeseed roots under Cd stress. Pectin methylesterase catalyzed pectin demethylation and released carboxyl groups that bound with Cd, fixing it in the roots and reducing the cadmium content in the shoots as a result. Additionally, GN6 inoculation accelerated rapeseed growth, thereby shortening the plant’s exposure time to cadmium [221]. In summary, PGPB reduced the bioavailability of HMs through extracellular precipitation, cellular adsorption, and intracellular accumulation, thereby alleviating heavy metal stress in the plants.
Extracellular precipitation refers to the process in which certain extracellular substances are produced by PGPB to chelate, complex, and precipitate heavy metal ions. This effectively reduces the movement of heavy metal ions and their absorption by plant roots. Cellular adsorption involves the cell wall structure and substances on the cell surface adsorbing heavy metal ions, thereby reducing the possibility of these ions being absorbed by the roots. Intracellular accumulation, on the other hand, is the transformation and precipitation of heavy metal ions in cells. These three mechanisms play a crucial role in promoting plant growth under conditions of heavy metal stress. However, cellular adsorption is particularly favored for its extensive surface area and strong adsorption capacity exhibited by bacteria. Therefore, cellular adsorption is a metabolism-independent mechanism that can occur both in living cells and dead microbial biomass [236].
3.5.3. Change of the Valence State of Heavy Metals
The toxicity of HMs depends on their valence state. PGPB can regulate the redox reaction of heavy metal ions through cellular metabolism, thereby transforming a highly toxic valence state to a less toxic valence state [237]. Some PGPB possess genes related to heavy metal transport, enabling them to uptake heavy metals into the cells. Once inside the cells, these heavy metals undergo oxidation-reduction reactions and transition from highly toxic valence states to less toxic valence states. Changing toxic Cr6+ to the non-toxic Cr3+ form is one of the mechanisms of chromium-resistant bacteria [238,239]. Acinetobacter bouvetii P1 not only solubilizes phosphate and produces IAA, GA, and SA but also converts unstable chromium into stable forms while increasing the antioxidant system in host sunflowers [240]. Staphylococcus arlettae MT4 effectively reduced chromate stress in sunflowers by converting Cr6+ to Cr3+ to relieve chromate stress [119,120].
The alteration of heavy metal valence states usually takes place within bacterial cells as a response to the stress induced by the entry of heavy metal ions into these cells. This phenomenon is particularly observed in heavy metal ions, such as chromium, whose toxicity varies with their valence state. PGPB employ this mechanism to safeguard themselves against heavy metal ion poisoning. However, it remains challenging to mitigate heavy metal toxicity through genetic manipulation aimed at controlling bacterial alterations in the chemical valence states of these metals.
3.5.4. Enhancement of the Antioxidant System
In addition to reducing the bioavailability of heavy metals and changing their valence states to reduce toxicity, PGPB can also enhance plant photosynthesis and bolster the antioxidant system [241]. Inoculation with Bradyrhizobium japonicum E109 alone did not alleviate arsenic stress in soybeans, but co-inoculation with free-living PGPB Azospirillum brasilense Cd alleviated the decline in the chlorophyll a and chlorophyll b levels under arsenic stress and increased nodulation by 15–19% [242]. Stemphylium lycopersici Cp1 and Stemphylium solani Cp2 supported the antioxidant system of soybean seedlings by enhancing the production of ascorbic acid oxidase (AAO), catalases (CAT), peroxidase (POD), and free radical scavenging enzymes under chromate stress [243]. Acinetobacter bouvetii P1 enhanced the host sunflower’s antioxidant system by stimulating the production of enzyme antioxidants, thereby improving sunflower growth under chromate stress [118]. Severe damage occurred to the sunflower seedlings growth when exposed to Cr6+. However, inoculation with Staphylococcus arletae MT4 caused the sunflower seedlings to produce antioxidant enzymes, effectively clearing the accumulated reactive oxygen species and enabling normal growth under toxic conditions [120].
Enhancing the host plant’s antioxidant system is the mechanism of PGPB under any stress. When plants are under unfavorable environmental conditions, an excessive accumulation of reactive oxygen species in their bodies can cause damage to the cell structure and function. Inoculation with PGPR enhances the activity of reactive oxygen species scavenging enzymes, thereby clearing excess reactive oxygen species and protecting plants from damage caused by reactive oxygen species.
4. Opportunities and Challenges
4.1. Opportunities
Based on the understanding of the interaction between oil crops and bacteria, the increasing research has focused on how to use bacteria for regulating the yield and quality of oil crops. There are some opportunities in practical applications. (i) High-throughput strain isolation technology provides the possibility for screening and identifying more beneficial strains. The method developed by Jingying Zhang et al. cultured and identified 69–72% of root bacterial taxa from rice roots in 8–9 weeks [244]. The machine learning-guided robotic strain isolation platform developed by Yiming Huang et al. used colony morphology and genome data to improve the diversity of isolated microorganisms [245]. Using efficient and precise methods for screening and exploring a greater variety of strains provides a prerequisite for the development of microbial inoculants. (ii) Due to the increasing maturity of omics technology, it is possible to use a combination of genomics, transcriptomics, and metabolomics to investigate the mechanisms of plant–microbial interactions [246]. (iii) The manipulation of bacteria can be achieved through molecular techniques to attain targeted characteristics [247]; for example, using clustered and regularly interspaced short palindromic repeats-associated protein 9 (CRISPR-Cas9) tools to edit the bacterial genome. The CRISPR-Cas9 technology is currently extensively employed in microbial catalysis and industrial metabolite production, as well as for the transformation of beneficial strains and the construction of engineered strains in the agricultural sector. (iv) The interactions among microorganisms are highly intricate, and the use of single strain inoculants in field conditions often leads to competition from indigenous microbial communities, thereby weakening or offsetting the potential benefits of the inoculants [248]. Consequently, in recent years, researchers have proposed research methods for synthetic communities (SynCom). Synthetic communities refer to the application of artificial synthesis techniques to accurately introduce microorganisms based on the proportion and quantity of each member within the community [72]. From the exploration of beneficial bacteria to mechanism research, targeted modification and synthetic community construction, and ultimately the development of microbial inoculants, all are indispensable links in microbial products.
4.2. Challenges
There are still many challenges that need to be addressed in the process of using PGPB as microbial inoculants in oil crops. (i) The isolation and purification of bacteria from extreme environments is difficult because some bacteria cannot be cultured under controlled laboratory conditions. The proportion of cultivable endophytic bacteria in plants accounts for less than 1% of the total endophytic bacteria population [249]. (ii) The primary contribution of oil crops lies in their ability to extract oil. Currently, the majority of the research has focused on improving the biomass and yield of oil crops through PGPB, while insufficient attention has been given to its impact on seed oil synthesis and metabolism. While the enhancement of biomass and yield is bound to exert an influence on the total oil content of oil crops, a boost in oil synthesis will also exert a significant impact on the total oil content. Therefore, more attention should be paid to the influence and regulation of PGPB on the oil synthesis and metabolism process of oil crops in future research. (iii) After thoroughly investigating the molecular mechanism of PGPB, researchers should consider how to maximize its efficacy in the subsequent stages. It is a necessary step to use the genetic engineering method to effectively express advantageous genes and construct genetically engineered strains. Furthermore, PGPB also synthesizes bioactive compounds that promote plant growth and enhance plant resistance. We should not only consider the utilization of live microorganisms as microbial inoculants but also explore the full potential of bioactive compounds for synthesizing biostimulant products. This can effectively address the issues related to the ineffective application of live microbial inoculants. Therefore, the synthesis of stable microbial inoculants and biostimulants using PGPB and its metabolites is a challenge. (iv) In addition, when applying a new microbial inoculant to soil, researchers must consider whether it will lead to biological invasion and the adverse impacts it may have on indigenous microorganisms and ecosystems. During the development, production, and market application of microbial inoculants and biostimulants, it is imperative for governments and relevant departments to establish comprehensive rules, regulations, and laws to effectively regulate and manage these processes. It is crucial for every country, and even the global community, to impose strict controls on the implementation of microbial inoculants to prevent biological invasion and escape.
5. Concluding Remarks
Oil crops are important economic crops in the world, and their yield and quality are limited by diseases, pests, drought, salinity, heavy metals, and other factors. Plant growth promoting bacteria have effectively improved the growth and alleviated biotic and abiotic stresses in oil crops. When excavating beneficial bacteria in oil crops, it is necessary to employ advanced technological means and optimize their efficacy through genetic engineering. When applying, it is essential to consider not only its growth promoting mechanism in laboratory conditions but also its growth promoting effect in the field and its impact on indigenous microorganisms and the ecological environment. In summary, beneficial bacteria and their metabolites as inoculants represent a sustainable and eco-friendly technology for improving nutrient uptake and stress tolerance in oil crops, thus holding great potential for widespread adoption and implementation in the future.
Author Contributions
Writing—original draft preparation and software, L.M.; resources, L.M. and Y.L. date curation, L.M. and H.L.; visualization, C.C. and N.L.; supervision, S.W.; formal analysis, Y.Y. and W.Y.; project administration, C.L. and N.L.; writing—review and editing, X.C. and N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD1900705), the National Natural Foundation of China (32072102; 31671728; 32130076), the Fundamental Research Funds for the Central Universities (SWU-XJLJ202308; XDJK2018AA005), and the Shuangcheng Cooperative Agreement Research Grant of Yibin of China (XNDX2022020003) for Nannan Li.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dong, Z.; Tian, Z.; Liu, B. Oil crops: From the classical traits to genetic improvement. J. Integr. Plant Biol. 2021, 63, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, G.; Wan, S. Oil Crops: A Potential Source of Biodiesel. Engineering, 2023; in press. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Krauss, R.M. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am. J. Clin. Nutr. 2020, 112, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Isanga, J.; Zhang, G. Soybean bioactive components and their implications to health—A review. Food Rev. Int. 2008, 24, 252–276. [Google Scholar] [CrossRef]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska-Czerniak, A. Rapeseed and its Products—Sources of Bioactive Compounds: A Review of their Characteristics and Analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330. [Google Scholar] [CrossRef]
- Nguyen, D.; Nguyen, T.T.; Le, H.; Nguyen, T.; Bach, L.G.; Nguyen, T.D.; Vo, D.; Tran, T.V. The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture: A review. Environ. Chem. Lett. 2021, 19, 3701–3726. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects. 2019. Available online: https://population.un.org/wpp2019/ (accessed on 12 December 2023).
- Food and Agriculture Organization. How to Feed the World in 2050. Available online: https://www.fao.org/about/about-fao/en/ (accessed on 12 December 2023).
- Falcon, W.P.; Naylor, R.L.; Shankar, N.D. Rethinking Global Food Demand for 2050. Popul. Dev. Rev. 2022, 48, 921–957. [Google Scholar] [CrossRef]
- Ray, D.K.; Gerber, J.S.; Macdonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Landau, C.A.; Hager, A.G.; Williams, M.M. Deteriorating weed control and variable weather portends greater soybean yield losses in the future. Sci. Total Environ. 2022, 830, 154764. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, T.; Guo, X.; Li, M.; Liu, X.; Cao, J.; Tan, X. Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, D.; Xiong, Q.; Bao, B.; Zhang, W.; Dai, T.; Zhao, Y.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium Bacillus velezensis FZB42 Controls the Soybean Pathogen Phytophthora sojae Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e01601-21. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; El-Badri, A.M.; Hassan, M.U.; Yang, H.Y.; Wang, C.Y.; Yan, Z.K.; Kuai, J.; Wang, B.; Zhou, G.S. Drought Stress in Brassica napus: Effects, Tolerance Mechanisms, and Management Strategies. J. Plant Growth Regul. 2023, 42, 21–45. [Google Scholar] [CrossRef]
- Elferjani, R.; Soolanayakanahally, R. Canola Responses to Drought, Heat, and Combined Stress: Shared and Specific Effects on Carbon Assimilation, Seed Yield, and Oil Composition. Front. Plant Sci. 2018, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Johnson, E.N.; Blackshaw, R.E.; Hossain, Z.; Gan, Y. Improving the productivity and stability of oilseed cropping systems through crop diversification. Field Crop. Res. 2019, 237, 65–73. [Google Scholar] [CrossRef]
- Hegewald, H.; Wensch-Dorendorf, M.; Sieling, K.; Christen, O. Impacts of break crops and crop rotations on oilseed rape productivity: A review. Eur. J. Agron. 2018, 101, 63–77. [Google Scholar] [CrossRef]
- Ding, L.; Li, M.; Guo, X.; Tang, M.; Cao, J.; Wang, Z.; Liu, R.; Zhu, K.; Guo, L.; Liu, S.; et al. Arabidopsis GDSL1 overexpression enhances rapeseed Sclerotinia sclerotiorum resistance and the functional identification of its homolog in Brassica napus. Plant Biotechnol. J. 2020, 18, 1255–1270. [Google Scholar] [CrossRef]
- Liu, T.; Ji, J.; Cheng, Y.; Zhang, S.; Wang, Z.; Duan, K.; Wang, Y. CRISPR/Cas9-mediated editing of GmTAP1 confers enhanced resistance to Phytophthora sojae in soybean. J. Integr. Plant Biol. 2023, 65, 1609–1612. [Google Scholar] [CrossRef]
- Kong, L.; Shi, X.; Chen, D.; Yang, N.; Yin, C.; Yang, J.; Wang, G.; Huang, W.; Peng, H.; Peng, D.; et al. Host-induced silencing of a nematode chitin synthase gene enhances resistance of soybeans to both pathogenic Heterodera glycines and Fusarium oxysporum. Plant Biotechnol. J. 2022, 20, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Xian, P.; Cai, Z.; Jiang, B.; Xia, Q.; Cheng, Y.; Yang, Y.; Zhou, Q.; Lian, T.; Ma, Q.; Wang, Y.; et al. GmRmd1 encodes a TIR-NBS-BSP protein and confers resistance to powdery mildew in soybean. Plant Commun. 2022, 3, 100418. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.; Goher, F.; Zhang, D.; Shi, P.; Li, Z.; Htwe, Y.M.; Wang, Y. Is CRISPR/Cas9 a way forward to fast-track genetic improvement in commercial palms? Prospects and limits. Front. Plant Sci. 2022, 13, 1042828. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Mamet, S.D.; Helgason, B.L.; Lamb, E.G.; Mcgillivray, A.; Stanley, K.G.; Robinson, S.J.; Aziz, S.U.; Vail, S.; Siciliano, S.D. Phenology-dependent root bacteria enhance yield of Brassica napus. Soil Biol. Biochem. 2022, 166, 108468. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Chatterjee, P.; Niinemets, U. Improving plant stress resistance by growth-promoting bacteria and evaluating the improvements by volatile emissions. Plant Soil 2022, 476, 403–419. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S.S. Mitigating abiotic stress: Microbiome engineering for improving agricultural production and environmental sustainability. Planta 2022, 256, 85. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Jan, S.; Bhat, M.A.; Kamal, M.A.; Rahman, S.; Shah, A.A.; Jan, A.T. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants 2023, 12, 629. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J. A brief from the leaf: Latest research to inform our understanding of the phyllosphere microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Tack, A.J.M.; Lobato, C.; Wassermann, B.; Berg, G. From seed to seed: The role of microbial inheritance in the assembly of the plant microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moenne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Wang, L.; Rengel, Z.; Zhang, K.; Jin, K.; Lyu, Y.; Zhang, L.; Cheng, L.; Zhang, F.; Shen, J. Ensuring future food security and resource sustainability: Insights into the rhizosphere. iScience 2022, 25, 104168. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270. [Google Scholar] [CrossRef] [PubMed]
- Kotoky, R.; Rajkumari, J.; Pandey, P. The rhizosphere microbiome: Significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil. J. Environ. Manag. 2018, 217, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; War, A.F.; Rafiq, I.; Reshi, Z.A.; Rashid, I.; Shouche, Y.S. Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 2022, 254, 126888. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Paasch, B.C.; Liber, J.A.; He, S.Y. Phyllosphere Microbiome. Annu. Rev. Plant Biol. 2023, 74, 539–568. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Cho, G.; Jeon, C.; Weller, D.M.; Thomashow, L.S.; Paulitz, T.C.; Kwak, Y. A mutualistic interaction between Streptomyces bacteria, strawberry plants and pollinating bees. Nat. Commun. 2019, 10, 4802. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mueller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Muench, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364. [Google Scholar] [CrossRef]
- Manirajan, B.A.; Ratering, S.; Rusch, V.; Schwiertz, A.; Geissler-Plaum, R.; Cardinale, M.; Schnell, S. Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environ. Microbiol. 2016, 18, 5161–5174. [Google Scholar] [CrossRef]
- Vannette, R.L. The Floral Microbiome: Plant, Pollinator, and Microbial Perspectives. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 363–386. [Google Scholar] [CrossRef]
- Liu, X.; Matsumoto, H.; Lv, T.; Zhan, C.; Fang, H.; Pan, Q.; Xu, H.; Fan, X.; Chu, T.; Chen, S.; et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat. Microbiol. 2023, 8, 1419. [Google Scholar] [CrossRef]
- Gupta, R.; Elkabetz, D.; Leibman-Markus, M.; Sayas, T.; Schneider, A.; Jami, E.; Kleiman, M.; Bar, M. Cytokinin drives assembly of the phyllosphere microbiome and promotes disease resistance through structural and chemical cues. ISME J. 2022, 16, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, Z.; Zhang, Y.; Xu, J.; Wang, H.; Wang, Z.; Li, H. The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Bringel, F.; Couee, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 2015, 6, 486. [Google Scholar] [CrossRef] [PubMed]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How Auxin and Cytokinin Phytohormones Modulate Root Microbe Interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef] [PubMed]
- Arun, D.K.; Sabarinathan, K.G.; Gomathy, M.; Kannan, R.; Balachandar, D. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress—Tolerant rice phyllosphere bacteria. J. Basic Microbiol. 2020, 60, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, E.J.N.; Vogel, C.M.; Ueoka, R.; Schaefer, M.; Ryffel, F.; Mueller, D.B.; Probst, S.; Kreuzer, M.; Piel, J.; Vorholt, J.A. Bipartite interactions, antibiotic production and biosynthetic potential of the Arabidopsis leaf microbiome. Nat. Microbiol. 2018, 3, 909–919. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant—Microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Zhang, W.; Mason, G.A. Modulating the rhizosphere microbiome by altering the cocktail of root secretions. Plant Physiol. 2022, 188, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Koprivova, A.; Kopriva, S. Plant secondary metabolites altering root microbiome composition and function. Curr. Opin. Plant Biol. 2022, 67, 102227. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Rocha, U.N.D.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cheng, L.; Liu, K.; Liu, Q.; Gong, Z.; Cai, Z.; Liu, J.; Zhao, X.; Nian, H.; Ma, Q.; et al. Transgenic soybean of GsMYB10 shapes rhizosphere microbes to promote resistance to aluminum (Al) toxicity. J. Hazard. Mater. 2023, 455, 131621. [Google Scholar] [CrossRef] [PubMed]
- Fazal, A.; Wen, Z.; Yang, M.; Wang, C.; Hao, C.; Lai, X.; Jie, W.; Yang, L.; He, Z.; Yang, H.; et al. Triple-transgenic soybean in conjunction with glyphosate drive patterns in the rhizosphere microbial community assembly. Environ. Pollut. 2023, 335, 122337. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, K.; Shi, L.; Sun, X.; Tan, Q.; Hu, C. Recruitment of specific microbes through exudates affects cadmium activation and accumulation in Brassica napus. J. Hazard. Mater. 2023, 442, 130066. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Nian, H.; Jin, J.; Lian, T. Linking plant functional genes to rhizosphere microbes: A review. Plant Biotechnol. J. 2022, 21, 902–917. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Penuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef]
- Carlstroem, C.; Field, C.M.; Bortfeld-Miller, M.; Mueller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y.; et al. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.A.; Novinscak, A.; Filion, M. Pseudomonas fluorescens LBUM677 differentially increases plant biomass, total oil content and lipid composition in three oilseed crops. J. Appl. Microbiol. 2020, 128, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b—2R. Biol. Fertil. Soils 2016, 52, 119–125. [Google Scholar] [CrossRef]
- Valetti, L.; Iriarte, L.; Fabra, A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl. Soil Ecol. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ghavami, N.; Alikhani, H.A.; Pourbabaei, A.A.; Besharati, H. Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J. Plant Nutr. 2017, 40, 736–746. [Google Scholar] [CrossRef]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Sundaram, S.; Sa, T. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul. 2015, 76, 327–332. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Silva, L.I.D.; Pereira, M.C.; de Carvalho, A.M.X.; Buttros, V.H.; Pasqual, M.; Doria, J. Phosphorus—Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zhang, M.; Li, X.; Wang, M.; Li, L.; Li, X.; Ding, Z.; Tian, H. Serratia marcescens PLR enhances lateral root formation through supplying PLR—Derived auxin and enhancing auxin biosynthesis in Arabidopsis. J. Exp. Bot. 2022, 73, 3711–3725. [Google Scholar] [CrossRef]
- Kechid, M.; Desbrosses, G.; Rokhsi, W.; Varoquaux, F.; Djekoun, A.; Touraine, B. The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM196. New Phytol. 2013, 198, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Kechid, M.; Desbrosses, G.; Gamet, L.; Castaings, L.; Varoquaux, F.; Djekoun, A.; Touraine, B. Arabidopsis Growth-Promotion and Root Architecture Responses to the Beneficial Rhizobacterium Phyllobacterium brassicacearum Strain STM196 Are Independent of the Nitrate Assimilatory Pathway. Plants 2022, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Gonzalez-Andres, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Nett, R.S.; Bender, K.S.; Peters, R.J. Production of the plant hormone gibberellin by rhizobia increases host legume nodule size. ISME J. 2022, 16, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z.; Pan, S.; Li, L.; Li, X. Characterization and Metabolism Effect of Seed Endophytic Bacteria Associated with Peanut Grown in South China. Front. Microbiol. 2019, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Meinke, D.W.; Cherry, J.M.; Dean, C.; Rounsley, S.D.; Koornneef, M. Arabidopsis thaliana: A model plant for genome analysis. Science 1998, 282, 662. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Yang, Z.; Cheng, X.; Zhao, Y.; Qin, L.; Bisseling, T.; Cao, Q.; Willemsen, V. Plant growth-promoting rhizobacterium Pseudomonas sp. CM11 specifically induces lateral roots. New Phytol. 2022, 235, 1575–1588. [Google Scholar] [CrossRef]
- Jimenez-Vazquez, K.R.; Garcia-Cardenas, E.; Barrera-Ortiz, S.; Ortiz-Castro, R.; Ruiz-Herrera, L.F.; Ramos-Acosta, B.P.; Coria-Arellano, J.L.; Saenz-Mata, J.; Lopez-Bucio, J. The plant beneficial rhizobacterium Achromobacter sp. 5B1 influences root development through auxin signaling and redistribution. Plant J. 2020, 103, 1639–1654. [Google Scholar] [CrossRef]
- Li, W.Q.; Nishiyama, R.; Watanabe, Y.; Ha, C.V.; Kojima, M.; An, P.; Tian, L.; Tian, C.J.; Sakakibara, H.; Tran, L. Effects of overproduced ethylene on the contents of other phytohormones and expression of their key biosynthetic genes. Plant Physiol. Biochem. 2018, 128, 170–177. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Burger, M.; Chory, J.; Wang, X. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef]
- Poor, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2022, 41, 675–698. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Trivellini, A.; Chhillar, H.; Chopra, P.; Ferrante, A.; Khan, N.A.; Ismail, A.M. The significance and functions of ethylene in flooding stress tolerance in plants. Environ. Exp. Bot. 2020, 179, 104188. [Google Scholar] [CrossRef]
- Czarny, J.C.; Grichko, V.P.; Glick, B.R. Genetic modulation of ethylene biosynthesis and signaling in plants. Biotechnol. Adv. 2006, 24, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Ali, S. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the Plant Growth-Promoting Enzyme ACC Deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Ma, Y.; Shadan, A. Perspective of ACC—Deaminase producing bacteria in stress agriculture. J. Biotechnol. 2022, 352, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Nascimento, F.X. Pseudomonas 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase and Its Role in Beneficial Plant-Microbe Interactions. Microorganisms 2021, 9, 2467. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.B.; Khan, M.S.; Singh, P.; Kumar, R.; Singh, R.N.; Kumar, A.; Singh, H.V.V. Bacterial ACC deaminase: Insights into enzymology, biochemistry, genetics, and potential role in amelioration of environmental stress in crop plants. Front. Microbiol. 2023, 14, 1132770. [Google Scholar] [CrossRef]
- Horstmann, J.L.; Dias, M.P.; Ortolan, F.; Medina-Silva, R.; Astarita, L.V.; Santarem, E.R. Streptomyces sp. CLV45 from Fabaceae rhizosphere benefits growth of soybean plants. Braz. J. Microbiol. 2020, 51, 1861–1871. [Google Scholar] [CrossRef]
- Roskova, Z.; Skarohlid, R.; Mcgachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-Producing Rhizobacteria as a Promising Tool for Empowering Plants to Cope with Iron Limitation in Saline Soils: A Review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, A.; Saati-Santamaria, Z.; Kostovcik, M.; Rivas, R.; Velazquez, E.; Mateos, P.F.; Menendez, E.; Garcia-Fraile, P. Selection of the Root Endophyte Pseudomonas brassicacearum CDVBN10 as Plant Growth Promoter for Brassica napus L. Crops. Agronomy 2020, 10, 1788. [Google Scholar] [CrossRef]
- Lipkova, N.; Medo, J.; Artimova, R.; Makova, J.; Petrova, J.; Javorekova, S.; Michalko, J. Growth Promotion of Rapeseed (Brassica napus L.) and Blackleg Disease (Leptosphaeria maculans) Suppression Mediated by Endophytic Bacteria. Agronomy 2021, 11, 1966. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Genomic Analysis of Endophytic Bacillus cereus T4S and Its Plant Growth-Promoting Traits. Plants 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Chlebek, D.; Pinski, A.; Zur, J.; Michalska, J.; Hupert-Kocurek, K. Genome Mining and Evaluation of the Biocontrol Potential of Pseudomonas fluorescens BRZ63, a New Endophyte of Oilseed Rape (Brassica napus L.) against Fungal Pathogens. Int. J. Mol. Sci. 2020, 21, 8740. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- Alleluya, V.K.; Arias, A.A.; Ribeiro, B.; De Coninck, B.; Helmus, C.; Delaplace, P.; Ongena, M. Bacillus lipopeptide-mediated biocontrol of peanut stem rot caused by Athelia rolfsii. Front. Plant Sci. 2023, 14, 1069971. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, D.; Chen, X.; Zhang, J.; Huang, H.; Wei, L. Isolation and characterization of Bacillus altitudinis JSCX-1 as a new potential biocontrol agent against Phytophthora sojae in soybean [Glycine max (L.) Merr.]. Plant Soil 2017, 416, 53–66. [Google Scholar] [CrossRef]
- Xia, C.; Zhao, Y.; Zhang, L.; Li, X.; Cheng, Y.; Wang, D.; Xu, C.; Qi, M.; Wang, J.; Guo, X.; et al. Myxobacteria restrain Phytophthora invasion by scavenging thiamine in soybean rhizosphere via outer membrane vesicle-secreted thiaminase I. Nat. Commun. 2023, 14, 5646. [Google Scholar] [CrossRef]
- Kim, M.; Radhakrishnan, R.; Kang, S.; You, Y.; Jeong, E.; Kim, J.; Lee, I. Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol. Mol. Biol. Plants 2017, 23, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.; Lee, I. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.K.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Kumar, A.; Tripti; Voropaeva, O.; Maleva, M.; Panikovskaya, K.; Borisova, G.; Rajkumar, M.; Bruno, L.B. Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosphere 2021, 266, 128983. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Irshad, M.; Ahmad, A.; Lodhi, M.A.; Lee, I. Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants 2021, 10, 1868. [Google Scholar] [CrossRef]
- Rathi, M.; Yogalakshmi, K.N. Brevundimonas diminuta MYS6 associated Helianthus annuus L. for enhanced copper phytoremediation. Chemosphere 2021, 263, 128195. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Husna; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, D.; Li, D.; Zhang, J.; Jiang, H.; Guo, L.; He, Q.; Zhang, T.; Macho, A.P.; Wang, E.; et al. Phytophthora sojae boosts host trehalose accumulation to acquire carbon and initiate infection. Nat. Microbiol. 2023, 8, 1561. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Identification and Characterization of Rhizoctonia Species Associated with Soybean Seedling Disease. Plant Dis. 2017, 101, 520–533. [Google Scholar] [CrossRef]
- Cruz, D.R.; Leandro, L.F.S.; Mayfield, D.A.; Meng, Y.; Munkvold, G.P. Effects of Soil Conditions on Root Rot of Soybean Caused by Fusarium graminearum. Phytopathology 2020, 110, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, F.; Hu, C.; Yang, X.; Zheng, A.; Wang, Y.; Tang, Y.; He, Y.; Lv, M. Production status and research advancement on root rot disease of faba bean (Vicia faba L.) in China. Front. Plant Sci. 2023, 14, 1165658. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Voegele, R.T.; Link, T.I. Diagnosis of Soybean Diseases Caused by Fungal and Oomycete Pathogens: Existing Methods and New Developments. J. Fungi 2023, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; El-Mageed, T.A.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Antil, S.; Kumar, R.; Pathak, D.V.; Kumari, A. Recent advances in utilizing bacteria as biocontrol agents against plant parasitic nematodes emphasizing Meloidogyne spp. Biol. Control 2023, 183, 105244. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; Hamss, H.E.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 105244. [Google Scholar] [CrossRef]
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Nazari, M.T.; Schommer, V.A.; Braun, J.C.A.; Santos, L.F.D.; Lopes, S.T.; Simon, V.; Machado, B.S.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Using Streptomyces spp. as plant growth promoters and biocontrol agents. Rhizosphere 2023, 27, 100741. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Zhu, X.; Wang, Y.; Liu, X.; Duan, Y.; Fan, H.; Chen, L. Isolation and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb1997 and Serratia plymuthica Sneb2001 for the biological control of root-knot nematode. Appl. Soil Ecol. 2021, 164, 103924. [Google Scholar] [CrossRef]
- Bashir, S.; Iqbal, A.; Hasnain, S.; White, J.F. Screening of sunflower associated bacteria as biocontrol agents for plant growth promotion. Arch. Microbiol. 2021, 203, 4901–4912. [Google Scholar] [CrossRef] [PubMed]
- Archana, T.; Rajendran, L.; Manoranjitham, S.K.; Santhana Krishnan, V.P.; Paramasivan, M.; Karthikeyan, G. Culture-dependent analysis of seed bacterial endophyte, Pseudomonas spp. EGN 1 against the stem rot disease (Sclerotium rolfsii Sacc.) in groundnut. Egypt. J. Biol. Pest Control 2020, 30, 119. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Baek, K. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Miao, S.; Liang, J.; Xu, Y.; Yu, G.; Shao, M. Bacillaene, sharp objects consist in the arsenal of antibiotics produced by Bacillus. J. Cell. Physiol. 2023, 238, 30974. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Rishi, N.; Singh, R. Microbial lipopeptides: Properties, mechanics and engineering for novel lipopeptides. Microbiol. Res. 2023, 271, 127363. [Google Scholar] [CrossRef]
- Wang, X.; Liang, G. Control Efficacy of an Endophytic Bacillus amyloliquefaciens Strain BZ6-1 against Peanut Bacterial Wilt, Ralstonia solanacearum. BioMed Res. Int. 2014, 2014, 465435. [Google Scholar] [CrossRef]
- Wei, D.; Zhu, D.; Zhang, Y.; Yang, Z.; Wu, X.; Shang, J.; Yang, W.; Chang, X. Characterization of rhizosphere Pseudomonas chlororaphis IRHB3 in the reduction of Fusarium root rot and promotion of soybean growth. Biol. Control 2023, 186, 105349. [Google Scholar] [CrossRef]
- Douglas, A.E. The microbial exometabolome: Ecological resource and architect of microbial communities. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190250. [Google Scholar] [CrossRef]
- Tyler, B.M. Phytophthora sojae: Root rot pathogen of soybean and model oomycete. Mol. Plant Pathol. 2007, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Oezkaya, O.; Kuemmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeno, L.R.; Orozco-Mosqueda, M.D.C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de Los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhao, H.; Zhang, H.; Wang, Q.; Yao, Y.; Cheng, J.; Lin, Y.; Xie, J.; Fu, Y.; Jiang, D. Bio-priming with a hypovirulent phytopathogenic fungus enhances the connection and strength of microbial interaction network in rapeseed. npj Biofilms Microbomes 2020, 6, 45. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, J.; Fu, Y.; Cheng, J.; Qu, Z.; Zhao, Z.; Cheng, S.; Chen, T.; Li, B.; Wang, Q.; et al. A 2-kb Mycovirus Converts a Pathogenic Fungus into a Beneficial Endophyte for Brassica Protection and Yield Enhancement. Mol. Plant. 2020, 13, 1420–1433. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Xu, H.; Wu, J.; Xu, Z.; Wang, Y. The Phytophthora effector Avh94 manipulates host jasmonic acid signaling to promote infection. J. Integr. Plant Biol. 2022, 64, 2199–2210. [Google Scholar] [CrossRef]
- Pokotylo, I.; Hodges, M.; Kravets, V.; Ruelland, E. A menage a trois: Salicylic acid, growth inhibition, and immunity. Trends Plant Sci. 2022, 27, 460–471. [Google Scholar] [CrossRef]
- Jia, S.; Song, C.; Dong, H.; Yang, X.; Li, X.; Ji, M.; Chu, J. Evaluation of efficacy and mechanism of Bacillus velezensis CB13 for controlling peanut stem rot caused by Sclerotium rolfsii. Front. Microbiol. 2023, 14, 1111965. [Google Scholar] [CrossRef]
- Zhu, M.; Monroe, J.G.; Suhail, Y.; Villiers, F.; Mullen, J.; Pater, D.; Hauser, F.; Jeon, B.W.; Bader, J.S.; Kwak, J.M.; et al. Molecular and systems approaches towards drought-tolerant canola crops. New Phytol. 2016, 210, 1169–1189. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, C.; Zhang, L.; Liu, H.; Yu, J.; Hu, Z.; Hua, W. Systematic Analysis of Hsf Family Genes in the Brassica napus Genome Reveals Novel Responses to Heat, Drought and High CO2 Stresses. Front. Plant Sci. 2017, 8, 1174. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Xu, Y.; Li, S.; Zhang, S.; Yuan, Z.; Li, J.; Ni, Y. Overexpression of BnKCS1-1, BnKCS1-2, and BnCER1-2 promotes cuticular wax production and increases drought tolerance in Brassica napus. Crop. J. 2020, 8, 26–37. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent Advances in PGPR and Molecular Mechanisms Involved in Drought Stress Resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429–430. [Google Scholar] [CrossRef]
- Sade, N.; Rubio-Wilhelmi, M.D.M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef]
- Verslues, P.E. ABA and cytokinins: Challenge and opportunity for plant stress research. Plant Mol. Biol. 2016, 91, 629–640. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmuelling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Q.; Jiang, W.; Xiao, N.; Zeng, F.; Chen, G.; Mak, M.; Chen, Z.; Deng, F. Molecular Regulation and Evolution of Cytokinin Signaling in Plant Abiotic Stresses. Plant Cell Physiol. 2023, 63, 1787–1805. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Meyer, K.M.; Porch, R.; Muscettola, I.E.; Vasconcelos, A.L.S.; Sherman, J.K.; Metcalf, C.J.E.; Lindow, S.E.; Koskella, B. Plant neighborhood shapes diversity and reduces interspecific variation of the phyllosphere microbiome. ISME J. 2022, 16, 1376–1387. [Google Scholar] [CrossRef]
- Singh, S.K.; Sun, Y.; Yang, Y.; Zuo, Z.; Wu, X.; Shao, C.; Peng, L.; Pare, P.W.; Zhang, H. Bacterial diacetyl suppresses abiotic stress-induced senescence in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1135–1139. [Google Scholar] [CrossRef]
- Salvi, P.; Mahawar, H.; Agarrwal, R.; Kajal; Gautam, V.; Deshmukh, R. Advancement in the molecular perspective of plant-endophytic interaction to mitigate drought stress in plants. Front. Microbiol. 2022, 13, 981355. [Google Scholar] [CrossRef]
- Trivedi, G.; Patel, P.; Saraf, M. Synergistic effect of endophytic selenobacteria on biofortification and growth of Glycine max under drought stress. S. Afr. J. Bot. 2020, 134, 27–35. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Vanacore, A.; Forgione, M.C.; Cavasso, D.; Nguyen, H.N.A.; Molinaro, A.; Saenz, J.P.; D’Errico, G.; Paduano, L.; Marchetti, R.; Silipo, A. Role of EPS in mitigation of plant abiotic stress: The case of Methylobacterium extorquens PA1. Carbohydr. Polym. 2022, 295, 119863. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Kim, I.; Chhetri, G.; So, Y.; Park, S.; Jung, Y.; Woo, H.; Seo, T. Characterization and Antioxidant Activity of Exopolysaccharides Produced by Lysobacter soyae sp. nov Isolated from the Root of Glycine max L. Microorganisms 2023, 11, 1900. [Google Scholar] [CrossRef]
- Zboralski, A.; Filion, M. Pseudomonas spp. can help plants face climate change. Front. Microbiol. 2023, 14, 1198131. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.; Yue, L.; Zhao, X.; Zhang, Y.; Xie, Z.; Wang, R. Epsc Involved in the Encoding of Exopolysaccharides Produced by Bacillus amyloliquefaciens FZB42 Act to Boost the Drought Tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 3795. [Google Scholar] [CrossRef]
- Suarez, R.; Wong, A.; Ramirez, M.; Barraza, A.; Orozco, M.D.C.; Cevallos, M.A.; Lara, M.; Hernandez, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant-Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, S.; Akhtar, N.; Ilyas, N.; Sayyed, R.Z.; Fitriatin, B.N.; Perveen, K.; Bukhari, N.A. Interactive effects of Pseudomonas putida and salicylic acid for mitigating drought tolerance in canola (Brassica napus L.). Heliyon 2023, 9, e14193. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Trusca, M.; Gadea, S.; Vidican, R.; Stoian, V.; Vatca, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vatca, S. Exploring the Research Challenges and Perspectives in Ecophysiology of Plants Affected by Salinity Stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs Feed-Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef]
- Ke, D.; He, Y.; Fan, L.; Niu, R.; Cheng, L.; Wang, L.; Zhang, Z. The soybean TGA transcription factor GmTGA13 plays important roles in the response to salinity stress. Plant Biol. 2022, 24, 313–322. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Jalal, R.S. Use of plant growth-promoting bacteria to enhance salinity stress in soybean (Glycine max L.) plants. Saudi J. Biol. Sci. 2021, 28, 3823–3834. [Google Scholar] [CrossRef]
- Mishra, A.K.; Das, R.; Kerry, R.G.; Biswal, B.; Sinha, T.; Sharma, S.; Arora, P.; Kumar, M. Promising management strategies to improve crop sustainability and to amend soil salinity. Front. Environ. Sci. 2023, 10, 962581. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Ma, Y.; Wang, X.; Zhang, B.; Zhang, G.; Bahadur, A.; Chen, T.; Liu, G.; Zhang, W.; et al. Research progress regarding the role of halophilic and halotolerant microorganisms in the eco-environmental sustainability and conservation. J. Clean Prod. 2023, 418, 138054. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 516. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hoque, M.I.U.; Woo, J.; Lee, I. Mitigation of Salinity Stress on Soybean Seedlings Using Indole Acetic Acid-Producing Acinetobacter pittii YNA40. Agriculture 2023, 13, 1021. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.; Kim, K.; Lee, I. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Park, E.; Glick, B.R. 1-aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation. Microbiology 2015, 84, 512–519. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Multifunctional Exopolysaccharides from Pseudomonas aeruginosa PF23 Involved in Plant Growth Stimulation, Biocontrol and Stress Amelioration in Sunflower Under Saline Conditions. Curr. Microbiol. 2014, 69, 484–494. [Google Scholar] [CrossRef]
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr. Sci. 2016, 111, 1961–1967. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, C.; Du, Z.; Yu, B. Pre-inoculation with Bradyrhizobium japonicum confers NaCl tolerance by improving nitrogen status and ion homeostasis in wild soybean (Glycine soja L. ) seedlings. Acta Physiol. Plant. 2022, 44, 21. [Google Scholar] [CrossRef]
- Riedelsberger, J.; Miller, J.K.; Valdebenito-Maturana, B.; Pineros, M.A.; Gonzalez, W.; Dreyer, I. Plant HKT Channels: An Updated View on Structure, Function and Gene Regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.; Sun, Y.; Dowd, S.E.; Shi, H.; Pare, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-Mediated Induction of Systemic Tolerance to Salinity with Expression of Stress Alleviating Enzymes in Soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Jia, M.; Lu, X.; Yue, L.; Zhao, X.; Jin, W.; Wang, Y.; Zhang, Y.; Xie, Z.; et al. Induction of Salt Tolerance in Arabidopsis thaliana by Volatiles from Bacillus amyloliquefaciens FZB42 via the Jasmonic Acid Signaling Pathway. Front. Microbiol. 2020, 11, 562934. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; et al. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Qiu, Z.; Shahzad, S.M.; Nawaz, M.F.; Huang, J.; Naveed, S.; Li, L.; Wang, X.; Cheng, H. Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: A review. Environ. Pollut. 2023, 325, 121433. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Rizvi, Z.F.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Pollut. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio-Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ahmad, Z.; Asgher, M.; Bhat, J.A.A.; Sharma, M.; Kumar, A.; Sharma, V.; Kumar, A.; Pant, S.; Lukatkin, A.S.S.; et al. Phytoremediation of Potentially Toxic Elements: Role, Status and Concerns. Plants 2023, 12, 429. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Zhao, X.; Joo, J.C.; Du, D.; Li, G.; Kim, J.Y. Modelling heavy-metal phytoextraction capacities of Helianthus annuus L. and Brassica napus L. Chemosphere 2023, 337, 139341. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.W.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminated soils using sunflower: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Sharma, P.; Parakh, S.K.; Singh, S.P.; Parra-Saldivar, R.; Kim, S.; Varjani, S.; Tong, Y.W. A critical review on microbes-based treatment strategies for mitigation of toxic pollutants. Sci. Total Environ. 2022, 834, 155444. [Google Scholar] [CrossRef]
- Saha, L.; Tiwari, J.; Bauddh, K.; Ma, Y. Recent Developments in Microbe-Plant-Based Bioremediation for Tackling Heavy Metal-Polluted Soils. Front. Microbiol. 2021, 12, 731723. [Google Scholar] [CrossRef]
- Wood, J.L.; Tang, C.; Franks, A.E. Microbial associated plant growth and heavy metal accumulation to improve phytoextraction of contaminated soils. Soil Biol. Biochem. 2016, 103, 131–137. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Chen, M.; Huang, J.; Liu, F.; Xie, S.; Kong, L.; Pan, Y. Reduced cadmium toxicity in rapeseed via alteration of root properties and accelerated plant growth by a nitrogen-fixing bacterium. J. Hazard. Mater. 2023, 449, 131040. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotox. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Hussain, S.; Hussain, B.; Khan, W.; Riaz, L.; Ashraf, M.N.; Khaliq, M.A.; Du, Z.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Mathur, P.; Tripathi, D.K.; Balus, F.; Mukherjee, S. Auxin-mediated molecular mechanisms of heavy metal and metalloid stress regulation in plants. Environ. Exp. Bot. 2022, 196, 104796. [Google Scholar] [CrossRef]
- Arshad, M.; Saleem, M.; Hussain, S. Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol. 2007, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, M.; Kowalchuk, G.A.; Jousset, A. Optimization of plant hormonal balance by microorganisms prevents plant heavy metal accumulation. J. Hazard. Mater. 2019, 379, 120787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tripti; Maleva, M.; Bruno, L.B.; Rajkumar, M. Synergistic effect of ACC deaminase producing Pseudomonas sp. TR15a and siderophore producing Bacillus aerophilus TR15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 2021, 276, 130038. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gangola, S.; Bhandari, G.; Bhandari, N.S.; Nainwal, D.; Rani, A.; Malik, S.; Slama, P. Rhizospheric bacteria: The key to sustainable heavy metal detoxification strategies. Front. Microbiol. 2023, 14, 1229828. [Google Scholar] [CrossRef]
- Fakhar, A.; Gul, B.; Gurmani, A.R.; Khan, S.M.; Ali, S.; Sultan, T.; Chaudhary, H.J.; Rafique, M.; Rizwan, M. Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1868–1914. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef]
- Lal, S.; Ratna, S.; Said, O.B.; Kumar, R. Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: An advancement in metal phytoremediation technology. Environ. Technol. Innov. 2018, 10, 243–263. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal -polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Irshad, M.; Khan, Z.H.; Islam, B.; Elansary, H.O.; Mahmoud, E.A.; et al. Pantoea conspicua promoted sunflower growth and engulfed rhizospheric arsenate by secreting exopolysaccharide. Plant Physiol. Biochem. 2023, 201, 107826. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, Q.; He, L.; Sheng, X. Increased biomass and reduced rapeseed Cd accumulation of oilseed rape in the presence of Cd—Immobilizing and polyamine-producing bacteria. J. Hazard. Mater. 2018, 353, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ayele, A.; Godeto, Y.G. Bioremediation of Chromium by Microorganisms and Its Mechanisms Related to Functional Groups. J. Chem. 2021, 2021, 7694157. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Hossan, S.; Hossain, S.; Islam, M.R.; Kabir, M.H.; Ali, S.; Islam, M.S.; Imran, K.M.; Moniruzzaman, M.; Mou, T.J.; Parvez, A.K.; et al. Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity. Int. J. Environ. Res. Public Health 2020, 17, 6013. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, N.; Singh, A.; Kumar, D.; Yadav, D.K.; Varshney, A.; Sharma, N. The Effect of Cadmium Tolerant Plant Growth Promoting Rhizobacteria on Plant Growth Promotion and Phytoremediation: A Review. Curr. Microbiol. 2023, 80, 153. [Google Scholar] [CrossRef]
- Vezza, M.E.; Pramparo, R.D.P.; Oller, A.L.W.; Agostini, E.; Talano, M.A. Promising co-inoculation strategies to reduce arsenic toxicity in soybean. Environ. Sci. Pollut. Res. 2022, 29, 88066–88077. [Google Scholar] [CrossRef]
- Husna, H.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Asim, S.; Lee, I. Stemphylium lycopersici and Stemphylium solani improved antioxidant system of soybean under chromate stress. Front. Microbiol. 2022, 13, 1001847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Guo, X.; Qin, Y.; Garrido-Oter, R.; Schulze-Lefert, P.; Bai, Y. High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat. Protoc. 2021, 16, 988–1012. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sheth, R.U.; Zhao, S.; Cohen, L.A.; Dabaghi, K.; Moody, T.; Sun, Y.; Ricaurte, D.; Richardson, M.; Velez-Cortes, F.; et al. High-throughput microbial culturomics using automation and machine learning. Nat. Biotechnol. 2023, 41, 1424. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Xu, X.; Fu, Y.V. Microbe social skill: The cell-to-cell communication between microorganisms. Sci. Bull. 2017, 62, 516–524. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).