Abstract
Despite the known benefits of integrated pest management, adoption in Australian broadacre crops has been slow, in part due to the lack of understanding about how pests and natural enemies interact. We use a previously developed process-based model to predict seasonal patterns in the population dynamics of a canola pest, the green peach aphid (Myzus persicae), and an associated common primary parasitic wasp (Diaeretiella rapae), found in this cropping landscape. The model predicted aphid population outbreaks in autumn and spring. Diaeretiella rapae was able to suppress these outbreaks, but only in scenarios with a sufficiently high number of female wasps in the field (a simulated aphid:wasp density ratio of at least 5:1 was required). Model simulations of aphid-specific foliar pesticide applications facilitated biological control. However, a broad-spectrum pesticide negated the control provided by D. rapae, in one case leading to a predicted 15% increase in aphid densities compared to simulations in which no pesticide was applied. Biological and chemical control could therefore be used in combination for the successful management of the aphid while conserving the wasp. This modelling framework provides a versatile tool for further exploring how chemical applications can impact pests and candidate species for biological control.
1. Introduction
Integrated pest management (IPM) aims to control invertebrate pests below damaging population densities, while minimising the detrimental impacts of chemicals on the environment [1,2]. Such IPM programs emphasise the maintenance of natural enemies (predators and parasitic wasps) in the system, which help to control pest outbreaks. Reducing reliance on chemical applications can have multiple benefits, including a reduction in secondary pest outbreaks within a growing season, slowing the rates of evolved chemical resistance among pests and, more generally, improving environmental sustainability [3,4].
Adoption of IPM in broadacre crops in Australia has been slow. This is partly due to relatively inexpensive broad-spectrum and easily applied chemical controls (often combined with fungicide or herbicide treatments [5,6]), and a paucity of baseline ecological knowledge for some pests [7]. Even for species for which baseline data do exist, the broad geographic (and climatic) extent across which crops are grown in Australia means that a “one-size-fits-all” approach to pest management yields limited success at local scales [8]. Indeed, developing dynamic decision support tools that can be applied across multiple locations is a substantial challenge for IPM in Australia.
The green peach aphid (Myzus persicae) (Sulzer) (Aphididae: Aphidinae) is a broadly distributed agricultural pest in Australia. It is a major pest of canola (Brassica napus), a winter crop grown throughout the grain-belt region of Australia. While direct feeding damage caused by M. persicae to canola is often of little concern, this species is the principal vector of turnip yellows virus (TuYV, a form of beet western yellows virus), a pathogen that causes substantial damage to the crop and reduces yield [9,10,11]. In response to this problem, farmers tend to apply pesticides prophylactically, including as seed treatments, to prevent aphid infestations during the early (and most vulnerable) growth stages of the crop. Frequent exposure to insecticides has resulted in M. persicae evolving resistance to several chemical groups in mainland Europe, the UK, and more recently Australia [12,13]. The long-term efficacy of many insecticide seed treatments is therefore not guaranteed, and it is expected that alternative options for pest control will soon be required.
A promising candidate species for biological (and augmentative) control of M. persicae, is the wasp Diaeretiella rapae (M’Intosh) (Hymenoptera: Braconidae: Aphidiinae). Laboratory studies confirmed that D. rapae readily parasitises M. persicae [14], and its potential as a biological control agent in grain crops across Australia has recently been recognized [15]. A number of studies have already modelled the population dynamics of M. persicae [16,17,18,19]. Of most relevance, a hybrid (mechanistic/statistical) model has been integrated with species-specific information for M. persicae and TuYV to track population densities and virus transmission in canola in Western Australia [20]. While there have also been some theoretical modelling studies exploring the capacity of D. rapae to act as a biological control agent [16,21,22], none have been used to explore the impacts of different pest control strategies on the population dynamics in the field. Furthermore, modelling studies that incorporate changes to agronomic practices associated with growing canola in the different contexts and their impact on pest and natural enemy dynamics are not available.
Forecasting the population dynamics of a pest, and understanding whether natural enemies can suppress outbreaks, is a key requirement for the development of decision-support tools, and IPM more generally. Process-based simulation models are well suited to this task because they can explicitly integrate multiple, interactive, drivers of a pest’s population growth, including local climate and cropping data; management practices such as the time of crop sowing or chemical application; and predation or parasitism by natural enemies [23,24,25]. While the relative impacts of these processes will differ among locations and between seasons, such models can account for this variability and provide insights into how pests can be managed in the field.
Here, we employ a previously developed process-based model that integrates information on local climate and crop growth, to track the population dynamics of M. persicae and D. rapae on daily timesteps [26]. The model has been validated with historic (1960s) field observation data of species abundances from several locations and time-series prior to the introduction of seed-treated crops, such that it can be used as a baseline ‘no pesticide’ scenario to new locations across the Australian grain-belt. We started by exploring the temporal dynamics of the aphid population with the model, to identify when and where chemical intervention is required to suppress a potential aphid outbreak. We then tested how three pest management scenarios affect these dynamics, involving: (1) biological control exerted by varying densities of D. rapae, (2) an aphid-specific chemical control, and (3) a broad-spectrum chemical control (which is assumed to be toxic to D. rapae). With these simulation outputs, we test the relative success of biological and/or chemical controls, addressing management challenges relevant to both species at selected locations. We then discuss the findings in the context of developing dynamic economic thresholds for M. persicae in canola.
2. Materials and Methods
2.1. Insect Models
Across Australia, M. persicae exhibits predominantly asexual reproduction [27], allowing colonies within canola to grow rapidly when abiotic conditions are favourable, generally in late autumn (April–June) and spring (September–November). As with all aphid species, M. persicae can develop into different adult morphs: one with apterous morphology characterized by high reproductive rates, and another with winged alates characterized by lower reproductive rates but enhanced dispersal ability. During the dry summer months, M. persicae in Australia take refuge on secondary host plants within the landscape [28]. Diaeretiella rapae is similarly distributed across the Mediterranean-type climate zone of Australia, although relatively little is known about its life-history and phenology [14,15,29,30].
The simulation model aims to predict how population abundances of M. persicae and D. rapae vary through time under different climatic and cropping conditions across Australia. The model is structured into cohorts, each containing multiple individuals that are tracked together through their lifecycle as the simulation progresses on daily timesteps (Figure 1). Population abundances of each species depend on the rates of development, reproduction, dispersal, and mortality (Figure 1). Each of these processes vary with temperature, rainfall, cohort age, population densities, and species interactions (i.e., rates of parasitism or age of the crop). All functions are deterministic, such that the physiological state of individuals within a cohort are assumed to be identical. The functions were parameterized with empirical data sourced from the literature, or previously published models where data could not be found (see [26] for further details). The model describes a population of aphids that occupy a 1 m2 “patch” within a canola paddock [16] and assumes that all M. persicae individuals are virginoparae [27]. While many models integrate spatial and temporal components, here we focus on the temporal patterns in population dynamics within a small area, given the lack of knowledge of the M. persicae and D. rapae spatial distribution and association across field crop environments [15].
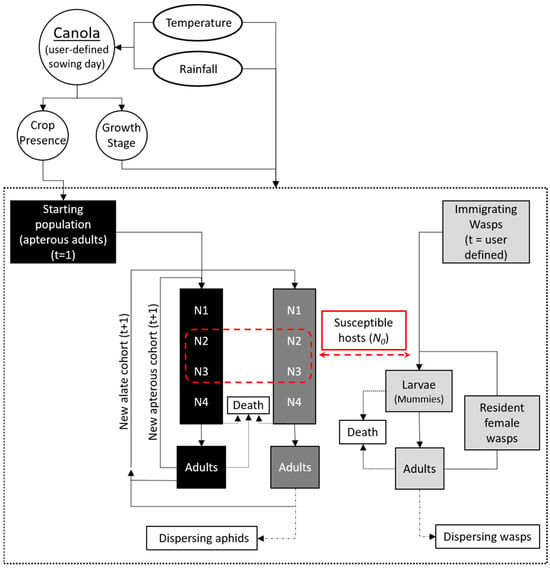
Figure 1.
Schematic of the aphid—wasp model with climate and host-plant inputs. Black: apterous (wingless) aphids, mid-grey: alate (winged) aphids; light grey: wasp, red: interactions between the two species through parasitism of second and third instar nymphs. White ovals show (daily) climate inputs used in the APSIM canola model (white circles) and the insect models (dashed rectangle). Days of the model simulation (t) on which immigrating wasps intercept the aphid population are user defined. Reprinted from [26].
2.2. Crop Model and Climate Inputs
To align the insect population dynamics to the growth and phenology of canola, we used an established crop modelling platform: Agricultural Predictions Systems Simulator modelling platform (APSIM [31]). APSIM integrates daily climate variables with information on soil and water availability to track the development of canola with growing degree-day calculations [32]. This canola model was run prior to the insect simulations, such that its outputs (predictions of crop ‘presence’, after germination, and developmental stage) were used as inputs to the insect models. There was no feedback to the APSIM model in terms of pest impacts on the crop; we assumed that an increased presence of aphids corresponds linearly to increased risk of crop damage (through virus transmission), and that biological control, if present, takes effect immediately (on the date specified by the user).
We assumed that the initial immigrating aphids cannot establish colonies until seedlings of the canola crop have emerged (dictating the commencement of the simulation), and that colonies cannot persist once the crop has been harvested (terminating the simulation). In addition, climate data that are included as outputs of the APSIM model (sourced from the Scientific Information for Land Owners online “Patched Point” database (SILO) provided by the Queensland Department Natural Resources and Mines [33]), including daily maximum and minimum temperature (°C) and rainfall (mm), were incorporated directly into the insect models as climate inputs (Figure 1).
2.3. Initial Conditions
We considered two locations where canola is grown in Australia: one site in the State of Victoria (Latitude: −36.35, Longitude: 144.63) and the other site in the State of South Australia (Latitude: −34.54, Longitude: 138.71). At these sites, the APSIM canola model was run for 10 years from 2005 until 2014 inclusive. All simulations described hereafter were repeated for each of these 10 years, and the predicted population densities of aphids, on each day of the year, were averaged across these 10-yearly repeats, providing an indication of the variability in population dynamics in response to the inter-annual variation in climate.
All scenarios commenced with a single cohort of 10 female apterous adult M. persicae in the crop (simulating a 1 m2 patch), which commence reproduction on the following day. While alate (winged) adults generally immigrate into the crop and initialise the population, apterous adults were used in previous (validated) iterations of the model [26], representing the first-generation post-immigration. Throughout the simulation, a background population of ten alate adults was also included, allowing for multiple outbreaks of aphids within the simulation period [26]. For example, if the initial population of aphids crashed under extreme temperature or parasitism load, the population could re-establish from these 10 background individuals when conditions were once again conducive to survival.
2.4. Timing of Canola Sowing and Aphid Arrival
Canola sowing time can vary from year to year, depending on various factors like seasonal rainfall patterns, crop rotations and canola variety [34]. In addition, the relative timing at which aphids arrive in the crop after it emerges will affect their population dynamics for the remainder of the season, as the dynamics will be influenced by the progressively colder winter temperatures. To explore how these temporal dynamics impact aphid population dynamics at both locations, we simulated three sowing dates, herein referred to as early (15 April), middle (1 May) and late (31 May). Within these three times of sowing (TOS) scenarios, ten model iterations were run in which aphids arrived at weekly intervals after the predicted crop emergence (Table 1). Model simulations ran until the date of canola harvest, as determined by the APSIM model. Total aphid density was used as a proxy for plant damage, defined by the area under the aphid population curve (AUC, including both alate and apterous aphids). This metric was compared among the TOS and aphid arrival scenarios. For these simulations, no biological or chemical controls were considered as we wanted to simulate the inherent crop–aphid interactions that would occur in the absence of factors such as the application of insecticides.

Table 1.
Summary of management scenarios explored with the model, with potential questions relevant to farmers in italics. Each iteration ran from aphid arrival to crop harvest. Aphid-specific and broad-spectrum pesticides were both applied to the aphids in the presence of wasps. TOS refers to time of sowing and SA is South Australia. Each scenario was run for 10 years, accounting for inter-annual variation in temperature, rainfall, and crop phenology from 2005 to 2014 (e.g., n = 600 for ‘Seasonal patterns’).
2.5. Biological Control
To simulate the biological control of M. persicae by D. rapae, the scenarios described above were repeated, but with the wasp population model activated. We assumed a background population of D. rapae was present in the field margins (of varying densities, see below), such that on each day of the simulation female wasp(s) could intercept the aphid colony and parasitise aphids, commencing on the day of aphid arrival.
Biological control of aphids by D. rapae and the population dynamics of the wasps were simulated in the same manner as described in [26]. Parasitism rates of 2nd and 3rd M. persicae nymphs were described by the random parasitoid equation [35]: a Type II response curve without replacement, assuming no interference between multiple wasps (see [26] for further details). Once oviposited into the aphids, wasp larvae developed and emerged from their hosts, and upon emergence either dispersed from the population, or remained for 1 day to lay offspring in aphids prior to dispersing.
To assess the relative densities required to suppress aphid population outbreaks, we ran five scenarios that differed only in the number of “intercepting” female wasps, including 0.5 (simulating one wasp every 2 days), 1, 2, 5, and 10 (or, a density ratio of background aphids:wasps of 20:1, 10:1, 5:1, 2:1, and 1:1, respectively; Table 1). Total aphid densities (AUC, defined above) during the canola growing season were calculated and compared with the simulation in which no wasps were present. The difference in aphid densities between these scenarios indicated the relative amount of biological control achieved by the wasps, where 0% is no control, and values approaching 100% indicated almost total suppression of the aphid population.
2.6. Chemical and Biological Controls
In the above scenarios, population outbreaks of M. persicae were predicted to be most severe in the South Australian site when aphids arrived on the day of crop emergence in the absence of control (see Figure 2a). Therefore, due to the outbreak severity, this location and timing scenario was selected to explore chemical control scenarios. In addition, for easier interpretation, we present simulations in which one female wasp intercepts the population each day (see Figure 3 and Table 1). There are many types of chemicals used to suppress aphid outbreaks in Australia, with different active ingredients, exposure times and rates of application (in frequency and dosage) [36]. Each chemical will impact aphid and wasp populations in different ways and to demonstrate the applicability of our modelling framework, two (hypothetical) chemical applications were simulated: (1) a broad-spectrum foliar application that is assumed to be toxic to both the aphid and wasp, and (2) an aphid-specific foliar application that is toxic to aphids only (Table 1). While in reality the toxicity of chemicals deteriorate over time [23,37,38], here we assumed that each insecticide resulted in the 70% mortality (cf. [23]) of one (aphid-specific) or both (broad-spectrum) species for the duration of their exposure. Application of chemical sprays was assumed to occur only once per canola season, which is a common approach used by farmers to manage M. persicae and TuYV risk in Australia [12]. For simplicity, we simulated insecticides being applied 14 days after initial aphid arrival, and the toxic effects of each insecticide persisting for 7 days for M. persicae and 21 days for D. rapae [37]. After this period, daily mortality rates were returned to those exerted by temperature, rainfall, senescence, and parasitism, as defined in the model [26]. We calculated the relative change in aphid densities for each of the three management scenarios in comparison to those without any intervention (see above). Insecticide seed treatments are commonplace in Australian canola; however, all registered products with aphid activity rely on neonicotinoids as the main active ingredient [12,36,39]. Given the regulatory pressures facing neonicotinoids globally and the emergence of P450-mediated resistance in Australian M. persicae [39], the future role of insecticide seed treatments is tenuous, and so we excluded these from our study, allowing us to explore alternative scenarios (e.g., the foliar application of chemicals and biological control), where seed treatments were not available.
As an additional measure of control “success”, we calculated the proportion of aphids that had been parasitised (number wasp larvae/(number of wasp larvae + aphid density) [40]) at the end of the simulation. Here we assumed that a relatively larger wasp population is a more sustainable management outcome, and that low variance in this metric among the 10-year climate replicates indicated a more reliable strategy. All data handling, modelling and analyses were conducted in R (version 4,3.1 [41])
3. Results
3.1. Timing of Canola Sowing and Aphid Arrival
In the absence of biological and chemical control, population outbreaks of M. persicae were predicted to occur in autumn and spring, separated by a period of reduced population growth in winter (Figure 2a). This variation in aphid densities reflects field observations and was expected [26]. The severity of these outbreaks, however, varied with location and time of sowing (TOS). For example, the population outbreak in autumn was most severe at the South Australian site when canola was sown early in the season, and progressively declined for the middle and late TOS scenarios (Figure 2a), with an early rapid increase in populations in early sown crops. In contrast, aphid densities at the Victorian site were the highest when canola was sown late in the season due to a high population peak prior to harvest, followed by middle and then early TOS. Overall, M. persicae densities were predicted to be higher at the South Australian site, in comparison to the Victorian site (Figure 2b).
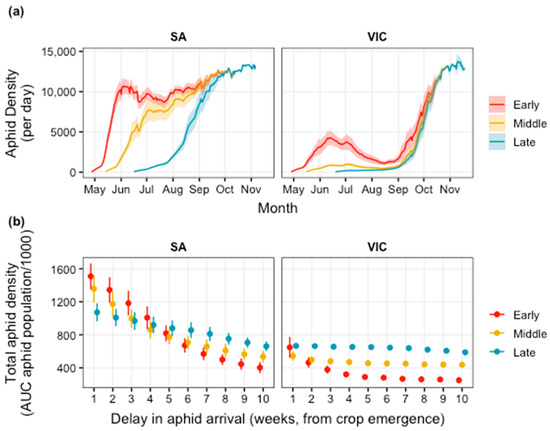
Figure 2.
Baseline simulations of Myzus persicae population growth dynamics in the absence of chemical or biological control, where (a) aphids arrive on the day of crop emergence for early, middle, and late time of sowing (TOS, Table 1) at South Australian (SA) and Victorian (VIC) sites. Total aphid densities (b) of the population (area under the population curves) varied depending on location, TOS, and delay of aphid arrival into the crop, where week 1 refers to aphids arriving on the day of seedling emergence. Shading and error bars denote variation among the 10-year (climate) replicates (2005–2014).
Moreover, at the South Australian site, aphid densities were the highest when aphids arrived on the day of seedling emergence (shown in Figure 2a) and tended to decline as aphids arrived progressively later in crop development. At the Victorian site, the impact of aphid arrival time in the crop was greatest in the early TOS scenario (Figure 2b); however, within the TOS scenarios, the timing of aphid arrival at the Victorian site did not impact aphid densities to the same extent as those at the South Australian site (Figure 2b). Inter-annual variation among the model predictions, reflecting differences in climate conditions between years, was highest at the South Australian site, and appeared to have relatively minor impacts on aphid densities throughout the season at the Victorian site (Figure 2b).
3.2. Biological Control
The presence of D. rapae in the system reduced population densities of M. persicae, although its impact varied with seasonal timing and the density of background wasps (Figure 3). Across all TOSs and both locations, a background aphid:wasp density ratio of 20:1 and 10:1 (wasp densities of 0.5 and 1) resulted in, at best, 27.1% and 55.7% control efficacy, compared with aphid densities when no wasps were present (0% control efficacy; Figure 2a). This level of control declined as aphids arrived later in crop development (i.e., wasps became less effective).
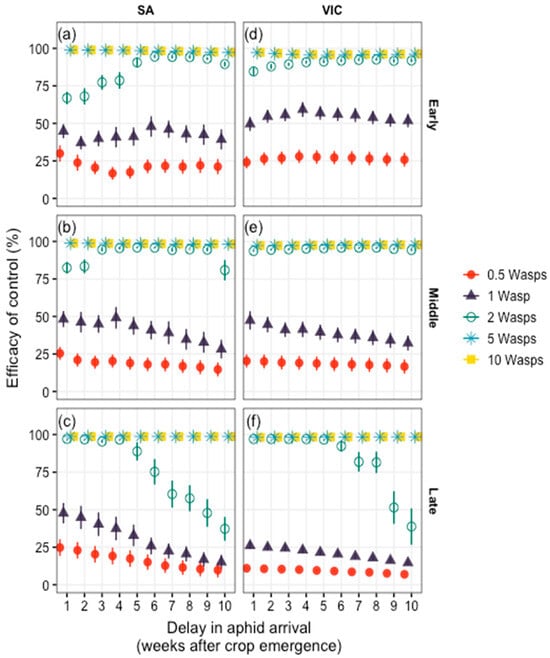
Figure 3.
Efficacy of control exerted by varying densities of Diaeretiella rapae on Myzus persicae, compared to simulations with no biological control. Here, an efficacy of 100% reflects complete suppression of the aphid population, while 0% reflects aphid densities in a system without any wasps (see Figure 2). Scenarios for early (a,d), middle (b,e) and late (c,f) TOS at South Australian (a–c) and Victorian (d–f) sites were considered. Error bars denote variation among the 10-year (climate) replicates (2005–2014). Note that the scenario with 10 wasps (yellow squares) is equivalent to that with 5 wasps (light-blue asterisks, both achieved near-full control).
At an intermediate wasp density, a ratio of 5:1 aphid:wasp background densities (i.e., wasp density of 2, Figure 3), achieved a control efficacy from 38.3% to 98.1% compared with simulations without wasps, but again this depended on seasonal timing and location. Here, biological control was greatest when ambient conditions were cold; control efficacy in early and middle TOS at the Victorian site reached 94.6% (Figure 3d,e). However, under warmer autumn and spring temperatures, impacts of parasitism on aphid densities was reduced, and more wasps were required in the system to suppress M. persicae (Figure 3a,c,f, explained by a higher rate of increase in reproduction for the aphids, in comparison to the wasps; see [26]). At relatively high wasp densities (aphid:wasp density ratios or 2:1 and 1:1), almost full suppression of the aphid populations could be achieved, with efficacy remaining above 98% (Figure 3).
3.3. Chemical and Biological Controls
The three management strategies impacted the predicted population dynamics of M. persicae in different ways, and these impacts were generally consistent among the three TOS scenarios at the South Australian site (Figure 4a–c). In the absence of chemical control, D. rapae failed to substantially reduce the autumn aphid outbreak in the early and middle TOS; however, once established, the wasp population increased as the season progressed, and total aphid densities were reduced (Figure 4a–c). In contrast, the application of a broad-spectrum insecticide suppressed the autumn population growth, but the efficacy of this control quickly declined, and aphid densities increased towards the end of the season (Figure 4a–c). In some years, total aphid densities in the broad-spectrum treatment were higher than the scenario in which “no control” was simulated at early TOS. Finally, the combined impacts of biological and an aphid-specific chemical control resulted in an almost-complete suppression of the aphid population for the full canola growing season (Figure 3).
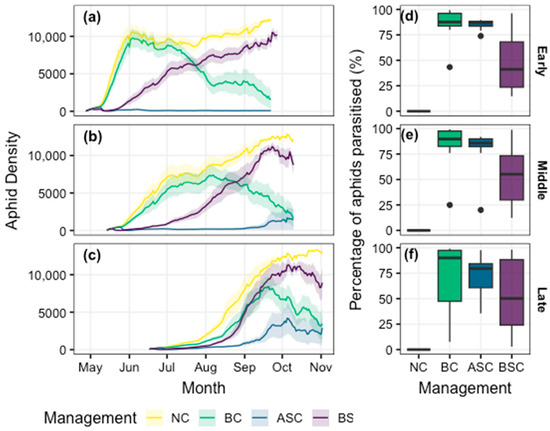
Figure 4.
Impacts of different management strategies on the population dynamics of Myzus persicae throughout the canola growing season with an (a) early, (b) middle, and (c) late sowing time at the South Australian site (where aphids arrive on the day of crop emergence; see Figure 2a). Management scenarios include no control (NC), biological control (BC), aphid-specific chemical control (ASC) and broad-spectrum chemical control (BSC). The proportion of parasitised aphids (wasp larvae/[wasp larvae + aphids]) on the day of crop maturation for the three sowing times (d–f) provides a measure of the relative success of the management approaches (black points are outlying values below 1.5 × inter-quartile range). Shaded regions (a–c) and box-plot whiskers (d–f) denote standard error of mean and the 75% interquartile range, respectively, where variation is generated from the 10-year (climate) replicates (see Table 1 for further details).
The relative “success” of these management strategies (proportion of aphids that were parasitised on the day of crop maturation) was highest in the early and middle TOS scenario (Figure 4d,e) and progressively declined in the late TOS (Figure 4f). In all three TOS, broad-spectrum chemical control was, on average, less successful than the biological and aphid-specific controls. In addition, reliability of these management scenarios (indicated by the variance across the 10-year replicates) differed among scenarios. Success of the broad-spectrum chemical application was less reliable than the biological and aphid-specific chemical controls across all TOS scenarios (Figure 4d–f). Of the two latter control scenarios, success became progressively less reliable (higher variance) as canola was planted later in the season (Figure 4d–f).
4. Discussion
The modelling approach used in our study enabled us to describe seasonal crop-aphid–parasitoid interactions for canola grown in an Australian context. Such patterns would be impossible to record empirically given the widespread use of insecticide seed treatments, foliar insecticide applications, and the geographic scope over which canola is grown in that country. This context necessitates a modelling approach to evaluating control options that can be later tested in the field. Our simulations illustrate that the application of a broad-spectrum foliar-spray early in the winter growing season can have long-lasting effects on the population dynamics of M. persicae and a major parasitoid, D. rapae. While such a chemical may appear highly effective in the short term, disrupting the interactions between pests and their natural enemies can have longer-lasting consequences. In our simulations, excessive mortality of D. rapae caused by the broad-spectrum chemical led to a secondary outbreak of M. persicae in spring. This outcome was avoided when management scenarios allowed D. rapae to persist within the aphid population. These findings support the general approach of IPM, whereby successful management includes not only the suppression of short-term pest outbreaks, but also maintaining the ongoing presence of natural enemies in a broader landscape.
The chemical-control scenarios modelled in this study were necessarily simplified versions of real-world conditions of a complex system and should be treated as such. For example, in reality, even highly soft aphicides like flonicamid and afidopyropen will cause some lethality or sub-lethality to parasitoid wasps [42], and so the ‘aphid-specific’ chemical control should be interpreted as a ‘best-case’ scenario. Moreover, different chemicals have different residual periods, and so the duration of exposure, and rates of mortality, are likely to differ considerably to our ‘generic’ values incorporated here. The model has been written such that these specific details can be altered, providing a versatile framework with which the impacts of different chemicals can be tested. Moreover, most canola currently grown in Australia is treated with neonicotinoid-based seed treatments, a control that we have not considered here (other than to interpret the ‘delayed aphid arrival’ scenario as such). In the context of our model, seed treatments would act to delay the arrival of aphids by up to ten weeks [39], and can impact wasps via several pathways (e.g., wasps developing within aphids, direct contact with the plant, or feeding on tainted plants and/or honeydew [43,44]). Importantly, our model indicates that if insecticide seed treatments in canola become unavailable (or restricted) in the future, there is potential for the greater utility of D. rapae to control M. persicae outbreaks and that using aphid-selective foliar insecticides could support this biological control.
In addition to the simplified chemical-control scenarios, other assumptions and processes we have not considered, may impact the population dynamics of M. persicae and D. rapae. For example, we assumed that fecund wasps intercept aphids immediately after their establishment in the crop, but in reality there is often a time-lag between aphid population outbreaks and wasp parasitism. This time-lag is likely to reduce the efficacy of the biological control levels predicted here. The health of the canola crop (and its carrying capacity) were not accounted for and doing so could result in a reduction in predicted aphid densities at the end of the growing season (more accurately reflecting field conditions). We also made assumptions as to the number of aphids and wasps in the “background” populations of the surrounding fields. While these values have been validated with field observations [26], our model could be coupled with a spatially explicit component which would allow researchers to track background densities and aphid dispersal (and potential virus transmission from one aphid colony to another) across the landscape.
5. Conclusions
While eliminating the use of chemical controls in Australian canola may be unrealistic, we demonstrate how the selection of chemicals can facilitate, or hinder, biological control provided by natural enemies. Indeed, the combination of biological and chemical control is an important step in transitioning into a more sustainable, IPM approach. By considering seasonal dynamics in aphid population growth, our simulations indicate prophylactic chemical controls targeting M. persicae could be avoided in many cases and point to the importance of other agronomic practices (i.e., TOS) in shaping pest population dynamics. While further work is required to validate our predictions in the field and to relate the predicted population dynamics to canola damage and yield loss, the modelling framework developed here is an important step to better understand population dynamics of pests and natural enemies in Australian agriculture.
Author Contributions
Conceptualization, M.G.B., H.P. and S.M.; methodology, M.G.B. and H.P.; formal analysis, M.G.B. and H.P.; data collection, S.W., T.H., D.S., M.R.B., J.H. and R.W.; writing—original draft preparation, M.G.B. and H.P.; writing—review and editing, M.G.B., H.P., S.M., P.A.U., T.H., S.W., A.A.H., M.V.H., D.S. and M.R.B.; project delivery, M.G.B., S.W., T.H., D.S., M.R.B., J.H., R.W., H.P., M.V.H., P.A.U., A.A.H. and S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grains Research and Development Corporation (CSE00059), and led by the Commonwealth Scientific and Industrial Research Organization in partnership with Cesar Australia, NSW Department of Primary Industries, the South Australian Research and Development Institute, the University of Melbourne, and the Western Australian Department of Primary Industries and Regional Development.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available from the authors on request, and code are available on git-hub repository: https://github.com/madeleine-barton/AphidWaspModel.git.
Acknowledgments
We would like to thank Julianne Lilley for assistance with APSIM (TBA), and two anonymous reviewers for their helpful comments.
Conflicts of Interest
Authors P.A.U. and S.M. were employed by the company cesar Autsralia.The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Stern, V.; Smith, R.; van den Bosch, R.; Hagen, K. The integration of chemical and biological control of the spotted alfalfa aphid: The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Adamson, D.; Furlong, M.J. The future of IPM: Whither or wither? Aust. J. Entomol. 2009, 48, 85–96. [Google Scholar] [CrossRef]
- Hill, M.P.; Macfadyen, S.; Nash, M.A. Broad spectrum pesticide application alters natural enemy communities and may facilitate secondary pest outbreaks. PeerJ 2017, 5, e4179. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Pesticides and pest control. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawa, A.K., Eds.; Springer: Dordrecht, Germany, 2009; Volume 1, pp. 83–87. [Google Scholar]
- Horne, P.A.; Page, J.; Nicholson, C. When will integrated pest management strategies be adopted? Example of the development and implementation of integrated pest management strategies in cropping systems in Victoria. Anim. Prod. Sci. 2008, 48, 1601–1607. [Google Scholar] [CrossRef]
- Micic, S.; Hoffmann, A.A.; Strickland, G.; Weeks, A.R.; Bellati, J.; Henry, K.; Nash, M.A.; Umina, P.A. Pests of germinating grain crops in southern Australia: An overview of their biology and management options. Aust. J. Exp. Agric. 2008, 48, 1560–1573. [Google Scholar] [CrossRef]
- Macfadyen, S.; Moradi-Vajargah, M.; Umina, P.A.; Hoffmann, A.; Nash, M.; Holloway, J.; Severtson, D.; Hill, M.; Van Helden, M.; Barton, M. Identifying critical research gaps that limit control options for invertebrate pests in Australian grain production systems. Austral Entomol. 2019, 58, 9–26. [Google Scholar] [CrossRef]
- McDonald, G.; Umina, P.A.; Macfadyen, S.; Mangano, P.; Hoffmann, A.A. Predicting the timing of first generation egg hatch for the pest redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Exp. Appl. Acar. 2015, 65, 259–276. [Google Scholar] [CrossRef]
- Schliephake, E.; Graichen, K.; Rabenstein, F. Investigations on the vector transmission of the Beet mild yellowing virus (BMYV) and the Turnip yellows virus (TuYV). J. Plant Dis. Protect. 2000, 107, 81–87. [Google Scholar]
- Jones, R.A.C.; Coutts, B.A. Yield-limiting potential of Beet western yellows virus in Brassica napus. Aust. J. Agric. Res. 2007, 58, 788–801. [Google Scholar] [CrossRef]
- Congdon, B.S.; Kehoe, M.A.; Filardo, F.F.; Coutts, B.A. In-field capable loop-mediated isothermal amplification detection of Turnip yellows virus in plants and its principal aphid vector Myzus persicae. J. Virol. Methods 2019, 265, 15–21. [Google Scholar] [CrossRef]
- Umina, P.A.; McDonald, G.; Maino, J.; Edwards, O.; Hoffmann, A.A. Escalating insecticide resistance in Australian grain pests: Contributing factors, industry trends and management opportunities. Pest Manag. Sci. 2019, 75, 1494–1506. [Google Scholar] [CrossRef]
- Pym, A.; Umina, P.A.; Reidy-Crofts, J.; Troczka, B.J.; Matthews, A.; Gardner, J.; Hunt, B.; van Rooyen, A.; Edwards, O.; Bass, C. Overexpression of UDP-glucuronosyltransferase and cytochrome P450 enzymes confers resistance to sulfoxaflor in field populations of the aphid, Myzus persicae. Insect Biochem. Molec. 2022, 143, 103743. [Google Scholar] [CrossRef]
- Ferreira, S.E.; Sampaio, M.V.; Silva de Oliveira, R.; Vasconcelos, H.L.D. Parasitism rate of Myzus persicae (Sulzer) by Diaeretiella rapae (McIntosh) in the presence of an alternative, resistant host. Rev. Bras. Entomol. 2018, 62, 13–18. [Google Scholar] [CrossRef]
- Ward, S.; Umina, P.A.; Parry, H.; Balfour-Cunningham, A.; Cheng, X.; Heddle, T.; Holloway, J.C.; Langley, C.; Severtson, D.; Van Helden, M.; et al. Is what you see what you get? The relationship between field observed and actual aphid parasitism rates in canola crops. Pest Manag. Sci. 2022, 78, 3596–3607. [Google Scholar] [CrossRef]
- Bannerman, J.A.; Roitberg, B.D. Impact of extreme and fluctuating temperatures on aphid–parasitoid dynamics. Oikos 2014, 123, 89–98. [Google Scholar] [CrossRef]
- Ro, T.H.; Long, G.E. GPA-Phenodynamics, a simulation model for the population dynamics and phenology of green peach aphid in potato: Formulation, validation, and analysis. Ecol. Model. 1999, 119, 197–209. [Google Scholar] [CrossRef]
- Trivedi, T.; Khurana, S.; Puri, S.; Bhar, L.; Mehta, S.; Jain, R.; Singh, G.; Chaudhari, S.; Mohasin, M.; Dhandapani, A. Development of forewarning system of potato aphid (Myzus persicae) on potato (Solanum tuberosum) in India. Indian J. Agric. Sci. 2002, 72, 341–345. [Google Scholar]
- Zhu, M.; Radcliffe, E.B.; Ragsdale, D.W.; MacRae, I.V.; Seeley, M.W. Low-level jet streams associated with spring aphid migration and current season spread of potato viruses in the U.S. northern Great Plains. Agric. Forest Meteorol. 2006, 138, 192–202. [Google Scholar] [CrossRef]
- Maling, T.; Diggle, A.J.; Thackray, D.J.; Siddique, K.H.M.; Jones, R.A.C. An epidemiological model for externally acquired vector-borne viruses applied to Beet western yellows virus in Brassica napus crops in a Mediterranean-type environment. Crop Pasture Sci. 2010, 61, 132–144. [Google Scholar] [CrossRef]
- Neuville, S.; Le Ralec, A.; Outreman, Y.; Jaloux, B. The delay in arrival of the parasitoid Diaeretiella rapae influences the efficiency of cabbage aphid biological control. BioControl 2016, 61, 115–126. [Google Scholar] [CrossRef]
- Stark, J.D.; Acheampong, S. A demographic and modeling approach to determine the suitability of two hosts, Brevicoryne brassicae (Linnaeus) and Myzus persicae (Sulzer) (Heteroptera: Aphididae) of the aphid parasitoid, Diaeretiella rapae (McIntosh) (Hymenoptera: Aphidiidae). Pan-Pac. Entomol. 2007, 83, 75–77. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Ives, A.R.; Shellhorn, N.A. Interactions between conventional and organic farming for biocontrol services across the landscape. Ecol. Appl. 2013, 23, 1531–1543. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Watt, M.S.; Withers, T.M.; Leriche, A.; Watson, M.C.A. process-based population dynamics model to explore target and non-target impacts of a biological control agent. Ecol. Model. 2009, 220, 2035–2050. [Google Scholar] [CrossRef]
- Sporleder, M.; Carhuapoma, P.; Juarez, H.; Gamarra, H.; Simon, R.; Kroschel, J. ILCYM-Insect Life Cycle Modeling. A Software Package for Developing Temperature-Based Insect Phenology Models with Applications for Local, Regional and Global Analysis of Insect Population and Mapping; International Potato Center: Lima, Peru, 2017. Available online: https://cgspace.cgiar.org/handle/10568/109024 (accessed on 3 March 2020).
- Barton, M.; Parry, H.; Ward, S.; Hoffmann, A.A.; Umina, P.A.; van Helden, M.; Macfadyen, S. Forecasting impacts of biological control under future climates: Mechanistic modelling of an aphid pest and a parasitic wasp. Ecol. Model. 2021, 457, 109679. [Google Scholar] [CrossRef]
- Moran, N.A. The evolution of aphid life cycles. Annu. Rev. Entomol. 1992, 37, 321–348. [Google Scholar] [CrossRef]
- Coutts, B.A.; Hawkes, J.R.; Jones, R.A.C. Occurrence of Beet western yellows virus and its aphid vectors in over-summering broad-leafed weeds and volunteer crop plants in the grainbelt region of south-western Australia. Aust. J. Agric. Res. 2006, 57, 975–982. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Nagasaka, K.; Moriya, S. Differences in the reproductive durations of Myzus persicae (Hemiptera: Aphididae) parasitized by three aphidid parasitoids. Appl. Entomol. Zool. 2016, 51, 297–304. [Google Scholar] [CrossRef]
- Heddle, T.; Van Helden, M.; Michael Nash, M.; Muirhead, K. Parasitoid communities and interactions with Diuraphis noxia in Australian cereal production systems. BioControl 2020, 65, 571–582. [Google Scholar] [CrossRef]
- Holzworth, D.P.; Huth, N.I.; de Voil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM–Evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Robertson, M.J.; Lilley, J.M. Simulation of growth, development and yield of canola (Brassica napus) in APSIM. Crop Pasture Sci. 2016, 67, 332–344. [Google Scholar] [CrossRef]
- Jeffrey, S.J.; Carter, J.O.; Moodie, K.B.; Beswick, A.R. Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environ. Model. Softw. 2001, 16, 309–330. [Google Scholar] [CrossRef]
- GRDC (Grain Research and Development Corporation). Victorian Winter Crop Growing Guide. Department of Economic Development, Jobs, Transport and Resources, Victoria. 2023. Available online: https://grdc.com.au/resources-and-publications/all-publications/nvt-crop-sowing-guides/vic-tas-crop-sowing-guide (accessed on 10 January 2023).
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- APVMA (Australian Pesticides and Veterinary Medicines Authority). APVMA Public Chemical Registration Information System Database. Available online: https://portal.apvma.gov.au/pubcris. (accessed on 21 October 2023).
- Abo El-Ghar, G.E.S.; El-Sayed, A.E.M. Long-term effects of insecticides on Diaeretiella rapae (M’Intosh), a parasite of the cabbage aphid. Pestic. Sci. 1992, 36, 109–114. [Google Scholar] [CrossRef]
- Abo El-Ghar, G.E.S.; El-Sayed, A.E.M. Impact of two synthetic pyrethroids and methomyl on management of the Cabbage Aphid, Brevicoryne brassicae (L.) and its Associated Parasitoid, Diaeretiella rapae (M’Intosh). Pestic. Sci. 1989, 25, 35–41. [Google Scholar] [CrossRef]
- Kirkland, L.S.; Chirgwin, E.; Ward, S.E.; Congdon, B.S.; van Rooyen, A.; Umina, P.A. P450-mediated resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae) reduces the efficacy of neonicotinoid seed treatments in Brassica napus. Pest Manag. Sci. 2023, 79, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 1 July 2023).
- Snyder, W.E.; Ives, A.R. Interactions between specialist and generalist natural enemies: Parasitoids, predators, and pea aphid biocontrol. Ecology 2003, 84, 91–107. [Google Scholar] [CrossRef]
- McDougall, R.; Mata, L.; Ward, S.; Hoffmann, A.; Umina, P.A. Assessing the sub-lethal impacts of insecticides on aphid parasitoids through laboratory-based studies. Austral. Entomol. 2023; in revision. [Google Scholar]
- Ward, S.; Hoffmann, A.A.; van Helden, M.; Umina, P.A. The effects of insecticide seed treatments on the parasitism and predation of green peach aphids, Myzus persicae (Homoptera: Aphididae). J. Econ. Entomol. 2023, accepted. [Google Scholar]
- Calvo-Agudo, M.; Dregni, J.; González-Cabrera, J.; Dicke, M.; Heimpel, G.E.; Tena, A. Neonicotinoids from coated seeds toxic for honeydew-feeding biological control agents. Environ. Pollut. 2021, 289, 117813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).