Abstract
The rhizome of Polygonatum, which has been consumed in China for nearly 2000 years, is rich in oligosaccharides, polysaccharides, saponins, and alkaloids and has various effects such as lowering blood sugar and anti-aging. Its aerial parts are often discarded as by-products and have been rarely studied; moreover, the nutritional composition and carbohydrate composition of these parts are not clear. In this study, the nutritional composition, amino acid evaluation, and the total phenolics content of the leaves, stems, fruits, and flowers of Polygonatum cyrtonema Hua were analyzed, and the composition and content of carbohydrates were determined by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC–PAD). The results suggested that the leaves and flowers of Polygonatum cyrtonema Hua (PC) contained 1.12–1.37% phenolic substances. The proteins of the aerial parts had high nutritional values, and the total amino acid content of the leaves and flowers, with the total leaf amino acids amounting to 11.77%, were followed by the flowers at 11.65%. The sugars in the flowers were mainly free monosaccharides at 10.38%, and the fruits were dominated by 9.44% fructo-oligosaccharides. This study provided new evidence for the utilization of the aerial parts of Polygonatum.
1. Introduction
Polygonatum is a perennial plant; it is also a traditional Chinese herb that can be eaten as a food or used medicinally. It was commonly reported that the rhizome of Polygonatum contains polysaccharides, flavonoids, saponins, and alkaloids, which make it have a variety of pharmacological effects, including antiglycation activity [1], immunomodulatory activity [2], and anti-diabetic activity [3]. The rhizomes of Polygonatum cyrtonema Hua (PC), one of the three species recorded in the pharmacopeia, are usually steamed and then dried several times before use. In ancient China, it was recorded that the leaves, flowers, fruits, and seedlings of Polygonatum had a history of being eaten [4,5], but these aerial parts were rarely studied and were usually discarded, making the whole plant underutilized.
Carbohydrates, especially functional polysaccharides, are an important class of biologically active substances. They are macromolecular compounds composed of several monosaccharides, which are widely present in living organisms and have the function of regulating cell growth, metabolism, and immune response, promoting the health of the organism and other physiological functions [6]. Numerous studies have been conducted to demonstrate that carbohydrates were the primary components of Polygonatum‘s aerial parts. Several studies compared the carbohydrate composition of the stems, leaves, and flowers, and it was found that the flowers had the highest polysaccharide content, and the stems had the highest content of fructose and glucose [7]. In addition, the seeds contained a large amount of starch, with a content proportion of about 60.9–65.2% of the total; moreover, the soluble sugar content in the seeds of PC was as high as 28.4% [8]. The rhizome, as an underground part, was also rich in carbohydrates, including sucrose, fructose, and about 30% fructo-oligosaccharides (FOSs). Interestingly, it contained almost no starch [9], so it is a good source of prebiotics, which is a type of nondigestible sugar broken down in the large intestine by probiotics, and it can contribute to intestinal microbial balance [10]. As a prebiotic, an FOS can selectively stimulate lactic acid bacteria and promote bacterial growth [11]. In addition, the structural information and biological activity of the rhizomes’ polysaccharides have been studied in depth [12,13]. Studies have also shown that Polygonatum leaf polysaccharides have the effect of regulating intestinal probiotics [14]. However, the sugar composition of the aerial parts of Polygonatum is not clear and comprehensive, and we speculate that FOSs also exist in these parts as an important prebiotic.
Other functionally active components were present in the aerial parts of Polygonatum. In comparison with the roots and rhizomes, the leaves and flowers contained higher levels of total phenolics, total flavonoids, and total saponins [15,16]. These components were shown to have antioxidative activity, free radical scavenging capacity, and anti-inflammatory and antimicrobial effects [17,18]. These functionally active components are the most important non-nutritional bioactive components in the human diet. However, a thorough analysis of the composition and content of these components in the aerial parts of Polygonatum has not yet been done.
Based on the amino acid composition of the human body, amino acids could be classified as essential amino acids (EAAs), conditionally essential amino acids (CEAAs), and nonessential amino acids (NEAAs). In addition, amino acids are classified according to their characteristics, such as flavor amino acids (FAAs) and medicinal amino acids (MAAs) [19]. Polygonatum seeds had 2.69% EAAs [20], which were necessary because they can only be obtained from food, and the flowers had 4.42% EAAs [15]. Additionally, the EAA/TAA score of the flowers was about three times that of the rhizomes, and FAAs were abundant in the flowers [15], making the flowers both tasty and nutritious.
Previous studies implied that collecting every ton of rhizomes yields about 400 kg of stems and leaves [21], meaning there was a considerable amount of aerial materials that remained unused. However, due to the lack of systematic analysis of its nutritional content and utilization value, the related products of the aerial parts of Polygonatum are very scarce in the market. Therefore, the aerial parts of Polygonatum need to be analyzed from the point of view of food nutrition, and their unclear carbohydrate composition needs to be accurately analyzed.
The rhizome of Polygonatum has been eaten in China for over 2000 years as a food and as a medicine [22]. It exhibits enormous market potential with more than 150 proprietary Chinese medicines and more than 350 health foods among the developments of its raw materials. However, the rest of the whole plant has not been utilized. Therefore, the aim of the present study was to evaluate the nutritional composition and active components of the leaves, stems, fruits, and flowers of Polygonatum cyrtonema Hua, with a focus on the fine analysis of amino acids and carbohydrates, in order to effectively improve the comprehensive consumption value of Polygonatum.
2. Materials and Methods
2.1. Materials and Chemicals
Fresh leaves, stems, flowers, and fruits of Polygonatum cyrtonema Hua (PC) were collected from Fujian province, China. These parts were cleaned separately and then freeze-dried under vacuum and then ground to an average particle size of 250 μm.
Monosaccharide standards [fucose (Fuc), rhamnose (Rha), arabinose (Ara), galactose (Gal), mannose (Man), xylose (Xyl), glucose (Glc), fructose (Fru), galacturonic acid (GalA), glucuronic acid (GlcA), and sucrose (Suc)] were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Trifluoroacetic acid (TFA) was supplied by Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Diethylene glycol was supplied by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Amino acid standards [histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), threonine (Thr), tryptophan (Try), valine (Val), aspartic acid (Asp), glutamate (Glu), glycine (Gly), proline (Pro), serine (Ser), alanine (Ala), tyrosine (Tyr), arginine (Arg), cystine (Cys)], 5-hydroxymethylfurfural (5-HMF), rutin, and gallic acid were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China); other reagents are analytically pure reagents.
2.2. Nutritional Content Analysis
According to the definition of the China Food Composition Tables Standard Edition [23], macronutrients include protein, amino acids, fat, carbohydrates, dietary fiber, and others.
Protein content was determined by the Kjeldahl method [24], which was on the basis of total nitrogen content multiplied by a conversion factor of 6.25. The composition and content level of total amino acids were determined by the National Standards for Food Safety of China—Determination of Amino Acids in Food (GB 5009.124-2016) [25]. Fat was extracted using petroleum ether by continuous refluxing for 6 h; the liquid was collected and weighed after it was dried.
The total carbohydrate content was calculated by the subtraction method of the Association of Official Analytical Chemists:
Total carbohydrate (%) = 100 − [(protein (%) + fat (%) + moisture (%) + ash (%)]
In addition, the amount of available carbohydrate could be calculated from the total carbohydrate minus the total dietary fiber (TDF). TDF, soluble dietary fiber (SDF), and insoluble dietary fiber (IDF) were calculated by the AOAC enzymatic–gravimetric method; TDF = SDF + IDF.
Fresh plant material was dried by oven dehydration at 105 °C for 12 h then cooled off and weighed to determine the moisture content. The dried powder was placed in a muffle furnace at 650 °C for 4 h, then the weight of the ash content was recorded [26].
All samples were analyzed in triplicate, and their nutritional contents were expressed as a percentage of the fresh weight (FW).
2.3. Amino Acid Nutritional Evaluation
EAAs include 9 amino acids, and NEAAs include 5 amino acids, and the content calculation formula were as follows:
EAAs (%) = His (%) + Ile (%) + Leu (%) + Lys (%) + Met (%) + Phe (%) + Thr (%) + Try (%) + Val (%)
NEAAs (%) = Asp (%) + Ser (%) +Glu (%) + Gly (%) + Pro (%)
FAAs include umami amino acids (Glu, Asp, Lys), bitter amino acids (Val, Ile, Leu, Tyr, Phe, Arg), and sweet amino acids (Thr, Ser, Pro, His, Gly, Ala) [27]. MAAs contain 9 amino acids [28,29]:
MAAs (%) = Glu (%) + Arg (%) + Gly (%) + Asp (%) + Tyr (%) + Met (%) + Phe (%) + Leu (%) + Lys (%)
According to the models of human amino acids in the World Health Organization/Food and Agriculture Organization of the United Nations/United Nations University (WHO/FAO/UNU) in 2007 [30], the amino acid score (AAS) is calculated to determine whether the amino acids of protein in a food are restricted amino acids, and the higher the AAS, the higher the nutritional value of the protein.
The amino acid with the lowest score is called the restriction amino acid, and its score is the AAS of the protein. In this study, the human amino acid pattern was used as the standard mode.
2.4. Determination of Phenolic Content
Petroleum ether was added to the powders of the leaves, flowers, fruits, and stems, and ultrasound extraction was performed for 30 min to remove the pigments; then, we repeated the extraction again. After the residue was dry, 80% methanol was added into it; ultrasound extraction was performed for 15 min and repeated twice. Then, we centrifuged and removed the insoluble matter to obtain the extract.
The total phenolic content was determined by the Folin–Ciocalteu colorimetric method [31] with minor modifications. We mixed 200 μL diluted extract and 800 μL 3% (w/v) Na2CO3, then added 20 μL Folin–Ciocalteu reagent, and the mixture was shaken vigorously. After a 30 min reaction at room temperature in the dark, the absorbance was measured at 765 nm. Using gallic acid as the standard product, the total phenolic content was expressed as mg gallic acid equivalents (GAE) per g−1 of dry weight (DW).
2.5. Extraction of Polysaccharides and the Low Molecular Weight Fraction
We added pure water to the sample powder treated with petroleum ether to obtain the extract at 90 °C for 2 h. After a centrifugation, the unprecipitated fraction was the extract. To achieve a concentration of 80% ethanol in the extract, absolute ethanol was added, then the extract was placed at 4 °C for alcoholic precipitation. The precipitated fraction was crude polysaccharide. After the ethanol was removed, the fraction was dissolved in pure water to obtain the polysaccharide solution. The unprecipitated fraction was concentrated under reduced pressure until the ethanol was removed, then diluted with ultrapure water to obtain the low molecular weight fraction containing monosaccharides and oligosaccharides.
2.6. Determination of Total Soluble Sugar and Polysaccharide Content
Total soluble sugar content was analyzed by the phenol–sulfuric acid method [32]. We diluted the extract and the polysaccharide solution to appropriate concentrations, respectively. Then, we took 1 mL and added 1 mL of 6% phenol solution and 5 mL of H2SO4. The same procedure was performed using glucose as a standard product. The absorbance was measured at 490 nm.
2.7. Determination of Monosaccharides, Sucrose, and Fructo-Oligosaccharides
Since fructose could not be derived by 1-phenyl-3-methyl-5-pyrazolone (PMP) [33], the HPAEC–PAD method was used to detect 10 monosaccharides, which include Fuc, Rha, Ara, Gal, Man, Xyl, GalA, GlcA, Glu, and Fru, and sucrose could also be detected.
The low molecular weight fraction was passed through 0.22 μm aqueous phase filter membranes. The content levels of Glu, Fru, and Suc were analyzed by HPAEC–PAD, and the fructo-oligosaccharides (FOSs) were analyzed using modified acidolysis [9]. Fructose and glucose could convert to 5-HMF during acidolysis, resulting in low FOS content [34]. Therefore, 5-HMF was also quantified in this study to convert to glucose and fructose.
Other free monosaccharides were determined by ion chromatography (IC) [35].
Free monosaccharides (%) = Fuc (%) + Rha (%) + Ara (%) + Gal (%) + Man (%) + Xyl (%) + Glc (%) + Fru (%)
All were calculated by dry weight.
2.8. Structural Analysis of Polysaccharides
The monosaccharide composition of the polysaccharides was also determined by the IC method. The weight-average molecular weight (Mw) and the polydispersity [Mw/the number-average molecular weight (Mn)] of the polysaccharides were determined by size-exclusion chromatography measurement coupled with a multi-angle laser light scattering detector (SEC–MALLS). The crude polysaccharides were dissolved in ultrapure water to obtain a concentration of 2–4 mg/mL then filtered through 0.22 μm membranes before injection. The detector was a MALLS instrument (DAWN HELEOS II, Wyatt Technology, Santa Barbara, CA, USA) combined with a differential refractive index (RI) detector [36]. The SB-804/806 HQ columns (7.8 mm × 300 mm; Showa Denko, Tokyo, Japan) were connected in series with 0.15 mol/L of NaCl as the mobile phase. Data were analyzed using ASTRA software (Version 7.3.2.17 64-bit, Wyatt Technology, Santa Barbara, CA, USA).
3. Results and Discussion
3.1. Nutritional Content Analysis
The content percentages of the moisture, protein, fat, ash, and total carbohydrates are shown in Figure 1a. In the fresh state, the flowers had the highest moisture content (83.8 ± 1.90%), followed by the stems (77.35 ± 2.48%) and leaves (74.04 ± 1.88); the lowest was in the fruits (62.21 ± 1.83).
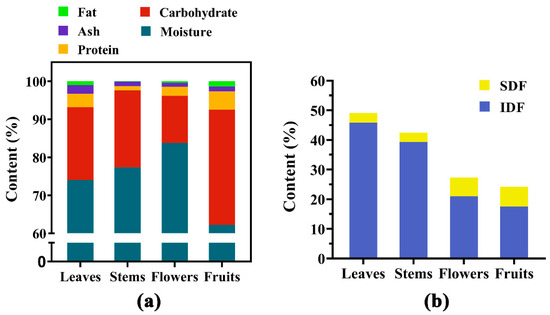
Figure 1.
(a) Nutrition content of the aerial parts of PC (by fresh weight). (b) The composition and content of dietary fiber (by dry weight). The sum of soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) was the total dietary fiber.
Carbohydrates were the main macronutrients. According to the dietary reference intakes (DRIs) in China (2013), carbohydrate intake accounts for 50–65% of the body’s total calorie intake. Daily intake of high-quality carbohydrates reduced the risk of all-cause mortality [37]. Calculated according to Formula (1), the fruits of PC had the highest carbohydrate content (30.32%) based on fresh weight (FW), followed by the stems (20.26%), leaves (19.17%), and flowers (12.36%). However, the results were higher than reality because lignin, organic acids, tannic acids, and other unknown compounds were included [38]. Dietary fiber was considered an important nutrient and refers to carbohydrates such as polysaccharides and oligosaccharides that are difficult to digest and absorb [39]. According to the method used, some low molecular soluble dietary fibers, including oligofructose, oligogalactose, polydextrose, resistant maltodextrin, and resistant starch, were not detected [40], and thus the dietary fiber content was lower than the actual values. Based on dry weight (DW) calculations, the IDF (45.81%) and TDF (49.08%) content of the leaves were highest (Figure 1b), which showed that in the dried leaves, insoluble dietary fiber was the predominant component. Fruits had the least TDF, but the highest SDF content, followed by flowers, indicating that they were rich in dietary fiber.
Protein was also one of the main macronutrients in the aerial parts of PC, and it was abundant in the leaves and the fruits. Its content level was 3.5–4.8% between them, which was roughly three times as much as in the stems and flowers. In addition, the fat content of PC aerial parts was extremely low, accounting for only 0.09–1.38%.
3.2. Amino Acid Profile and Nutritional Evaluation
When determining the nutritional value of food, the quality of the protein is a crucial component that is typically determined by the composition and content of amino acids [19]. The four aerial parts of PC, as shown in Table 1, contained nine essential amino acids. Glutamate was the most abundant amino acid in flowers, stems, and leaves, and it significantly affected the memory and the functioning of the central nervous system [41]. In PC fruits, tryptophan was found in the highest concentration, and it also had potential physiological activity that could modulate neuroendocrine and intestinal immune responses [42].

Table 1.
The composition and content percentages of amino acids from the aerial parts of PC.
The highest EAA and TAA contents were found in PC leaves, and the EAAs were also abundant in flowers. According to the ideal amino acid composition proposed by WHO/FAO, an EAA content/NEAA content > 0.6 and an EAA content/TAA content ≈ 0.4 are better for the human body [19]. The four parts of PC were all close to the values, so the proteins of the aerial parts were high-quality protein. Among the parts, the fruits had the highest ratios of EAAs/TAAs (0.51) and EAAs/MAAs (1.33), suggesting that its protein had the higher proportion of essential amino acids and the higher nutritional value. The abundance of MAAs in the leaves (6.85% content) hinted at their potential medicinal properties. The flowers and leaves contained the largest amount of FAAs, reaching more than 10%. The amount of umami and sweet amino acids in the flowers was found to be about 63.7% of the TAAs, which was regarded to be the cause of the nice flavor of the flowers.
The amino acid score (AAS) recommended by WHO/FAO/UNU is a widely used method for protein quality evaluation. The protein quality was evaluated by comparing the AAS of the tested sample with WHO/FAO/UNU standard AAS. Of the nine essential amino acids, the amino acid with the lowest AAS is called the first-limiting amino acid, and its AAS value is also used as the amino acid score for this protein. As shown in Table 2, Leu was the first-limiting amino acid for stems and flowers, and methionine and valine for leaves and fruits, respectively. Lack of limiting amino acids may lead to inadequate utilization of other amino acids by the body, thus reducing the nutritional value of the protein. Therefore, noting the compatibility of indispensable amino acids in one’s diet can prevent an amino acid imbalance [43].

Table 2.
Amino acid score (AAS) of the aerial parts of PC.
3.3. Total Phenolics Content
The phenolics are known as the most abundant secondary metabolites in plants and have medicinal properties, with medicinal plants and aromatic plants being their important sources [27]. As shown in Table 3, the total phenolic content in the leaves was the highest, and in the flowers, it was also higher, being 3–4 times that of the stems and fruits. It was speculated that the leaves and flowers had some medicinal properties, such as antidiabetic, anti-inflammatory, and anticancer [44].

Table 3.
Total phenolic and soluble sugars content of the aerial parts of PC (%).
In this experiment, it was observed that the methanol extract of the four parts showed varying degrees of yellow-green, indicating the significant presence of flavonoids, which can modify flowers’ colors, enhance plant resistance, and provide nutritional value for human diets [45]. However, due to the complex structure of phenolic substances, the results of using this colorimetric method are not accurate enough. Other substances such as ascorbic acid, aromatic amines, and sugar may also be measured [46]. Therefore, more advanced methods, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), or combined mass spectrometry (MS), should be used to further analyze the particular phenolic composition, which plays an important role in the utilization of the medicinal value of the aerial parts of Polygonatum.
3.4. Sugar Composition and Content
Sugar is the most important energy substance in plants. Clarifying the content and composition of small molecule sugars and polysaccharides is conducive to the processing and utilization of some aerial raw materials. The results of the phenol–sulfuric acid method showed that fruits had the highest total soluble sugar and polysaccharide content (Table 3), with 25.35% and 3.08%, respectively, whereas the polysaccharide contents in the leaves and stems were lower, less than 0.5%. Since colorimetric and chromatographic methods were used for the determination of the sugar composition, their results may slightly differ. IC was used to determine the content of free monosaccharides in samples before acidolysis (the blue lines in Figure 2a,c,e,g), and the data were shown in Table 3.
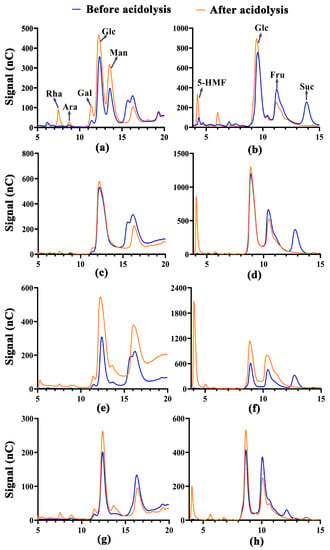
Figure 2.
Signals of monosaccharides and sucrose before and after acidolysis under IC and HPAEC –PAD analysis. (a) Monosaccharides in leaves determined by IC; (b) 5-HMF, Glc, Fru, Suc in leaves determined by HPAEC–PAD; (c) monosaccharides in stems determined by IC; (d) 5-HMF, Glc, Fru, Suc in stems determined by HPAEC–PAD; (e) monosaccharides in fruits determined by IC; (f) 5-HMF, Glc, Fru, Suc in fruits determined by HPAEC–PAD; (g) monosaccharides in flowers determined by IC; (h) 5-HMF, Glc, Fru, Suc in flowers determined by HPAEC–PAD.
The free monosaccharide content in the four parts is about 5.05–10.38%, illustrating that the free monosaccharide was the main soluble sugar in the leaves, stems, and flowers. On the other hand, the content percentages of Rha, Ara, Gal, and Man increased slightly with the low molecular weight sugars that were revealed under acidolysis, as shown by the orange lines in Figure 2a,c,e,g, indicating the presence of oligosaccharides composed of these monosaccharides.
HPAEC–PAD was used to determine the content percentages of Glu, Fru, Suc, and 5-HMF, which was necessary for the quantification of FOSs. FOSs are oligosaccharides composed of Glu and Fru [47], so the Glu and Fru produced by the sample after acidolysis were considered to be degraded by FOS. However, under acidic or high temperature conditions, Glu and Fru were dehydrated to produce 5-HMF, especially the latter, and the 5-HMF was further degraded [48]. Therefore, in this study, the conversion of 5-HMF to Glu/Fru according to the concentration of the amount of substance could reduce the error, but it was not possible to calculate the 5-HMF that has been degraded. And different samples under the condition of acidolysis may also be over or under acidolysis. So, the calculation results would be lower than the actual value. The results were shown in Table 3. It was found that about 9.44% fructo-oligosaccharides were present in the fruits, but some substances were still not identified.
3.5. Structural Analysis of Polysaccharides
Monosaccharide composition was a primary structure characterization for polysaccharides, and it can reflect the relative molar ratios of various monosaccharides in polysaccharides. The results showed that the monosaccharide composition of the polysaccharides in the four parts was slightly different (Table 4). The leaves‘ polysaccharides were mainly composed of Gal, Ara, and Man. Gal and Man were also the highest in the polysaccharides of the stems and flowers. However, a considerable portion of Glc and GalA were present in the fruits. Since GalA is the main component of pectin, it meant that the fruits contained more pectin structure than other parts.

Table 4.
Monosaccharide composition of polysaccharides of the aerial parts from PC.
The fruits had the smallest polysaccharide Mw of 155.1 kDa, and the leaves had the largest of 809.2 kDa. The leaves were richer in dietary fiber and had the highest molecular weight. Since the main chain of hemicellulose consists of galactans, mannans, and branched chains that have arabinose or galactose [49], and because of the information on the composition of the monosaccharides, it was assumed that the leaves were rich in hemicellulose, which is also contained in the stems and flowers. The larger the polydispersity index (PDI) of the polysaccharide (Mw/Mn), the wider the Mw distribution. Table 3 suggested that the Mw distribution of polysaccharides in the stems was relatively narrow, while the polysaccharides in other parts had a wide Mw distribution.
4. Conclusions
This present study found that the leaves and flowers of Polygonatum cyrtonema Hua were rich in amino acids and had some nutritional value. Additionally, the aerial parts of Polygonatum contained small molecule weight sugars that have not been carefully studied, including free monosaccharides, sucrose, and fructo-oligosaccharides. Further, there was about 9.44% fructo-oligosaccharide in the Polygonatum fruits, providing evidence for its potential prebiotic effects. This was an important reference for the utilization of the whole plant of Polygonatum and for the development of new resources.
Author Contributions
Writing—original draft and Formal analysis, J.X.; Investigation, X.M.; Project administration, Supervision, and Funding acquisition, H.C., S.C., X.Y. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ningbo Municipal Bureau of Science and Technology (2022Z174), the Major Agricultural Technology Collaborative Promotion Plan of Zhejiang Province (2022XTTGZYC03), and the Key Research and Development Program of Zhejiang Province (2021C02001).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The dataset is available from the first author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, P.; Li, X.; Wang, Y.; Zhang, X.; Jia, H.; Guo, L.; Huang, L.; Gao, W. Comparative studies on characterization, saccharide mapping and antiglycation activity of polysaccharides from different Polygonatum ssp. J. Pharm. Biomed. Anal. 2020, 186, 113243. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Kong, X.; Li, H. Characterization and Immunological Activities of Polysaccharides from Polygonatum sibiricum. Biol. Pharm. Bull. 2020, 43, 959–967. [Google Scholar] [CrossRef]
- Shi, Y.; Si, D.; Chen, D.; Zhang, X.; Han, Z.; Yu, Q.; Liu, J.; Si, J. Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives. Food Chem. 2023, 408, 135183. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Yang, M.; Zhang, J. Herbal Textual Research on Polygonati Rhizoma and Ethnic Usage. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 237–250. (In Chinese) [Google Scholar]
- Liu, J.; Si, J. Herbal textual research on Chinese medicine “Huangjing” (Polygonati Rhizoma) and some enlightenments. China J. Chin. Mater. Med. 2018, 43, 631–636. (In Chinese) [Google Scholar]
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring Carbohydrates for Therapeutics: A Review on Future Directions. Front. Pharmacol. 2021, 12, 756724. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Liu, Y.; Zu, Y.; Zhang, F.; Du, L.; Chen, J.; Li, L.; Wang, K.; Wang, Y.; et al. Identification of key gene networks controlling polysaccharide accumulation in different tissues of Polygonatum cyrtonema Hua by integrating metabolic phenotypes and gene expression profiles. Front. Plant. Sci. 2022, 13, 1012231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, J.; Li, C.; Wang, R.; Shi, G.; Li, J. Nutritional Components and Antioxidant Enzyme Activity of Polygonatum cyrtonema and Polygonatum kingianum Seeds. Guizhou Agric. Sci. 2022, 50, 90–95. (In Chinese) [Google Scholar]
- Xia, J.; Zhang, C.; Zhu, K.; Mei, X.; Cheng, H.; Chen, S.; Ye, X.; Chen, J. Identification of carbohydrate in Polygonatum sibiricum: Fructo-oligosaccharide was a major component. Food Qual. Saf. 2023, 7, fyad029. [Google Scholar] [CrossRef]
- Gibson, G.; Scott, K.; Rastall, R.; Tuohy, K.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, G.; Gan, X.; Li, T.; Qu, Z.; Xu, S.; Liu, C.; Qian, C. Study on the varied content of Polygonatum cyrtonema polysaccharides in the processing of steaming and shining for nine times based on HPLC-MS/MS and chemometrics. Microchem. J. 2020, 159, 105352. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; Nie, X.; Wang, D.; Zhang, Q.; Peng, T.; Zhang, C.; Wu, D.; Zhang, J. Recent advances in polysaccharides from edible and medicinal Polygonati rhizoma: From bench to market. Int. J. Biol. Macromol. 2022, 195, 102–116. [Google Scholar] [CrossRef]
- Yang, J.; Yan, P.; Luo, Y.; Mu, Y.; Liu, M.; Gao, P. Optimization of water extraction process for polysaccharides from leaves of polygonatum by response surface methodology. J. Sichuan Univ. Nat. Sci. Edi. 2020, 57, 791–796. (In Chinese) [Google Scholar]
- Zhang, Z.; Huang, S.; Liu, J.; Zhang, X.; Si, J. Main nutrients and functional ingredients in flowers of Polygonatum cyrtonema and P. filipes. China J. Chin. Mater. Med. 2020, 45, 1329–1333. (In Chinese) [Google Scholar]
- Wang, T.; Wang, H.; Li, D.; Chen, S.; Pan, K. Comparison of active components and antioxidant activity of non-medicinal parts of Polygonatum cyrtonema. Food Mach. 2022, 38, 57–60. (In Chinese) [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the Hurdles and Potential Health Benefits in Value-Added Use of Plant Food Processing By-Products as Sources of Phenolic Compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Shi, J.; Tomás-barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant. Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef]
- Li, G.; Ji, J.; Zhang, Y. Measurement and Analysis of the Nutrients of Polygonatum sibiricum Seeds. Acta Bot. Boreali-Occident. Sin. 2009, 29, 1692–1696. (In Chinese) [Google Scholar]
- Luo, Y.; Fang, Q.; Lai, Y.; Lei, H.; Zhang, D.; Niu, H.; Wang, R.; Song, C. Polysaccharides from the leaves of Polygonatum sibiricum Red. regulate the gut microbiota and affect the production of short-chain fatty acids in mice. Amb. Express 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, J.; Si, D.; Golding, J.B.; Pristijono, P.; Li, Y.; He, F.; Zhang, X.; Han, Z.; Wu, L. Huangjing—From medicine to healthy food and diet. Food Front. 2023, fft2.231. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition Table (2002 No. 1) (Chinese Edition); Beijing Medical University Pub.: Beijing, China, 2000. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds: Block Digestion Method Using Copper Catalyst and Steam Distillation into Boric Acid, Official Method of Analysis of AOAC International, 22nd ed.; AOAC: Rockville, MD, USA, 2001. [Google Scholar]
- GB 5009.124-2016; The National Standards for Food Safety of China—Determination of Amino Acids in Food. Standards Press of China: Beijing, China, 2016.
- Gupta, E.; Purwar, S.; Jaiswal, P.; Chaturvedi, R.; Rai, G.K. Sensory Evaluation and Nutritional Composition of Developed Papaya-Gooseberry Jam. Food Nutr. Sci. 2016, 7, 600–608. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Tian, X.; Yang, Y.; Duan, G.; Gao, P.; Liu, Q. Study on Flavor Characteristics and Nutritional Evaluation of Free Amino Acids in Walnut Pellicle. Sci. Technol. Food Ind. 2023, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Shi, F.; Yin, X.; Zhang, H.; Zhang, M.; Zhang, Z.; Zhang, J.; Zhou, J.; Sun, L. Determination and Analysis of Free Amino Acid Composition of Xinjiang Black Bee Honey in Nilka. Sci. Technol. Food Ind. 2022, 43, 276–283. (In Chinese) [Google Scholar] [CrossRef]
- Chen, M.; Huang, Z.; Zhou, D.; Lu, Y.; Tang, Q.; Zhou, H.; Shi, X.; Xie, H.; Zeng, J.; Zheng, Y. Effect of processing on amino acid composition and nutritional value of protein from Polygonatum. Food Ferment. Ind. 2023, 1–11. (In Chinese) [Google Scholar] [CrossRef]
- Weltgesundheitsorganisation; FAO; Vereinte Nationen (Eds.) Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; [Geneva, 9–16 April 2002]; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- DuBois Michel Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Chen, F.; Zheng, F. Comparison of determination of sugar-PMP derivatives by two different stationary phases and two HPLC detectors: C18 vs. amide columns and DAD vs. ELSD. J. Food Compos. Anal. 2021, 96, 103715. [Google Scholar] [CrossRef]
- Li, R.; Lin, Q.; Liu, Y.; Wang, X.; Liu, C.; Peng, F.; Ren, J. Insights into solvent effect on selective production of furfural and 5-hydroxymethylfurfural from fructose. J. Catal. 2023, 424, 162–172. [Google Scholar] [CrossRef]
- Hu, X.; Yu, C.; Ahmadi, S.; Wang, Y.; Ye, X.; Hou, Z.; Chen, S. Optimization of high-pressure processing-assisted extraction of pectic polysaccharides from three berries. Food Qual. Saf. 2022, 6, fyac051. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocoll. 2021, 112, 106160. [Google Scholar] [CrossRef]
- Hou, W.; Han, T.; Sun, X.; Chen, Y.; Xu, J.; Wang, Y.; Yang, Y.; Jiang, W.; Sun, C. Relationship Between Carbohydrate Intake (Quantity, Quality, and Time Eaten) and Mortality (Total, Cardiovascular, and Diabetes): Assessment of 2003–2014 National Health and Nutrition Examination Survey Participants. Diabetes Care. 2022, 45, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, L.; Song, L.; Sommerfeld, M.; Hu, Q. An improved phenol-sulfuric acid method for the quantitative measurement of total carbohydrates in algal biomass. Algal Res. 2023, 70, 102986. [Google Scholar] [CrossRef]
- An, Y.; Lu, W.; Li, W.; Pan, L.; Lu, M.; Cesarino, I.; Li, Z.; Zeng, W. Dietary fiber in plant cell walls—The healthy carbohydrates. Food Qual. Saf. 2022, 6, fyab037. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Zhou, R.; Bian, L.; Wang, G. Determination of total soluble and insoluble dietary fiber in foods by enzymatic-gravimetric method. J. Hyg. Res. 2001, 30, 377–378+390. (In Chinese) [Google Scholar]
- Huang, X.; He, X.; Yang, Q.; Gu, W.; Zhou, X.; Zhang, H.; Zhou, N. Determination of 17 amino acids in the dried rhizome of Lycopus lucidus Turcz. var. hirtus regel from different habitats. Food Sci. 2021, 42, 255–261. (In Chinese) [Google Scholar]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-C. Amino Acid Imbalance-Biochemical Mechanism and Nutritional Aspects. Asian-Australas. J. Anim. Sci. 2006, 19, 1361–1368. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.M.; Yao, L.N.; Shang, Y.Y.; Liu, S.; Meng, J.X.; Zhang, S.Y.; Li, H.H. Advances in the application of biosynthesis and metabolic engineering of flavonoids in plants. Biol. Plant. 2022, 66, 163–171. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Shi, S.; Wang, H.; Yan, J.; Wang, S. An inulin-type fructan isolated from Artemisia japonica and its anti-arthritic effects. J. Funct. Foods 2017, 29, 29–36. [Google Scholar] [CrossRef]
- Li, R. The Conversion of Hexose to Pentose and Furfural. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2021. [Google Scholar]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, chemical modification, and application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).