Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling
Abstract
:1. Introduction
2. Materials and Methods
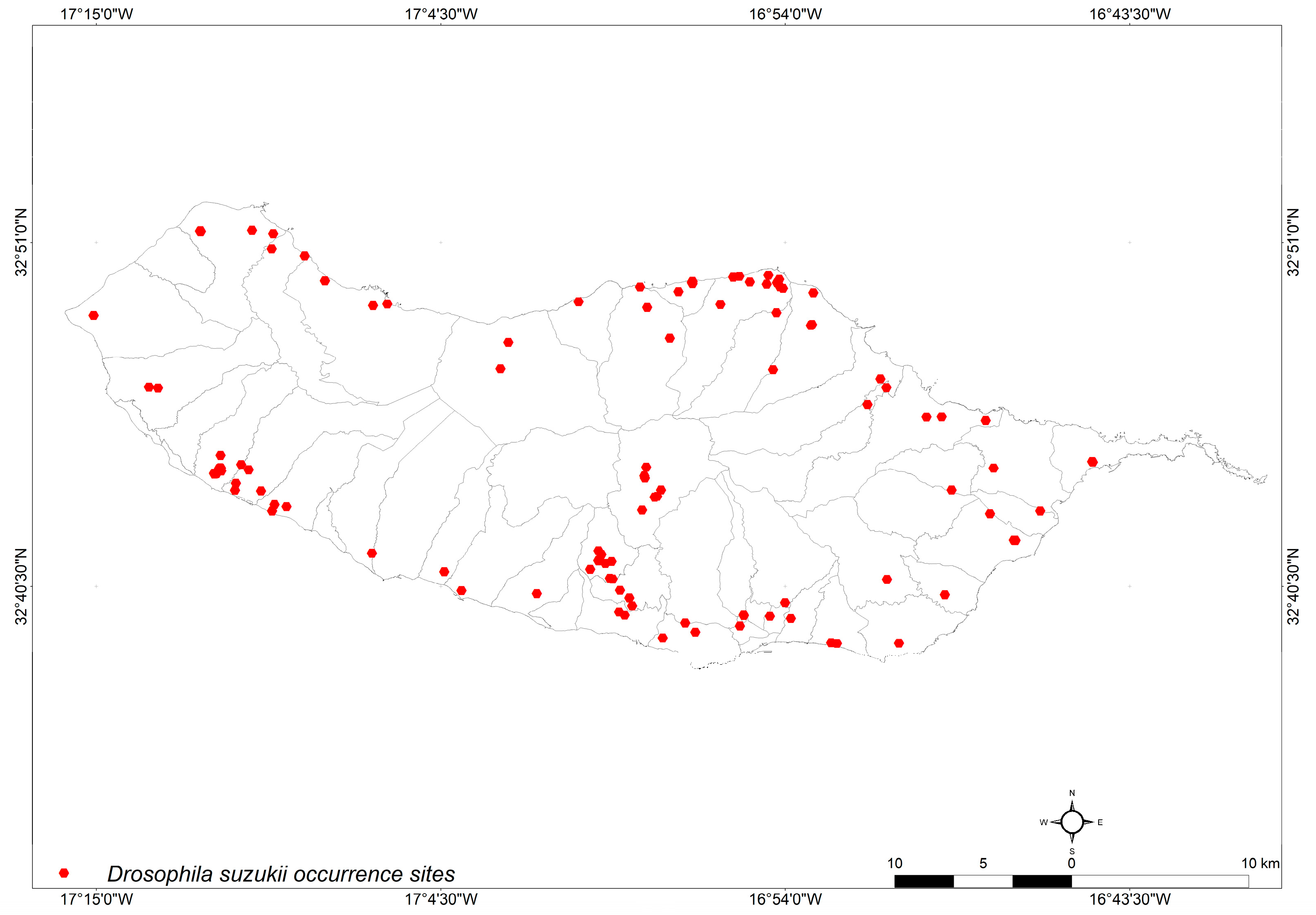
2.1. Occurrence Sites
2.2. Environmental Variables
2.3. Data Modeling
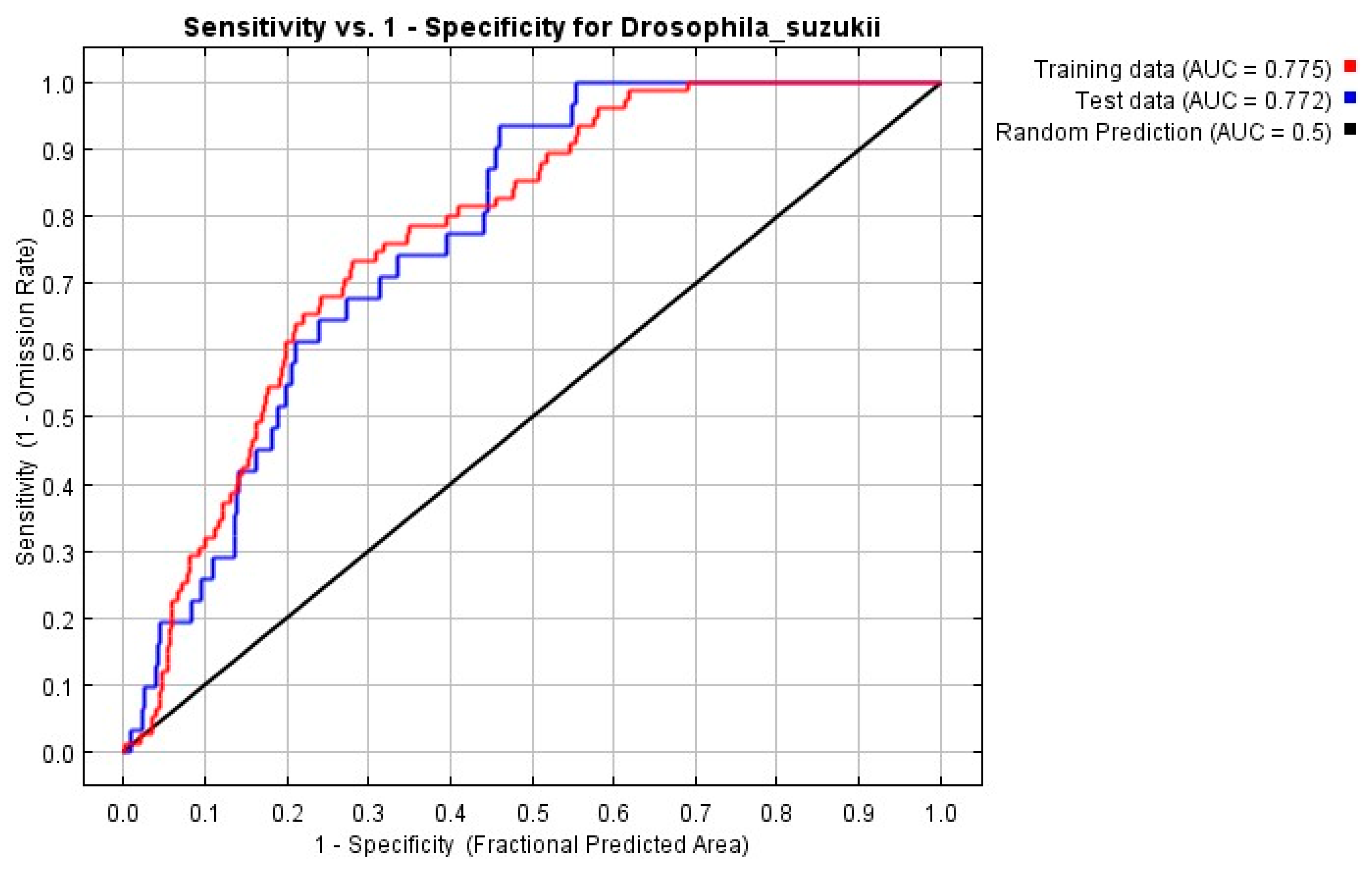
2.4. Evaluation of the Model
2.5. Statistical Procedures
3. Results and Discussion
3.1. Modeling Results
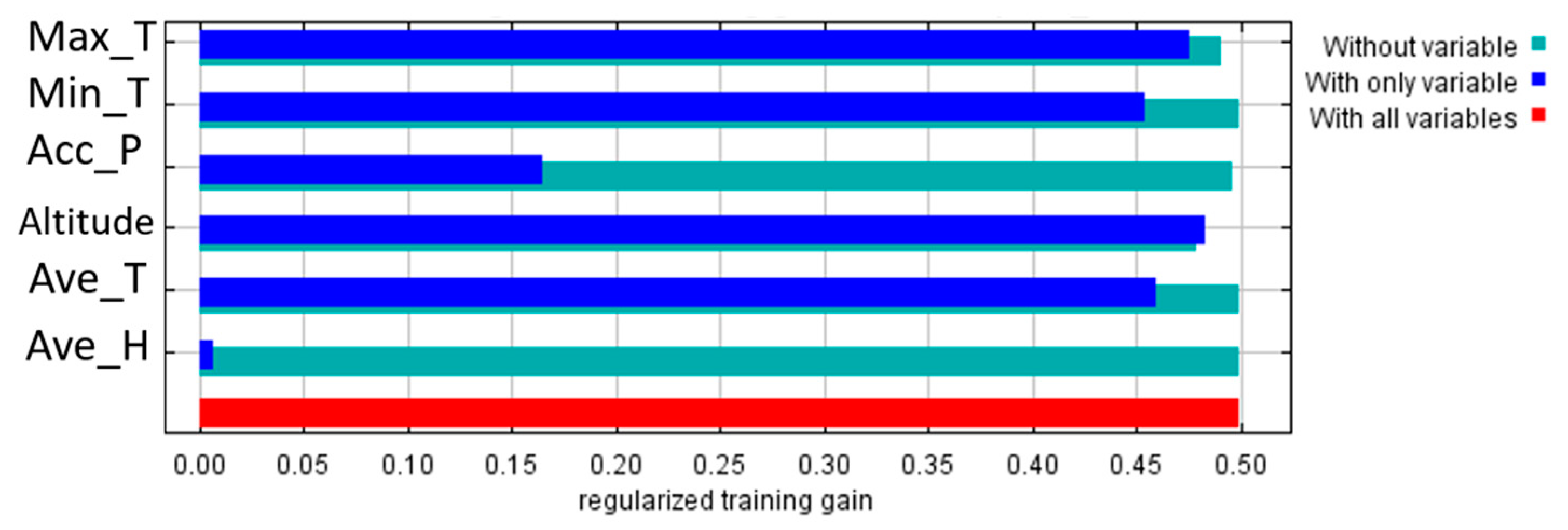
3.2. Importance of Environmental Variables
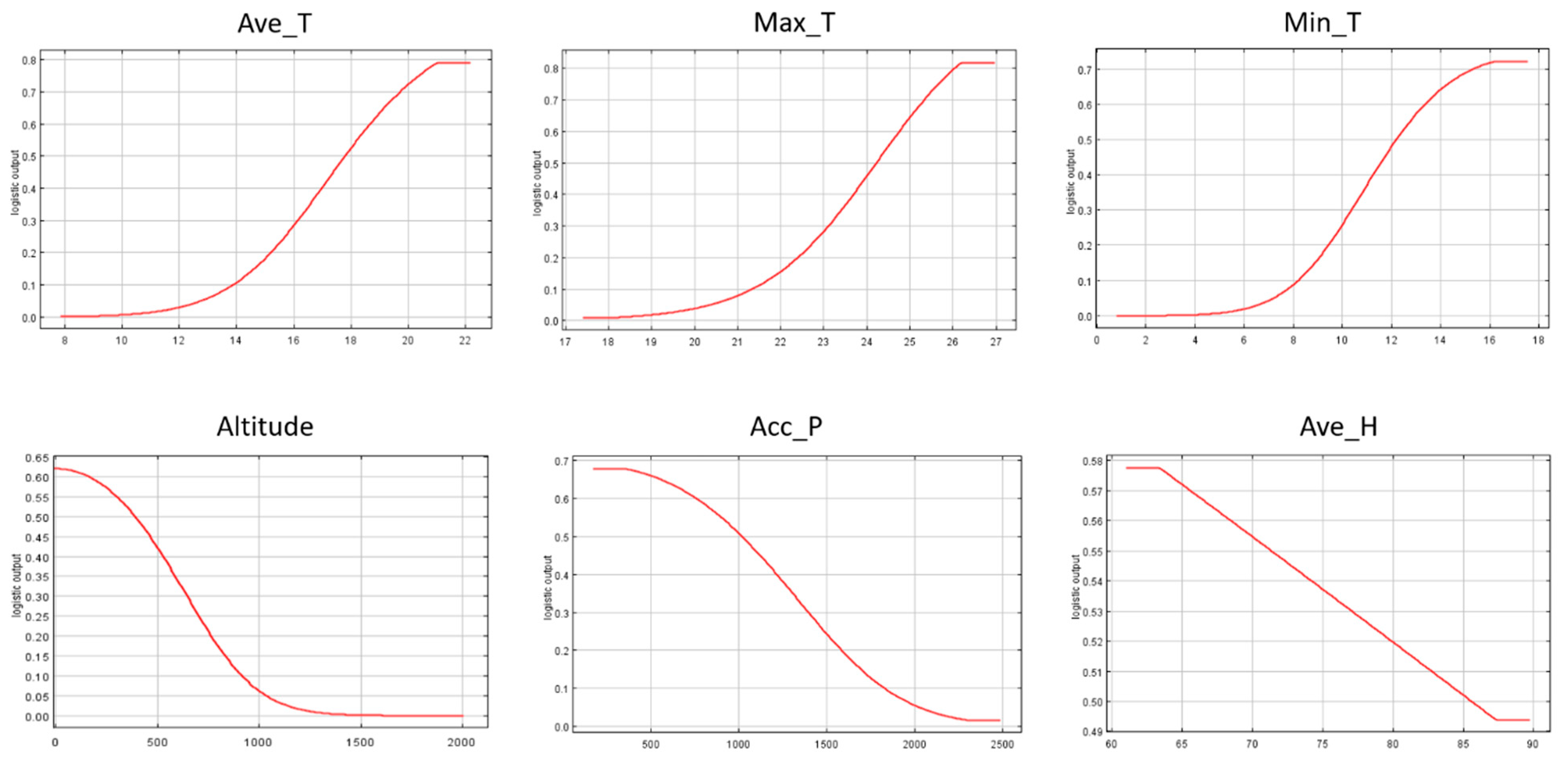
3.3. Individual Response Curves
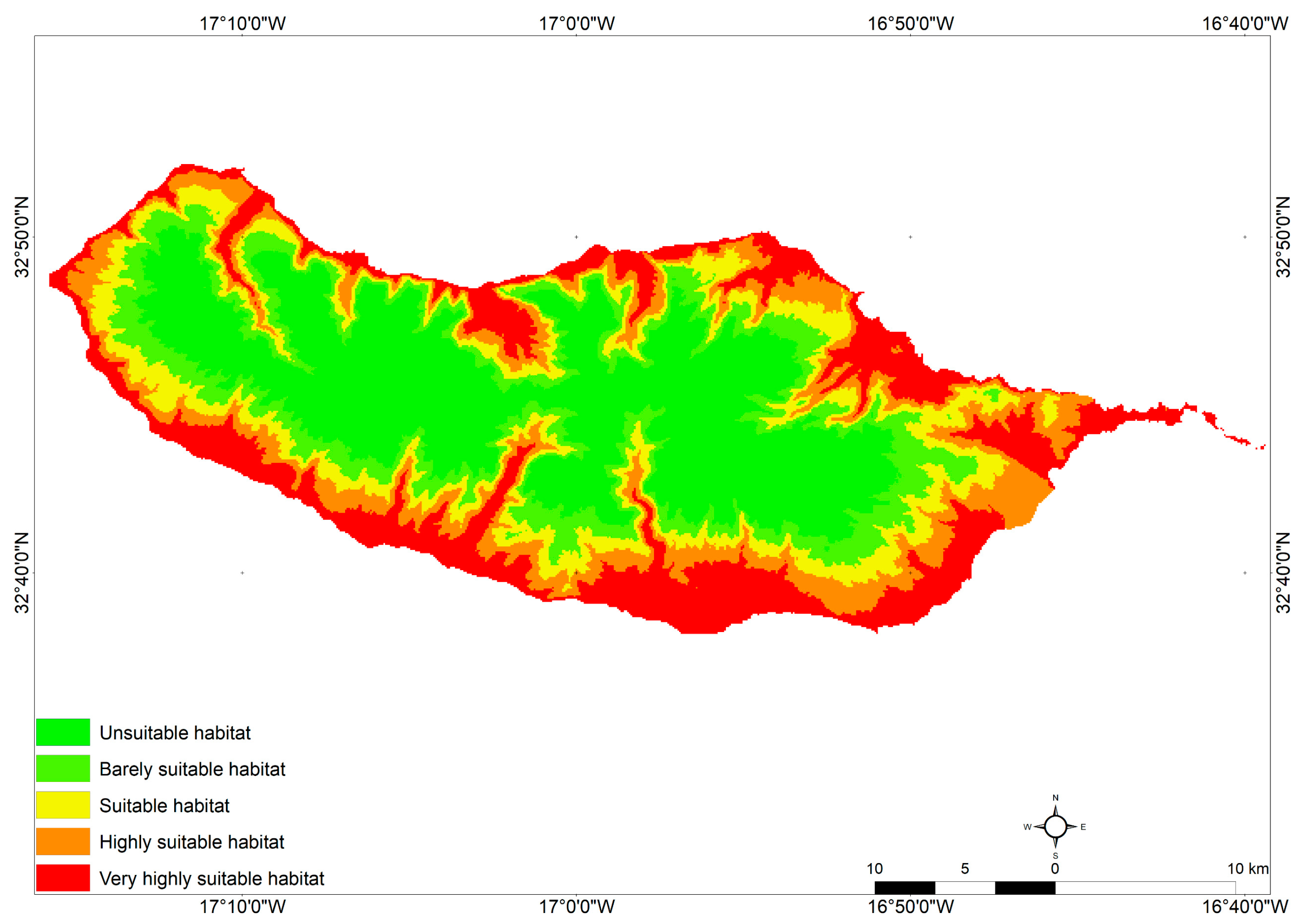
3.4. Potential Distribution of Drosophila suzukii
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanzawa, T. Research into the fruit-fly Drosophila suzukii Matsumura (preliminary report). In Yamanashi Prefecture Agricultural Experiment Station Report; Hoshino Printing: Kofu-Shi, Japan, 1935. [Google Scholar]
- Bächli, G. TaxoDros: The Database on Taxonomy of Drosophilidae. Available online: https://www.taxodros.uzh.ch/ (accessed on 10 May 2023).
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The Making of a Pest: The Evolution of a Fruit-Penetrating Ovipositor in Drosophila suzukii and Related Species. Proc. R. Soc. B 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.P.; Grunwald Kadow, I.C.; Gompel, N.; Prud’homme, B. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Crava, C.M.; Zanini, D.; Amati, S.; Sollai, G.; Crnjar, R.; Paoli, M.; Rossi-Stacconi, M.V.; Rota-Stabelli, O.; Tait, G.; Haase, A.; et al. Structural and Transcriptional Evidence of Mechanotransduction in the Drosophila suzukii ovipositor. J. Insect Physiol. 2020, 125, 104088. [Google Scholar] [CrossRef]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the Biological Control of Drosophila suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- O’Grady, P.M.; Beardsley, J.W.; Perreira, W.D. New Records for Introduced Drosophilidae (Diptera) in Hawaii. Bishop Mus. Occas. Pap. 2002, 69, 34–35. [Google Scholar]
- Hauser, M.A. Historic Account of the Invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the Continental United States, with Remarks on Their Identification. Pest. Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First Records of the Potential Pest Species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organisation (EPPO). First Report of Drosophila suzukii in the United Kingdom. Available online: https://gd.eppo.int/reporting/article-2414 (accessed on 10 May 2023).
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The First Records of the Invasive Pest Drosophila suzukii in the South American Continent. J. Pest. Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Rego, C.; Aguiar, A.F.; Boieiro, M.; Cravo, D. Invasive Fruit Flies (Diptera: Drosophilidae) Meet in a Biodiversity Hotspot. J. Entomol. Res. 2017, 19, 61–69. [Google Scholar]
- Rosa, P.J.G. Determinação da Curva de voo e Possíveis Hospedeiros Alternativos da Praga Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) na Cultura da Framboesa na Região do Algarve. Master’s Thesis, Escola Superior Agrária—Instituto Politécnico de Beja, Beja, Portugal, 27 July 2016. [Google Scholar]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef]
- Consejería de Agricultura, Ganadería, Pesca y Aguas Gobierno de Canarias (CAPMA) Drosophila Suzukii; Gestión de Medio Rural de Canarias, SAU Área de Agricultura—División de Proyectos Dirección General de Agricultura. Available online: https://gmrcanarias.com/wp-content/uploads/2020/10/REVISTA-A5-DROSOPHILA-SUZUKII_WEB.pdf (accessed on 10 May 2023).
- Pearson, R.G. Species distribution modeling for conservation educators and practitioners. Synthesis. Am. Mus. Nat. Hist. 2007, 50, 54–89. [Google Scholar]
- Kearney, M.R.; Wintle, B.A.; Porter, W.P. Correlative and Mechanistic Models of Species Distribution Provide Congruent Forecasts under Climate Change: Congruence of Correlative and Mechanistic Distribution Models. Conserv. Lett. 2010, 3, 203–213. [Google Scholar] [CrossRef]
- Trindade, W.C.F. Modelagem de Distribuição das Formações Vegetais do Estado do Paraná: Passado, Presente e Futuro. Master’s Dissertation, Universidade Estadual de Ponta Grossa, Ponta Grossa, Brazil, 11 February 2019. [Google Scholar]
- Ricklefs, R.E. A Economia Da Natureza, 6th ed.; Grupo Gen—Guanabara Koogan: Rio de Janeiro, Brazil, 2010; 546p. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, J. Geography and Evolution. Ecology 1924, 5, 225–229. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Cold Spring Harbor Symposium on Quantitative Biology. In Concluding Remarks, 1st ed.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1957; Volume 22, pp. 415–427. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Castro-Sosa, R.; Castillo-Peralta, M.D.R.; Monterroso-Rivas, A.I.; Gomez-Díaz, J.D.; Flores-González, E.; Rebollar-Alviter, Á. Potential Distribution of Drosophila suzukii (Diptera: Drosophilidae) in Relation to Alternate Hosts in Mexico. Fla. Entomol. 2017, 100, 787–794. [Google Scholar] [CrossRef]
- De La Vega, G.J.; Corley, J.C. Drosophila Suzukii (Diptera: Drosophilidae) Distribution Modelling Improves Our Understanding of Pest Range Limits. Int. J. Pest Manag. 2019, 65, 217–227. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global Potential Distribution of Drosophila Suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef]
- Ørsted, I.V.; Ørsted, M. Species Distribution Models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in Its Native and Invasive Range Reveal an Ecological Niche Shift. J. Appl. Ecol. 2019, 56, 423–435. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.P.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, J.R.; Huettmann, F.; Leathwick, R.J.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Santos, A.R.; Ribeiro, C.A.A.S.; Sediyama, G.C.; Peluzio, J.B.E.; Pezzopane, J.B.M.; Bragança, R. Espacialização de Dados Meteorológicos No ArcGIS 10.3: Passo a Passo; CAUFES—Centro Agropecuária da Universidade Federal do Espírito Santo: Vitória, Brazil, 2015. [Google Scholar]
- ESRI Inc Esri Support ArcMap 2021. Available online: https://desktop.arcgis.com/en/quick-start-guides/10.6/arcgis-desktop-quick-start-guide.htm (accessed on 10 May 2023).
- Farr, T.G.; Kobrick, M. Shuttle Radar Topography Mission Produces a Wealth of Data. Eos Trans. AGU 2000, 81, 583–585. [Google Scholar] [CrossRef]
- Pinto, M.A.D.S.; Gonçalves, A.P.S.; Santos, S.A.P.; de Almeida, M.R.L.; Azevedo, J.C.M. de Invasão biológica de Corythucha ciliata em espaços verdes urbanos de portugal: Modelação do nicho ecológico com o método de máxima entropia. Ciênc. Florest. 2014, 24, 597–607. [Google Scholar] [CrossRef]
- Plasencia-Vázquez, A.H.; Escalona-Segura, G.; Esparza-Olguín, L.G. Modelación de la distribución geográfica potencial de dos especies de psitácidos neotropicales utilizando variables climáticas y topográficas. AZM 2014, 30, 471–490. [Google Scholar] [CrossRef]
- Krzanowski, W.J.; Hand, D.J. ROC Curves for Continuous Data, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009; p. 332. [Google Scholar]
- Chang, Y.L.; Xia, Y.; Peng, M.W.; Chu, G.M.; Wang, M. MaxEnt modelling for predicting impacts of climate change on the potential distribution of Anabasis aphylla in northwestern China. Appl. Ecol. Env. Res. 2020, 18, 1637–1648. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 2.3.16) 2023. Available online: https://www.jamovi.org (accessed on 15 August 2023).
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.P.; Ponti, L.; Dalton, D.T. Analysis of the Invasiveness of Spotted Wing Drosophila (Drosophila suzukii) in North America, Europe, and the Mediterranean Basin. Biol. Invasions 2016, 18, 3647–3663. [Google Scholar] [CrossRef]
- Esfanjani, J.; Ghorbani, A.; Zare Chahouki, M.A. MaxEnt Modeling for Predicting Impacts of Environmental Factors on the Potential Distribution of Artemisia aucheri and Bromus tomentellus-Festuca ovina in Iran. Pol. J. Environ. Stud. 2018, 27, 1041–1047. [Google Scholar] [CrossRef]
- Yan, H.; He, J.; Xu, X.; Yao, X.; Wang, G.; Tang, L.; Feng, L.; Zou, L.; Gu, X.; Qu, Y.; et al. Prediction of Potentially Suitable Distributions of Codonopsis Pilosula in China Based on an Optimized MaxEnt Model. Front. Ecol. Evol. 2021, 9, 773396. [Google Scholar] [CrossRef]
- Mahatara, D.; Acharya, A.; Dhakal, B.; Sharma, D.; Ulak, S.; Paudel, P. Maxent Modelling for Habitat Suitability of Vulnerable Tree Dalbergia Latifolia in Nepal. Silva Fenn. 2021, 55. [Google Scholar] [CrossRef]
- Pinheiro de Carvalho, M.Â.A.; Ragonezi, C.; Oliveira, M.C.O.; Reis, F.; Macedo, F.L.; de Freitas, J.G.R.; Nóbrega, H.; Ganança, J.F.T. Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem. Agronomy 2022, 12, 2201. [Google Scholar] [CrossRef]

| Weather Station Location | Latitude (Decimal) | Longitude (Decimal) | Altitude (m) |
|---|---|---|---|
| Funchal/Observatório | 32.65 | −16.89 | 58 |
| Funchal/Lido | 32.64 | −16.93 | 25 |
| Santa Catarina/Aeroporto | 32.69 | −16.77 | 58 |
| Lugar de Baixo/P. do Sol | 32.68 | −17.09 | 40 |
| Calheta/P. do Pargo | 32.81 | −17.26 | 298 |
| Santana/São Jorge | 32.83 | −16.91 | 257 |
| Chão do Areeiro | 32.72 | −16.92 | 1.590 |
| Caniçal/P. de São Lourenço | 32.75 | −16.71 | 133 |
| Lombo da Terça | 32.84 | −17.21 | 931 |
| Santana | 32.81 | −16.89 | 380 |
| Bica da Cana | 32.76 | −17.06 | 1.560 |
| São Vicente | 32.80 | −17.05 | 97 |
| Santo da Serra | 32.73 | −16.82 | 660 |
| Quinta Grande | 32.66 | −17.00 | 580 |
| Pico Alto | 32.69 | −16.90 | 1.118 |
| Pico do Areeiro | 32.74 | −16.93 | 1.799 |
| Porto Moniz | 32.87 | −17.17 | 35 |
| Bioclimatic Variables | Percent Contribution |
|---|---|
| Max_T | 8.9 |
| Min_T | 7.6 |
| Acc_P | 2.8 |
| Altitude | 71.2 |
| Ave_T | 7.6 |
| Ave_H | 1.8 |
| Altitude | Ave_T | Max_T | Min_T | Acc_P | Ave_H | |
|---|---|---|---|---|---|---|
| Altitude | − | − | − | − | − | − |
| Ave_T | −0.969 *** | − | − | − | − | − |
| Max_T | −0.952 *** | 0.987 *** | − | − | − | − |
| Min_T | −0.971 *** | 0.987 *** | 0.952 *** | − | − | − |
| Acc_P | 0.890 *** | −0.913 *** | −0.875 *** | −0.924 *** | − | − |
| Ave_H | −0.006 | −0.055 | −0.046 | −0.070 | 0.120 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, F.L.; Ragonezi, C.; Reis, F.; de Freitas, J.G.R.; Lopes, D.H.; Aguiar, A.M.F.; Cravo, D.; Carvalho, M.A.A.P.d. Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture 2023, 13, 1764. https://doi.org/10.3390/agriculture13091764
Macedo FL, Ragonezi C, Reis F, de Freitas JGR, Lopes DH, Aguiar AMF, Cravo D, Carvalho MAAPd. Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture. 2023; 13(9):1764. https://doi.org/10.3390/agriculture13091764
Chicago/Turabian StyleMacedo, Fabrício Lopes, Carla Ragonezi, Fábio Reis, José G. R. de Freitas, David Horta Lopes, António Miguel Franquinho Aguiar, Délia Cravo, and Miguel A. A. Pinheiro de Carvalho. 2023. "Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling" Agriculture 13, no. 9: 1764. https://doi.org/10.3390/agriculture13091764
APA StyleMacedo, F. L., Ragonezi, C., Reis, F., de Freitas, J. G. R., Lopes, D. H., Aguiar, A. M. F., Cravo, D., & Carvalho, M. A. A. P. d. (2023). Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture, 13(9), 1764. https://doi.org/10.3390/agriculture13091764