Abstract
The cellulose synthase (CesA) and cellulose synthase-like (Csl) superfamily encodes critical enzymes involved in processing plant cellulose and hemicellulosic polysaccharides. The alfalfa (Medicago sativa L.) genome was sequenced in recent years, but this superfamily remains poorly understood at the genome-wide level. We identified 37 members of the CesA/Csl family from the alfalfa genome in this study as well as their chromosomal locations and synteny. We uncovered 28 CesA/Csl expressed across all tissues and CslD genes specifically expressed in the root. In addition, cis-acting element analysis showed that CesA/Csl contained several abiotic stress-related elements. Moreover, transcriptomic analysis of alfalfa seedlings demonstrated the involvement of this superfamily in responses to cold, drought, and salt stresses. Specifically, CslD increased expression in cold conditions and decreased under osmotic stress, highlighting its potential role in stress adaptation. The findings offer valuable information for the practical exploration of the functions of CesA/Csl during plant development and the development of enhanced tolerance to different stress conditions.
1. Introduction
Polysaccharide polymers, including cellulose, hemicellulose, and pectin, form the nanoscale network structure of the cell wall that determines plant shape, size, and specific physicochemical properties [1]. Cellulose is composed of β-1,4-linked chains of glucan units that are synthesized by cellulose synthase complexes (CSC) located at the plasma membrane and organized into microfibrils. Cellulose constitutes the main load-bearing element of the cell wall, making up approximately 20~30% of the dry weight of the primary cell wall and 50% of the secondary wall [2,3], respectively. Native cellulose primarily occurs in para-crystalline structures resulting from inter- and intra-chain hydrogen bonds and van der Waals forces [4,5].
The cellulose synthase gene superfamily can be found across all land plants. The first candidate gene belonging to this superfamily was found in embryophytes and identified by homology analysis to bacterial cellulose synthase genes [6,7]. The machinery necessary for cell wall synthesis evolved multiple times in prokaryotes while, in eukaryotes, this process was acquired from cyanobacteria via numerous lateral gene transfers during endosymbiosis [8]. In higher plants, cellulose synthase genes were first isolated from developing cotton (Gossypium hirsutum) fibers [6]. Later, the gene family identified from cotton and rice (Oryza sativa) was named CesA [2].
The cellulose synthase gene superfamily also includes the cellulose synthase-like gene family and is composed of membrane-embedded glycosyltransferases and 18 enzymatically active CesA proteins that form a cellulose synthase complex (CSC) which moves through the plane of the plasma membrane as it synthesizes a cellulose microfibril [3,9,10]. The CesAs have conserved motifs around three aspartic residues that, along with the conserved QXXRW motif, construct the active site on the cytoplasmic surface of the plasma membrane. The new glucan chain is synthesized inside a cavity created by a total of eight transmembrane domains. In addition, the cytoplasmic N-terminal region of the proteins contains two zinc finger domains [11,12].
Angiosperms typically contain 10–20 CesA genes [13]. Arabidopsis thaliana has 10 CesA genes, including 3 CesA isoforms essential for the synthesis of primary (AtCesA1, AtCesA3, and AtCesA6-like (CesA2, CesA5, CesA6, and CesA9)) and secondary (AtCesA4, AtCesA7, and AtCesA8) cell walls [14,15]. CesA2, CesA5, and CesA9 are partially redundant with CesA6, as demonstrated by mutant and co-immunoprecipitation analysis [16,17].
The Csl family has nine subfamilies, CslA to CslH and CslJ [18,19]. While CslA, CslC, and CslD are common to all land-growing plants, some are restricted to certain groups of plants: CslB and CslG are exclusive to dicotyledons and gymnosperms, whereas CslF and CslH are specific to monocots [20,21]. Initially, CslJ was solely found in cereals; however, recent studies have demonstrated its extensive occurrence in both dicots and monocots [2,18]. In Arabidopsis, there are six families consisting of 30 Csl genes that are classified into them. These families are known as CslA, CslB, CslC, CslD, CslE, and CslG, respectively [22]. Finally, Csl proteins encode processive glycosyl transferases (GTs) based on the familiar motifs DXD, D, and QXXRW [23].
Alfalfa is commonly referred to as the ‘Queen of Forages’ due to its high nutritional value. It contains significant levels of crude protein, secondary metabolites, and mineral composition [24]. High-quality forage production is the fundamental basis of both livestock and dairy industries. There are several component traits to forage quality, including protein concentration, percentage of non-digestible protein, animal intake as indicated by low levels of neutral detergent fiber (NDF), digestibility of the NDF fraction as well as other indicators of feed digestibility, such as fiber content, like acid detergent fiber (ADF), and acid detergent lignin (ADL) [25]. NDF is associated with the structural components of plants, particularly the cell wall. Generally, low NDF values are desirable as they tend to increase as forage matures. ADF is the least digestible plant constituent, mostly composed of cellulose and lignin, whereby forages with lower ADF concentrations are generally better sources of energy. In contrast, ADL affects the ability of animals to digest grass. As plants mature, their lignin content increases and their digestibility decreases. The fiber content and digestibility are significant factors affecting alfalfa quality [26]; reducing fiber content should improve digestibility and animal performance.
In this study, we identified and studied 37 protein-coding genes in alfalfa using phylogenetics, gene structure, chromosomal distribution, and collinearity analyses between species. Moreover, we investigated the unique expressions of CesA/Csl genes in diverse tissues and alfalfa’s response to abiotic stress through transcriptomic analysis. Finally, to simulate cellulose synthase gene family expression in alfalfa at different cutting intervals, we employed reverse transcription quantitative PCR (qRT–PCR) analysis on alfalfa stems and leaves at different phenological stages. Our findings revealed the features of CesA/Csl superfamily members in alfalfa and provide a foundation for further investigations on their functions.
2. Materials and Methods
2.1. Plant Material Preparation
The alfalfa plants of the Zhongmu No.1 cultivar were grown under standard greenhouse conditions. Stem cuttings were rooted in vermiculite for 14 days and then transferred to 15 cm diameter pots containing pasteurized soil. We set conditions as follows: vermiculite (1:1) mixture with a photoperiod of 16 h of light and 8 h of darkness, day/night temperature of 25 ± 2 °C, and water as needed. Plants were divided into two groups: one harvested at the branching stage and the other harvested during the first flowering stage. During leaf harvesting, new and old leaves were differentiated by selecting leaves more than two nodes down from the stem tip as new and leaves two nodes up from the stem base as old. The stems collected were categorized into those in elongation and those after elongation. All samples were immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
2.2. Identification and Characterization of Cellulose Synthase Genes
The alfalfa (cv. Zhongmu No.1) reference genome sequences and protein database were downloaded from Figshare [27]. The sequences of the Arabidopsis CesA/Csl comprising 10 CesAs and 30 Csls were obtained from the TAIR database (https://www.arabidopsis.org/ (accessed on 11 February 2023)) and subsequently used as search queries in BLAST with a cutoff E-value of 1 × 10−20 against the alfalfa genome. Subsequently, the feature domain of the CesA/Csl family (PF00535 and PF03552) was retrieved from Pfam (http://pfam-legacy.xfam.org/ (accessed on 12 February 2023)) and utilized as a query sequence. The HMMER3 [28] (E value = 1 × 10−10) was then used to query the CesA/Csl genes from the alfalfa genome. The verification of all candidate genes was confirmed utilizing Pfam (http://pfam.xfam.org/ (accessed on 15 February 2023)) and SMART (http://smart.embl-heidelberg.de/ (accessed on 15 February 2023)) to verify that the core domains were present. Then, genes were named following the sequence of their arrangement on the chromosomes. TBtools was used to identify coding sequences (CDSs) [29]. The physicochemical properties of the proteins of CesA/Csl genes were calculated using the ExPASy-ProtParam tool (http://web. expasy.org/protparam/ (accessed on 2 March 2023)).
2.3. Phylogenetic, Chromosomal Location and Synteny Analysis
The MUSCLE software (v5.1) was used to perform multiple sequence alignments with default settings. We constructed a phylogenetic tree from this alignment using the neighbor-joining method with a bootstrap value of 1000, as implemented in MEGA11 [30]. The tree was visualized and annotated in the R package Ggtree [31]. TBtools was utilized to analyze and visualize the chromosomal distribution of identified CesA/Csl family genes as well as the syntenic relationships among M. Sativa, M. Truncatula, and Arabidopsis.
2.4. Gene Structure and Motif Composition Analysis
The alfalfa reference genome (cv. Zhongmu No.1) was used to extract the genome sequences of CesA/Csl genes and predict their exon–intron structures. The conserved motifs of CesA/Csls were predicted using the MEME Suite 5.5.1 (http://meme-suite.org/tools/meme (accessed on 8 March 2023)) with the motif length in the range from 6 to 300 residues and maximum number of motifs to 5 and allowed any number of repetitions [32].
2.5. Promoter Cis-Acting Element Analysis
The putative promoter region was extracted as 2000-bp sequences upstream of the start codon of each CesA/Csl gene and was submitted to the PlantCARE [33] database to predict cis-acting elements.
2.6. Transcriptome Data Analysis
Transcriptome data for six alfalfa tissues (flowers, leaves, elongating stems, pre-elongating stems, nodules, and roots) and for treatments of low temperature (4 °C, 2, 24, and 48 h), drought (400 mM mannitol, 1, 3, and 12 h) or salinity (250 mM NaCl, 1, 3, and 12 h) exposed at the seedling stage were obtained from the SRA database on NCBI (SRP055547, SRP144299, SRP145557) [34,35]. HISAT2 [36] was used to map the alfalfa reference genome (cv. ZhongmuNo.1) and obtain the SAM files. The count value of the transcriptome data that matched the genome data was calculated using featureCounts software (v2.0.1) [37]. Gene expression levels were estimated using the FPKM value obtained through the R package DESeq2 (v1.40) [38].
2.7. qRT-PCR
RNA was extracted from the leaf and stem at different growing stages using the Eastep® super total RNA extraction kit (Promega, Shanghai, China). The corresponding cDNA was then obtained using the HiScript® III All-in-one RT Super Mix (Vazyme, Nanjing, China). The NCBI Primer-BLAST tool was utilized to design primers for the CesA/Csl genes. The qRT–PCR experiment was performed using the CFX384 Touch Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). cDNA was diluted at a concentration of 100 ng/µL. The qRT–PCR reaction system consisted of 10 µL of Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China), 0.2 mM of each primer, and 2 µL of template in a volume of 20 µL. The primers used to amplify each gene are shown in Table S1. The gene expression of MsActin was used for data normalization. Relative gene expression levels were calculated using the 2−∆∆t method [39]. Three replicates were designed for each experiment. Post hoc Duncan’s multiple range test for multiple comparisons was used for analysis of variance (ANOVA).
3. Results
3.1. Basic Information on the CesA/Csl Genes in Alfalfa
In all, 37 CesA and Csl genes were identified in alfalfa. Table 1 shows that the protein sequences varied in length, ranging from 371 aa (MsCslB1) to 1572 aa (MsCesA1). Likewise, the protein molecular weights range between 41.98 kDa (MsCslB1) and 175.18 kDa (MsCesA1).

Table 1.
The sequence of 37 CesA/Csl genes identified in alfalfa.
The locations of CesA/Csl genes were obtained and mapped to the different chromosomes (Figure 1). The results showed the 37 genes are distributed across the eight chromosomes, mostly on chromosomes 1, 2, 3, and 4. We also found a small cluster on chromosome 7 and at least eight genes on chromosome 1, mostly clustered on the lower arm of the chromosome. Interestingly, we found only a single gene on chromosomes 5 and 6, in the upper arm of each chromosome.
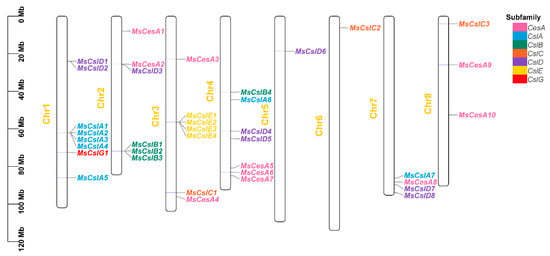
Figure 1.
Chromosomal location of CesA/Csl members in the alfalfa genome. Genes from the same subfamily are shown in the same color, corresponding to the phylogenetic tree of Figure 2.
3.2. Phylogenetic Analysis
In order to investigate the evolutionary relationships among CesA/Csl proteins in alfalfa, we constructed a phylogenetic tree of the 37 genes with 40 CesA/Csl protein sequences obtained from the Arabidopsis database using MEGA11. The cellulose synthase homologues of both species were divided into a single CesA subfamily and six Csl families (i.e., CslA-CslE and CslG, according to their classification in Arabidopsis), as depicted in Figure 2.
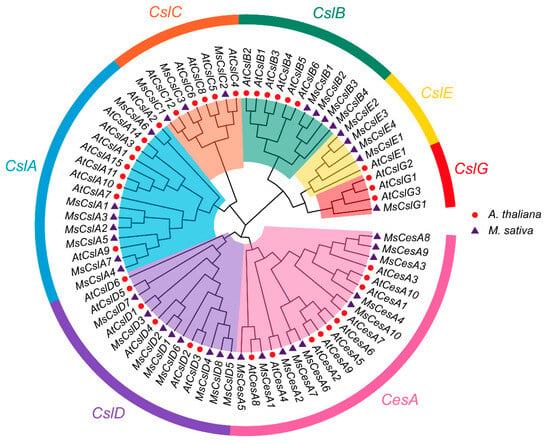
Figure 2.
Phylogenetic relationships and subfamily CesA/Csl protein designations in alfalfa and Arabidopsis.
The 37 CesA/Csl proteins were unevenly distributed among the seven clades, indicating that they experienced dynamic changes since they last shared a common ancestor. We found 10 alfalfa CesA, which are essential for cell wall synthesis in Arabidopsis. This indicates CesA members might also be responsible for cell wall synthesis in alfalfa.
3.3. Motifs and Gene Structure
We predicted and confirmed conserved motifs of the CesA/Csl proteins using the MEME software (v5.5.1). Motif 1, including QXXRW, is the most conserved motif and found in most CesA/Csl proteins. Motif 9, also including QXXRW, is expected in CslAs. We found that there are similar motifs present in both CesA and CslD proteins, including motifs 1 to 8 and 10, which have the highest number among all subfamilies (9). The majority of proteins in the CslDs share a comparable motif composition with the CesAs.
The conserved domains of the cellulose synthase are contained within the 27 proteins belonging to the CesA, CslB, CslD, CslE, and CslG subfamilies (PLN02638, PLN02400, PLN02915, PLN02915, PLN02436, PLN2189, PLN2248, PLN02190, and PLN02893). The CslA and CslC clades, which were compared, included 10 proteins with GT2 domains (CesA_CaSu_A2 and Glyco_tranf_GTA_type) (Figure S1). The CslA and CslC clades, which had a similar motif composition, had a distinct composition from other subfamilies (Figure 3A). Most proteins with similar motif compositions were classified in the same subfamilies and could be responsible for similar functions.
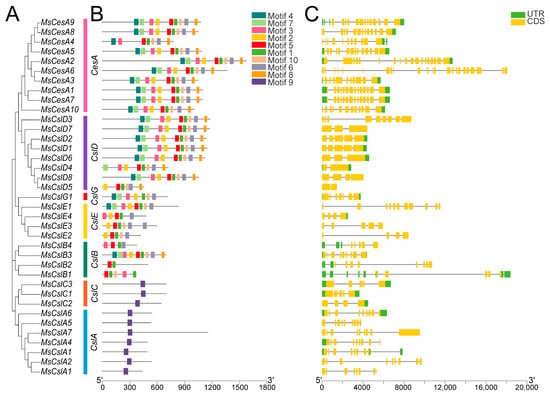
Figure 3.
Structural characteristics of CesA/Csls in alfalfa. (A) Phylogenetic analysis of CesA/Csl proteins. (B) Predicted motifs of CesA/Csl proteins. (C) Exon–intron structures of CesA/Csl genes. UTRs will be displayed as green rectangles, exons will be shown as yellow rectangles, and solid lines will denote introns. Additionally, the length of the respective proteins and genes is indicated by the scale bars.
3.4. Promoter Analysis
The promoter region of each alfalfa CesA/Csl gene was extracted by taking 2000-bp sequences upstream of their start codon. These sequences were submitted to the PlantCARE database to predict cis-acting elements (Figure 4). Assembling the common cis-elements, we found that almost all CesA/Csl gene promoters contain light-responsive elements. This suggests they may be involved in the regulation of the light response regulatory network. We also discovered several stress response elements, such as MREs, MBSs, LTRs, and TC-rich repeats. These elements are related to various stress factors, namely light, drought, cold, defense, and stress responsiveness. In addition, hormone-regulating elements, such as the CGTAC motifs, ABREs, and TCA elements, were also identified and may be associated with MeJA, ABA, and SA response. These results suggest that these factors may affect the transcriptional levels of CesA/Csl.
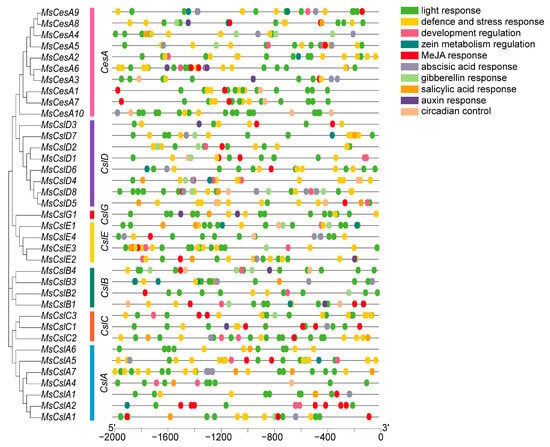
Figure 4.
Analysis of cis-acting elements in CesA/CSL. Boxes filled with different colors represent different functions.
3.5. Collinearity Analysis
In order to gain a deeper understanding of the possible evolutionary events related to the CesA/Csls across various plants, three comparative syntenic maps were constructed for M. sativa, A. thaliana, and M. truncatula (Figure 5). Fifteen collinear pairs of CesA/Csl genes were identified between M. sativa and A. thaliana, and 30 were identified between M. sativa and M. truncatula. The amount of homologous gene pairs between the genes CesA/Csl of M. sativa and M. truncatula was twice that of the homologous gene pairs between M. sativa CesA/Csls and AtCesA/Csls.
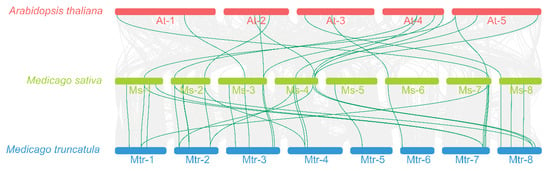
Figure 5.
Collinearity analysis between A. thaliana, M. sativa and M. truncatula. Grey lines in the background indicate collinear blocks within A. thaliana. The green lines of A. thaliana, M. sativa, and M. truncatula highlight syntenic CesA/Csl gene pairs. Chromosomes of these representative plant species are shown in different colors. The species names with the prefixes ‘At’, ‘Ms,’ and ‘Mtr’ represent A. thaliana, M. sativa, and M. truncatula genomes, respectively.
3.6. Analysis of the Expression of CesA/Csl Genes in Various Tissues
We investigated the function of CesA/Csls by analyzing their expression patterns in six tissues from an RNA-seq database. As shown in Figure 6A, 3 CslA genes were not expressed in any of the examined tissues. These genes might be upregulated in other tissues or under specific biotic or abiotic conditions. Other 34 expressed genes showed varying transcript abundance across tissues, suggesting their functions may be different. We also identified two genes (MsCslD2, MsCslD1) expressed in a single tissue, respectively, in stem and root (Figure 6A); a single gene expressed in two different tissues (MsCslB4); and 28 genes expressed in all tissues. These findings indicate that these genes play a crucial role in the growth and development of lucerne. We performed qRT–PCR to further validate their expression patterns at different harvest stages (Figure 6B–E). The MsCesA5 gene was expressed in all tissues but was mostly in the old stem (Figure 6B), in particular in cut alfalfa as compared to flowering one (Figure 6C). We found contrasting results in the case of MsCslD7 (Figure 6D). These results were consistent with transcriptomic data.
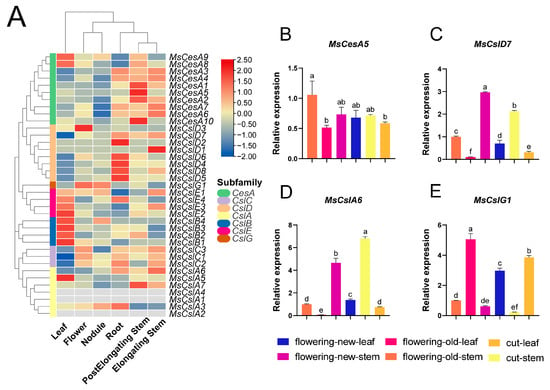
Figure 6.
Expression patterns of CesA/Csls in different alfalfa tissues. (A): Heatmap of the CesA/Csl genes in various tissues (leaf, elongating stem, post-elongating stem, root, flower, and nodule). The Z-score algorithm was used to normalize expression levels by row. The relative expression level is indicated by the color scale on the right side of the heatmap. The gradient from blue to red represents an increase in the level of expression. (B–E): Expression patterns of CesA/Csls in different tissues. Relative expression of target genes in different cuts. Each tissue’s expression level was normalized by expression level of the flowering old stem. The values represent the means ± SEM of three biological replications. Statistically significant differences were indicated by different lowercase letters (p < 0.05).
3.7. Expression Analysis of CesA/Csls under Abiotic Stress
We found CesA/Csl promoter sequences contain several abiotic stress-related elements and thus aimed to understand the possible involvement of the identified CesA/Csl genes in stress response. To achieve this, we analyzed the expression patterns of the identified genes in response to cold, drought, and salt using an RNA-seq database at the alfalfa seeding stage. As shown in Figure 7, we found two CslD genes (MsCslD1, MsCslD2) that were not expressed in any of the treatment and control groups. Under cold stress (Figure 7A), the expression levels of CesA/Csls were observed to form two distinct clusters: one group composed of nine genes with continuously increasing expression and eight genes with increased and then decreased expression; and another group with nine genes with increased and then decreased expression and six genes with a slowly increased expression before reaching equilibrium. In the mannitol treatment (Figure 7B), the genes were divided into two groups: one group containing two genes with sharply decreased expression and fifteen genes that changed slightly before decreasing expression; and another group with eight genes showing fluctuating and then sharply increased expression and seven genes with increased and then decreased expression. Finally, under salt stress (Figure 7C), two distinct clusters were observed in the expression levels of CesA/Csl genes: one group composed of eighteen genes decreasing in expression at different time points and five genes increasing and then decreasing expression slightly; and another group including six genes increasing slowly and then increasing sharply at 24 h and two genes decreasing and then increasing slowly in expression.
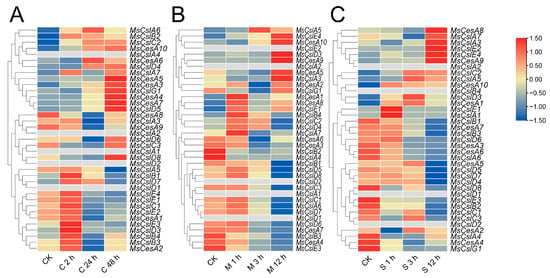
Figure 7.
Expression analysis of CesA/Csl response to different abiotic stresses. (A): cold stress, (B): drought stress, (C): salt stress. The Z-score algorithm was used to normalize expression levels by row. The relative expression level is indicated by the color scale on the right side of the heatmap. Increasing expression levels are represented by a color gradient from blue to red.
We also found two CesA genes (MsCesA5, MsCesA10), one CslG gene (MsCslG1), one CslC gene (MsCslC2) and one CslA gene (MsCslA7) with elevated expression under both cold and drought stress; and two CesA genes (MsCesA9, MsCesA8) and one CslE gene (MsCslE4) with elevated expression in both salt and drought stress.
4. Discussion
The plant CesA/Csl belongs to the glycosyltransferase type 2 (GT2) family, which is widely distributed across plants and participates in cell wall biogenesis by synthesizing cellulose and hemicellulose [22]. The gene families responsible for cellulose synthesis, namely cellulose synthase (CesA) and cellulose synthase-like (Csl), have received extensive research and have been well-documented in various plants, such as rice (Oryza sativa) [40], Arabidopsis (Arabidopsis thaliana) [17], bread wheat [41], cotton (Gossypium hirsutum) [42], and tomato (Solanum lycopersicum) [15]. Although the complete genome sequence for alfalfa is available, there has not been a comprehensive analysis of the whole genome or an extensive study of the CesA/Csl gene family in this species. In this study, we have, for the first time, discovered 37 genes from the alfalfa genome (Table 1). Our study involved basic bioinformatical, phylogenetic, evolutionary, and expression analyses, which verified the crucial contribution of the CesA/Csl genes to alfalfa’s growth, development, and tolerance to stress.
Characterizing the phylogeny of homologs from different plant species has immense potential for predicting gene function. This study’s multiple sequence alignments and neighbor-joining phylogenetic tree construction revealed seven CesA/Csl subfamilies (Figure 2), which is consistent with the number of subfamilies of soybean (Glycine max) studied previously [43]. Of these, ten CesA/Csl genes were clustered as CesA gene family and eight were classified in the CslD gene family, making them the two largest subfamilies of the gene family. The CslDs are is phylogenetically similar to the CesAs, suggesting that the two subfamilies have a shared ancestry, which is in line with previous reports [44]. The CslA and CslC subfamilies are closely related to single-copy gene homologs found in six chlorophyte green algae species that have an evolutionary origin distinct from the other Csl clades [45]. This confirms that the evolution of these genes likely occurred several times independently and led to functional diversification [46].
The analysis of gene structure and replication allows the identification of conserved introns and exons essential for evolution and helps understand the evolution and differentiation of gene family members, their conservation levels, or episodes of functional diversification [47,48,49]. We showed that most CesA/Csl genes have a similar structure across sub-families, suggesting evolutionary conservation (Figure 3). The examination of motifs and conserved domains also revealed strong conservation among the subfamilies in alfalfa. The CesA and CslB, CslD, CslE, and CslG subfamilies, among the most highly conserved, contained the cellulose synthase-conserved domains. In addition, the CslA and CslC subfamilies containing the GT2 domains were distinct from those of the other subfamilies (Figure S1). The genes of the CesA, CslB, CslD, CslE, and CslG subfamilies share similar structures. However, CslA and CslC subfamilies contain a single motif, leading to major structural differences when compared to the other five subfamilies, confirming the findings of previous studies [50,51].
Transcriptional regulation plays the largest role in the activation and repression of expression and is largely controlled by gene promoters and cis-acting elements [52]. We found that a large number of cis-acting elements were discovered in the predicted promoter regions of the CesA/Csl genes, including the light-responsive, several types of stress-responsive, and hormone-regulating elements.
The biosynthesis of cellulose and hemicellulose in the plant cell walls during growth is significantly influenced by CesA/Csl genes. MsCslD2, a member of the CslD clade, was present only in the root and showed tissue-specific expression, while other CslD subfamily genes were highly expressed in the root. This observation implies that CslD is important for root development. In contrast, MsCslD1 is not found in the root but is highly expressed in the elongating stem. Previous studies indicated that CslD proteins may be involved in cellulose synthesis in root hairs and stem growth [53,54,55,56], which is in agreement with our results. In addition, CesA/Csl genes promote plant response to abiotic stress [51,57]. Our RNA-Seq data indicated most of the cellulose synthase family genes appear to respond differently to distinct abiotic stresses (Figure 7). In particular, the CslD genes (MsCslD7, MsCslD6, MsCslD4, MsCslD8, and MsCslD5) are down-regulated in osmotic stress and up-regulated in cold stress. Previous studies addressed this topic by investigating abiotic stress responses. Specifically, AtCslD5 plays a key role in osmotic stress tolerance, a function that may involve regulating ROS under stress [57] and OsCslD4, homologous to the AtCslD5 gene, is involved in salt stress response in rice by mediating abscisic acid (ABA) levels to enhance osmotic stress tolerance [58]. The modulation of cellulose synthesis is likely to have been an important factor in polysaccharide metabolism and plant adaptation to salt stress [59]. Cellulose synthase gene mutations or RNAi interference can reduce cellulose content and increase salt tolerance [59,60]. These processes both lead to an increase in soluble sugars, while drought and salt tolerance can be increased by increasing soluble sugar accumulation in plants [61].
5. Conclusions
In this study, 37 CesA/Csl genes were identified from the alfalfa genome and classified into CesA and 5 Csl subfamilies (CslA, CslB, CslD, CslE, and CslG). Phylogenetic analysis revealed that the MsCesA/Csl gene families exhibit significant homology to that found in in Arabidopsis. Expression pattern analysis showed that the CesA/Csls have distinct expression patterns in different tissues and when experiencing various abiotic stresses. Most CesA/Csl genes were expressed in all tissues while CslDs were primarily expressed in the roots and stems, indicating their possible involvement in cellulose synthesis during growth. In addition, cis-acting element analysis showed that CesA/Csl contain several abiotic stress-related elements. Transcriptomic analysis of alfalfa seedlings under abiotic stress showed 32, 32, and 33 CesA/Csl genes that respond to cold, drought, and salt stress, respectively. The expression levels of the CslD genes were elevated under cold stress and decreased in drought and salt conditions. The subfamily CslD may participate in stress adaptation. These results will aid in identifying the role of MsCesA/Csl in modifying cell wall composition in alfalfa to improve forage quality.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture13091658/s1, Figure S1: Conserved domains of CesA/Csl genes; Table S1: Primers used to amplify CesA/Csl and reference genes using qRT-PCR.
Author Contributions
Conceptualization, B.S., R.L., M.L., Q.Y. and J.K.; methodology, B.S., R.L. and F.H.; investigation, B.S., L.X., Y.L. and Y.X.; original draft, B.S.; review and editing, T.Y. and T.G.; supervision, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Key Projects in Science and Technology of Inner Mongolia (Grant No. 2021ZD0031), Key Research and Development Project of Ningxia Hui Autonomous Region (Grant No. 2022BBF02029), the China Agriculture Research System of MOF and MARA (Grant No. CARS-34), and the Biological Breeding Project (2022ZD04011). The funding body played no role in the design of the study, the collection, analysis and interpretation of the data, or the writing of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon reasonable request, the corresponding author will provide the data supporting the conclusions of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Little, A.; Schwerdt, J.G.; Shirley, N.J.; Khor, S.F.; Neumann, K.; O’Donovan, L.A.; Lahnstein, J.; Collins, H.M.; Henderson, M.; Fincher, G.B.; et al. Revised Phylogeny of the Cellulose Synthase Gene Superfamily: Insights into Cell Wall Evolution. Plant Physiol. 2018, 177, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Kumar, M. Cellulose Synthase Complex Organization and Cellulose Microfibril Structure. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170048. [Google Scholar] [CrossRef] [PubMed]
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2018, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Pear, J.R.; Kawagoe, Y.; Schreckengost, W.E.; Delmer, D.P.; Stalker, D.M. Higher Plants Contain Homologs of the Bacterial CelA Genes Encoding the Catalytic Subunit of Cellulose Synthase. Proc. Natl. Acad. Sci. USA 1996, 93, 12637–12642. [Google Scholar] [CrossRef] [PubMed]
- Arioli, T.; Peng, L.; Betzner, A.S.; Burn, J.; Wittke, W.; Herth, W.; Camilleri, C.; Höfte, H.; Plazinski, J.; Birch, R.; et al. Molecular Analysis of Cellulose Biosynthesis in Arabidopsis. Science 1998, 279, 717–720. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef]
- Nixon, B.T.; Mansouri, K.; Singh, A.; Du, J.; Davis, J.K.; Lee, J.-G.; Slabaugh, E.; Vandavasi, V.G.; O’Neill, H.; Roberts, E.M.; et al. Comparative Structural and Computational Analysis Supports Eighteen Cellulose Synthases in the Plant Cellulose Synthesis Complex. Sci. Rep. 2016, 6, 28696. [Google Scholar] [CrossRef]
- Duncombe, S.G.; Chethan, S.G.; Anderson, C.T. Super-Resolution Imaging Illuminates New Dynamic Behaviors of Cellulose Synthase. Plant Cell 2022, 34, 273–286. [Google Scholar] [CrossRef]
- Kurek, I.; Kawagoe, Y.; Jacob-Wilk, D.; Doblin, M.; Delmer, D. Dimerization of Cotton Fiber Cellulose Synthase Catalytic Subunits Occurs via Oxidation of the Zinc-Binding Domains. Proc. Natl. Acad. Sci. USA 2002, 99, 11109–11114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhugga, K.S.; Gill, K.; Singh, J. Novel Structural and Functional Motifs in Cellulose Synthase (CesA) Genes of Bread Wheat (Triticum Aestivum, L.). PLoS ONE 2016, 11, e0147046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pancaldi, F.; van Loo, E.N.; Schranz, M.E.; Trindade, L.M. Genomic Architecture and Evolution of the Cellulose Synthase Gene Superfamily as Revealed by Phylogenomic Analysis. Front. Plant Sci. 2022, 13, 870818. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.G.; Howells, R.M.; Huttly, A.K.; Vickers, K.; Turner, S.R. Interactions among Three Distinct CesA Proteins Essential for Cellulose Synthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1450–1455. [Google Scholar] [CrossRef]
- Song, X.; Xu, L.; Yu, J.; Tian, P.; Hu, X.; Wang, Q.; Pan, Y. Genome-Wide Characterization of the Cellulose Synthase Gene Superfamily in Solanum Lycopersicum. Gene 2019, 688, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Höfte, H.; Gonneau, M.; Vernhettes, S. Organization of Cellulose Synthase Complexes Involved in Primary Cell Wall Synthesis in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar] [CrossRef]
- Persson, S.; Paredez, A.; Carroll, A.; Palsdottir, H.; Doblin, M.; Poindexter, P.; Khitrov, N.; Auer, M.; Somerville, C.R. Genetic Evidence for Three Unique Components in Primary Cell-Wall Cellulose Synthase Complexes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 15566–15571. [Google Scholar] [CrossRef]
- Yin, Y.; Johns, M.A.; Cao, H.; Rupani, M. A Survey of Plant and Algal Genomes and Transcriptomes Reveals New Insights into the Evolution and Function of the Cellulose Synthase Superfamily. BMC Genom. 2014, 15, 260. [Google Scholar] [CrossRef]
- Marcotuli, I.; Colasuonno, P.; Blanco, A.; Gadaleta, A. Expression Analysis of Cellulose Synthase-like Genes in Durum Wheat. Sci. Rep. 2018, 8, 15675. [Google Scholar] [CrossRef]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant Cell Wall Biosynthesis: Genetic, Biochemical and Functional Genomics Approaches to the Identification of Key Genes. Plant Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [CrossRef]
- Keegstra, K.; Walton, J. β-Glucans—Brewer’s Bane, Dietician’s Delight. Science 2006, 311, 1872–1873. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.A.; Somerville, C.R. The Cellulose Synthase Superfamily. Plant Physiol. 2000, 124, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, K.; Li, Y.; Tu, Y.; Hu, H.; Wang, B.; Cui, X.; Peng, L. Expression Profiling and Integrative Analysis of the CESA/CSL Superfamily in Rice. BMC Plant Biol. 2010, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Suwignyo, B.; Aristia Rini, E.; Helmiyati, S. The Profile of Tropical Alfalfa in Indonesia: A Review. Saudi J. Biol. Sci. 2023, 30, 103504. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Evaluation of the Importance of the Digestibility of Neutral Detergent Fiber from Forage: Effects on Dry Matter Intake and Milk Yield of Dairy Cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Lin, S.; Medina, C.A.; Norberg, O.S.; Combs, D.; Wang, G.; Shewmaker, G.; Fransen, S.; Llewellyn, D.; Yu, L.-X. Genome-Wide Association Studies Identifying Multiple Loci Associated with Alfalfa Forage Quality. Front. Plant Sci. 2021, 12, 648192. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The Chromosome-Level Genome Sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms Provide Genomic Resources for Alfalfa Research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.-Y. Ggtree: An r Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Fu, F.; Bucciarelli, B.; Yang, S.S.; Samac, D.A.; Lamb, J.F.S.; Monteros, M.J.; Graham, M.A.; Gronwald, J.W.; Krom, N.; et al. The Medicago Sativa Gene Index 1.2: A Web-Accessible Gene Expression Atlas for Investigating Expression Differences between Medicago Sativa Subspecies. BMC Genom. 2015, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Deng, H.; Ma, W.; Zhou, Q.; Liu, Z. Genome-Wide Identification of the MADS-Box Transcription Factor Family in Autotetraploid Cultivated Alfalfa (Medicago Sativa L.) and Expression Analysis under Abiotic Stress. BMC Genom. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhu, W.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR Rice Genome Annotation Resource: Improvements and New Features. Nucleic Acids Res. 2007, 35, D883–D887. [Google Scholar] [CrossRef]
- Kaur, S.; Dhugga, K.S.; Beech, R.; Singh, J. Genome-Wide Analysis of the Cellulose Synthase-like (Csl) Gene Family in Bread Wheat (Triticum Aestivum L.). BMC Plant Biol. 2017, 17, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, T.; Dai, D.; Hu, Y.; Guo, X.; Guo, H. Evolution, Gene Expression Profiling and 3D Modeling of CSLD Proteins in Cotton. BMC Plant Biol. 2017, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Rehman, H.M.; Baloch, F.S.; Ijaz, B.; Ali, M.A.; Khan, I.A.; Lee, J.D.; Chung, G.; Yang, S.H. Genome and Transcriptome-Wide Analyses of Cellulose Synthase Gene Superfamily in Soybean. J. Plant Physiol. 2017, 215, 163–175. [Google Scholar] [CrossRef]
- Ermawar, R.A.; Collins, H.M.; Byrt, C.S.; Henderson, M.; O’Donovan, L.A.; Shirley, N.J.; Schwerdt, J.G.; Lahnstein, J.; Fincher, G.B.; Burton, R.A. Genetics and Physiology of Cell Wall Polysaccharides in the Model C4 Grass, Setaria Viridis spp. BMC Plant Biol. 2015, 15, 236. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, J.; Xu, Y. The Cellulose Synthase Superfamily in Fully Sequenced Plants and Algae. BMC Plant Biol. 2009, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Bulone, V.; Schwerdt, J.G.; Fincher, G.B. Co-Evolution of Enzymes Involved in Plant Cell Wall Metabolism in the Grasses. Front. Plant Sci. 2019, 10, 1009. [Google Scholar] [CrossRef]
- Betts, M.J.; Guigó, R.; Agarwal, P.; Russell, R.B. Exon Structure Conservation despite Low Sequence Similarity: A Relic of Dramatic Events in Evolution? EMBO J. 2001, 20, 5354–5360. [Google Scholar] [CrossRef]
- Carmel, L.; Rogozin, I.B.; Wolf, Y.I.; Koonin, E.V. Evolutionarily Conserved Genes Preferentially Accumulate Introns. Genome Res. 2007, 17, 1045–1050. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Liang, Y.; Zhang, Y.; Cheng, X.; Cai, Y. Genome-Wide Characterization of the Cellulose Synthase Gene Superfamily in Pyrus Bretschneideri and Reveal Its Potential Role in Stone Cell Formation. Funct. Integr. Genom. 2020, 20, 723–738. [Google Scholar] [CrossRef]
- Daras, G.; Templalexis, D.; Avgeri, F.; Tsitsekian, D.; Karamanou, K.; Rigas, S. Updating Insights into the Catalytic Domain Properties of Plant Cellulose Synthase (CesA) and Cellulose Synthase-like (Csl) Proteins. Molecules 2021, 26, 4335. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Lin, W.; Deng, B.; Lin, L.; Lv, X.; Hu, Q.; Liu, K.; Fatima, M.; He, B.; et al. Genome-Wide Identification and Adaptive Evolution of CesA/Csl Superfamily among Species with Different Life Forms in Orchidaceae. Front. Plant Sci. 2022, 13, 994679. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and Validation of Promoters and Cis-Acting Regulatory Elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Favery, B.; Ryan, E.; Foreman, J.; Linstead, P.; Boudonck, K.; Steer, M.; Shaw, P.; Dolan, L. KOJAK Encodes a Cellulose Synthase-like Protein Required for Root Hair Cell Morphogenesis in Arabidopsis. Genes Dev. 2001, 15, 79–89. [Google Scholar] [CrossRef]
- Wang, X.; Cnops, G.; Vanderhaeghen, R.; De Block, S.; Van Montagu, M.; Van Lijsebettens, M. AtCSLD3, a Cellulose Synthase-like Gene Important for Root Hair Growth in Arabidopsis. Plant Physiol. 2001, 126, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.J.; Jensen, J.K.; Harholt, J.; Sørensen, S.; Moller, I.; Blaukopf, C.; Johansen, B.; de Lotto, R.; Pauly, M.; Scheller, H.V.; et al. Disruption of ATCSLD5 Results in Reduced Growth, Reduced Xylan and Homogalacturonan Synthase Activity and Altered Xylan Occurrence in Arabidopsis. Plant J. 2007, 52, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Park, S.H.; Je, B.I.; Park, S.H.; Park, S.J.; Piao, H.L.; Eun, M.Y.; Dolan, L.; Han, C. OsCSLD1, a Cellulose Synthase-like D1 Gene, Is Required for Root Hair Morphogenesis in Rice. Plant Physiol. 2007, 143, 1220–1230. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, B.-H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.-K. A Cellulose Synthase-like Protein Is Required for Osmotic Stress Tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Wang, Y.; Wang, J.; Xiao, M.; Liu, H.; Quan, R.; Zhang, H.; Huang, R.; Zhu, L.; et al. Cellulose Synthase-like Protein OsCSLD4 Plays an Important Role in the Response of Rice to Salt Stress by Mediating Abscisic Acid Biosynthesis to Regulate Osmotic Stress Tolerance. Plant Biotechnol. J. 2022, 20, 468–484. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wang, Y.; Xu, F.; Liu, M.; Lin, P.; Ren, S.; Ma, R.; Guo, Y.-D. Knockdown of a Cellulose Synthase Gene BoiCesA Affects the Leaf Anatomy, Cellulose Content and Salt Tolerance in Broccoli. Sci. Rep. 2017, 7, 41397. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, S.; Wu, K.; Ren, Y.; Jiang, H.; Chen, J.; Tao, L.; Fu, X.; Liu, B.; Wu, Y. A Semi-Dominant Mutation in OsCESA9 Improves Salt Tolerance and Favors Field Straw Decay Traits by Altering Cell Wall Properties in Rice. Rice 2021, 14, 19. [Google Scholar] [CrossRef]
- Cao, H.; Guo, S.; Xu, Y.; Jiang, K.; Jones, A.M.; Chong, K. Reduced Expression of a Gene Encoding a Golgi Localized Monosaccharide Transporter (OsGMST1) Confers Hypersensitivity to Salt in Rice (Oryza Sativa). J. Exp. Bot. 2011, 62, 4595–4604. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).