Effects of Nitrogen Fertilization and Plant Density on Proso Millet (Panicum miliaceum L.) Growth and Yield under Mediterranean Pedoclimatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Data Collection and Statistical Analysis
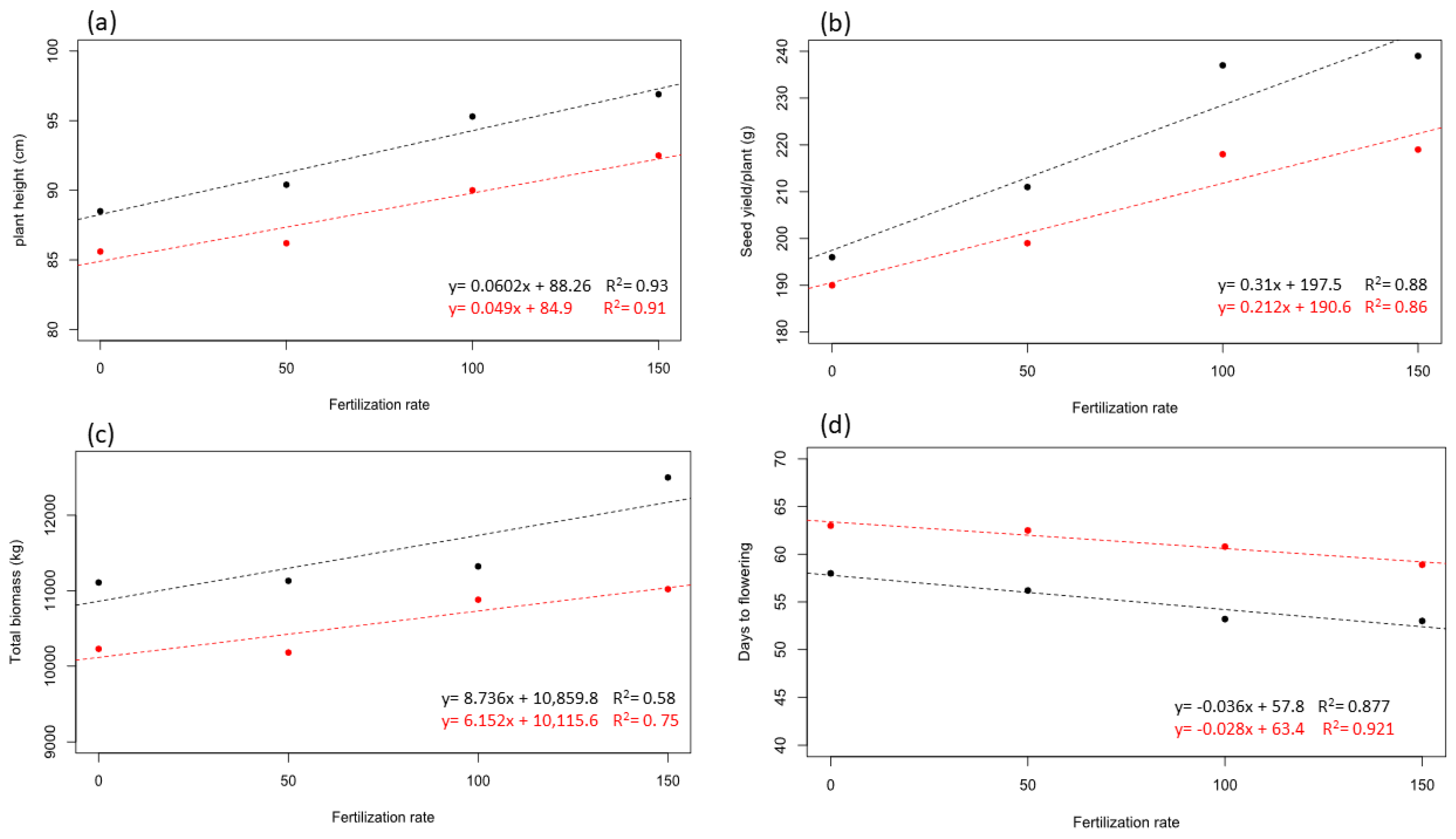
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saruhan, V.; Kuşvuran, A.; Babat, S. The Effect of Different Humic Acid Fertilization on Yield and Yield Components Performances of Common Millet (Panicum miliaceum L.). Sci. Res. Essays 2011, 6, 663–669. [Google Scholar] [CrossRef]
- Crawford, G.W. East Asian Plant Domestication. In Archaeology of Asia; Stark, M.T., Ed.; Blackwell Publishing: Victoria, BC, Canada, 2005; pp. 77–95. ISBN 978-1-4051-0212-4. [Google Scholar]
- De Wet, J.M.J. Origin, Evolution and Systematics of Minor Cereals. In Small Millets in Global Agruculture; Seetharam, A., Riley, K.W., Harinarayana, G., Eds.; Oxford & IBH Publishing Co.: New Delhi, India, 1986; pp. 19–30. [Google Scholar]
- Flajsman, M.; Stajner, N.; Acko, D.K. Genetic Diversity and Agronomic Performance of Slovenian Landraces of Proso Millet (Panicum miliaceum L.). Turk. J. Bot. 2019, 43, 185–195. [Google Scholar] [CrossRef]
- Murage, A.W.; Midega, C.A.O.; Pittchar, J.O.; Pickett, J.A.; Khan, Z.R. Determinants of Adoption of Climate-Smart Push-Pull Technology for Enhanced Food Security through Integrated Pest Management in Eastern Africa. Food Secur. 2015, 7, 709–724. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.; Muthamilarasan, M.; Prasad, M. Millets for next Generation Climate-Smart Agriculture. Front. Plant Sci. 2017, 8, 1266. [Google Scholar] [CrossRef]
- Ventura, F.; Poggi, G.M.; Vignudelli, M.; Bosi, S.; Negri, L.; Fakaros, A.; Dinelli, G. An Assessment of Proso Millet as an Alternative Summer Cereal Crop in the Mediterranean Basin. Agronomy 2022, 12, 609. [Google Scholar] [CrossRef]
- Habiyaremye, C.; Matanguihan, J.B.; Guedes, J.D.; Ganjyal, G.M.; Whiteman, M.R.; Kidwell, K.K.; Murphy, K.M. Proso Millet (Panicum miliaceum L.) and Its Potential for Cultivation in the Pacific Northwest, U.S.: A Review. Front. Plant Sci. 2017, 7, 1961. [Google Scholar] [CrossRef]
- Anderson, R.L.; Bowman, R.A.; Nielsen, D.C.; Vigil, M.F.; Aiken, R.M.; Benjamin, J.G. Alternative crop rotations for the central Great Plains. J. Prod. Agric. 1999, 12, 95–99. [Google Scholar] [CrossRef]
- Baltensperger, D.D. Foxtail and Proso Millet. In Progress in New Crops; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1996; pp. 182–190. ISBN 09-615-02738. [Google Scholar]
- Tadele, Z. Orphan Crops: Their Importance and the Urgency of Improvement. Planta 2019, 250, 677–694. [Google Scholar] [CrossRef]
- Lágler, R.; Gyulai, G.; Humphreys, M.; Szabó, Z.; Horváth, L.; Bittsánszky, A.; Kiss, J.; Holly, L.; Heszky, L. Morphological and Molecular Analysis of Common Millet (P. miliaceum) Cultivars Compared to an ADNA Sample from the 15th Century (Hungary). Euphytica 2005, 146, 77–85. [Google Scholar] [CrossRef]
- Li, X.; Siddique, K.H.M. Future Smart Food: Harnessing the Potential of Neglected and Underutilized Species for Zero Hunger. Matern. Child Nutr. 2020, 16, e13008. [Google Scholar] [CrossRef]
- Saha, D.; Gowda, M.C.; Arya, L.; Verma, M.; Bansal, K.C. Genetic and Genomic Resources of Small Millets. Crit. Rev. Plant Sci. 2016, 35, 56–79. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Alternative Pathways 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- Turgut, I.; Duman, A.; Wietgrefe, G.W.; Acikgoz, E. Effect of Seeding Rate and Nitrogen Fertilization on Proso Millet Under Dryland and Irrigated Conditions. J. Plant Nutr. 2006, 29, 2119–2129. [Google Scholar] [CrossRef]
- Patil, J.V. Millets and Sorghum: Biology and Genetic Improvement, 1st ed.; John Wiley & Sons: West Sussex, UK, 2017; ISBN 9781119123057. [Google Scholar]
- Vetriventhan, M.; Upadhyaya, H.D. Diversity and Trait-Specific Sources for Productivity and Nutritional Traits in the Global Proso Millet (Panicum miliaceum L.) Germplasm Collection. Crop J. 2018, 6, 451–463. [Google Scholar] [CrossRef]
- Boukail, S.; Macharia, M.; Miculan, M.; Masoni, A.; Calamai, A.; Palchetti, E.; Dell’ Acqua, M. Highly Efficient Generation of Bacterial Leaf Blight-Resistant and Transgene-Free Rice Using a Genome Editing and Multiplexed Selection System. BMC Plant Biol. 2021, 2021, 330. [Google Scholar] [CrossRef]
- Vischi, M.; Zorzin, N.; Bernhart, M.; Winkler, J.; Santra, D.; Pappalardo, C.; Marchetti, S. Morphophysiological and Molecular Characterization of Millet (Panicum miliaceum L.) Varieties for Crop Improvement in Western Europe. CABI Agric. Biosci. 2021, 2, 27. [Google Scholar] [CrossRef]
- Gomashe, S.S. Proso Millet, Panicum miliaceum (L.): Genetic Improvement and Research Needs. In Millets and Sorghum: Biology and Genetic Improvement; Patil, J.V., Ed.; John Wiley & Sons: Chennai, India, 2017; pp. 150–169. ISBN 9781119130789. [Google Scholar]
- Shi, J.; Ma, X.; Zhang, J.; Zhou, Y.; Liu, M.; Huang, L.; Sun, S.; Zhang, X.; Gao, X.; Zhan, W. Chromosome conformation capture resolved near complete genome assembly of broomcorn millet. Nat. Commun. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, J.; Ma, H.; Chen, G.; Dang, K.; Yang, P.; Wang, M.; Feng, B. Nitrogen Deficiency Induced a Decrease in Grain Yield Related to Photosynthetic Characteristics, Carbon–Nitrogen Balance and NitrogenUse Efficiency in Proso Millet (Panicum miliaceum L.). Arch. Agron. Soil Sci. 2020, 66, 398–413. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, K.; Luo, Y.; Vus, N.; Feng, B. Plant-Type Characteristics and Seed Yield of Proso Millet (Panicum miliaceum L.) Response to Plant Density. Russ. J. Agric. Socio-Econ. Sci. 2020, 98, 152–160. [Google Scholar] [CrossRef]
- Tan, M.; Olak, H.; Öztaş, T. Effects of Nitrogen Doses on Yield and Some Traits of Proso Millet (Panicum miliaceum L.) in Highlands. J. Adv. Agric. Technol. 2016, 3, 301–304. [Google Scholar] [CrossRef]
- Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Hess, M.; Klose, R.; Lancashire, P.D.; Meier, U.; Stauss, R.; van den Boom, T.; et al. Growth Stages of Mono- and Dicotyledonous Plants; BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Braunschweig, Germany, 2001; p. 158. [Google Scholar]
- AACC. Crude Protein—Improved Kjeldahl Method. In AACC International Approved Methods; AACC: St. Paul, MN, USA, 2002; Volume 46-10. [Google Scholar]
- Ozili, P.K. The Acceptable R-Square in Empirical Modelling for Social Science Research. In Social Research Methodology and Publishing Results: A Guide to Non-Native English Speakers; IGI Global: Hershey, PA, USA, 2023; pp. 134–143. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of Greenhouse Gas Emissions from Crop Production Systems and Fertilizer Management Effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Santra, D.K.; Heyduck, R.F.; Baltensperger, D.D.; Graybosch, R.A.; Nelson, L.A.; Frickel, G.; Nielsen, E. Registration of “Plateau” Waxy (Amylose-Free) Proso Millet. J. Plant Regist. 2015, 9, 41–43. [Google Scholar] [CrossRef]
- Masoni, A.; Calamai, A.; Marini, L.; Benedettelli, S.; Palchetti, E. Constitution of Composite Cross Maize (Zea mays L.) Populations Selected for the Semi-Arid Environment of South Madagascar. Agronomy 2020, 10, 54. [Google Scholar] [CrossRef]
- Di Paolo, E.; Rinaldi, M. Yield Response of Corn to Irrigation and Nitrogen Fertilization in a Mediterranean Environment. Field Crops Res. 2008, 105, 202–210. [Google Scholar] [CrossRef]
- Seghatoleslami, M.J.; Kafi, M.; Majidi, E. Effect of Drought Stress at Different Growth Stages on Yield and Water Use Efficiency of Five Proso Millet (Panicum miliaceum L.) Genotypes. Pak. J. Bot. 2008, 40, 1427–1432. [Google Scholar]
- Bradford, K.J. Water Stress and the Water Relations of Seed Development: A Critical Review. Crop Sci. 1994, 34, 1–11. [Google Scholar] [CrossRef]
- Bidinger, F.R.; Mahalakshmi, V.; Rao, G.D.P. Assessment of Drought Resistance in Pearl Millet [Pennisetum americanum (L.) Leeke]. II* Estimation of Genotype Response to Stress. J. Agric. Res. 1987, 38, 49–59. [Google Scholar] [CrossRef]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different Mechanisms of Adaptation to Cyclic Water Stress in Two South Australian Bread Wheat Cultivars. J. Exp. Bot. 2008, 59, 3327–3346. [Google Scholar] [CrossRef] [PubMed]
- Bidinger, F.R. Field Screening for Drought Tolerance: Principles and Illustrations. In Field Screening for Drought Tolerance in Crop Plants with Emphasis on Rice; Sexena, N.P., O’Toole, J., Eds.; International Crops Research Institute for the Semi-Arid Tropics (ICRISAT): Patanchereu, India, 2002; pp. 109–124. [Google Scholar]
- Craufurdf, P.Q.; Peacock, J.M. Effect of Heat and Drought Stress on Sorghum (Sorgum bicolor). II. Gran Yield. Exp. Agric. 1993, 29, 77–86. [Google Scholar] [CrossRef]
- Sabadin, P.K.; Malosetti, M.; Boer, M.P.; Tardin, F.D.; Santos, F.G.; Guimarães, C.T.; Gomide, R.L.; Andrade, C.L.T.; Albuquerque, P.E.P.; Caniato, F.F. Studying the Genetic Basis of Drought Tolerance in Sorghum by Managed Stress Trials and Adjustments for Phenological and Plant Height Differences. Theor. Appl. Genet. 2012, 124, 1389–1402. [Google Scholar] [CrossRef]
- Kalinova, J.; Moudry, J. Content and Quality of Protein in Proso Millet (Panicum miliaceum L.) Varieties. Plant Foods Hum. Nutr. 2006, 6, 43–47. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the Reliance on Nitrogen Fertilizer for Wheat Production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. The Influence of Temperature and Light Intensity on Grain Growth in Relation to the Carbohydrate and Nitrogen Economy of the Wheat Plant. Neth. J. Agric. Sci. 1977, 25, 182–197. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; et al. Improving Nitrogen Fertilization in Rice by Site-Specific N Management. A Review. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Hassan, S.M.E.; Rahman, M.S.; Hossain, M.F.; Amin, M.R.; Alam, M.M. Evaluation of Planting Density and Nitrogen on the Performance of Kaon (Setaria italica (L.) Beauv.). Pak. J. Biol. Sci. 2000, 3, 1863–1894. [Google Scholar] [CrossRef]
- Sloane, D. Early Vigour—Its Role in Enhancing the Productivity of Wheat Grown in South Australia. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 1999. [Google Scholar]
- He, W.-M.; Zhang, H.; Dong, M. Plasticity in Fitness and Fitness-Related Traits at Ramet and Genet Levels in a Tillering Grass Panicum miliaceum under Patchy Soil Nutrients. Plant Ecol. 2004, 172, 1–10. [Google Scholar] [CrossRef]
- Langseth, W.; Stabbetorp, H. The Effect of Lodging and Time of Harvest on Deoxynivalenol Contamination in Barley and Oats. J. Phytopathol. 1996, 144, 241–245. [Google Scholar] [CrossRef]
- Amanullah; Khattak, R.A.; Khalil, S.K. Plant Density and Nitrogen Effects on Maize Phenology and Grain Yield. J. Plant Nutr. 2009, 32, 246–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Li, J.; Wang, R. Optimum Planting Density Improves Resource Use Efficiency and Yield Stability of Rainfed Maize in Semiarid Climate. Front. Plant Sci. 2021, 12, 752606. [Google Scholar] [CrossRef]
- Agdag, M.; Nelson, L.; Baltensperger, D.; Lyon, D.; Kachman, S. Row Spacing Affects Grain Yield and Other Agronomic Characters of Proso Millet. Commun. Soil Sci. Plant Anal. 2001, 32, 2021–2032. [Google Scholar] [CrossRef]
- Papadopoulos, A.P.; Pararajasingham, S. The Influence of Plant Spacing on Light Interception and Use in Greenhouse Tomato (Lycopersicon esculentum Mill.): A Review. Sci. Hortic. 1997, 69, 1–29. [Google Scholar] [CrossRef]
- Dong, H.; Kong, X.; Li, W.; Tang, W.; Zhang, D. Effects of Plant Density and Nitrogen and Potassium Fertilization on Cotton Yield and Uptake of Major Nutrients in Two Fields with Varying Fertility. Field Crops Res. 2010, 119, 106–113. [Google Scholar] [CrossRef]
- Fahad, S.; Hasanuzzaman, M.; Mukhtar, A.; Ullah, H.; Saeed, M.; Khan, I.A.; Adnan, M. Environment, Climate, Plant and Vegetation Growth, 1st ed.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Tokatlidis, I.S.; Has, V.; Melidis, V.; Has, I.; Mylonas, I.; Evgenidis, G.; Copandean, A.; Ninou, E.; Fasoula, V.A. Maize Hybrids Less Dependent on High Plant Densities Improve Resource-Use Efficiency in Rainfed and Irrigated Conditions. Field Crops Res. 2011, 120, 345–351. [Google Scholar] [CrossRef]
| Properties | Units | Value |
|---|---|---|
| Sand | % | 25.4 |
| Silt | % | 30.1 |
| Clay | % | 44.5 |
| Total organic matter | % | 1.66 |
| Total nitrogen | % | 0.12 |
| Available phosphorous | ppm | 11 |
| pH | 7.1 | |
| Electrical conductivity (EC) | mS cm−1 | 0.154 |
| Cation exchange capacity (CEC) | meq 100 g−1 | 27.46 |
| Ca exchangeable | ppm | 21.25 |
| Mg exchangeable | ppm | 5.17 |
| Na exchangeable | ppm | 0.58 |
| K exchangeable | ppm | 0.46 |
| Morphological Data | Productive Data | Phenological Data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | Basal Tiller (n) | Peduncle Length (mm) | Inflorescence Length (mm) | Seed Yield per Plant (g) | 1000 Seed Weight (g) | Grain Yield (kg) | Total Biomass (kg) | Harvest Index | Protein (%) | Days to Flowering | Days to Maturity | |
| Year (Y) | ** | * | ** | ** | ** | ns | ** | ** | ns | * | ** | ns |
| 2018 | 89.35 b | 5.20 b | 105.85 b | 215.78 b | 206.87 b | 6.46 | 2847 b | 10,565 b | 0.26 | 9.77 b | 62.70 a | 99.1 |
| 2019 | 92.03 a | 5.60 a | 107.71 a | 221.67 a | 220.86 a | 6.85 | 3147 a | 11,525 a | 0.27 | 10.26 a | 55.20 b | 99.4 |
| Plant density (D) | * | * | * | ** | ** | ns | ** | ** | ** | ** | ** | ** |
| D55 | 91.73 a | 6.45 a | 110.04 a | 230.29 a | 236.55 a | 6.77 | 2758 c | 10,559 b | 0.26 c | 10.43 a | 61.10 a | 102.30 a |
| D111 | 90.19 b | 4.91 b | 107.31 b | 215.21 b | 206.73 b | 6.67 | 3020 b | 11,113 ab | 0.27 b | 9.81 b | 57.90 b | 98.80 b |
| D222 | 90.13 b | 4.85 b | 102.98 c | 210.67 c | 198.32 c | 6.49 | 3211 a | 11,464 a | 0.28 a | 9.79 b | 56.30 c | 96.20 c |
| Nitrogen (N) | ** | ** | ** | ** | ** | ns | ** | ** | ** | * | ** | ** |
| N0 | 87.06 b | 3.86 c | 97.39 b | 207.89 c | 193.32 c | 6.46 | 2705 d | 10,668 c | 0.25 c | 9.78 b | 60.50 a | 101.20 a |
| N50 | 88.32 b | 3.75 c | 101.36 b | 215.06 b | 205.33 b | 6.62 | 2896 c | 10,655 c | 0.27 b | 10.03 a | 59.30 a | 98.50 b |
| N100 | 92.67 ab | 6.67 b | 112.50 a | 224.28 a | 227.73 a | 6.71 | 3123 b | 11,101 b | 0.27 b | 10.11 a | 57.10 b | 97.20 b |
| N150 | 94.72 a | 7.34 a | 115.86 a | 227.67 a | 229.08 a | 6.78 | 3263 a | 11,760 a | 0.28 a | 10.14 a | 55.90 b | 95.70 c |
| D × N | ** | ns | ** | ** | ** | ns | ** | ** | ** | ** | ** | ** |
| D × Y | ns | ns | ns | ns | * | ns | * | ** | * | ns | ns | ns |
| N × Y | ns | ns | ns | ns | ns | ns | * | * | ** | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palchetti, E.; Moretta, M.; Calamai, A.; Mancini, M.; Dell’Acqua, M.; Brilli, L.; Armanasco, P.; Masoni, A. Effects of Nitrogen Fertilization and Plant Density on Proso Millet (Panicum miliaceum L.) Growth and Yield under Mediterranean Pedoclimatic Conditions. Agriculture 2023, 13, 1657. https://doi.org/10.3390/agriculture13091657
Palchetti E, Moretta M, Calamai A, Mancini M, Dell’Acqua M, Brilli L, Armanasco P, Masoni A. Effects of Nitrogen Fertilization and Plant Density on Proso Millet (Panicum miliaceum L.) Growth and Yield under Mediterranean Pedoclimatic Conditions. Agriculture. 2023; 13(9):1657. https://doi.org/10.3390/agriculture13091657
Chicago/Turabian StylePalchetti, Enrico, Michele Moretta, Alessandro Calamai, Marco Mancini, Matteo Dell’Acqua, Lorenzo Brilli, Paolo Armanasco, and Alberto Masoni. 2023. "Effects of Nitrogen Fertilization and Plant Density on Proso Millet (Panicum miliaceum L.) Growth and Yield under Mediterranean Pedoclimatic Conditions" Agriculture 13, no. 9: 1657. https://doi.org/10.3390/agriculture13091657
APA StylePalchetti, E., Moretta, M., Calamai, A., Mancini, M., Dell’Acqua, M., Brilli, L., Armanasco, P., & Masoni, A. (2023). Effects of Nitrogen Fertilization and Plant Density on Proso Millet (Panicum miliaceum L.) Growth and Yield under Mediterranean Pedoclimatic Conditions. Agriculture, 13(9), 1657. https://doi.org/10.3390/agriculture13091657










