Morpho-Physiological and Antioxidative Responses of Wheat Seedlings to Different Forms of Selenium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Condition, and Treatment
2.2. Total Se Determination
2.3. Morpho-Physiological Analyses
2.3.1. Seed Germination
2.3.2. Determination of Shoot and Root Biomass
2.3.3. Determination of Photosynthetic Pigment Concentration
2.4. Indicators of Oxidative Stress
2.4.1. Determination of Lipid Peroxidation Level
2.4.2. Determination of Hydrogen Peroxide
2.5. Extraction and Assays of Enzymes
2.6. Statistical Analyses
3. Results
3.1. Se Concentration in Shoots and Roots
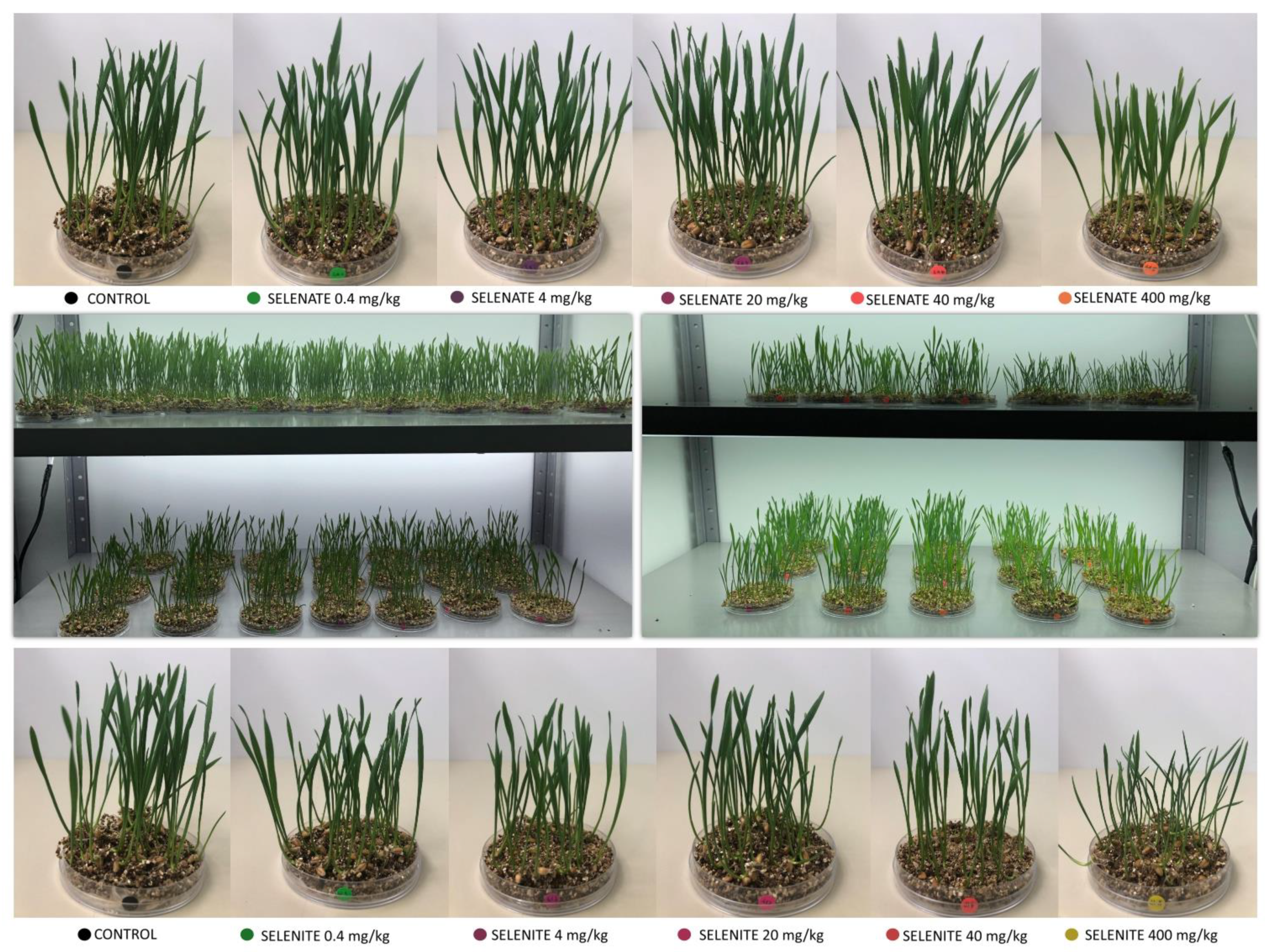
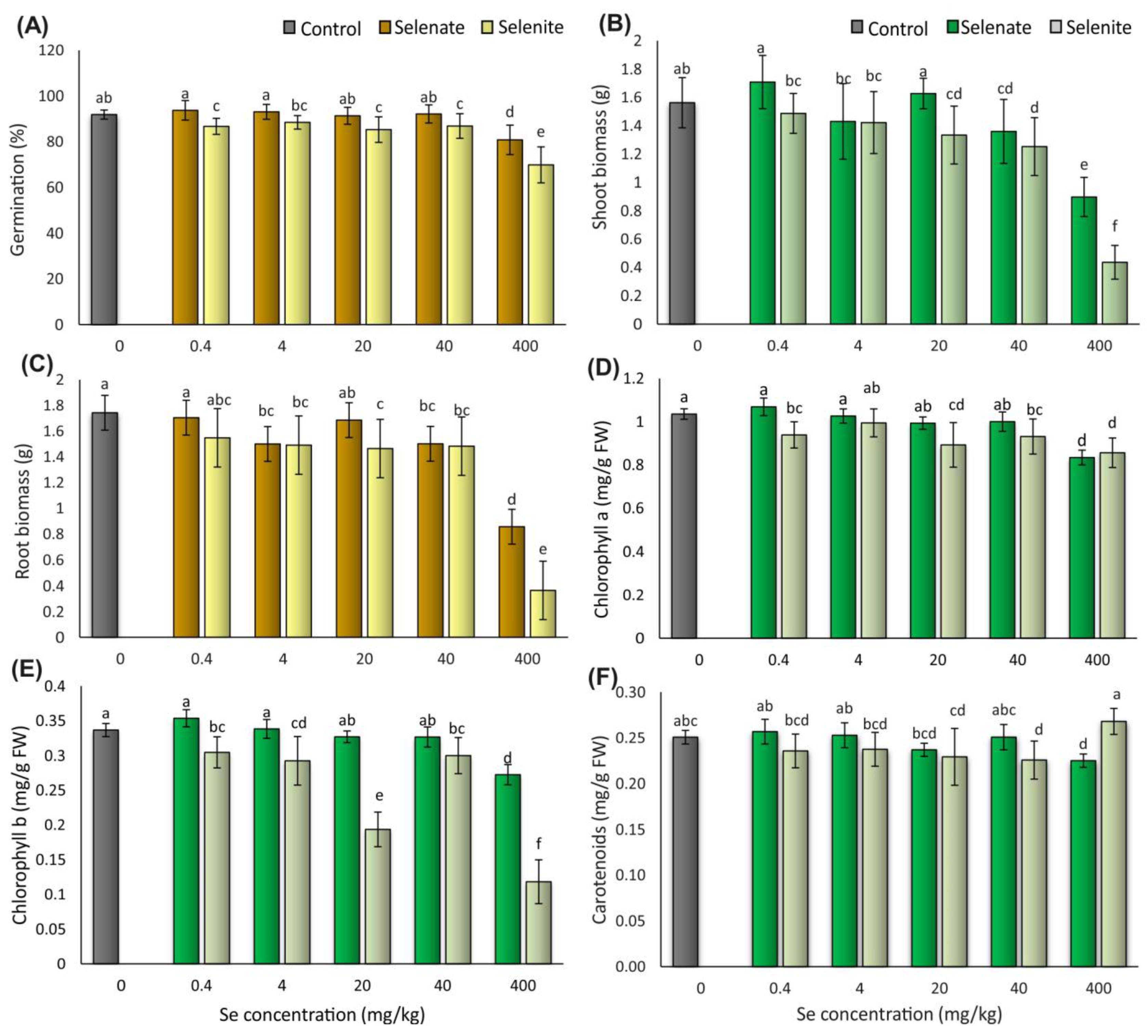
3.2. Morpho-Physiological Traits
3.2.1. Grain Germination
3.2.2. Shoot and Root Biomass
3.2.3. Concentrations of Photosynthetic Pigments
3.3. Indicators of Oxidative Stress
3.3.1. Lipid Peroxidation Levels in Wheat Shoots and Roots
3.3.2. The Concentration of H2O2 in Wheat Shoots and Roots
3.4. Antioxidative Enzyme Activities
3.4.1. Catalase Activity in Wheat Shoots and Roots
3.4.2. Guaiacol Peroxidase Activity in Wheat Shoots and Roots
4. Discussion
4.1. Se Effect on Its Accumulation in Wheat
4.2. Se Effect on Wheat Morpho-Physiological Characteristics
4.3. Se Effect on Wheat Oxidative Status and Antioxidative Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; Sztrik, A.; Elhawat, N.; El-Marsafawy, S.; Shams, M.S. Selenium in soils under climate change, implication for human health. Environ. Chem. Lett. 2015, 13, 1–19. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Zhu, D.; Wang, W.; Liu, H.; Li, J.; Shi, N.; Ma, L.; Fu, S. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Yin, X.A.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ. 2021, 771, 144751. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- Arshad, M.A.; Ebeid, H.M.; Hassan, F.U. Revisiting the Effects of Different Dietary Sources of Selenium on the Health and Performance of Dairy Animals: A Review. Biol. Trace Elem. Res. 2021, 199, 3319–3337. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiaoli, L.; Li, Y.; Shen, R.; Qiangwen, C.; Zhenzhou, Z.; Xin, C.; Weiwei, Z.; Jibao, Y.; Shuiyuan, C.; et al. Combined metabolome and transcriptome analysis reveal the mechanism of selenate influence on the growth and quality of cabbage (Brassica oleracea var. capitata L.). Food Res. Int. 2022, 156, 111135. [Google Scholar]
- Chen, L.; Yang, F.; Xu, J.; Hu, Y.; Hu, Q.; Zhang, Y.; Pan, G. Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food Chem. 2002, 50, 5128–5130. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Pandey, A.; Akkaya, M.S.; Gezgin, S.; Hamurcu, M.; Hakki, E.E. Wheat biofortification—A potential key to human malnutrition. J. Elem. 2017, 22, 937–944. [Google Scholar] [CrossRef]
- Lara, T.S.; Lessa, J.H.D.L.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Galinha, C.; Sánchez-Martínez, M.; Pacheco, A.M.G.; Freitas, M.D.C.; Coutinho, J.; Maçãs, B.; Almeida, A.S.; Pérez-Corona, M.T.; Madrid, Y.; Wolterbeek, H.T. Characterization of selenium-enriched wheat by agronomic biofortification. J. Food Sci. Technol. 2014, 52, 4236–4245. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- De Lima Lessa, J.H.; Araujo, A.M.; Ferreira, L.A.; da Silva Júnior, E.C.; de Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; de Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Štolfa, I.; Velki, M.; Vuković, R.; Ečimović, S.; Katanić, Z.; Lončarić, Z. Effect of different forms of selenium on the plant-soil-earthworm system. J. Plant Nutr. Soil Sci. 2017, 180, 231–240. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Guilherme, L.R.G.; Castro, E.M.; Ávila, F.W.; Carvalho, G.S.; Bastos, C.E.A.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Ximénez-Embún, P.; Alonso, I.; Madrid-Albarrán, Y.; Cámara, C. Establishment of Selenium Uptake and Species Distribution in Lupine, Indian Mustard, and Sunflower Plants. J. Agric. Food Chem. 2004, 52, 832–838. [Google Scholar] [CrossRef]
- Cabannes, E.; Buchner, P.; Hawkesford, M.J. Identification and Sequence Analysis of Sulfate/Selenate Transporters in Selenium Hyper- and Non-accumulating Astragalus Plant Species. In Sulfur Metabolism in Plants; De Kok, L.J., Tabe, L., Tausz, M., Hawkesford, M., Hoefgen, R., McManus, M., Schnug, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–162. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon, M.; Malagoli, M.; Pilon-Smits, E.A.H. Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—A comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front. Plant Sci. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Shrift, A. Exclusion of Selenium from Proteins of Selenium-Tolerant Astragalus Species. Plant Physiol. 1981, 67, 1051–1053. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Siddiqui, M.N.; Fujita, M.; Tran, L.S.P. Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere 2017, 178, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Luo, H.; He, L.; Zhang, L.; Liu, Y.; Mo, Z.; Pan, S.; Tian, H.; Duan, M.; Tang, X. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 2019, 9, 4311. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Wang, J.; Huang, Q.; Aziz, R.; Zhou, W. Dual behavior of selenium: Insights into physio-biochemical, anatomical and molecular analyses of four Brassica napus cultivars. Chemosphere 2019, 225, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Cabral Gouveia, G.C.; Galindo, F.S.; Dantas Bereta Lanza, M.G.; Caroline da Rocha Silva, A.; Pereira de Brito Mateus, M.; Souza da Silva, M.; Rimoldi Tavanti, R.F.; Tavanti, T.R.; Lavres, J.; dos Reis, A.R. Selenium toxicity stress-induced phenotypical, biochemical and physiological responses in rice plants: Characterization of symptoms and plant metabolic adjustment. Ecotoxicol. Environ. Saf. 2020, 202, 110916. [Google Scholar] [CrossRef]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef]
- Prins, C.N.; Hantzis, L.J.; Quinn, C.F.; Pilon-smits, E.A.H. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot. 2011, 62, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; dos Santos, F.L.M.; Putti, F.F.; Furlani, E., Jr.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Blasco, B.; Cervilla, L.M.; Rosales, M.A.; Sanchez-Rodriguez, E.; Romero, L.; Ruiz, J.M. Production and detoxification of H2O2 in lettuce plants exposed to selenium. Ann. Appl. Biol. 2009, 154, 107–116. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Y.; Ma, L.; Jin, X.; Guo, G.; Tan, R.; Liu, Z.; Zheng, L.; Ye, F.; Liu, W. Transcriptome analysis of differentially expressed genes involved in selenium accumulation in tea plant (Camellia sinensis). PLoS ONE 2018, 13, e0197506. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Wójcik, M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012, 147, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant Enzymatic Activities and Growth Response of Quinoa (Chenopodium quinoa Willd) to Exogenous Selenium Application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Łabanowska, M.; Filek, M.; Kościelniak, J.; Kurdziel, M.; Kuliś, E.; Hartikainen, H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings-EPR, enzymatic and fluorescence studies. J. Plant Physiol. 2012, 169, 275–284. [Google Scholar] [CrossRef]
- Akbulut, M.; Çakir, S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings. Plant Physiol. Biochem. 2010, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Ye, S.; Luo, Y.; Zhou, X. Effects of Selenium on Serotonin Synthesis and the Glutathione Redox Cycle in Plum Leaves. J. Soil Sci. Plant Nutr. 2020, 20, 2212–2221. [Google Scholar] [CrossRef]
- Katanić, Z.; Mlinarić, S.; Katanić, N.; Ćosić, J.; Španić, V. Photosynthetic efficiency in flag leaves and ears of winter wheat during fusarium head blight infection. Agronomy 2021, 11, 2415. [Google Scholar] [CrossRef]
- Spanic, V.; Sunic, K.; Duvnjak, J.; Babic, J.; Drezner, G. Winter wheat grain yield response to fungicide application at different stages and fusarium head blight is rather influenced by variety and year. Rom. Agric. Res. 2023, 40, 1–13. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agriculture Experimental Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Galić, L.; Galić, V.; Ivezić, V.; Zebec, V.; Jović, J.; Đikić, M.; Filipović, A.; Manojlović, M.; Almås, Å.R.; Lončarić, Z. Modelling Leverage of Different Soil Properties on Selenium Water-Solubility in Soils of Southeast Europe. Agronomy 2023, 13, 824. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Siegel, B.Z. The Isoperoxidases of Pisum sativum. Plant Physiol. 1967, 42, 221–226. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, W.; Weihong, X.; Chai, Y.; Xie, W.; Chi, S. Effects of selenium on activity of glutathione peroxidase and expression of selenium metabolism-related genes in Brassica. Toxicol. Environ. Chem. 2018, 100, 191–204. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Arvy, M.P. Some factors influencing the uptake and distribution of selenite in the bean plant (Phaseolus vulgaris). Plant Soil 1989, 117, 129–133. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Influence of selenite and selenate on growth, leaf physiology and antioxidant defense system in wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, P.F.; Faquin, V.; Clemente, A.D.C.S.; de Andrade, T.; Guilherme, L.R.G. Genotypic Variation and Biofortification with Selenium in Brazilian Wheat Cultivars. J. Environ. Qual. 2018, 47, 1371–1379. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Geng, M.; Wahid, A.; Ur Rehman, H. Seed priming with selenium: Consequences for emergence, seedling growth, and biochemical attributes of rice. Biol. Trace Elem. Res. 2015, 166, 236–244. [Google Scholar] [CrossRef]
- Moulick, D.; Ghosh, D.; Chandra Santra, S. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef]
- Molnárová, M.; Fargašová, A. Se(IV) phytotoxicity for monocotyledonae cereals (Hordeum vulgare L., Triticum aestivum L.) and dicotyledonae crops (Sinapis alba L., Brassica napus L.). J. Hazard. Mater. 2009, 172, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Lapaz, A.D.M.; Santos, L.F.D.M.; Yoshida, C.H.P.; Heinrichs, R.; Campos, M.; Reis, A.R.D. Physiological and toxic effects of selenium on seed germination of cowpea seedlings. Bragantia 2019, 78, 498–508. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Quinn, C.F.; Pilon-Smits, E.A.H. Effects of selenium hyperaccumulation on plant-plant interactions: Evidence for elemental allelopathy? New Phytol. 2011, 191, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Nithyananthan, S.; Somenath, S.; Sreenadh, B.; Thirunavukkarasu, C.; Othman Bahakim, N.; Shahid, M.; Hassan Abdelzaher, M.; Peer Mohideen, A.; Ramesh, T.; Lokanatha, V. Selenium conditioning decreases antioxidant enzyme activity and delays germination potency of Macrotyloma uniflorum and Vigna radiate. J. King Saud Univ. Sci. 2023, 35, 102501. [Google Scholar] [CrossRef]
- Sreekala, M.; Lalitha, K. Selenium-Mediated Differential Response of 13-Glucosidase and 13-Galactosidase of Germinating Trigonella foenum-graecum. Biol. Trace Elem. Res. 1998, 64, 247–258. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Zhang, Z. Responses of Wheat Seedlings to Exogenous Selenium Supply Under Cold Stress. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual effects of different selenium species on wheat. Plant Physiol. Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Cheema, S.A.; Farooq, M.; Wakeel, A. Selenium nutrition for yield enhancement and grain biofortification of wheat through different application methods. Int. J. Agric. Biol. 2018, 20, 1701–1709. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, F.; Cheng, N.; Chen, P.; Ma, Y.; Zhai, H.; Qi, M.; Liu, N.; Liu, Y. Soil and foliar selenium application: Impact on accumulation, speciation, and bioaccessibility of selenium in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 988627. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Fargašová, A. Toxicity comparison of some possible toxic metals (Cd, Cu, Pb, Se, Zn)on young seedlings of Sinapis alba L. Plant Soil Environ. 2004, 50, 33–38. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, W.; Chai, Y.; Zhou, X.; Zhang, M.; Xie, W. Differences in selenium uptake, distribution and expression of selenium metabolism genes in Tomatoes. Int. J. Agric. Biol. 2017, 19, 528–534. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Qi, M.; Peng, Q.; Wang, M.; Ba, G.S.; Miao, S.; Li, Z.; Toan, Q.; Liang, D. Ecotoxicology and Environmental Safety Insights into uptake, accumulation, and subcellular distribution of selenium among eight wheat (Triticum aestivum L.) cultivars supplied with selenite and selenate. Ecotoxicol. Environ. Saf. 2021, 207, 111544. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Zeka, D.; Fetahu, S.; Rusinovci, I.; Kaul, H.-P. Selenium supply affects chlorophyll concentration and biomass production of maize (Zea mays L.). J. Land Manag. Food Environ. 2018, 69, 249–255. [Google Scholar] [CrossRef]
- Cartes, P.; Gianfreda, L.; Mora, M.L. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 2005, 276, 359–367. [Google Scholar] [CrossRef]
- Sindireva, A.; Golubkina, N.; Bezuglova, H.; Fedotov, M.; Alpatov, A.; Erdenotsogt, E.; Agnieszka, S. Effects of High Doses of Selenate, Selenite and Nano-Selenium on Biometrical Characteristics, Yield and Biofortification Levels of Vicia faba L. Cultivars. Plants 2023, 12, 2847. [Google Scholar] [CrossRef]
- Tian, M.; Hui, M.; Thannhauser, T.W.; Pan, S.; Li, L. Selenium-induced toxicity is counteracted by sulfur in broccoli (Brassica oleracea L. var. italica). Front. Plant Sci. 2017, 8, 1425. [Google Scholar] [CrossRef]
- Hurd-Karrer, A.M. Comparative Toxicity of Selenates and Selenites to Wheat. Am. J. Bot. 1937, 24, 720. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- Coppa, E.; Celletti, S.; Sestili, F.; Mimmo, T.; Dolores, M.; Molina, G.; Cesco, S.; Astolfi, S. Interaction between Sulfate and Selenate in Tetraploid Wheat (Triticum turgidum L.) Genotypes. Int. J. Mol. Sci. 2023, 24, 5443. [Google Scholar] [CrossRef]
- Lyi, S.M.; Heller, L.I.; Rutzke, M.; Welch, R.M.; Kochian, L.V.; Li, L. Molecular and biochemical characterization of the selenocysteine Se-methyltransferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiol. 2005, 138, 409–420. [Google Scholar] [CrossRef]
- Boldrin, P.F.; de Figueiredo, M.A.; Yang, Y.; Luo, H.; Giri, S.; Hart, J.J.; Faquin, V.; Guilherme, L.R.G.; Thannhauser, T.W.; Li, L. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol. Plant. 2016, 158, 80–91. [Google Scholar] [CrossRef]
- Dalla Vecchia, F.; Nardi, S.; Santoro, V.; Pilon-Smits, E.; Schiavon, M. Brassica juncea and the Se-hyperaccumulator Stanleya pinnata exhibit a different pattern of chromium and selenium accumulation and distribution while activating distinct oxidative stress-response signatures. Environ. Pollut. 2023, 320, 121048. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Azizi, I.; Esmaielpour, B.; Fatemi, H. Effect of foliar application of selenium on morphological and physiological indices of savory (Satureja hortensis) under cadmium stress. Food Sci. Nutr. 2020, 8, 6539–6549. [Google Scholar] [CrossRef]
- Haghighi, M.; Sheibanirad, A.; Pessarakli, M. Effects of selenium as a beneficial element on growth and photosynthetic attributes of greenhouse cucumber. J. Plant Nutr. 2016, 39, 1493–1498. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of iron homeostasis and use in chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, K.; Prasad, D.D.K.; Prasad, A.R.K. Effect of selenium on chlorophyll biosynthesis in mung bean seedlings. Phytochemistry 1989, 28, 3321–3324. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Hartikainen, H. Association of antioxidative enzymes with the synergistic effect of selenium and UV irradiation in enhancing plant growth. Agric. Food Sci. 2000, 9, 177–186. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xu, H.J.; Liu, T. Effect of Selenium on Ascorbate-Glutathione Metabolism During PEG-induced Water Deficit in Trifolium repens L. J. Plant Growth Regul. 2011, 30, 436–444. [Google Scholar] [CrossRef]
- Filek, M.; Zembala, M.; Hartikainen, H.; Miszalski, Z.; Kornaś, A.; Wietecka-Posłuszny, R.; Walas, P. Changes in wheat plastid membrane properties induced by cadmium and selenium in presence/absence of 2,4-dichlorophenoxyacetic acid. Plant Cell Tissue Organ Cult. 2009, 96, 19–28. [Google Scholar] [CrossRef]
- Gzyl-Malcher, B.; Filek, M.; Brezesinski, G. Mixed DPPC/DPTAP monolayers at the air/water interface: Influence of indolilo-3-acetic acid and selenate ions on the monolayer morphology. Langmuir 2011, 27, 10886–10893. [Google Scholar] [CrossRef]
- Gzyl-Malcher, B.; Filek, M.; Rudolphi-Skórska, E.; Sieprawska, A. Studies of Lipid Monolayers Prepared from Native and Model Plant Membranes in Their Interaction with Zearalenone and Its Mixture with Selenium Ions. J. Membr. Biol. 2017, 250, 273–284. [Google Scholar] [CrossRef]
- Gzyl-Malcher, B.; Filek, M.; Brezesinski, G. Influence of cadmium and selenate on the interactions between hormones and phospholipids. Langmuir 2009, 25, 13071–13076. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- El-Hadary, A.A. Kinetic Studies of Catalase And Peroxidase Enzymes Extracted From Garlic Cloves (Allium sativum L.). Ann. Agric. Sci. Moshtohor 2021, 59, 331–338. [Google Scholar] [CrossRef]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; Reis, A.R. dos Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Chioti, V.; Zervoudakis, G. Is root catalase a bifunctional catalase-peroxidase? Antioxidants 2017, 6, 39. [Google Scholar] [CrossRef]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2001, 51, 51–106. [Google Scholar] [CrossRef]
- Gayatridevi, S.; Jayalakshmi, S.K.; Mulimani, V.H.; Sreeramulu, K. Salicylic acid and salicylic acid sensitive and insensitive catalases in different genotypes of chickpea against Fusarium oxysporum f. sp. ciceri. Physiol. Mol. Biol. Plants 2013, 19, 529–536. [Google Scholar] [CrossRef]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed Cell Death in Plants. New Insights into Redox Regulation and the Role of Hydrogen Peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Dey, J.; Patra, S.; Pothal, D. Changes in the antioxidative enzyme activities and lipid peroxidation in wheat seedlings exposed to cadmium and lead stress. Braz. J. Plant Physiol. 2007, 19, 53–60. [Google Scholar] [CrossRef]
- Mazej, D.; Osvald, J.; Stibilj, V. Selenium species in leaves of chicory, dandelion, lamb’s lettuce and parsley. Food Chem. 2008, 107, 75–83. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković Popović, A.; Štolfa Čamagajevac, I.; Vuković, R.; Matić, M.; Gupta, D.K.; Lončarić, Z. Morpho-Physiological and Antioxidative Responses of Wheat Seedlings to Different Forms of Selenium. Agriculture 2023, 13, 1632. https://doi.org/10.3390/agriculture13081632
Vuković Popović A, Štolfa Čamagajevac I, Vuković R, Matić M, Gupta DK, Lončarić Z. Morpho-Physiological and Antioxidative Responses of Wheat Seedlings to Different Forms of Selenium. Agriculture. 2023; 13(8):1632. https://doi.org/10.3390/agriculture13081632
Chicago/Turabian StyleVuković Popović, Ana, Ivna Štolfa Čamagajevac, Rosemary Vuković, Magdalena Matić, Dharmendra K. Gupta, and Zdenko Lončarić. 2023. "Morpho-Physiological and Antioxidative Responses of Wheat Seedlings to Different Forms of Selenium" Agriculture 13, no. 8: 1632. https://doi.org/10.3390/agriculture13081632
APA StyleVuković Popović, A., Štolfa Čamagajevac, I., Vuković, R., Matić, M., Gupta, D. K., & Lončarić, Z. (2023). Morpho-Physiological and Antioxidative Responses of Wheat Seedlings to Different Forms of Selenium. Agriculture, 13(8), 1632. https://doi.org/10.3390/agriculture13081632





