Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Layers Management
2.2. Sampling Collection
2.3. Serum Biochemistry
2.4. Antioxidant Capacity of Serum and Jejunum
2.5. Jejunal Morphological Observation
2.6. Quantitative Real-Time PCR Analysis
2.7. Cecal Microbial Sequencing
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
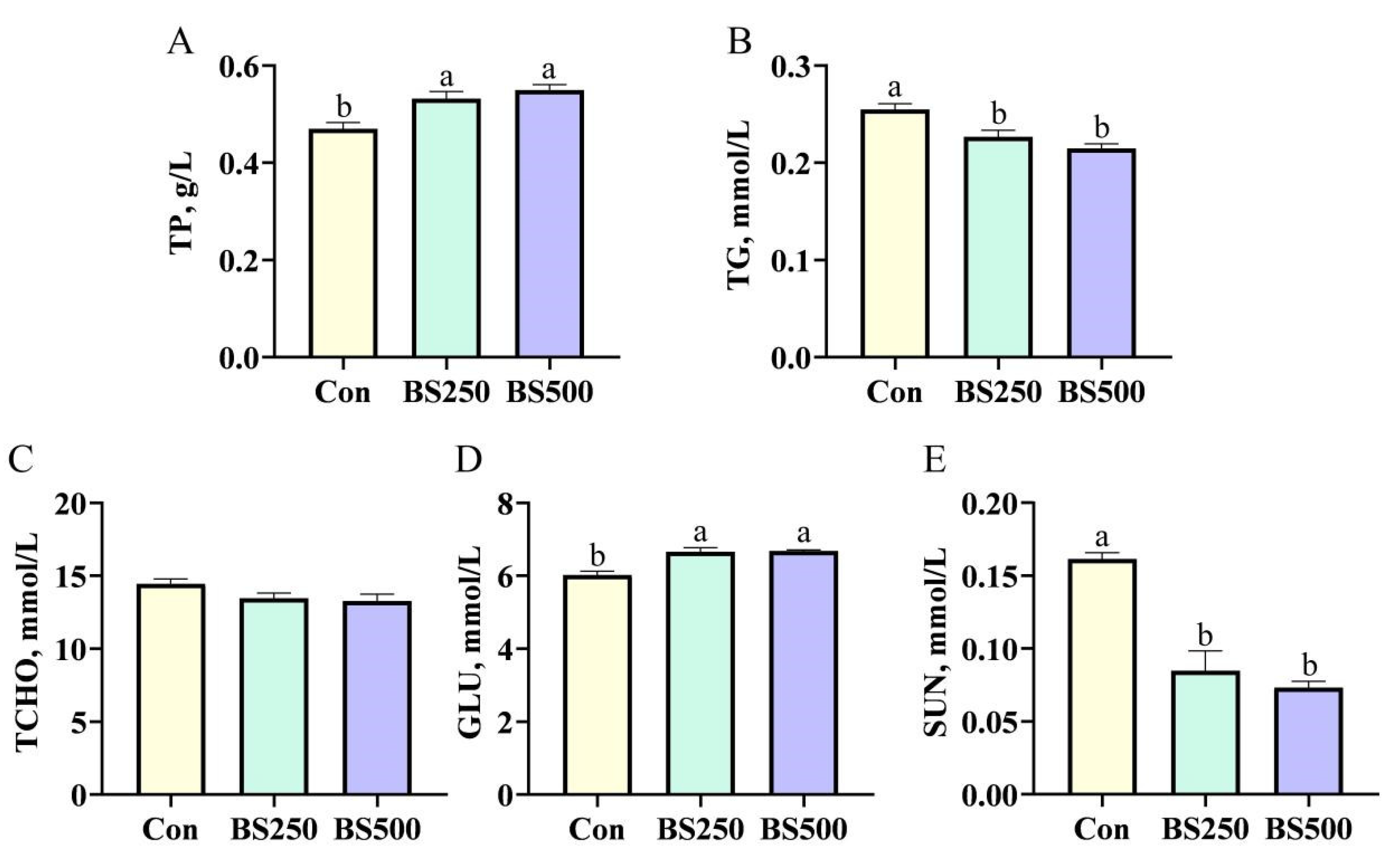
3.2. Serum Biochemistry
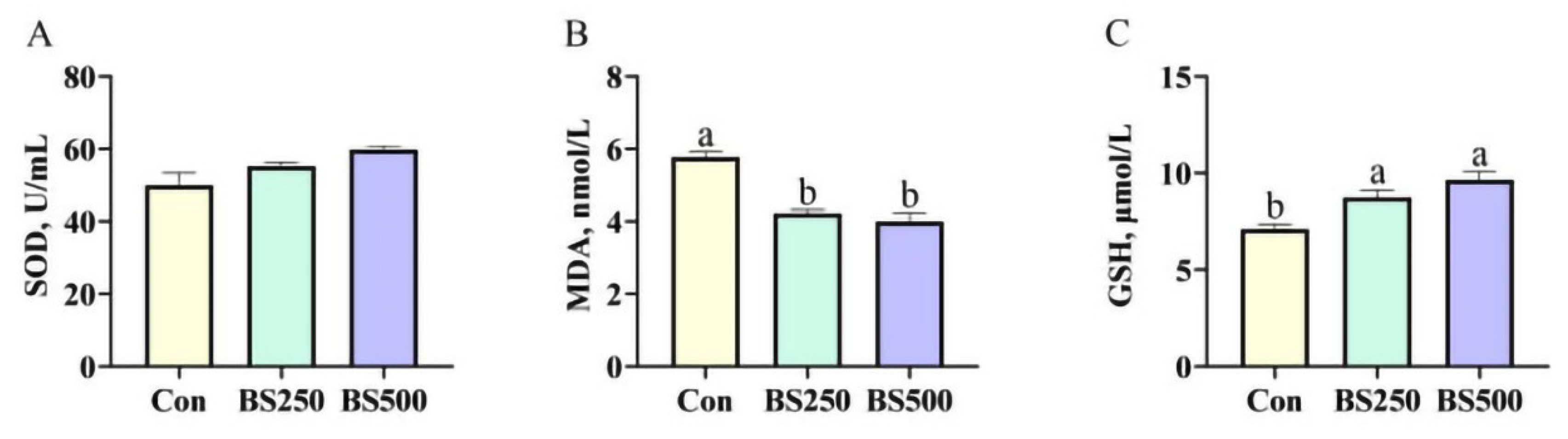
3.3. Serum Antioxidant
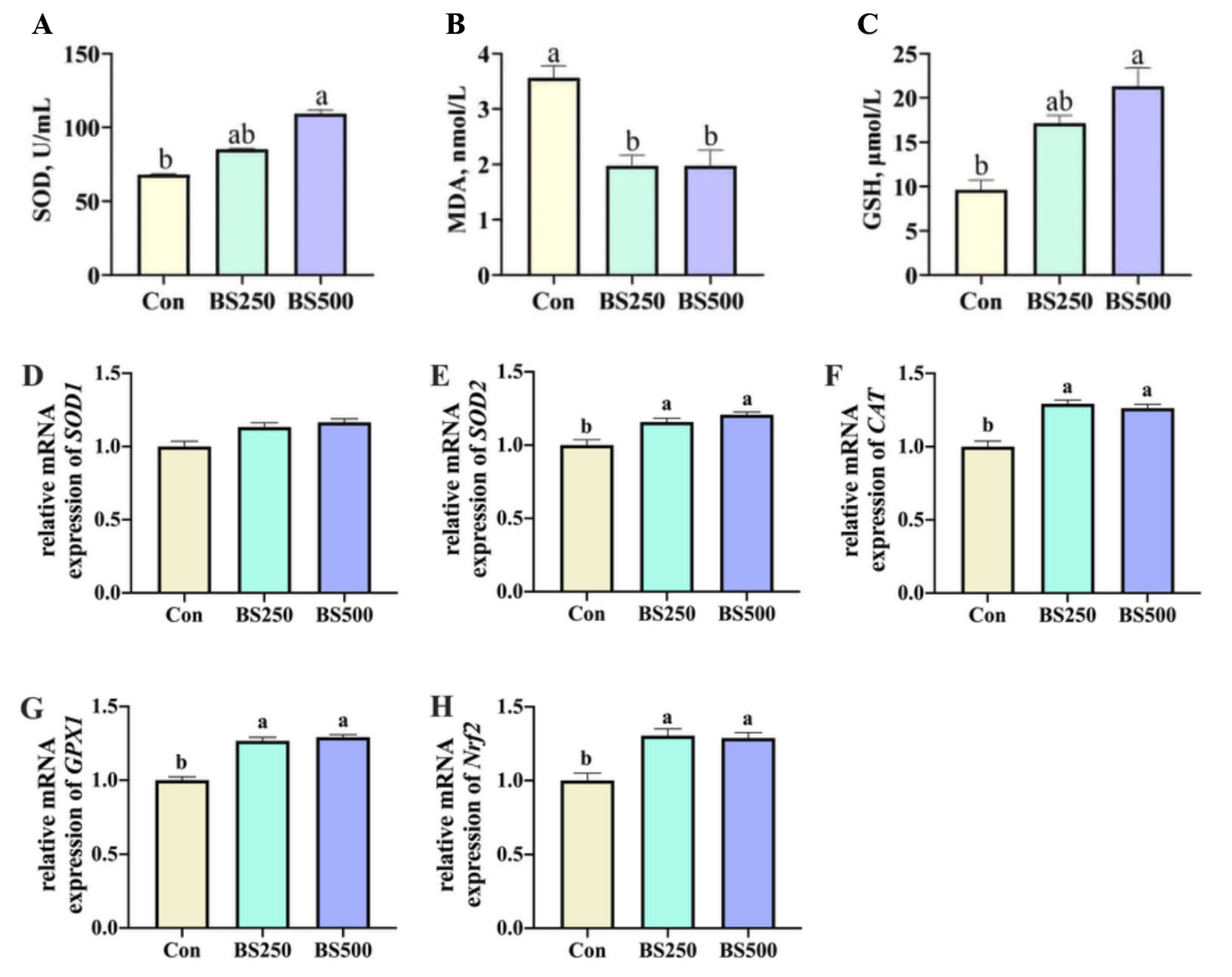
3.4. Jejunum Antioxidant
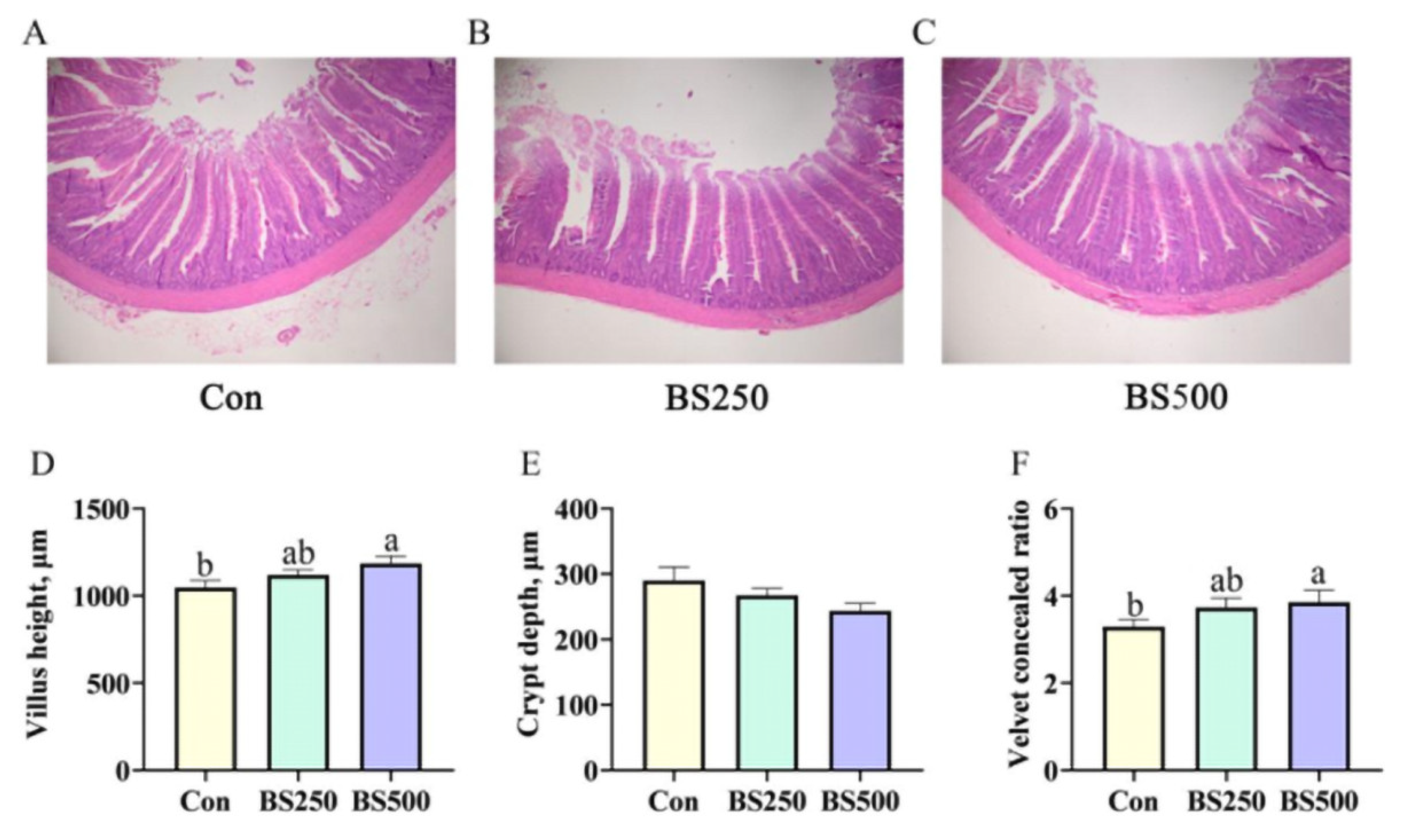
3.5. Jejunum Morphological Observation
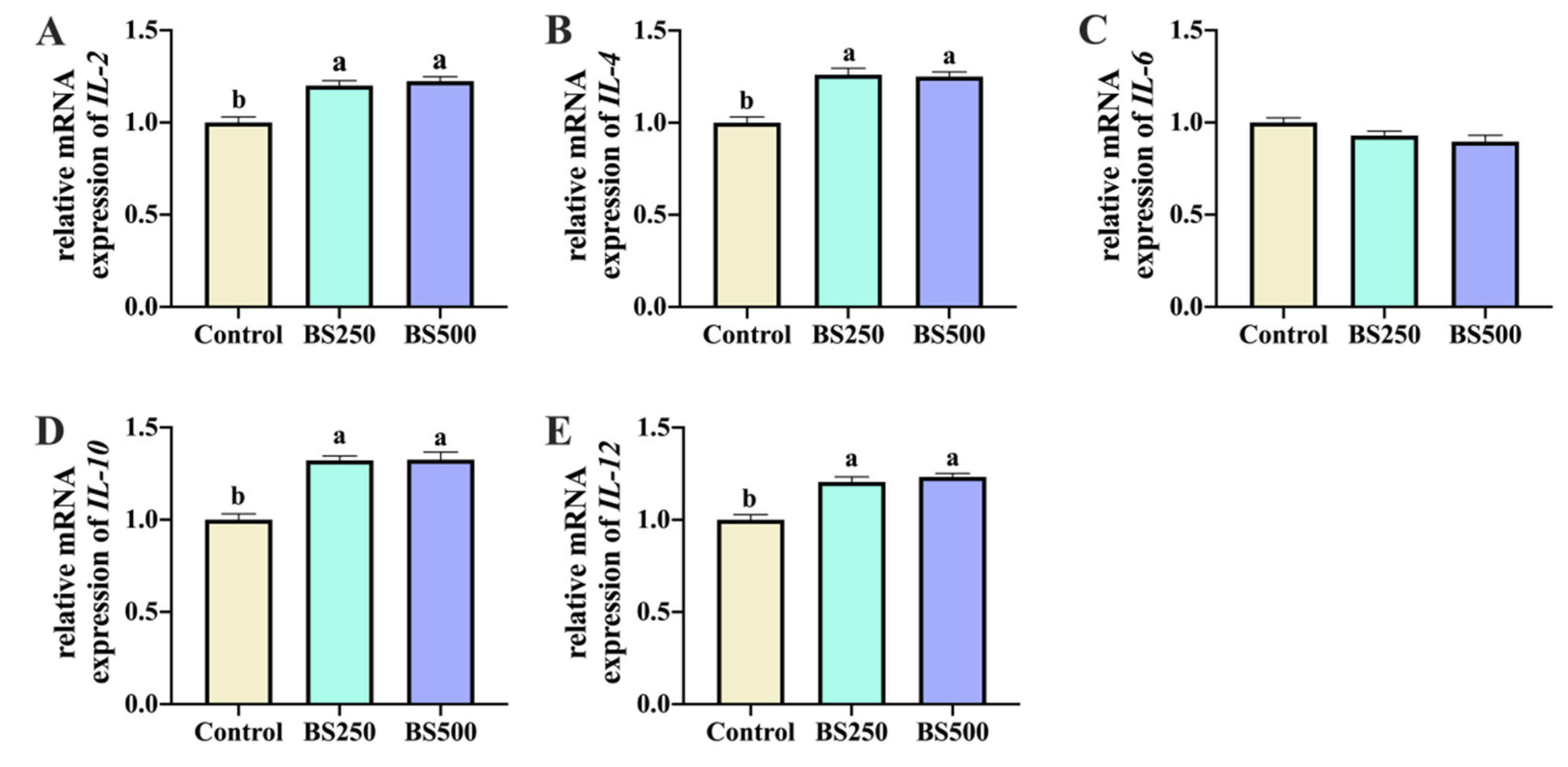
3.6. Jejunum Inflammatory Factor mRNA Expression
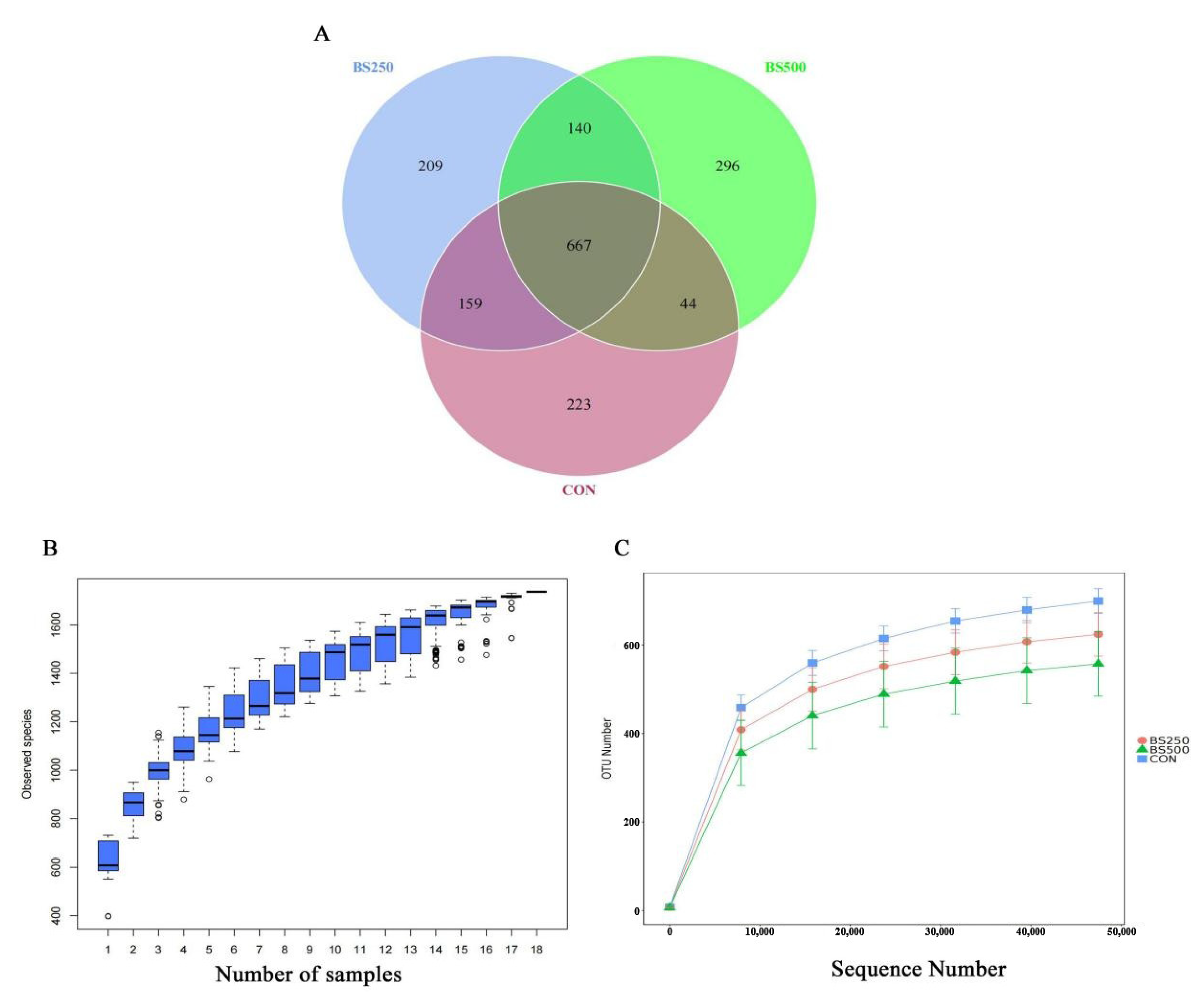
3.7. Assessment of Microbial Diversity in Broilers
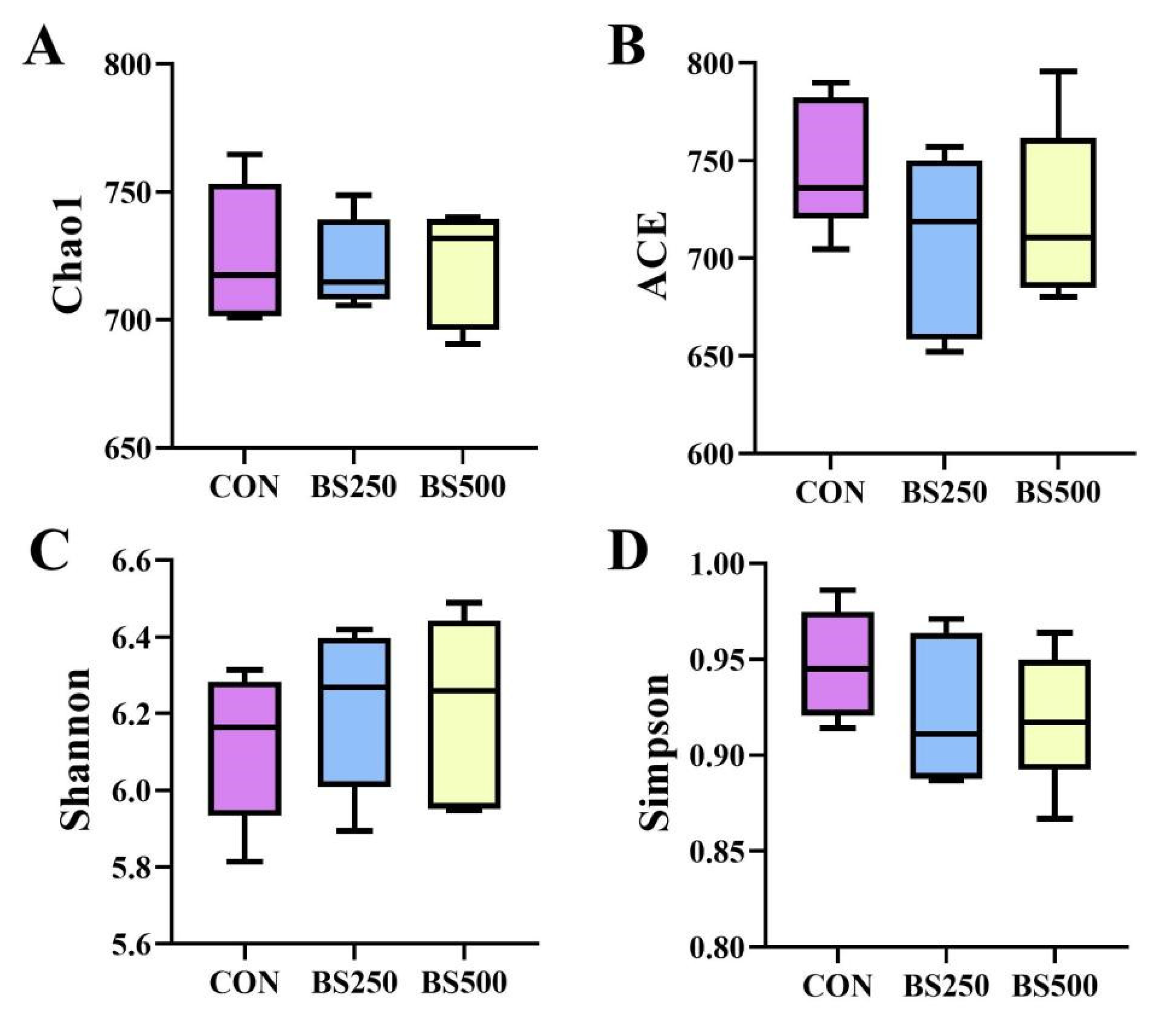
3.8. Alpha Diversity Index
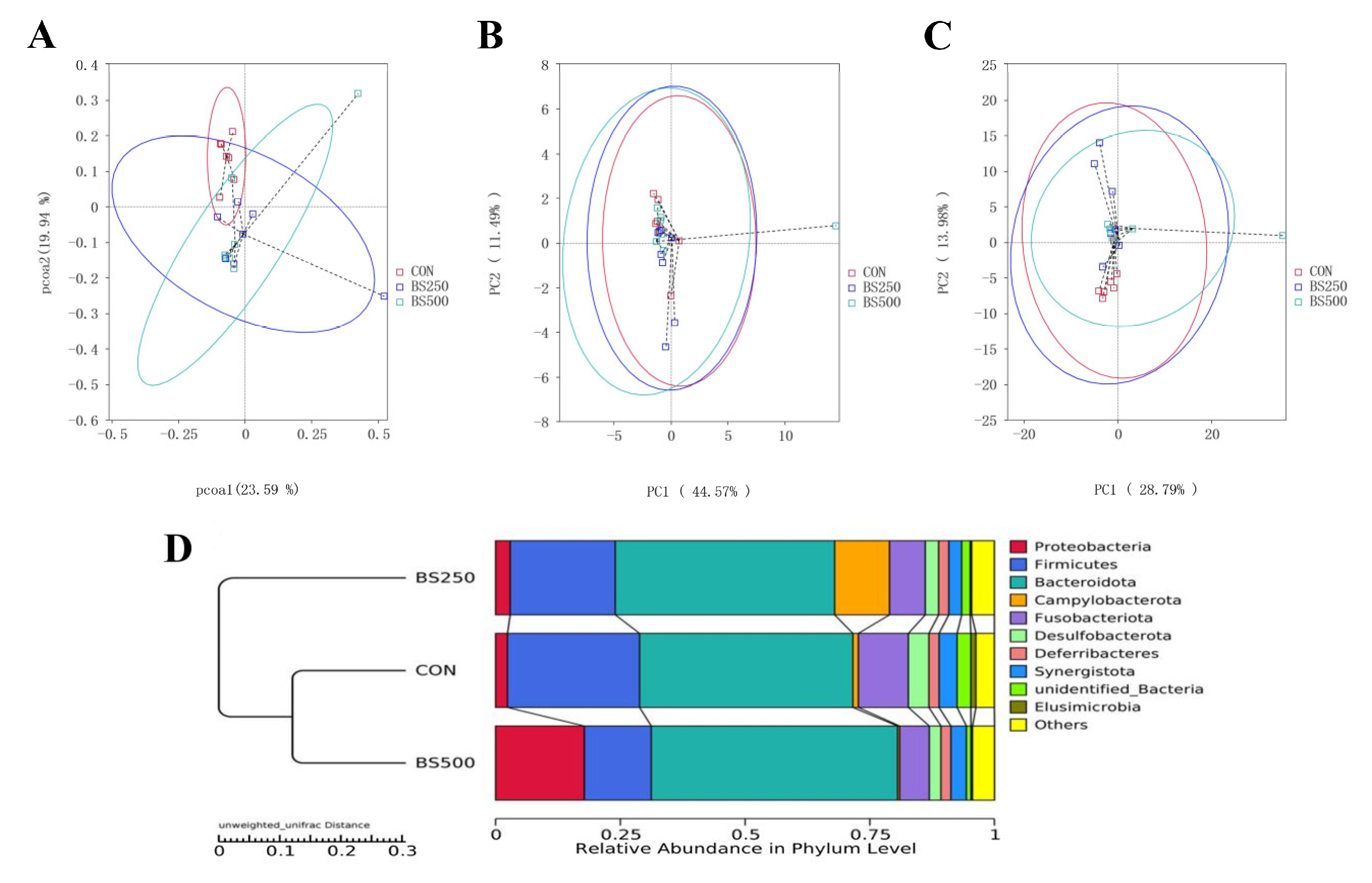
3.9. Beta Diversity Index Analysis
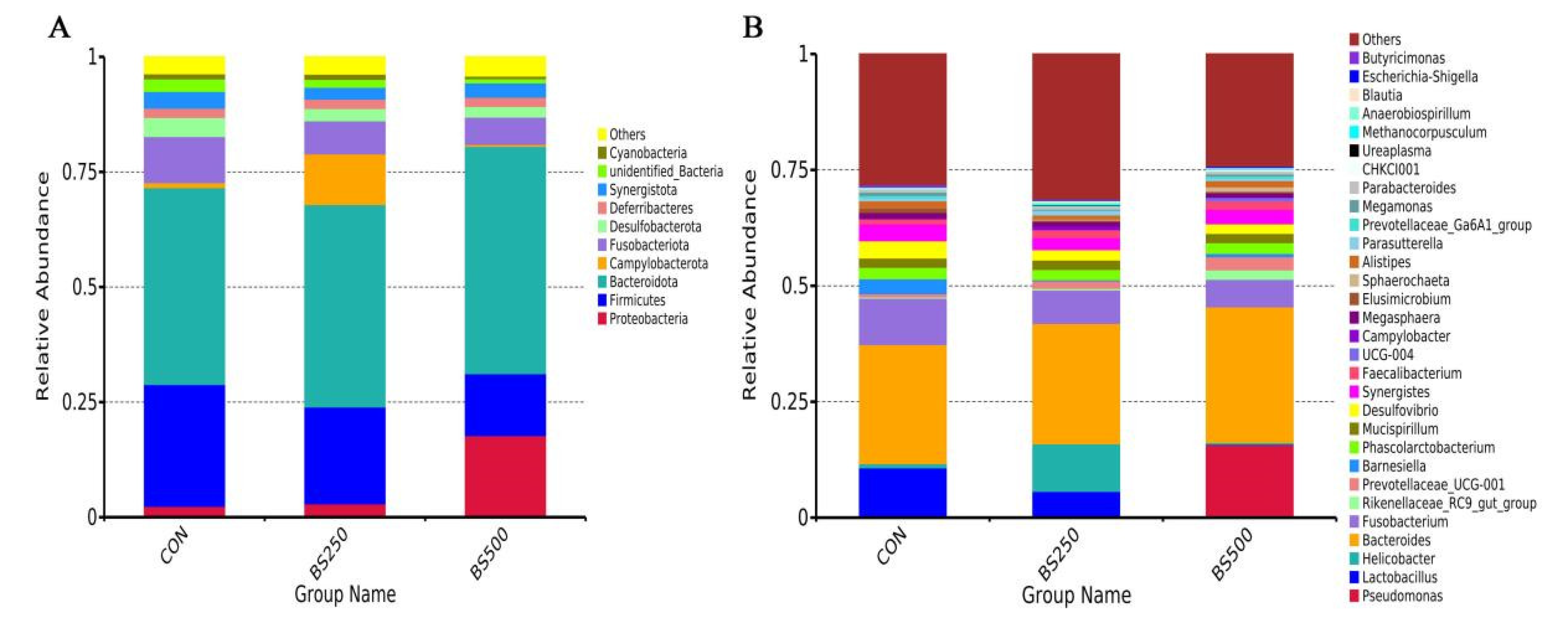
3.10. Species Relative Abundance Column Chart
3.11. Similarity Percentage Analysis
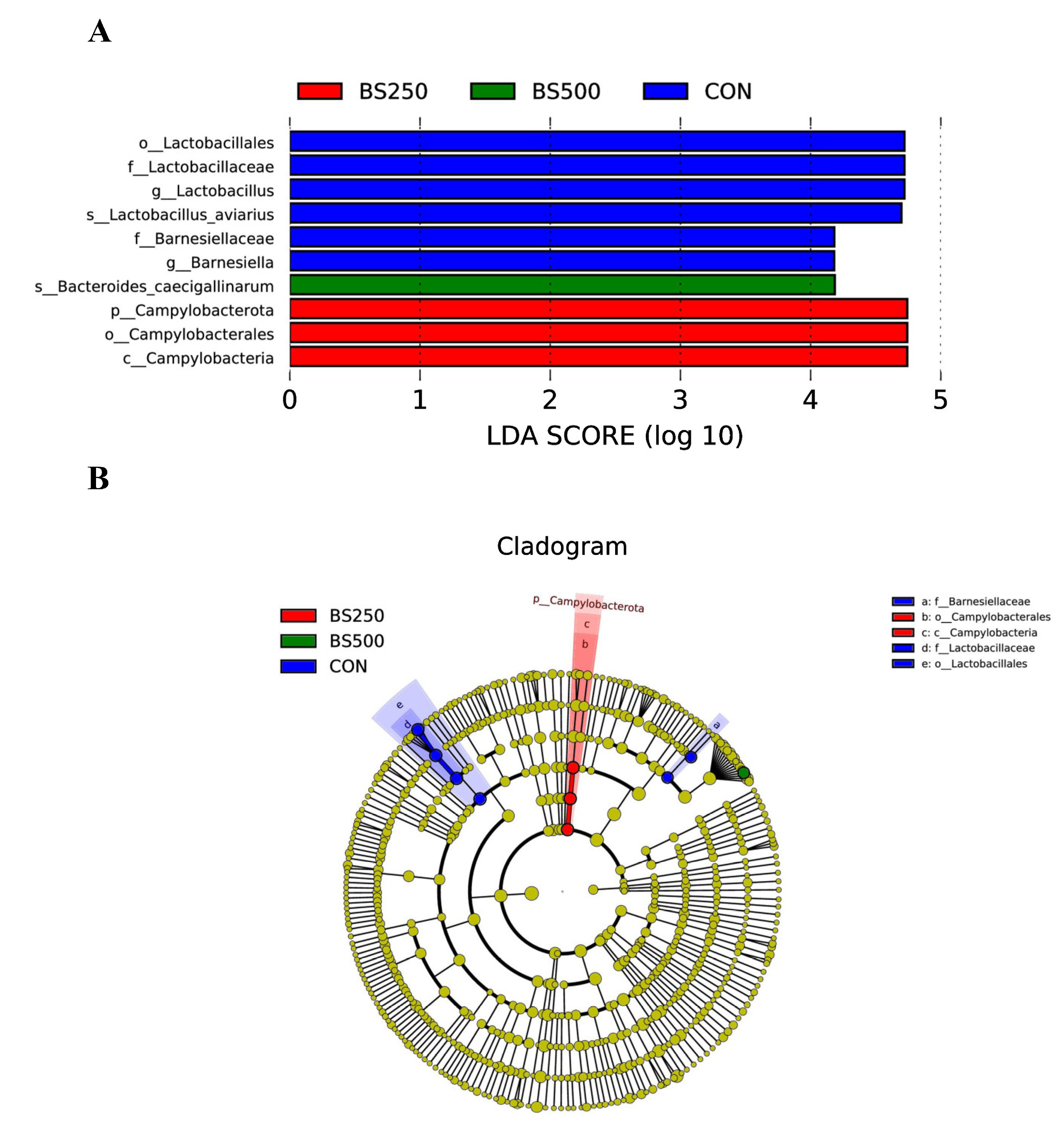
3.12. LDA Effect Size Analysis
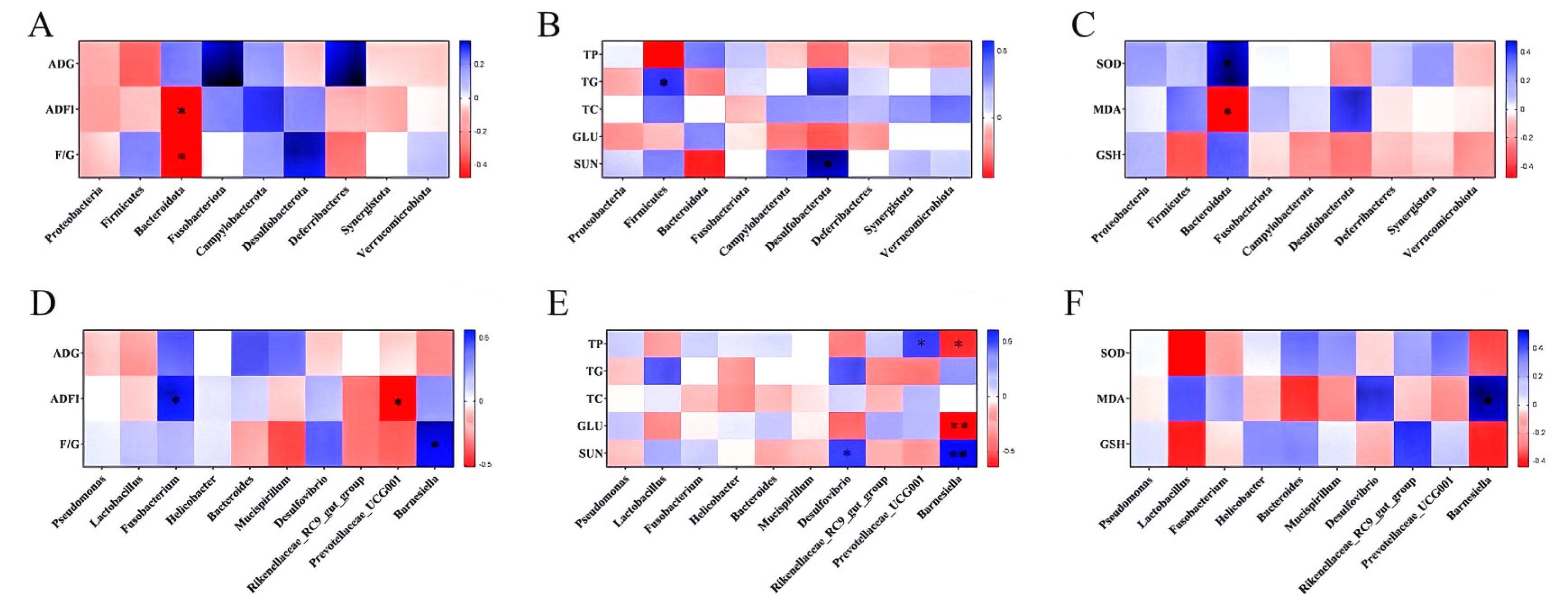
3.13. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 protects intestinal epithelium against Salmonella infection. Front. Cell Infect. Microbiol. 2022, 12, 837886. [Google Scholar]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens-authors’ response. J. Antimicrob. Chemother. 2019, 74, 536–537. [Google Scholar] [CrossRef]
- Selaledi, L.A.; Hassan, Z.M.; Manyelo, T.G.; Mabelebele, M. The current status of the alternative use to antibiotics in poultry production: An African perspective. Antibiotics 2020, 9, 594. [Google Scholar]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Nami, Y.; Haghshenas, B.; Abdullah, N.; Barzegari, A.; Radiah, D.; Rosli, R.; Yari Khosroushahi, A. Probiotics or antibiotics: Future challenges in medicine. J. Med. Microbiol. 2015, 64, 137–146. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães, A.I., Jr.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Du, Y.; Xu, Z.; Yu, G.; Liu, W.; Zhou, Q.; Yang, D.; Li, J.; Chen, L.; Zhang, Y.; Xue, C.; et al. A newly isolated Bacillus subtilis srain named WS-1 inhibited diarrhea and death caused by pathogenic Escherichia coli in Newborn Piglets. Front. Microbiol. 2019, 10, 1248. [Google Scholar] [PubMed]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [PubMed]
- Zhang, L.; Bai, K.; Zhang, J.; Xu, W.; Huang, Q.; Wang, T. Dietary effects of Bacillus subtilis fmbj on the antioxidant capacity of broilers at an early age. Poult. Sci. 2017, 96, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Jeong, J.S.; Lee, S.I.; Kim, I.H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult. Sci. 2016, 95, 2829–2835. [Google Scholar] [CrossRef]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Tsuruta, T.; Nakanishi, N.; Hikita, C.; Mochizuki, M.; Nakayama, K. The preventive effect of Bacillus subtilus strain DB9011 against experimental infection with enterotoxcemic Escherichia coli in weaning piglets. Anim. Sci. J. 2013, 84, 316–321. [Google Scholar] [PubMed]
- Xing, Y.; Wang, S.; Fan, J.; Oso, A.O.; Kim, S.W.; Xiao, D.; Yang, T.; Liu, G.; Jiang, G.; Li, Z.; et al. Effects of dietary supplementation with lysine-yielding Bacillus subtilis on gut morphology, cecal microflora, and intestinal immune response of Linwu ducks. J. Anim. Sci. 2015, 93, 3449–3457. [Google Scholar] [PubMed]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets1. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [PubMed]
- Algburi, A.; Al-Hasani, H.M.; Ismael, T.K.; Abdelhameed, A.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial activity of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against staphylococcus aureus biofilms isolated from wound infection. Probiotics Antimicrob. Proteins. 2021, 13, 125–134. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and function of chicken gut microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Hong, Y.; Cheng, Y.; Li, Y.; Li, X.; Zhou, Z.; Shi, D.; Li, Z.; Xiao, Y. Preliminary study on the effect of Bacillus amyloliquefaciens TL on cecal bacterial community structure of broiler chickens. BioMed Res. Int. 2019, 2019, 5431354. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef]
- Gadde, U.; Oh, S.T.; Lee, Y.S.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. The Effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal lmmune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. 2017, 9, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Rhayat, L.; Jacquier, V.; Brinch, K.S.; Nielsen, P.; Nelson, A.; Geraert, P.A.; Devillard, E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult. Sci. 2017, 96, 2274–2280. [Google Scholar] [CrossRef]
- NY/T 33-2004; Nutrient Requirements of Chinese Feeding Standard of Chicken. The Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004.
- Huntsville, A.L. Arbor Acres Broiler Breeder Manual; Acbor Acres Farm, Inc.: Iron Bridge, ON, Canada, 1996. [Google Scholar]
- Liu, Y.; Wang, Q.; Liu, H.; Niu, J.; Jiao, N.; Huang, L.; Jiang, S.; Guan, Q.; Yang, W.; Li, Y. Effects of dietary Bopu powder supplementation on intestinal development and microbiota in broiler chickens. Front. Microbiol. 2022, 13, 1019130. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Niu, J.; Liu, H.; Jiao, N.; Huang, L.; Jiang, S.; Yan, L.; Yang, W. Effects of dietary Macleaya cordata extract containing isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth performance and liver health of broiler chickens. Front. Vet. Sci. 2022, 9, 950174. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.M.; Yang, C.W.; Jiang, S.J.; Huang, L.B.; Li, Y.; Zhang, F.; Jiao, N.; Yang, W.R. Low level of dietary organic trace elements improve the eggshell strength, trace element utilization, and intestinal function in late-phase laying hens. Front. Vet. Sci. 2022, 9, 903615. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 2012, 25, 682–689. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef]
- Bilal, M.; Achard, C.; Barbe, F.; Chevaux, E.; Ronholm, J.; Zhao, X. Bacillus pumilus and Bacillus subtilis promote early maturation of cecal microbiota in broiler chickens. Microorganisms 2021, 9, 1899. [Google Scholar] [CrossRef]
- Sen, S.; Ingale, S.L.; Kim, Y.W.; Kim, J.S.; Kim, K.H.; Lohakare, J.D.; Kim, E.K.; Kim, H.S.; Ryu, M.H.; Kwon, I.K.; et al. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012, 93, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lillehoj, H.S.; Jang, S.I.; Lee, S.H. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res. Vet. Sci. 2014, 97, 304–308. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Nihira, T.; Suzuki, E.; Kitaoka, M.; Nishimoto, M.; Ohtsubo, K.; Nakai, H. Discovery of β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase involved in the metabolism of N-glycans. J. Biol. Chem. 2013, 288, 27366–27374. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Heinken, A.; Kutt, L.; Ravcheev, D.A.; Bauer, E.; Noronha, A.; Greenhalgh, K.; Jäger, C.; Baginska, J.; Wilmes, P.; et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 2017, 35, 81–89. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019, 9, 1968. [Google Scholar] [CrossRef]
- Medvecky, M.; Cejkova, D.; Polansky, O.; Karasova, D.; Kubasova, T.; Cizek, A.; Rychlik, I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 2018, 19, 561. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.M.; Argolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar]
- Ahmat, M.; Cheng, J.; Abbas, Z.; Cheng, Q.; Fan, Z.; Ahmad, B.; Hou, M.; Osman, G.; Guo, H.; Wang, J.; et al. Effects of Bacillus amyloliquefaciens LFB112 on growth performance, carcass traits, immune, and serum biochemical response in broiler chickens. Antibiotics 2021, 10, 1427. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Liu, W.; Ju, T.; Zhan, X. Effects of different methionine sources on production and reproduction performance, egg quality and serum biochemical indices of broiler breeders. Asian-Australas. J. Anim. Sci. 2017, 30, 828–833. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; et al. Effect of dietary protein on growth performance, and serum biochemical index in late pregnant Hu ewes and their offspring. Anim. Biotechnol. 2023, 34, 97–105. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Selim, D.A.; Basuony, H.A.; Sabic, E.M.; Saleh, A.A.; Ebeid, T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020, 52, 671–680. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
- Müller, J.A.; Galushko, A.S.; Kappler, A.; Schink, B. Initiation of anaerobic degradation of p-cresol by formation of 4-hydroxybenzylsuccinate in desulfobacterium cetonicum. J. Bacteriol. 2001, 183, 752–757. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Unno, T.; Kim, J.M.; Guevarra, R.B.; Nguyen, S.G. Effects of antibiotic growth promoter and characterization of ecological succession in Swine gut microbiota. J. Microbiol. Biotechnol. 2015, 25, 431–438. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, B.G.; Misiakos, E.P.; Fotiadis, C.; Stoidis, C.N. Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Dig. Dis. Sci. 2011, 56, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Wang, L.; He, H.Y.; Chen, J.; Yu, Y.R. Expression of neutrophil gelatinase-associated lipocalin in low osmolar contrast-induced nephropathy in rats and the effect of N-acetylcysteine. Exp. Ther. Med. 2016, 12, 3175–3180. [Google Scholar] [CrossRef][Green Version]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, S.; Li, X.; Yan, T.; Duan, Y.; Yang, X.; Duan, Y.; Sun, Q.; Yang, X. Simultaneous supplementation of Bacillus subtilis and antibiotic growth promoters by stages improved intestinal function of pullets by altering gut microbiota. Front. Microbiol. 2018, 9, 2328. [Google Scholar] [CrossRef]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e919412014. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Renz, H. The mucosal microbiome in shaping health and disease. F1000Prime Rep. 2014, 6, 11. [Google Scholar] [CrossRef]
- Piao, C.S.; Gao, S.; Lee, G.H.; Kim, D.S.; Park, B.H.; Chae, S.W.; Chae, H.J.; Kim, S.H. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial K(ATP) channels. Pharmacol. Res. 2010, 61, 342–348. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Zhu, Y.; Cui, H.; Yang, J.; Lu, M.; Cheng, H.; Gu, L.; Xu, T.; Xu, L. Effect of lycopene on the growth performance, antioxidant enzyme activity, and expression of gene in the Keap1-Nrf2 signaling pathway of arbor acres broilers. Front. Vet. Sci. 2022, 9, 833346. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus subtilis delivery route: Effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021, 100, 100809. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.L.; Wang, J.; Qi, G.H.; Gao, J.; Zhang, H.J.; Wu, S.G. Effects of dietary supplementation with bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, H.P.; He, Y.Y.; Chen, W.L.; Chen, J.W.; Gao, L.; Hu, H.Y.; Wang, J. ffects of rich-polyphenols extract of dendrobium loddigesii on anti-diabetic, anti-inflammatory, anti-oxidant, and gut microbiota modulation in db/db mice. Molecules 2018, 23, 3245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Spatial heterogeneity and Co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front. Microbiol. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, G.; Shao, D.; Wang, Q.; Hu, Y.; Wu, T.; Ji, C.; Shi, S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021, 100, 100935. [Google Scholar]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with campylobacter jejuni infection. Front. Cell Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef]

| Ingredients | 1–14 d | 15–28 d | 29–42 d | Nutrient Levels 2 | 1–14 d | 15–28 d | 29–42 d |
|---|---|---|---|---|---|---|---|
| Corn | 33.60 | 33.90 | 34.20 | ME, MJ/kg | 12.13 | 12.76 | 13.39 |
| Wheat | 29.00 | 29.00 | 29.00 | CP | 22.00 | 21.00 | 20.00 |
| 46% Puffed soybean meal | 23.80 | 21.40 | 19.00 | Ca | 0.96 | 0.9 | 0.84 |
| Cottonseed meal | 4.00 | 4.00 | 4.00 | Phosphorus | 0.66 | 0.61 | 0.56 |
| Corn protein flour | 2.00 | 2.00 | 2.00 | Nonphytate phosphorus | 0.45 | 0.43 | 0.4 |
| Hydrolyzed feather meal | 1.00 | 1.00 | 1.00 | Lysine, % | 1.47 | 1.45 | 1.43 |
| 16.5% Calcium hydrogen phosphate | 0.90 | 0.85 | 0.80 | Methionine, % | 0.59 | 0.55 | 0.51 |
| Limestone | 1.70 | 1.65 | 1.60 | Threonine, % | 0.92 | 0.87 | 0.82 |
| Soybean oil | 2.00 | 4.20 | 6.40 | ||||
| Premix 1 | 2.00 | 2.00 | 2.00 | ||||
| Total | 100 | 100 | 100 |
| Target Genes 2 | Primer Sequence 1 (5′ to 3′) | Product Size | Accession No. |
|---|---|---|---|
| SOD1 | F: CGCAGGTGCTCACTTCAATCC R: CAGTCACATTGCCGAGGTCAC | 89 | NM_205064.2 |
| SOD2 | F: GCTGTATCAGTTGGTGTTCAAGGA R: GCAATGGAATGAGACCTGTTGTTC | 130 | NM_204211.2 |
| CAT | F: GGAGGTAGAACAGATGGCGTATG R: CGATGTCTATGCGTGTCAGGAT | 114 | NM_001031215.2 |
| CAT1 | F: CTCTGGCTTGGTGGTGAACATCT R: CGTGCTTGGCTTGAGGGTAGT | 88 | NM_001145490.2 |
| GPX1 | F: CGGCTTCAAACCCAACTTCAC R: CTCTCTCAGGAAGGCGAACAG | 85 | NM_001277853.3 |
| Keap1 | F: GCATCACAGCAGCGTGGAGAG R: GCGTACAGCAGTCGGTTCAGC | 108 | NC_028739.2 |
| Nrf2 | F: CGCAGAGCACAGATACTTCAA R: CTGGAGAAGCCTCATTGTCATCTA | 109 | NM_001396902.1 |
| IL-2 | F: GCAGTGTTACCTGGGAGAAGT R: GGTGTGATTTAGACCCGTAAGACT | 133 | NM_204153.2 |
| IL-4 | F: GTCTTCCTCAACATGCGTCAG R: CCATTGAAGTAGTGTTGCCTGCT | 93 | NM_001007079.2 |
| IL-6 | F: AACAACCTCAACCTGCCCAAG R: AGGTAGGTCTGAAAGGCGAACA | 116 | NM_204628.2 |
| IL-10 | F: GGGTGAAGTTTGAGGAAATTAAGGA R: TCATCTGTAGAAGCGCAGCA | 148 | NM_001004414.4 |
| IL-12 | F: ATGTCTCACCTGCTATTTGCCTTA R: GTCTCATCGTTCCACTCAGATTCT | 116 | NM_213571.2 |
| β-actin | F: ATTGTCCACCGCAAATGCTTC R: AAATAAAGCCATGCCAATCTCGTC | 113 | NM_205518.1 |
| Items 2 | Con | BS250 | BS500 | SEM 3 | p-Value |
|---|---|---|---|---|---|
| 0–14 d | |||||
| 1 d-LBW, g | 42.62 | 42.77 | 42.38 | 0.334 | 0.905 |
| 14 d-LBW, g | 361.88 b | 385.50 a | 396.27 a | 4.731 | 0.017 |
| ADG, g | 23.54 b | 24.60 a | 24.81 a | 0.206 | 0.015 |
| ADFI, g | 31.30 a | 29.85 a,b | 28.62 b | 0.316 | <0.001 |
| F/G | 1.33 a | 1.21 b | 1.16 b | 0.019 | <0.001 |
| 14–28 d | |||||
| 28 d-LBW, g | 774.66 | 775.51 | 729.01 | 18.070 | 0.514 |
| ADG, g | 64.22 | 64.77 | 64.85 | 0.790 | 0.734 |
| ADFI, g | 119.15 | 118.00 | 116.72 | 1.139 | 0.711 |
| F/G | 1.86 | 1.83 | 1.81 | 0.021 | 0.328 |
| 29–42 d | |||||
| 42 d-LBW, g | 1220.2 | 1152.8 | 1158.4 | 55.832 | 0.875 |
| ADG, g | 65.35 | 66.18 | 66.91 | 1.072 | 0.787 |
| ADFI, g | 157.99 | 152.80 | 149.34 | 2.277 | 0.533 |
| F/G | 2.43 | 2.31 | 2.24 | 0.035 | 0.151 |
| 0–28 d | |||||
| ADG, g | 43.87 | 44.69 | 44.83 | 0.442 | 0.417 |
| ADFI, g | 75.27 | 73.93 | 72.67 | 0.631 | 0.269 |
| F/G | 1.59 a | 1.52 a,b | 1.48 b | 0.016 | <0.001 |
| 0–42 d | |||||
| ADG, g | 51.03 | 51.85 | 52.19 | 0.485 | 0.423 |
| ADFI, g | 102.84 | 100.22 | 98.22 | 0.886 | 0.210 |
| F/G | 1.87 a | 1.78 a,b | 1.73 b | 0.018 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhang, Y.; Lu, H.; Jiao, N.; Jiang, S.; Li, Y.; Li, J.; Yang, W. Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers. Agriculture 2023, 13, 1561. https://doi.org/10.3390/agriculture13081561
Ren X, Zhang Y, Lu H, Jiao N, Jiang S, Li Y, Li J, Yang W. Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers. Agriculture. 2023; 13(8):1561. https://doi.org/10.3390/agriculture13081561
Chicago/Turabian StyleRen, Xiaojie, Yan Zhang, Hai Lu, Ning Jiao, Shuzhen Jiang, Yang Li, Junxun Li, and Weiren Yang. 2023. "Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers" Agriculture 13, no. 8: 1561. https://doi.org/10.3390/agriculture13081561
APA StyleRen, X., Zhang, Y., Lu, H., Jiao, N., Jiang, S., Li, Y., Li, J., & Yang, W. (2023). Effects of Dietary Bacillus subtilis BC02 Supplementation on Growth Performance, Antioxidant Capacity, and Cecal Microbes in Broilers. Agriculture, 13(8), 1561. https://doi.org/10.3390/agriculture13081561






