Parameterization of the Response Function of Sesame to Drought and Salinity Stresses
Abstract
1. Introduction
2. Materials and Methods
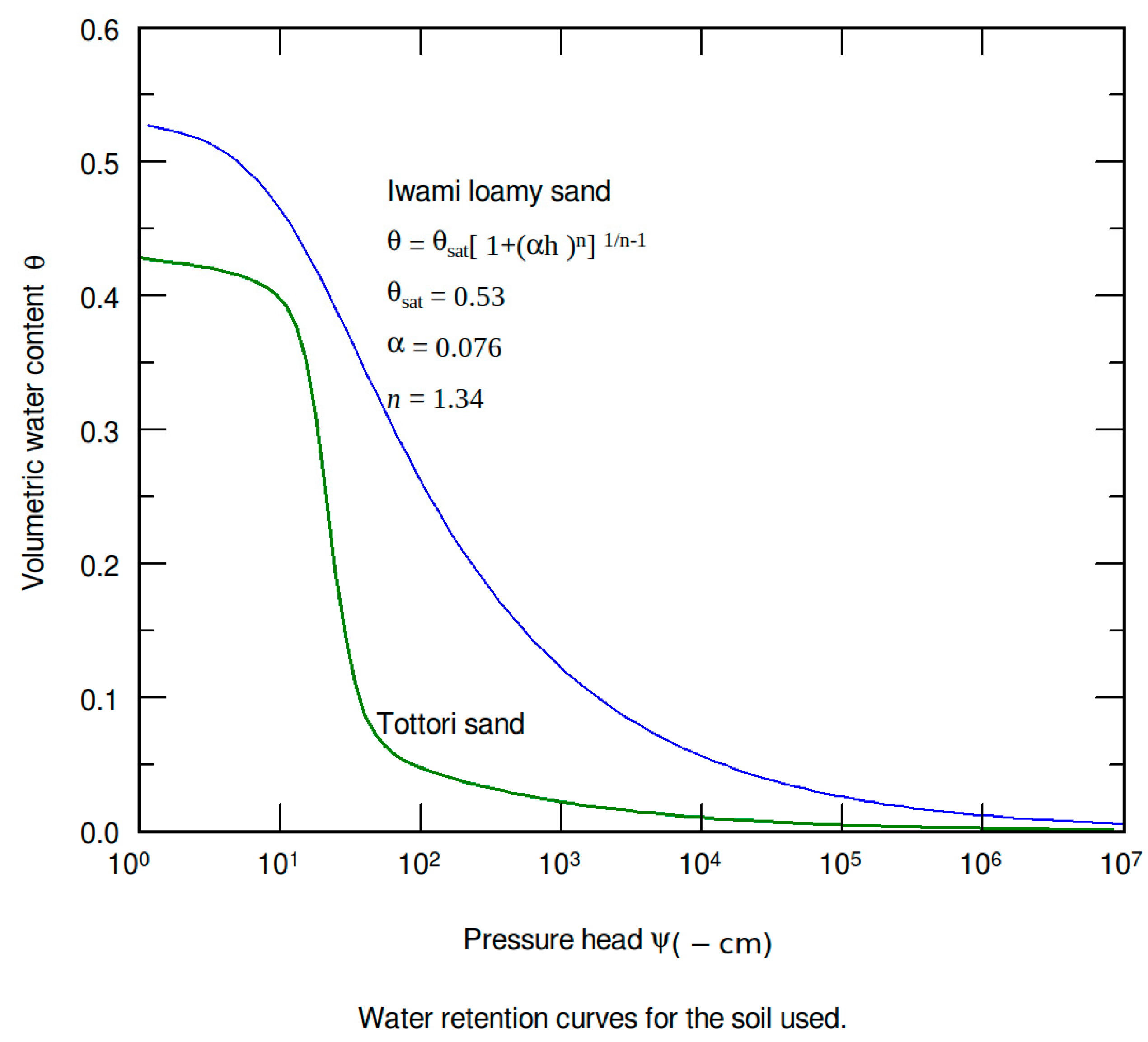
2.1. Crop Response Function
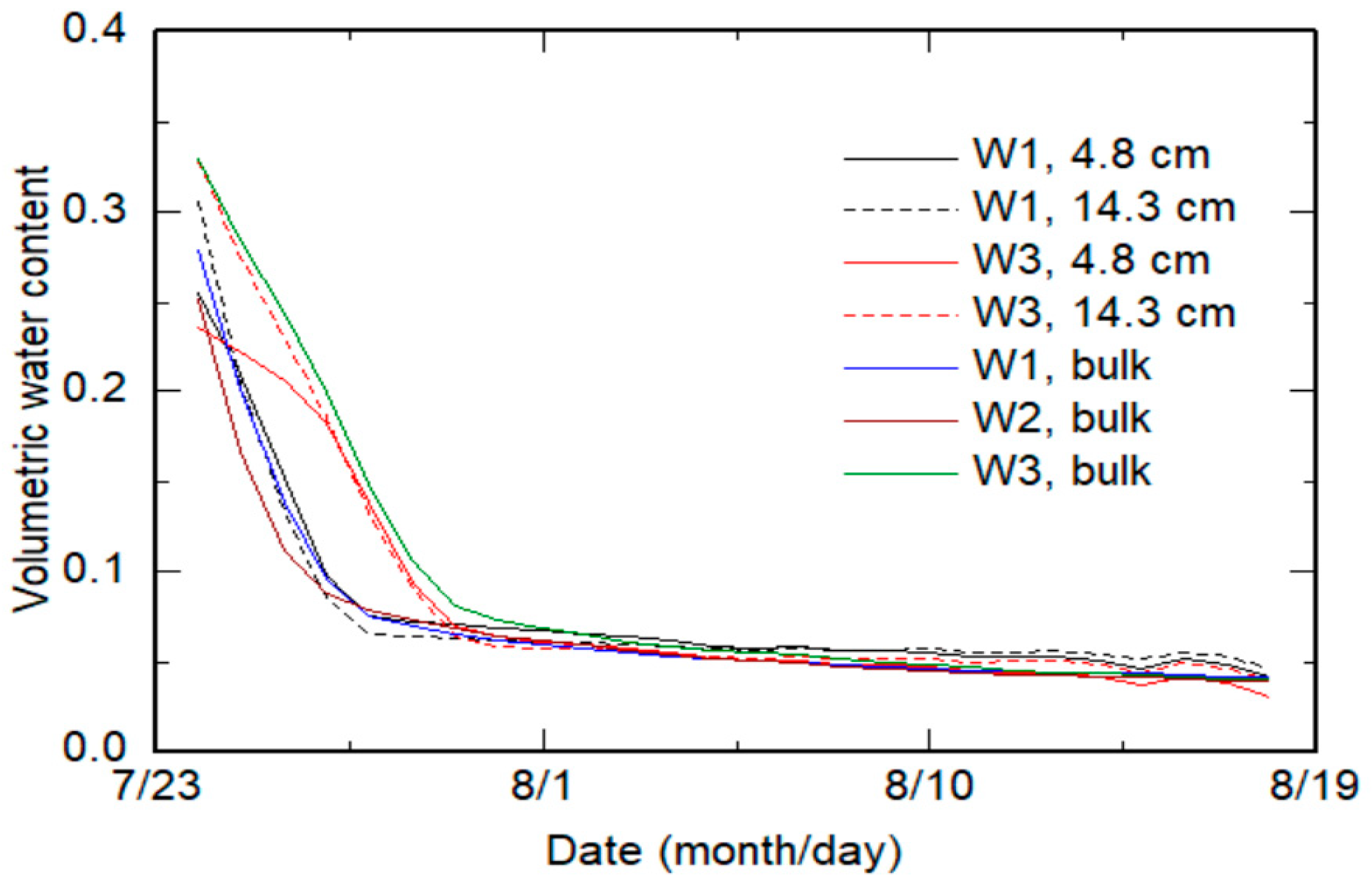
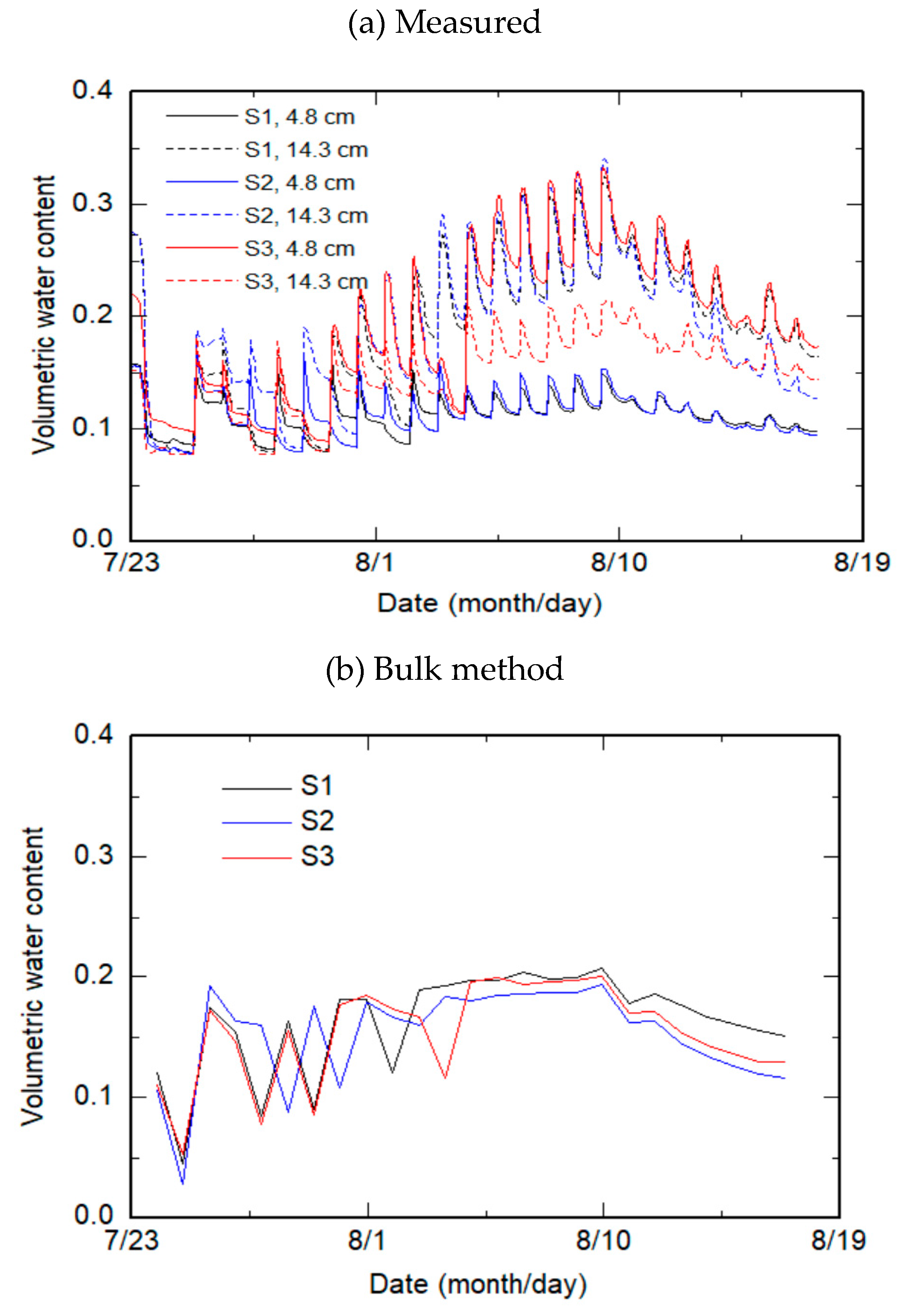
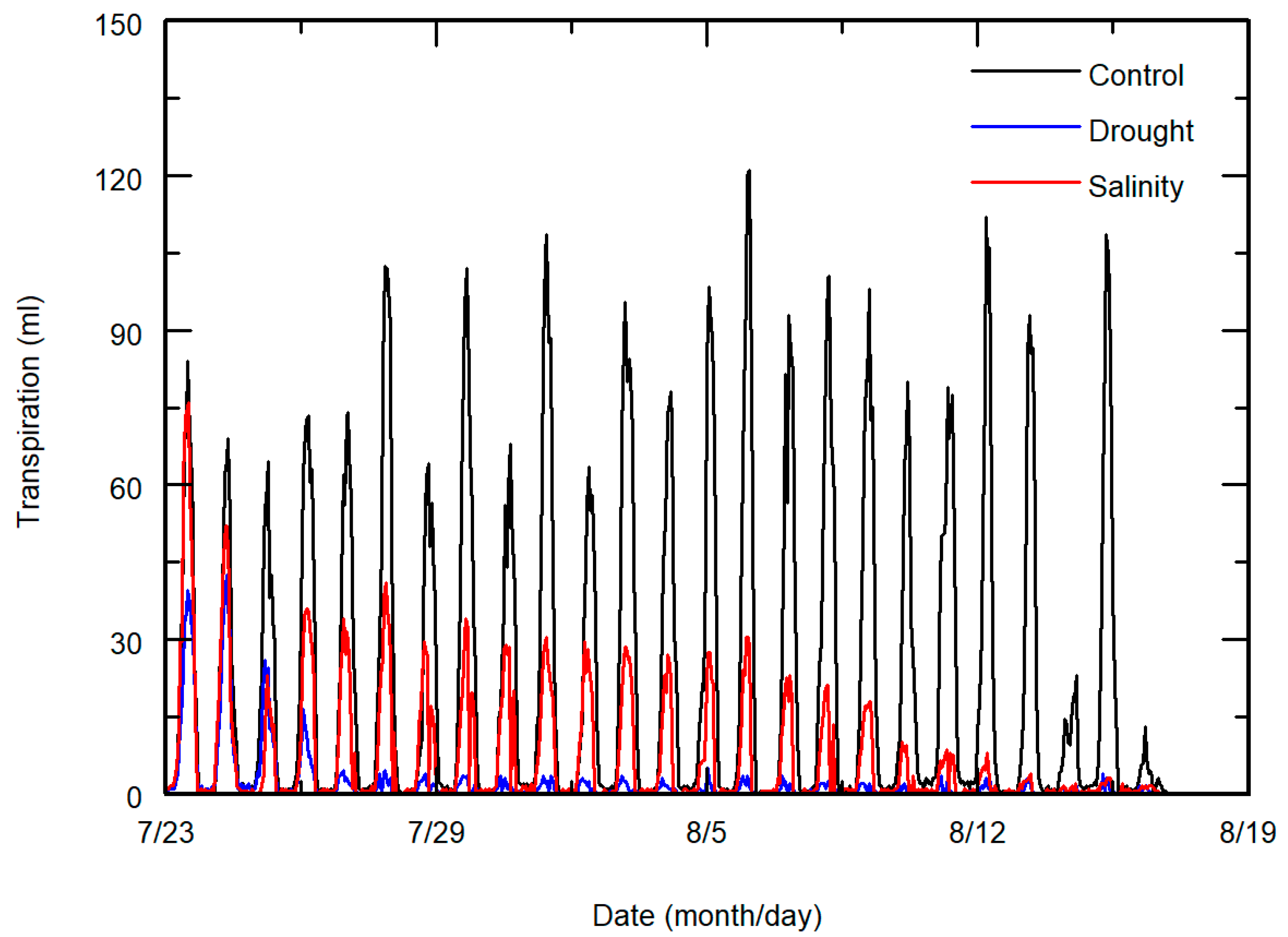
2.2. Experiment
2.3. Estimation Methods
2.3.1. Bulk Method
2.3.2. Inverse Method
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujimaki, H.; Abd El Baki, H.M.; Mohamad Mahdavi, S.; Ebrahimian, H. Optimization of Irrigation and Leaching Depths Considering the Cost of Water Using WASH_1D/2D Models. Water 2020, 12, 2549. [Google Scholar] [CrossRef]
- Hassanli, M.; Ebrahimian, H. Cyclic use of saline and non-saline water to increase water use efficiency and soil sustainability on drip irrigated maize in a semi-arid region. Span. J. Agric. Res. 2016, 14, e1204. [Google Scholar] [CrossRef]
- Teklu, D.H.; Shimelis, H.; Abady, S. Genetic improvement in sesame (Sesamum indicum L.): Progress and outlook: A review. Agronomy 2022, 12, 2144. [Google Scholar] [CrossRef]
- Raes, D.; Steduto, P.; Hsiao, T.C.; Fereres, E. AquaCrop—The FAO crop model to simulate yield response to water: II. Main algorithms and software description. Agron. J. 2009, 101, 438–447. [Google Scholar] [CrossRef]
- Fujimaki, H.; Tokumoto, I.; Saito, T.; Inoue, M.; Shibata, M.; Okazaki, M.; Nagaz, K.; El-Mokh, F. Determination of irrigation depths using a numerical model and quantitative weather forecasts and comparison with an experiment. In Practical Applications of Agricultural System Models to Optimize the Use of Limited Water; Ahuja, L.R., Ma, L., Lascano, R.J., Eds.; ACSESS: Madison, WI, USA, 2014; Volume 5, pp. 209–235. [Google Scholar]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. The HYDRUS Software Package for Simulating the Two- and Three Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media; PC Progress: Prague, Czech Republic, 2006. [Google Scholar]
- van Dam, J.C.; Huygen, J.; Wesseling, J.G.; Feddes, R.A.; Kabat, P.; van Walsum, P.E.V.; Groenendijk, P.; van Diepen, C.A. Theory of SWAP, Version 2.0: Simulation of Water Flow, Solute Transport and Plant Growth in the Soil-Water-Atmosphere-Plant Environment; Rep. No. 71; Department of Water Resources, Wageningen Agricultural University: Wageningen, The Netherlands, 1997. [Google Scholar]
- Holzworth, D.P.; Huth, N.I.; deVoil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM–evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalic, P.J.; Zaradny, H. Simulation of Field Water Use and Crop Yield; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Homaee, M.; Feddes, R.A.; Dirksen, C. A macroscopic water extraction model for nonuniform transient salinity and water stress. Soil Sci. Soc. Am. J. 2002, 66, 1764–1772. [Google Scholar] [CrossRef]
- Fujimaki, H.; Ando, Y.; Cui, Y.; Inoue, M. Parameter estimation of a root water uptake model under salinity stress. Vadose Zone J. 2008, 7, 31–38. [Google Scholar] [CrossRef]
- Fujimaki, H.; Kikuchi, N. Drought and salinity tolerances of young Jatropha. Int. Agrophys. 2010, 24, 121–127. [Google Scholar]
- Yanagawa, A.; Fujimaki, H. Tolerance of canola to drought and salinity stresses in terms of root water uptake model parameters. J. Hydrol. Hydromech. 2013, 61, 73–80. [Google Scholar] [CrossRef]
- Wang, Q.; Huo, Z.; Feng, S.; Yuan, C.; Wang, J. Comparison of spring maize root water uptake models under water and salinity stress validated with field experiment data. Irrig. Drain. 2015, 64, 669–682. [Google Scholar] [CrossRef]
- Cai, G.; Vanderborght, J.; Couvreur, V.; Mboh, C.M.; Vereecken, H. Parameterization of root water uptake models considering dynamic root distributions and water uptake compensation. Vadose Zone J. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Massoud, E.C.; Purdy, A.J.; Christoffersen, B.O.; Santiago, L.S.; Xu, C. Bayesian inference of hydraulic properties in and around a white fir using a process-based ecohydrologic model. Environ. Model. Softw. 2019, 115, 76–85. [Google Scholar] [CrossRef]
- Dainese, R.; Tarantino, A. Measurement of plant xylem water pressure using the high-capacity tensiometer and implications for the modelling of soil–atmosphere interaction. Géotechnique 2021, 71, 441–454. [Google Scholar] [CrossRef]
- Rabbel, I.; Bogena, H.; Neuwirth, B.; Diekkrüger, B. Using sap flow data to parameterize the Feddes water stress model for Norway spruce. Water 2018, 10, 279. [Google Scholar] [CrossRef]
- de Melo, M.L.A.; van Lier, Q.D.J. Revisiting the Feddes reduction function for modeling root water uptake and crop transpiration. J. Hydrol. 2021, 603, 126952. [Google Scholar] [CrossRef]
- Zech-Matterne, V.; Tengberg, M.; Van Andringa, W. Sesamum indicum L. (sesame) in 2nd century BC Pompeii, southwest Italy, and a review of early sesame finds in Asia and Europe. Veg. Hist. Archaeobotany 2015, 24, 673–681. [Google Scholar] [CrossRef]
- De Lima, G.S.; De Lacerda, C.N.; Dos Anjos Soares, L.A.; Gheyi, H.R.; Araújo, R.H.C.R. Production characteristics of sesame genotypes under different strategies of saline water application. Rev. Caatinga 2020, 33, 490–499. [Google Scholar] [CrossRef]
- Hamedani, N.G.; Gholamhoseini, M.; Bazrafshan, F.; Habibzadeh, F.; Amiri, B. Yield, irrigation water productivity and nutrient uptake of arbuscular mycorrhiza inoculated sesame under drought stress conditions. Agric. Water Manag. 2022, 266, 107569. [Google Scholar] [CrossRef]
- Sun, J.; Rao, Y.; Yan, T.; Yan, X.; Zhou, H. Effects of drought stress on sesame growth and yield characteristics and comprehensive evaluation of drought tolerance. Chin. J. Oil Crop Sci. 2010, 32, 525–533. [Google Scholar]
- Bahrami, H.; Razmjoo, J. Effect of salinity stress (NaCl) on germination and early seedling growth of ten sesame cultivars (Sesamum indicum L.). Int. J. AgriSci. 2012, 2, 529–537. [Google Scholar]
- El Harfi, M.; Hanine, H.; Rizki, H.; Latrache, H.; Nabloussi, A. Effect of drought and salt stresses on germination and early seedling growth of different color-seeds of sesame (Sesamum indicum). Int. J. Agric. Biol. 2016, 18, 1088–1094. [Google Scholar] [CrossRef]
- Suassuna, J.F.; Fernandes, P.D.; Brito, M.E.B.; Arriel, N.H.C.; de Melo, A.S.; Fernandes, J.D. Tolerance to Salinity of Sesame Genotypes in Different Phenological Stages. Am. J. Plant Sci. 2017, 8, 1904–1920. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A comprehensive review of nutritional value, phytochemical composition, health benefits, development of food, and industrial applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. The Use of Saline Waters for Crop Production; Irrigation and Drainage Paper 48; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; p. 133. [Google Scholar]
- Li, D.; Dossa, K.; Zhang, Y.; Wei, X.; Wang, L.; Zhang, Y.; Liu, A.; Zhou, R.; Zhang, X. GWAS uncovers differential genetic bases for drought and salt tolerances in sesame at the germination stage. Genes 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Li, D.; Zhou, R.; Yu, J.; Wang, L.; Zhang, Y.; You, J.; Liu, A.; Mmadi, M.A.; Fonceka, D.; et al. The genetic basis of drought tolerance in the high oil crop Sesamum indicum. Plant Biotechnol. J. 2019, 17, 1788–1803. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Numerical Model for Water and Solute Movement in and below the Root Zone; Research Report; US Salinity Lab: Riverside, CA, USA, 1987. [Google Scholar]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Stewart, B.A.; Steiner, J.L. Water-use efficiency. In Advances in Soil Science; Springer: New York, NY, USA, 1990; pp. 151–173. [Google Scholar]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- He, N.; Gao, X.; Zhao, L.; Hu, P.; Zhao, X. Modeling root water uptake patterns of oil crops grown on semiarid loess. Agric. For. Meteorol. 2023, 330, 109306. [Google Scholar] [CrossRef]
- Svoboda, P.; Kurešová, G.; Raimanová, I.; Kunzová, E.; Haberle, J. The effect of different fertilization treatments on wheat root depth and length density distribution in a long-term experiment. Agronomy 2020, 10, 1355. [Google Scholar] [CrossRef]
- Metselaar, K.; Pinheiro, E.A.R.; de Jong van Lier, Q. Mathematical Description of Rooting Profiles of Agricultural Crops and its Effect on Transpiration Prediction by a Hydrological Model. Soil Syst. 2019, 3, 44. [Google Scholar] [CrossRef]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root distribution by depth for temperate agricultural crops. Field Crop. Res. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Sainju, U.M.; Good, R.E. Vertical root distribution in relation to soil properties in New Jersey Pinelands forests. Plant Soil 1993, 150, 87–97. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Chanduvi, F.; Lesch, S.M. Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurements; Irrigation and Drainage Paper 57; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999; pp. 1–150. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Irrigation and Drainage Paper 29 (Rev. 1); Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; p. 186. [Google Scholar]

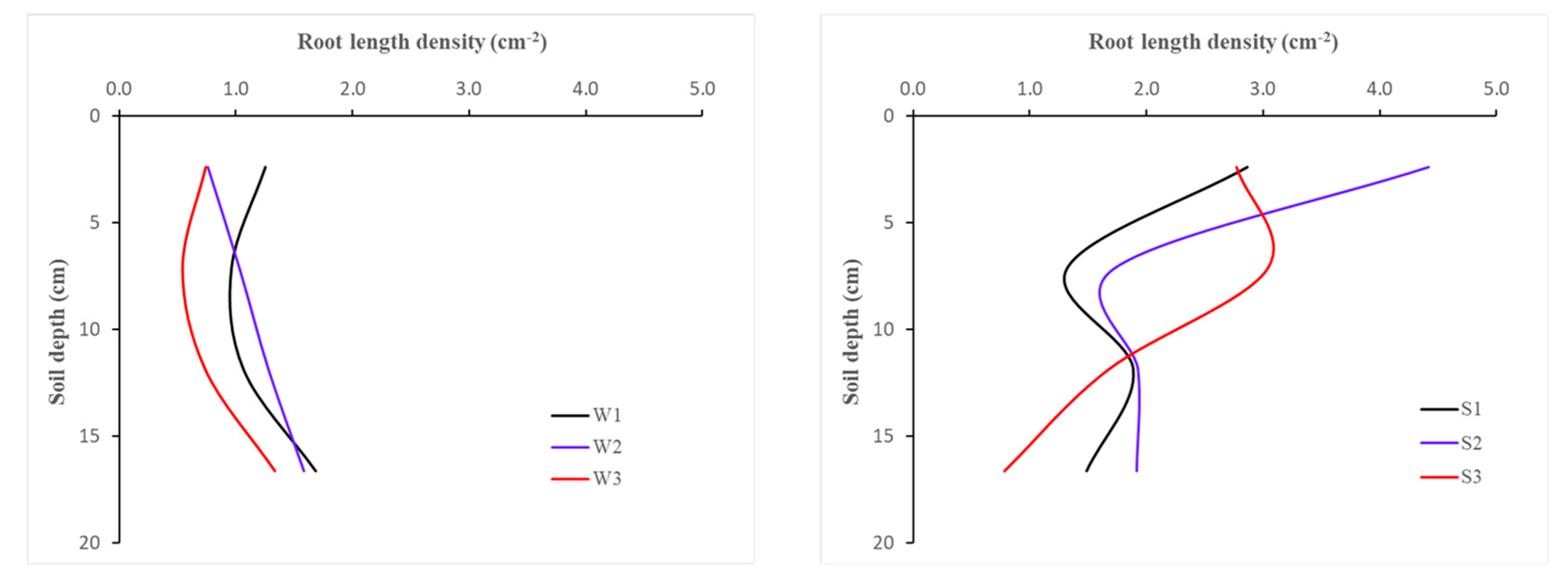
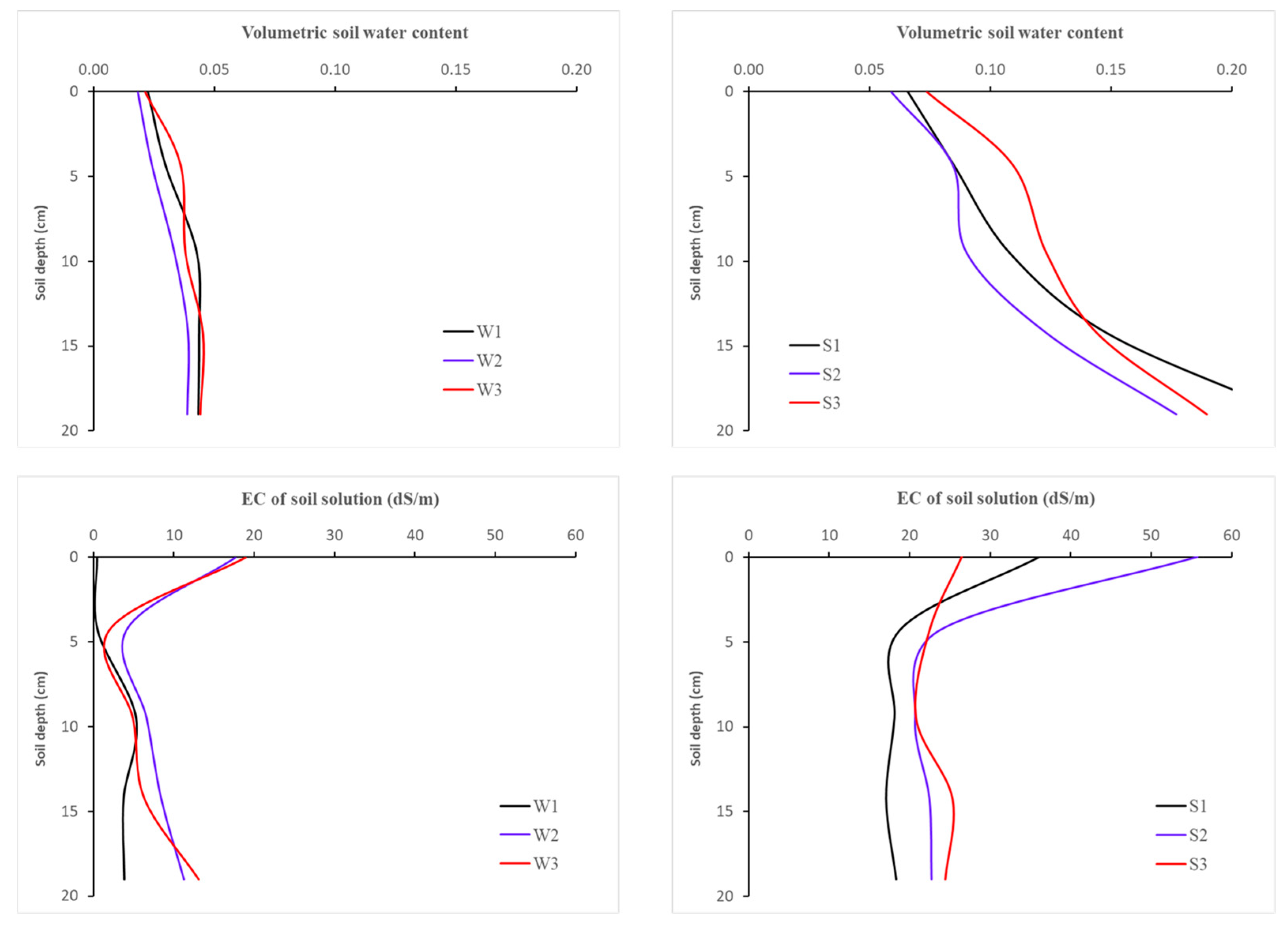

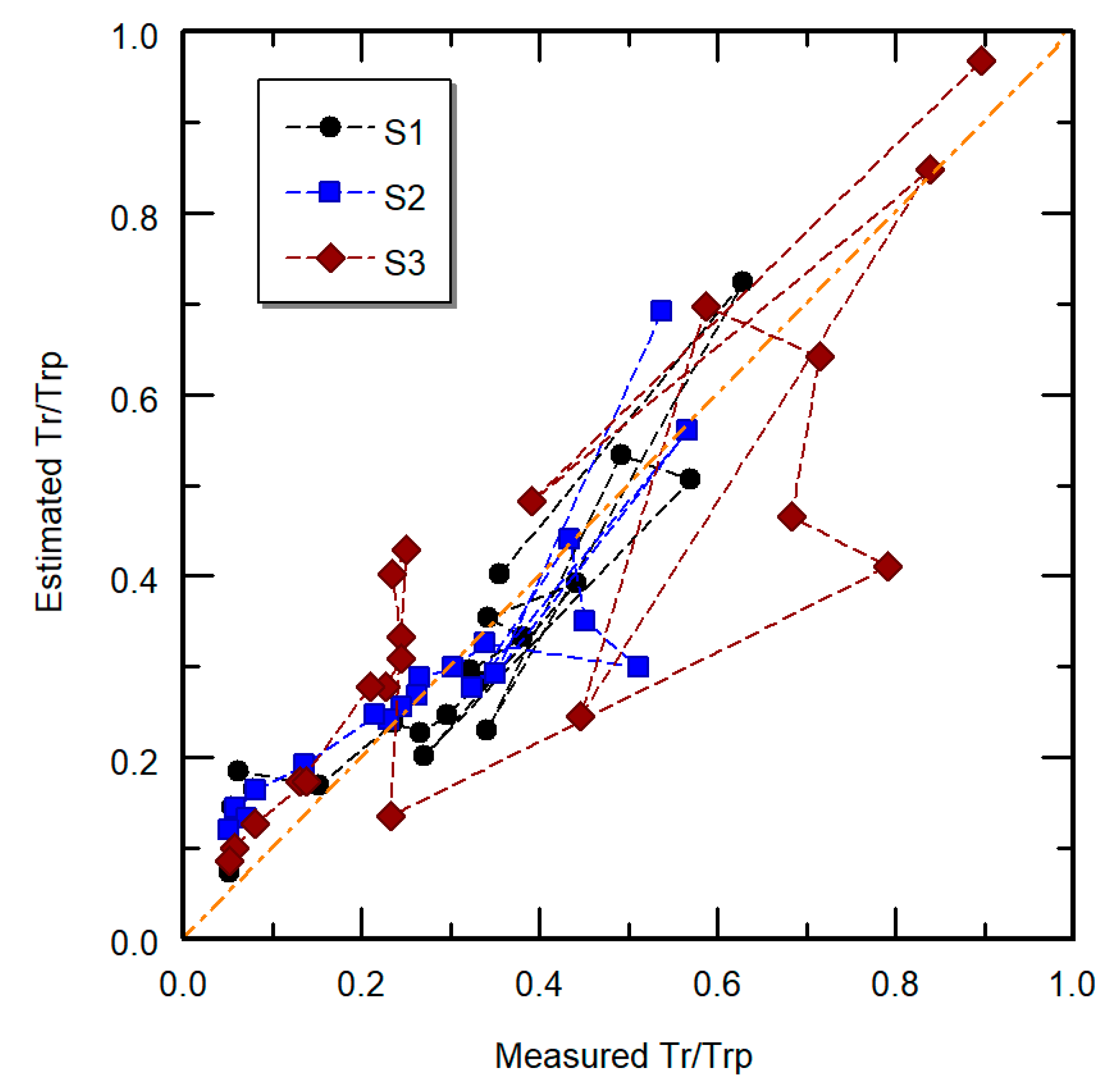

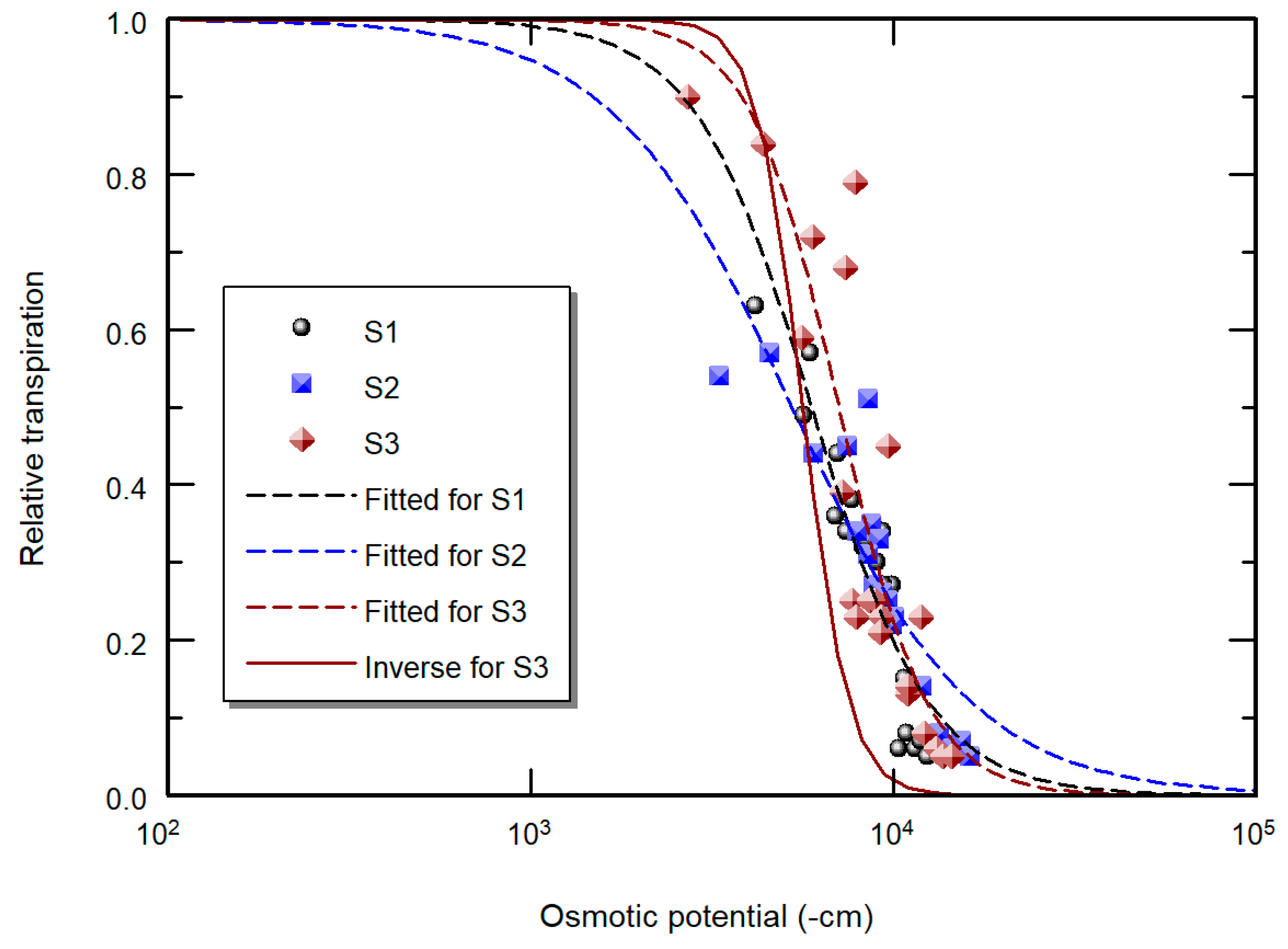
| Stress | Method | Pot | Parameter | ||
|---|---|---|---|---|---|
| ho50 (cm) | h50 (cm) | p | |||
| Drought | Bulk | W1 | – | 1539.0 | 2.29 |
| W2 | – | 1533.1 | 3.27 | ||
| W3 | – | 1601.0 | 1.85 | ||
| Mean | – | 1557.7 | 2.47 | ||
| Drought | Inverse | W1 | – | 1537.0 | 6.89 |
| W2 | – | – * | – * | ||
| W3 | – | 1747.0 | 3.34 | ||
| Mean | – | 1642.0 | 5.12 | ||
| Salinity | Bulk | S1 | 5986.5 | – | 2.68 |
| S2 | 5281.5 | – | 1.75 | ||
| S3 | 7115.1 | – | 3.56 | ||
| Mean | 6127.7 | – | 2.66 | ||
| Salinity | Inverse | S3 | 5659.0 | – | 6.89 |
| Treatment | Pot (Replication) | Aboveground Biomass (g) | WUE (kg/m3) | |
|---|---|---|---|---|
| Fresh | Dry | |||
| Control | C1 | 155.0 | 31.1 | 2.30 |
| C2 | 193.0 | 31.6 | 2.35 | |
| C3 | 177.0 | 28.0 | 2.08 | |
| Mean | 175.0 a | 30.2 a | 2.24 a | |
| Salinity | S1 | 88.0 | 15.3 | 3.74 |
| S2 | 100.0 | 17.2 | 4.06 | |
| S3 | 107.0 | 16.5 | 4.15 | |
| Mean | 98.3 b | 16.3 b | 3.99 b | |
| Drought | W1 | 33.0 | 3.5 | 3.54 |
| W2 | 47.0 | 3.2 | 3.46 | |
| W3 | 28.0 | 2.5 | 2.27 | |
| Mean | 36.0 c | 3.1 c | 3.09 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimian, H.; Fujimaki, H.; Toderich, K. Parameterization of the Response Function of Sesame to Drought and Salinity Stresses. Agriculture 2023, 13, 1516. https://doi.org/10.3390/agriculture13081516
Ebrahimian H, Fujimaki H, Toderich K. Parameterization of the Response Function of Sesame to Drought and Salinity Stresses. Agriculture. 2023; 13(8):1516. https://doi.org/10.3390/agriculture13081516
Chicago/Turabian StyleEbrahimian, Hamed, Haruyuki Fujimaki, and Kristina Toderich. 2023. "Parameterization of the Response Function of Sesame to Drought and Salinity Stresses" Agriculture 13, no. 8: 1516. https://doi.org/10.3390/agriculture13081516
APA StyleEbrahimian, H., Fujimaki, H., & Toderich, K. (2023). Parameterization of the Response Function of Sesame to Drought and Salinity Stresses. Agriculture, 13(8), 1516. https://doi.org/10.3390/agriculture13081516








