Abstract
New solutions are compulsorily needed to reconcile the enormous and ever-growing request for protein for human nutrition and, at the same time, reduce conventional meat production. This epochal challenge can find a valuable aid to a winning solution in insect rearing. The use of insects as feed and food, far from being a definitive solution to global food shortages, can offer new protein sources and perfectly fit circular economy precepts, yet more so when insects feed on by-products from the agri-food industry. In this scenario, Tenebrio molitor (TM) is a concrete alternative. Therefore, making its rearing more sustainable is a prime objective. In this paper, we evaluated the possibility of replacing usual plant sources of wet supplementation used in TM rearing with sustainable alternatives, including the cladodes of prickly pear (Opuntias ficus indica, OFI), to reduce the frequency of administration, thus minimizing related labor costs. The alternatives were tested for water content, dehydration, and shelf life to select the best-performing ones. On the selected matrices, we evaluated the preference of the larvae and their palatability because a matrix may be convenient and sustainable but not appreciated by consumers. The results showed that OFI cladodes have high moisture and a long shelf life and are appreciated by the larvae that prefer them to other matrices. Thus, OFI can replace the conventional wet source in TM rearing, at least in areas where this cactus grows wild and is not difficult to obtain.
1. Introduction
The world’s challenge today is to feed the entire human population sustainably. In fact, the increase in the population to over 9 billion by 2050 [1] and the consequent increase in the demand for food will lead to the excessive exploitation of animals and habitats, with deforestation and increased greenhouse gas (GHG) emissions [2]. In this context, the food system releases more than a quarter of all GHG emissions, most of which are due to livestock [3].
Reducing the consumption of emissions-intensive animal-based foods can improve our dramatic environmental and climatic scenario. However, the trend towards plant-based diets cannot assure either health results or higher sustainability [4]. There is a need to find alternative, more sustainable protein sources—best if produced using a circular economy perspective.
Given their biological characteristics and nutritional profile, insects lend themselves to being a solution to global food shortages [5,6]. In nature, about 2000 species of edible insects can provide food for both humans and farm animals such as fish, poultry, etc. [7].
Among edible insects, Tenebrio molitor (TM), a beetle of the Tenebrionidae family, also named yellow mealworm, has been extensively studied in the last few years in scientific circles as an alternative source of protein, especially since the European Commission approved it as a Novel Food and deemed it fit for human consumption in June 2021 [8].
TM has several advantages: it has a higher efficiency in converting food to body weight than meat animals, it reproduces and grows quickly, and its edible fraction is almost 100% compared to chicken and pigs (55%) and cattle (40%) [9]. It is currently widely used for animal feed such as fish, birds, and poultry, both as a whole larva and as a processed product (protein meals, protein extracts, and oils) [10,11].
TM rearing is much more sustainable than traditional livestock farming, as it emits fewer GHGs and requires less space and water [12]. Moreover, TM fits perfectly into a circular economy logic as it can grow and efficiently convert agro-industrial by-products into protein, thus valorizing secondary feedstocks and reducing food loss and waste [13].
TM can exploit organic matter as a source of nutrients and water. Water supply is crucial in insect rearing. It is known that TM larvae (TMLs), unlike adults [14], can recover water from atmospheric moisture, but only at high levels (88–92%) [15]. Such values, on the other hand, positively influence larval growth, causing their weight to increase already in the early period of development [16].
Humidifying the rearing environment, however, is not energetically sustainable as it requires additional water and, in the case of facilities like climatic chambers (especially on large-scale farms), high energy consumption. Moreover, highly humid environments favor the growth of mold and bacteria.
The most feasible alternative is to provide water using discrete doses of vegetables (potatoes, carrots, pumpkin, red cabbage, and red beet) distributed 2–3 times a week [17]. Such additional feeding does not harm rearing and even reduces the larval development time [9], increases the average weight of larvae of the same age [18], and decreases the feed conversion ratio (i.e., the amount of feed it takes to grow one kilogram of larvae) [19].
To ensure the economic and environmental sustainability of small- and medium-scale TM farming, it is preferable to use “water-supplying vegetable matrices” (hereinafter VMs) such as agri-food by-products that are inexpensive, seasonally available, and do not compete with human nutrition [20].
For our TM rearing, we focused on the cladodes of the prickly pear cactus (Opuntia ficus-indica (L.) Mill., hereinafter OFI). This cactus is a rustic crop, cultivated in warm climate areas for its fruit, but it is also very common on poor, dry soils, or marginal lands due to its easy adaptation. For non-productive purposes, it is used as a defensive hedge (due to the presence of spines) and windbreak. It is widely used in soil erosion protection and degraded area restoration programs, providing food resources during drought [21]. Mexico is the world’s largest producer of prickly pears, while Italy (specifically Sicily) is the biggest European producer [22].
In specialized productive orchards, annual pruning produces a large amount of waste of cladodes/immature fruits which is usually wasteful and costly to farmers [23]. However, recent studies have shown that these by-products can be sources of high-value components with antioxidant activity, exploitable as anticancer, anti-inflammatory, hypoglycemic, lipid-lowering, and cholesterol-lowering agents [24,25].
OFI has several advantages as a water source for small- to medium-scale insect farming. It is widely spread and cultivated in the Mediterranean area and semi-arid climate areas of South Africa, Australia, and Central and South America. OFI cladodes are available throughout the year, even in semi-arid areas, and store well at room temperature, thus allowing to reduce energy costs for storage [26].
This article’s principal objectives are to evaluate OFI as a replacement for the most common VMs, at least in areas where OFI is widespread, and demonstrate whether it is possible to reduce the wet supplementation frequency to reduce related labor costs. To achieve these goals, we compared the performance of OFI to other selected fresh VMs, in terms of dehydration and shelf life in the rearing chamber, the choice of TMLs, the palatability by TMLs after 6 days under rearing conditions, and the larval weight gain and survival.
2. Materials and Methods
One-year-old OFI cladodes were collected in the ENEA—Trisaia research centre (in the Basilicata region, in the south of Italy), where they grow wild. The cladodes are harvested when they reach a size of about 40 × 20 cm2 and are brushed thoroughly to remove thorns and stored at room temperature. They were provided to TMLs with the peel because its presence protects them from molding and slows down dehydration, mainly due to their fatty acids that keep out water-soluble molecules [27].
The other tested VMs were purchased from local retail suppliers. All experiments were conducted in a rearing room and on TMLs reared at the Trisaia research centre under standard conditions of temperature (28.0 ± 0.1 °C), relative humidity (60 ± 5%), and photoperiod (0L:24D) [28]. The Larvae’s standard diet consisted of 95% bran and 5% zootechnical yeast, purchased from local suppliers of livestock products. The larvae used in the various tests were reared in standard conditions and fed with the standard diet, adding slices of carrot twice a week, as a source of water, vitamins, and minerals.
2.1. Dehydration Test of Some VMs
One of the aims of this article is to look for an alternative to reduce the frequency of administration of VMs to TMLs. The VM chosen, however, must have particular characteristics, including that it does not dry out or go moldy when administered (i.e., under rearing conditions) and does not require removal within a few days of administration. For this purpose, the suitability of vegetables as a wet supplement was tested over time, under standard rearing conditions. The tested VMs are carrot (C), used as a control, OFI, potato (P), white apple (WA), red apple (RA), and red beet (RB). Specifically, 2 cm × 2 cm × 1 cm cubes of each VM, weighing on average 4.46 ± 0.41 g, without peel, were placed in the rearing chamber for 4 observation times, i.e., 3, 6, 9, and 12 days (respectively indicated as T3, T6, T9, and T12), according to a completely randomized design with 10 pieces (replicates) per treatment. Weight loss was used to estimate water loss over time. The presence of mold/rot was verified for each observation time.
In addition, for non-moldy VMs after three days, the initial percentage of water content was determined using a thermobalance (Gibertini Eurotherm, mod. EUJ; precision ± 1 mg).
2.2. Choice Test by TMLs
The 60-day-old TMLs (kept for 24 h without wet supplementation) were weighed and divided into 10 groups each of 15 larvae, balancing the weights of the replicates to avoid larger and more ravenous larvae all ending up in the same group.
The protocol for the choice tests was adapted from Morales-Ramos et al. (2020) [29]. In detail, multiple-choice arenas (12 cm-diameter Petri dishes) were set up with cubes of three VMs (OFI, C, and P), weighing enough to provide 3 g of water/cube of VMs, according to the percentages of water contained in each VM previously determined using the thermobalance. Thus, the water content, which was the same for all the matrices, could not influence the choice of larvae. The average cube weights were OFI = 2.68 ± 0.08 g, C = 2.44 ± 0.05 g, and P = 2.65 ± 0.04 g.
Fifteen larvae/arena were released simultaneously into the center of the arena (Figure 1). The number of larvae present on each VM was observed after 20 min (the maximum time defined by the preliminary tests). The test was performed under standard rearing conditions using larvae reared on a standard diet with the wet supplement in the first case consisting of carrots (larvae taken as our control, CtL) and in the second case, it consisted of various vegetable matrices (VvL). This was carried out to test whether the habit of a particular wet supplement influenced the larvae in their choice. The choice tests were repeated 10 times (replicates) for each group of larvae (CtL and VvL).

Figure 1.
Choice test. Arena prepared with the OFI-P-C equally spaced from the center and 15 TMLs in the choice phase.
2.3. Palatability Test of the VMs by the TMLs
60-day-old TMLs (0.75 ± 0.09 g), kept for 24 h without wet supplementation, were used to investigate the palatability of the VMs, both fresh (“Fresh”) and stored in a rearing room for 6 days (“Stored”). In detail, larvae fed on the standard diet were administered fresh portions (0.56 ± 0.07 g) and stored portions (0.17 ± 0.05 g) of the VMs (carrot (C), potato (P), and OFI). After 96 h, the residual weight of the VMs is measured to evaluate the amount of VMs eaten by the larvae. Ten replicates of each VM were conducted using ten larvae/replicate.
In addition, we calculated the percentage of moisture in the three VMs, both in the “Fresh” and “Stored” samples, by using the thermobalance (Gibertini Eurotherm, mod. EUJ; precision ±1 mg).
2.4. Performance of the TMLs Fed on the Standard Diet and the VMs
Next, 30-day-old TMLs (6.2 ± 0.5 mg), previously reared under standard conditions, were used to evaluate the effect of three VMs (carrot (C), potato (P), and OFI), added to the standard diet, on larval weight gain. Complete randomization was applied to the experimental design, with 10 replicates/treatments and adding about 1 g/replica of different VMs to the standard diet on a weekly basis. Each replicate consisted of groups of 20 larvae, according to Rumbos et al. [17].
After one month, the trial was stopped, and larval survival was evaluated. The final larval weight was determined with an analytical balance (Gibertini, mod. E42S-B; precision ±0.1 mg), and the weight gain/larva was calculated.
2.5. Statistical Analyses
For all tests, the data collected were statistically processed using the software GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). The values are shown as the means ± standard deviation (SD). Homogeneity of variance and normality was verified prior to the statistical analysis. If this was confirmed, one-way ANOVA was used, followed by Tukey’s post hoc test to identify the differences between the multiple theses. Alternatively, the Kruskal–Wallis test with pairwise multiple comparisons (Dunn’s test) was applied. An unpaired t-test was used to compare the means of two groups of independent values with the homogeneity of variance and normality. Significance was assumed at p < 0.05.
3. Results
3.1. Dehydration Test of Some VMs
The dehydration test in the rearing chamber provided interesting data on both weight loss (Table 1) and mold and rot resistance (Figure 2) at four observation times: 3–6–9–12 days (named T3, T6, T9, and T12, respectively).

Table 1.
Percentage of weight loss in the six VMs during four times of observation.

Figure 2.
Evolution of the six VMs tested during the dehydration test in the rearing room under standard conditions.
Comparing the weight loss of the various matrices at the four observation times using the unpaired t-test (p < 0.05), it can be observed that the values at T9 and T12 for all the VMs are unchanged, while the values at T3, T6, and T9 are significantly different, probably because after 9 days, they have no more water to lose, and only their dry weight remains. The comparison between the results for the different VMs for each observation time using Tukey’s multiple comparison tests shows that at T3, OFI is significantly different from all matrices except P, and C is different from every other VM except P. At T6, OFI, P, and C are significantly different from WA, RB, and RA. At T9, P equals C and WA equals RA. Finally, at T12, OFI is still significantly different from all the matrices.
What happens during the dehydration test in the rearing chamber to two of the six VMs is evident in Figure 2. The white apple (WA) and the red apple (RA) develop mold after only 6 days and show clear signs of rotting already at 3 days; their texture also becomes immediately too soft, making them very difficult to handle.
At T3, clear signs of desiccation also begin to appear on the potato samples. The other 3 VMs (OFI, C, and RB), although showing small changes, are still usable at T6. At 9 days (T9), OFI and C also show clear signs of desiccation, while, apparently, RB seems to resist better, probably because it has a higher dry residue and lower water content than the other three VMs at the start (Table 2). At T12, all matrices are strongly altered and their use as a source of water is certainly not recommended.

Table 2.
Percentage of dry matter and moisture in the four VMs calculated by the thermobalance.
Although RB has shown excellent resistance to dehydration, given its lower water content than other VMs, a larger amount of RB should be added to the standard diet to guarantee the appropriate water supplement, or it should be added twice a week, as is usually carried out with carrots. In addition, it is not a convenient alternative wet supplement, because of its cost and use for human nutrition. Therefore, we ruled it out in subsequent tests.
3.2. Choice Test by TMLs
At the end of the choice test, the number of larvae on the three VMs was detected, both for larvae that received carrots (CtL) and various vegetable matrices (VvL) as their water source.
The unpaired t-test shows that the choice of VM is not influenced by the type of wet supplement habitually consumed, as there is no statistically significant difference between the values in the same column, as shown in Table 3.

Table 3.
Number of TMLs on the three VMs.
3.3. Palatability Test of the VMs by the TMLs
In this test, we observed both groups of larvae (“Fresh” and “Stored”) for 96 h and noticed that as soon as we provided the ‘Fresh’ VMs, the larvae pounced on them, whereas as soon as we provided the ‘Stored’ VMs, the larvae took a while to approach the VMs.
The results on the VMs’ consumption are shown in Figure 3.
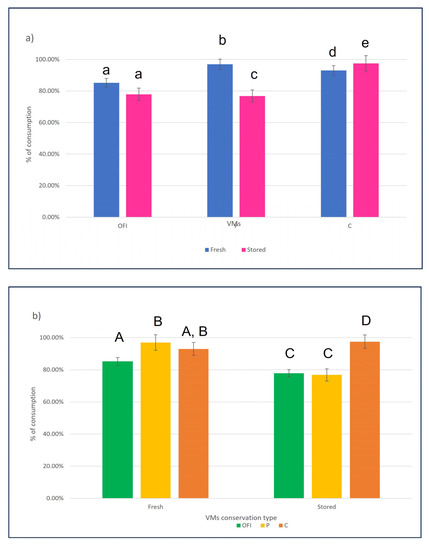
Figure 3.
Percentage of VMs consumed by the TMLs after 96 h. (a) Percentage of consumption for each VM, administered as “fresh” (blue bars) and “stored” (pink bars). Bars with different lowercase letters are significantly different according to the unpaired t-test (p < 0.05). (b) Percentage consumption of the three tested VMs for each administrative condition. Bars with different uppercase letters are significantly different according to Dunn’s test (p < 0.05).
The data were analyzed using an unpaired t-test to detect differences between the types of administration for each VM (Figure 3a) and Dunn’s test to evaluate differences between the VMs by the type of administration (Figure 3b).
Figure 3a shows no significant difference between the consumption percentages of ‘fresh’ and ‘stored’ OFI. In contrast, the percentages of consumption between the ‘Fresh’ and ‘Stored’ matrices are significantly different for both P and C. Figure 3b shows that among the “Fresh” VMs, the consumption percentage of OFI is different from that of P, while C is not different from the other two. Furthermore, among the “Stored” VMs, the consumption percentage of C is significantly different from that of OFI and P.
The analysis of the three VMs with the thermobalance provided interesting results (Table 4).

Table 4.
Percentage of moisture in the three VMs calculated by the thermobalance. The analyses were conducted on both fresh samples (“Fresh”) (see Table 2) and those stored in a rearing room for 6 days (“Stored”).
Tukey’s test for the comparison between the moisture data for the three VMs shows that the three fresh samples have similar percentages of moisture content, except for P, which has a slightly lower percentage. After 6 days, the moisture percentage is similar only for P and C, while OFI has approximately twice as much moisture as the other two matrices.
3.4. Performance of the TMLs Fed on the Standard Diet and the VMs
The weekly supply of the tested VMs, as a moist supplement to the standard diet, ensured a larval survival rate of 98% (Table 5). Furthermore, after one month of observation, larval survival was not significantly different between the treatments. Conversely, the weight gain/larva was differently influenced by the provided VM (F = 34.907; df = 3.36; p ≤ 0.0001). In fact, the weight gain in the P-fed larvae was significantly less than in the other treatments. In the C-fed larvae and OFI-fed larvae, the weight gain/larva was significantly higher than in the P-fed larvae, but they were not different from each other.

Table 5.
Effects of different VMs on larval survival and weight gain; VMs were added as a wet supplement to the standard diet for one month of rearing.
The lower weight gain in the P-fed larvae could be because we fed them the same quantity of wet supplement per week (1 g/replicate) instead of the same amount of water. Indeed, the potato contains a lower water percentage than the other two administered VMs (see Table 2).
4. Discussion
We tested OFI cladodes as a wet supplement both to test the possibility of using a more sustainable alternative in areas where it is spontaneous and widespread and to decrease the frequency of administration and, thus, the costs of managing the rearing activity.
The need to supplement the TM’s dry diet with a vegetable matrix providing water and minerals, as well as some vitamins, is reported by many authors, who mostly used potato slices [29,30] once or twice a week [17] or sliced carrots [31] once a week [32]. Ruschioni et al. (2020) tried feeding olive pomace in addition to the dry diet [33].
Wet supplementation is so important in TM rearing, both in terms of survival and larval weight gain, that Morales-Ramos et al. established an optimal diet consisting of 20% of a mixed substrate, which in turn consists of 83% moist integration, i.e., more than 16% of the total diet consists of water [34]. Deruytter et al. (2021) studied the influence of wet supplement distribution on the weight gain of TMLs, by testing the different distances between wet supplement pieces and different numbers of pieces per larval density [35].
Although the comparison between TM and meat animals (pigs, chickens, and cattle) sees TM as the winner in terms of lower water consumption per gram of protein [36], the TM’s water footprint can certainly be reduced by appropriately choosing the water-supplying matrix. In this sense, the same study by Miglietta et al. (2015), which focused on medium–large industrial rearing of TM that uses a diet of mixed cereals with small percentages of brewer’s yeast (like our standard diet) and carrots as VMs, calculated an annual food consumption of 182 t of mixed grains and 260 t of carrots. These quantities correspond to the virtual water consumption of approximately 310,000 m3 and 51,000 m3, respectively. In addition, carrots need much more watering than mixed grain feed. The peculiar characteristics of OFI, a cactus with low water and cultural needs, make it ideal for significantly reducing the water footprint of medium-scale TM rearing [36].
In addition to the water footprint, the choice of the water-supplying vegetable is a critical point in increasing the whole sustainability of mealworm rearing. Coudron et al. (2022) calculated that 1.35 kg of VMs is required for each kg of worms produced. The authors pointed out that the use of VMs requires frequent procurement, energy costs for their transport and storage, and a lot of labor, which obviously affects the production costs and economic sustainability of TM rearing [37].
For this purpose, we chose to test the prickly pear cladodes because they have the necessary requisites for their valorization as by-products, are available all year round, are not subtracted from the human diet, are sufficiently inexpensive, and require little energy or economic expenditure for supply and storage [20]. From this point of view, the most frequently used matrices (potatoes, carrots, and apples) are usually by-products of the agri-food chain, which could be valorized in the same sector for juices, extracts, etc. Conversely, the cladodes are the by-product derived from pruning OFI orchards for fruit production, which is not intended for human nutrition. The absence of competition between resources and human needs is therefore underlined.
Moreover, energy consumption for storage is one of the most critical aspects for the other water-suppling vegetables. In our trials, we observed that apples rot very quickly (Figure 2). Red beets are more resistant to dehydration and molding but contain too little water (Table 2) to be a sustainable alternative, as they need to be added more frequently to the standard diet. Potatoes have a water content slightly lower than carrots and OFI and are quite palatable to TMLs. However, they show significant signs of dehydration as early as day 3 in the rearing chamber, thus requiring more frequent feeding. Finally, carrots, normally used as a control, have a water content comparable to OFI and a similar dehydration/molding pattern. However, they are less attractive to the larvae. Testing the palatability of “fresh” and 6-day-old matrices under standard rearing conditions (“stored”), we observed that TMLs like both “fresh” and “stored” OFI equally. Among the “fresh” VMs, they like the potato slightly more, whereas, among the “stored” ones, the preference is definitely for the carrot (Figure 3). What makes us favor the use of OFI is its water content after 6 days, which is about twice that of the other two VMs tested. We have also shown that providing OFI as a wet supplement to the standard diet guarantees a very good survival rate for the TMLs (equal to that provided by carrots and potatoes) and a larval weight gain comparable to that obtained by feeding them carrots (Table 5). The larval weight gain due to potato feeding is slightly lower, as expected, due to the lower water content of potatoes per gram of VMs fed. This demonstrates that the amount of water supplied to the TMLs by the wet supplement influences their weight gain and suggests that wet supplementation also provides important nutrients for larval growth.
Our results suggest that OFI represents a valid and easily available alternative VM, which is particularly attractive to TMLs. The cladodes have an interesting water content, which makes it possible to replenish the TM herds even every 10 days.
To our knowledge, this is the first time that OFI has been considered a possible wet substitute in TM rearing, or at least, there are no other scientific articles on its use in this context. For this reason, no literature data are available to compare with our results. The current study represents a novelty in introducing and evaluating OFI as a cost-effective, easily available, and more sustainable alternative VM.
On the other hand, based on our experience, using OFI may have limitations in medium-scale TM rearing, which would merit further investigation. For example, when OFI is administered in the rearing vessels, the larvae pounce on the water-rich pulp and leave the peel intact, which then dries up until the next addition. These peels end up in the leftover feed and must be removed before the new OFI administration. Another limitation could be the number of cladodes needed, which, by projecting our results for a medium-scale farm, could be around 30 kg/month. As a result, TM rearing should have an orchard or a marginal field large enough to provide such quantities.
On the other hand, based on our experience, using OFI may have limitations in medium-scale TM rearing, which would merit further investigation. For example, when OFI is administered in the rearing vessels, the larvae pounce on the water-rich pulp and leave the peel intact that dry up until the next addition. These peels add to leftover feed and must be removed before adding new OFI administration. Another limitation could be the number of cladodes needed, which, projecting our results for a medium-scale farm, could be around 30 kg/month. As a result, TM rearing should have an orchard or a marginal field large enough to provide such quantities.
All this is crucial with a view to setting up medium–small TML rearing that is increasingly sustainable, especially in anticipation of the ever-increasing use of TMLs as a source of protein in human nutrition.
5. Conclusions
The tests conducted in this study allow us to suggest the use of OFI as a water source in TM rearing, especially in the Mediterranean area, where this cactus is widespread, but its cladodes are little used for human consumption. We have demonstrated that OFI maintains its overall qualities for more days than apple and potato and, at the same time, remains palatable for mealworms (therefore, the frequency of OFI administrations is lower). OFI does not require preservation at low temperatures (therefore, there are no costs for the investment of facilities for its conservation nor costs for energy consumption), and OFI administration like supplying water increases larval growth. In addition, residual cladodes from pruning can be used, thus enhancing what would otherwise have no value and saving water that would have been necessary for producing VMs with high water demand. Based on our results, OFI is an advantageous alternative water source that could further reduce the ecological footprint of TM rearing at the small–medium production level.
Author Contributions
Conceptualization, S.E. and F.B.; methodology, S.E, P.S. and F.B.; software, P.S.; formal analysis, S.E., P.S., A.V., A.S. and F.B.; investigation, S.E., S.M., S.D. and F.B.; resources, S.E., S.M., A.V., A.S. and S.D.; writing—original draft preparation, S.E., P.S., S.M., S.D. and F.B.; writing—review and editing, S.E. and P.S.; visualization, S.E.; supervision, S.E. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hertog, G.P.; Wheldon, S.; Kantorova, M.; Gu, V.; Gonnella, D.; Williams, G.; Zeifman, I.; Bay, L.; Castanheira, G.; Kamiya, H.; et al. World Population Prospects 2022: Summary of Results; United Nations: New York, NY, USA, 2022.
- Ordoñez-Araque, R.; Egas-Montenegro, E. Edible Insects: A Food Alternative for the Sustainable Development of the Planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food Systems Are Responsible for a Third of Global Anthropogenic GHG Emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.I. The Food System and Climate Change: Are Plant-Based Diets Becoming Unhealthy and Less Environmentally Sustainable? Proc. Nutr. Soc. 2022, 81, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible Insects as Innovative Foods: Nutritional and Functional Assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can Insects Help to Ease the Problem of World Food Shortage? Search 2014, 6, 261–262. [Google Scholar]
- Imathiu, S. Benefits and Food Safety Concerns Associated with Consumption of Edible Insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/882; European Commission: Brussels, Belgium, 2012.
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food by-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a Source of Interesting Natural Compounds, Their Recovery Processes, Biological Effects, and Safety Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of Insect Use for Feed and Food: Life Cycle Assessment Perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Derler, H.; Lienhard, A.; Berner, S.; Grasser, M.; Posch, A.; Rehorska, R. Use Them for What They Are Good at: Mealworms in Circular Food Systems. Insects 2021, 12, 40. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Insect Mass Production Technologies; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128028568. [Google Scholar]
- Hansen, L.L.; Ramløv, H.; Westh, P. Metabolic Activity and Water Vapour Absorption in the Mealworm Tenebrio molitor L. (Coleoptera, Tenebrionidae): Real-Time Measurements by Two-Channel Microcalorimetry. J. Exp. Biol. 2004, 207, 545–552. [Google Scholar] [CrossRef]
- Johnsen, N.S.; Andersen, J.L.; Offenberg, J. The Effect of Relative Humidity on the Survival and Growth Rate of the Yellow Mealworm Larvae (Tenebrio molitor, Linnaeus 1758). J. Insects Food Feed 2021, 7, 311–318. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Fasce, B.; Ródenas, L.; López, M.C.; Moya, V.J.; Pascual, J.J.; Cambra-López, M. Nutritive Value of Wheat Bran Diets Supplemented with Fresh Carrots and Wet Brewers’ Grains in Yellow Mealworm. J. Insect Sci. 2022, 22, 7. [Google Scholar] [CrossRef]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Valorisation of Agri-Food Waste and Mealworms Rearing Residues for Improving the Sustainability of Tenebrio molitor Industrial Production. J. Insects Food Feed 2022, 8, 509–524. [Google Scholar] [CrossRef]
- Gouhis, F.; Louhaichi, M.; Nefzaoui, A. Cactus (Opuntia ficus-indica) Utilization for Rehabilitating Rangelands in Arid Regions of Tunisia. Acta Hortic. 2019, 1247, 95–101. [Google Scholar] [CrossRef]
- Palmieri, N.; Suardi, A.; Stefanoni, W.; Pari, L. Opuntia ficus-indica as an Ingredient in New Functional Pasta: Consumer Preferences in Italy. Foods 2021, 10, 803. [Google Scholar] [CrossRef]
- Procacci, S.; Bojórquez-Quintal, E.; Platamone, G.; Maccioni, O.; Vecchio, V.L.; Morreale, V.; Alisi, C.; Balducchi, R.; Bacchetta, L. Opuntia ficus-indica Pruning Waste Recycling: Recovery and Characterization of Mucilage from Cladodes. Nat. Resour. 2021, 12, 91–107. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Sipango, N.; Ravhuhali, K.E.; Sebola, N.A.; Hawu, O.; Mabelebele, M.; Mokoboki, H.K.; Moyo, B. Prickly Pear (Opuntia spp.) as an Invasive Species and a Potential Fodder Resource for Ruminant Animals. Sustainability 2022, 14, 3719. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, Á.; Legua, P.; Hernández, F. Fatty Acid Profile of Fruits (Pulp and Peel) and Cladodes (Young and Old) of Prickly Pear [Opuntia ficus-indica (L.) Mill.] from Six Spanish Cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Ribeiro, N.; Abelho, M.; Costa, R. A Review of the Scientific Literature for Optimal Conditions for Mass Rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2018, 53, 434–454. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-Selection of Agricultural by-Products and Food Ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and Impact on Food Utilization and Nutrient Intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef]
- Mlček, J.; Adámek, M.; Adámková, A.; Matyáš, J.; Bučková, M.; Mrázková, M.; Vícha, R.; Vychodil, R.; Knížková, I.; Volek, Z. Feed Parameters Influencing the Breeding of Mealworms (Tenebrio molitor). Sustainability 2021, 13, 12992. [Google Scholar] [CrossRef]
- Rovai, D.; Ortgies, M.; Amin, S.; Kuwahara, S.; Schwartz, G.; Lesniauskas, R.; Garza, J.; Lammert, A. Utilization of Carrot Pomace to Grow Mealworm Larvae (Tenebrio molitor). Sustainability 2021, 13, 9341. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Self-Selection of Feeding Substrates by Tenebrio molitor Larvae of Different Ages to Determine Optimal Macronutrient Intake and the Influence on Larval Growth and Protein Content. Insects 2022, 13, 657. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of Olive Pomace to Feeding Substrate Affects Growth Performance and Nutritional Value of Mealworm (Tenebrio molitor L.) Larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Llan, D.I.; Tedders, W.L. Use of Nutrient Self-Selection as a Diet Refining Tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudron, C.L.; Claeys, J. The Influence of Wet Feed Distribution on the Density, Growth Rate and Growth Variability of Tenebrio molitor. J. Insects Food Feed 2021, 7, 141–149. [Google Scholar] [CrossRef]
- Miglietta, P.P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for Food: A Water Footprint Perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Coudron, C.L.; Deruytter, D.; Claeys, J. The Influence of Wet Feed PH on the Growth of Tenebrio molitor Larvae. Sustainability 2022, 14, 7841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).