Soil Sulfur Deficiency Restricts Canola (Brassica napus) Productivity in Northwestern Russia Regardless of NPK Fertilization Level
Abstract
1. Introduction
2. Materials and Methods
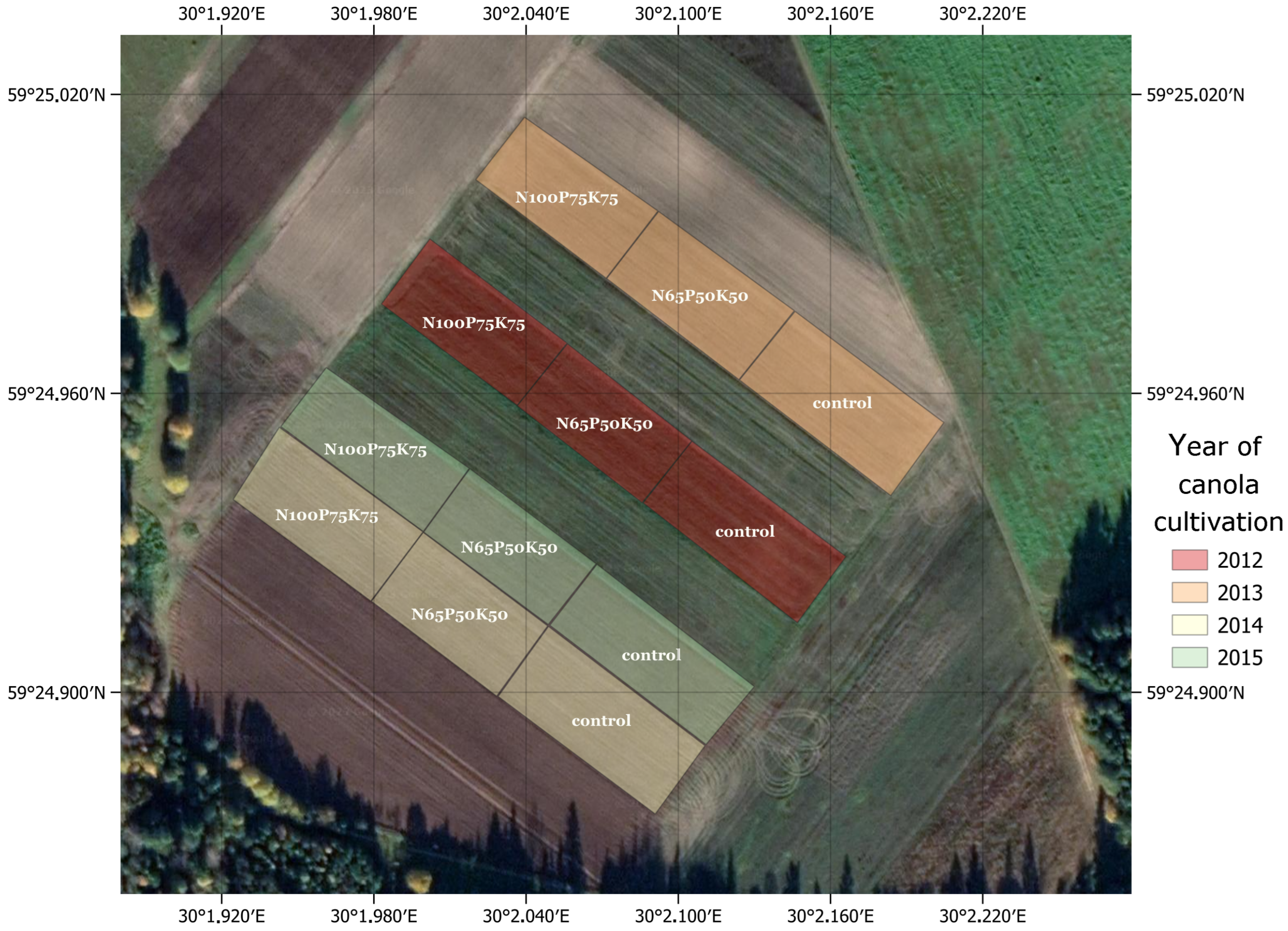
2.1. General Characteristics of Experimental Field
2.2. Experimental Design
2.3. Assessing Canola Yield and Soil Analysis
2.4. FAO-AquaCrop Model Calibration and Input Data
2.5. Statistical Analysis
3. Results
3.1. Soil Fertility Parameters at Studied Sites
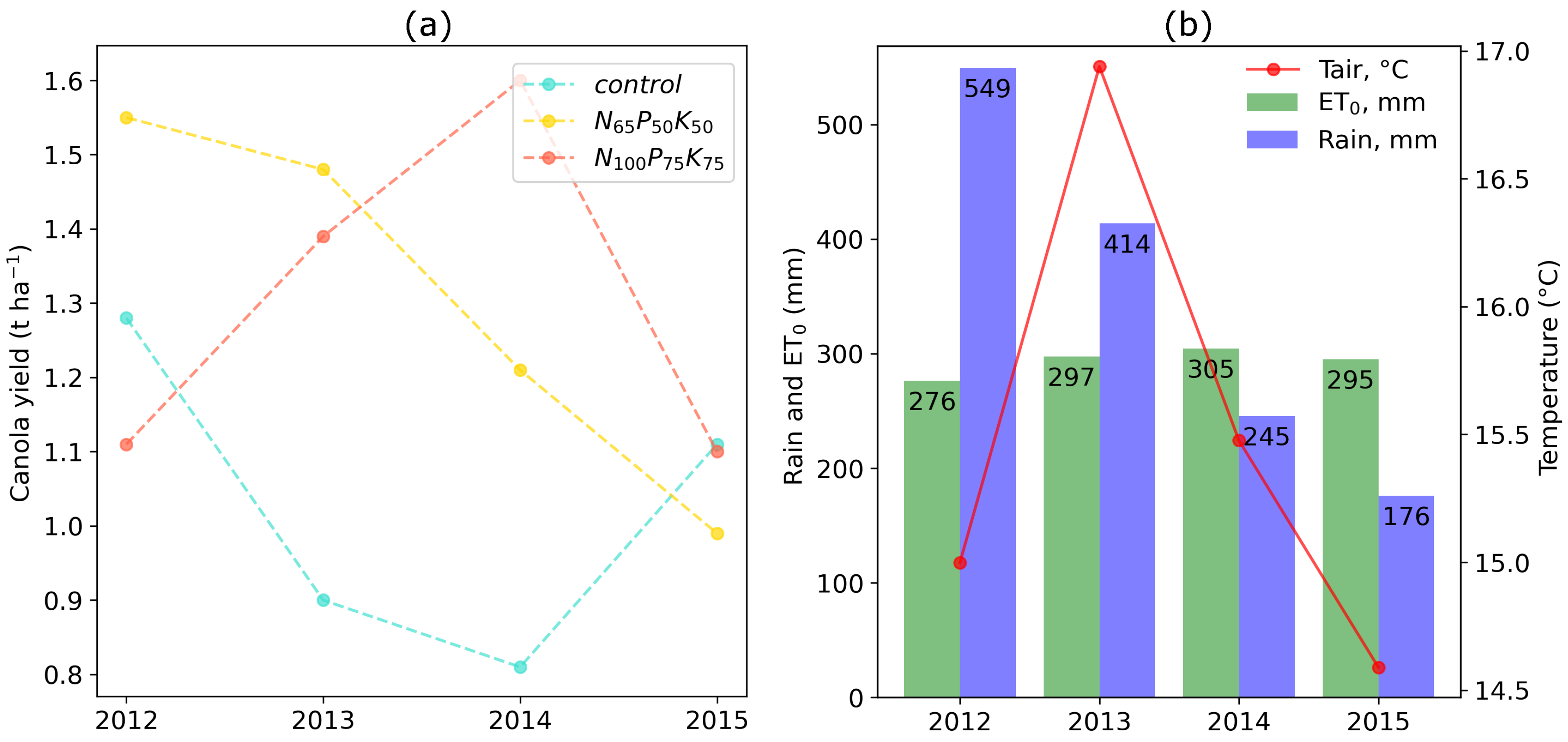
3.2. Temporal Dynamics of Canola Yield and Climatic Characteristics
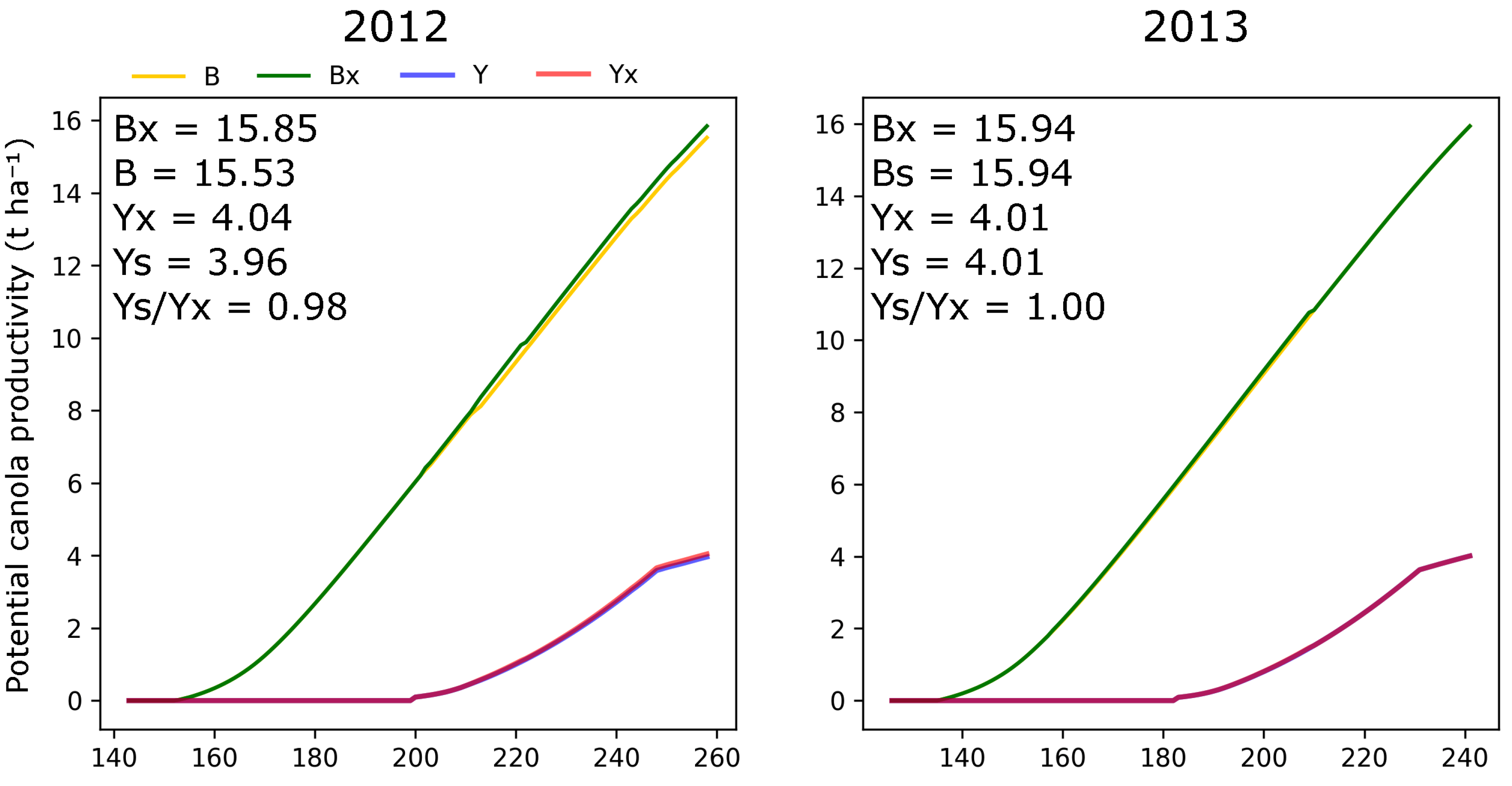
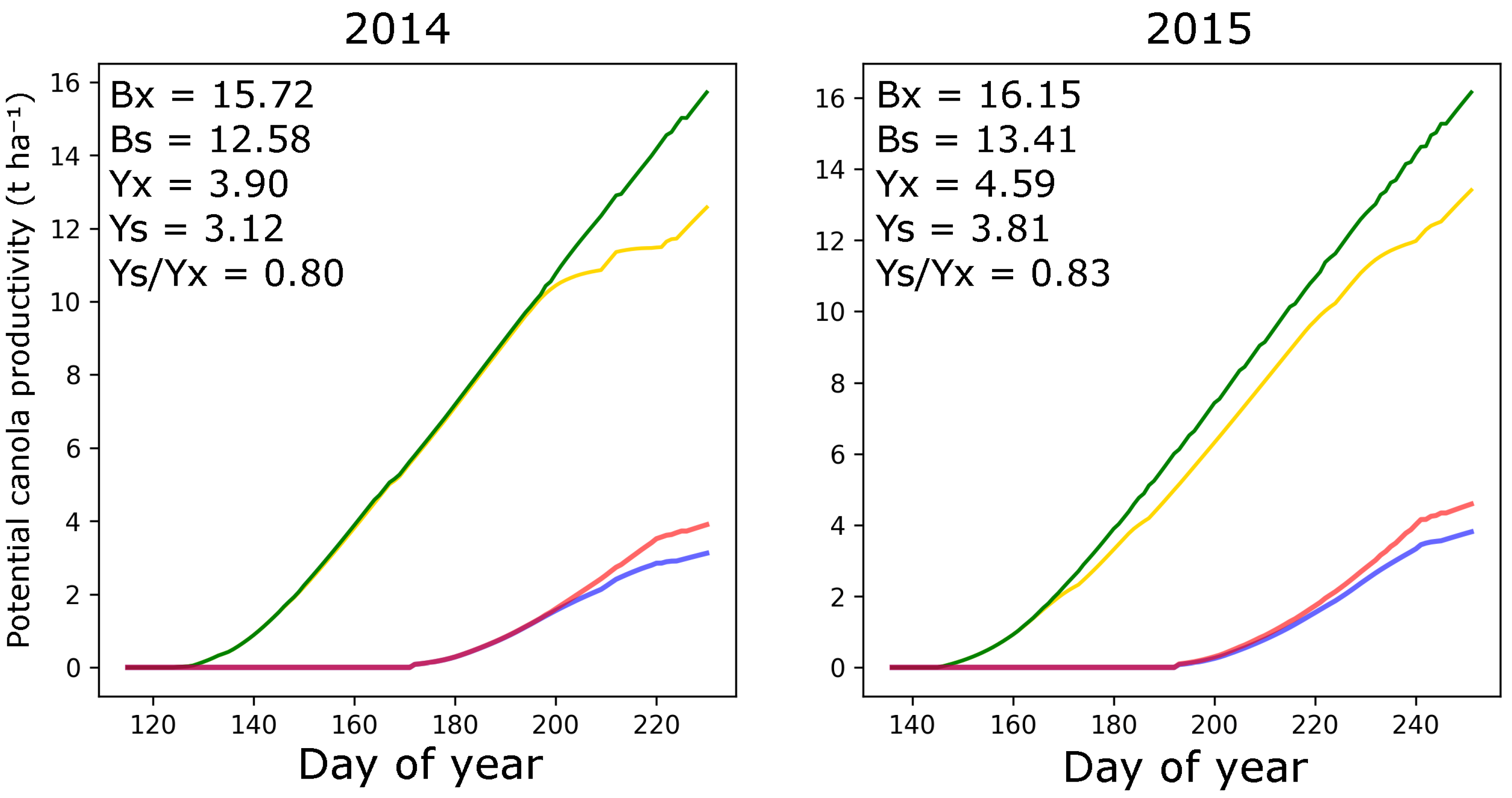
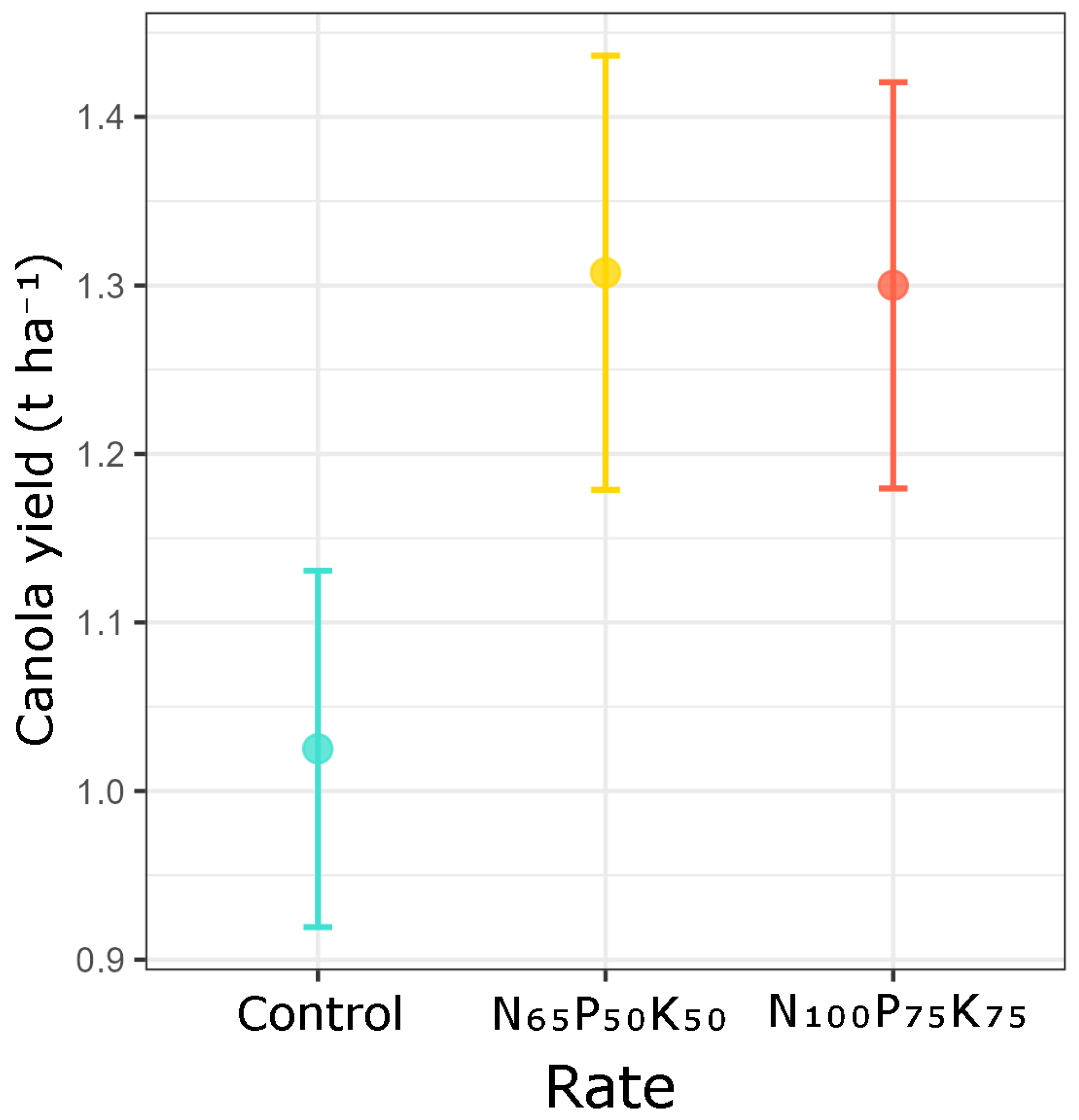
3.3. Effect of NPK Treatment and Soil Fertility on Canola Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McVetty, P.B.; Duncan, R.W. Canola, rapeseed, and mustard: For biofuels and bioproducts. In Industrial Crops Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; pp. 133–156. [Google Scholar]
- USDA. World Agricultural Production Circulars. 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 1 July 2023).
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The critical period for yield and quality determination in canola (Brassica napus L.). Field Crop. Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Hussain, M.A.; Mehmood, S.S.; Zhang, X.; Cheng, Y.; Zou, X.; Lv, Y. Integrated analysis of metabolome and transcriptome reveals insights for cold tolerance in rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 1796. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield, oil, and protein: A meta-analysis. Field Crop. Res. 2023, 293, 108848. [Google Scholar] [CrossRef]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef] [PubMed]
- Sharmina, M.; Anderson, K.; Bows-Larkin, A. Climate change regional review: Russia. Wiley Interdiscip. Rev. Clim. Chang. 2013, 4, 373–396. [Google Scholar] [CrossRef]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Cherenkova, E.; Kononova, N.; Muratova, N. Summer drought 2010 in the European Russia. Geogr. Environ. Sustain. 2013, 6, 55–66. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Fasullo, J.T. Climate extremes and climate change: The Russian heat wave and other climate extremes of 2010. J. Geophys. Res. Atmos. 2012, 117, D17103. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; update 2015; FAO: Rome, Italy, 2015; pp. 95–108. [Google Scholar]
- Reuter, D.; Robinson, J.B. Plant Analysis: An Interpretation Manual, 2nd ed.; CSIRO Publishing: Collingwood, Australia, 1997; pp. 102–270. [Google Scholar]
- Haneklaus, S.; Bloem, E.; Schnug, E.; De Kok, L.J.; Stulen, I. Sulfur. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 183–238. [Google Scholar]
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef]
- Liyanage, D.W.; Bandara, M.S.; Konschuh, M.N. Main factors affecting nutrient and water use efficiencies in spring canola in North America: A review of literature and analysis. Can. J. Plant Sci. 2022, 102, 799–811. [Google Scholar] [CrossRef]
- Gan, Y.; Malhi, S.S.; Brandt, S.; Katepa-Mupondwa, F.; Stevenson, C. Nitrogen use efficiency and nitrogen uptake of juncea canola under diverse environments. Agron. J. 2008, 100, 285–295. [Google Scholar] [CrossRef]
- Hocking, P.J.; Randall, P.J.; DeMarco, D. The response of dryland canola to nitrogen fertilizer: Partitioning and mobilization of dry matter and nitrogen, and nitrogen effects on yield components. Field Crop. Res. 1997, 54, 201–220. [Google Scholar] [CrossRef]
- Malhi, S.S.; Schoenau, J.J.; Grant, C.A. A review of sulphur fertilizer management for optimum yield and quality of canola in the Canadian Great Plains. Can. J. Plant Sci. 2005, 85, 297–307. [Google Scholar] [CrossRef]
- Al-Solaimani, S.G.; Alghabari, F.; Ihsan, M.Z. Effect of different rates of nitrogen fertilizer on growth, seed yield, yield components and quality of canola (Brassica napus L.) under arid environment of Saudi Arabia. Int. J. Agron. Agric. Res. 2015, 6, 268–274. [Google Scholar]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Grant, C.A.; Monreal, M.A.; Irvine, R.B.; Mohr, R.M.; McLaren, D.L.; Khakbazan, M. Crop response to current and previous season applications of phosphorus as affected by crop sequence and tillage. Can. J. Plant Sci. 2009, 89, 49–66. [Google Scholar] [CrossRef]
- Bélanger, G.; Ziadi, N.; Pageau, D.; Grant, C.; Lafond, J.; Nyiraneza, J. Shoot growth, phosphorus–nitrogen relationships, and yield of canola in response to mineral phosphorus fertilization. Agron. J. 2015, 107, 1458–1464. [Google Scholar] [CrossRef]
- Vicianová, M.; Ducsay, L.; Ryant, P.; Provazník, M.; Zapletalová, A.; Slepčan, M. Oilseed rape (Brassica Napus L.) nutrition by nitrogen and phosphorus and its effect on yield of seed, oil and higher fatty acids content. Acta Univ. Agric. Silvic. Mendel. Brun. 2020, 68, 129–136. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 1st ed.; Academic Press: San Diego, CA, USA, 1995; pp. 135–190. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Pan, X.Y.; Li, J.Y.; Deng, K.Y.; Xu, R.K.; Shen, R.F. Four-year effects of soil acidity amelioration on the yields of canola seeds and sweet potato and N fertilizer efficiency in an ultisol. Field Crop. Res. 2019, 237, 1–11. [Google Scholar] [CrossRef]
- Lofton, J.; Godsey, C.B.; Zhang, H. Determining aluminum tolerance and critical soil pH for winter canola production for acidic soils in temperate regions. Agron. J. 2010, 102, 327–332. [Google Scholar] [CrossRef]
- Baquy, M.; Li, J.Y.; Xu, C.Y.; Mehmood, K.; Xu, R.K. Determination of critical pH and Al concentration of acidic Ultisols for wheat and canola crops. Solid Earth 2017, 8, 149–159. [Google Scholar] [CrossRef]
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Jones, C.; Olson-Rutz, K. Montana State University Extension. Soil Nutrient Management for Canola. 2016. EB0224. Available online: https://agresearch.montana.edu/wtarc/producerinfo/agronomy-nutrient-management/Canola/MSUExtensionBulletin.pdf (accessed on 1 July 2023).
- Gaines, T.P.; Gaines, S.T. Soil texture effect on nitrate leaching in soil percolates. Commun. Soil Sci. Plant Anal. 1994, 25, 2561–2570. [Google Scholar] [CrossRef]
- Sitthaphanit, S.; Limpinuntana, V.; Toomsan, B.; Panchaban, S.; Bell, R.W. Fertiliser strategies for improved nutrient use efficiency on sandy soils in high rainfall regimes. Nutr. Cycl. Agroecosyst. 2009, 85, 123–139. [Google Scholar] [CrossRef]
- Kidin, V.V.; Shibalkin, A.E.; Kagirova, M.V. Imbalance of nutritional substances of the soil at the modern stage of development of agricultural production in Russia. Eurasian J. Soil Sci. 2019, 8, 167–175. [Google Scholar] [CrossRef]
- Monastyrsky, O.A.; Glinushkin, A.P.; Sokolov, M.S. Problems of ensuring food security of Russia and ways to solve it. Agrochemistry 2016, 11, 3–11. [Google Scholar]
- FAO. Measuring and Modelling Soil Carbon Stocks and Stock Changes in Livestock Production Systems: Guidelines for Assessment (Version 1), 1st ed.; Livestock Environmental Assessment and Performance (LEAP) Partnership: Rome, Italy, 2018; pp. 9–37. [Google Scholar]
- Sahrawat, K.L. Correlations between indexes of soil nitrogen availability and nitrogen percent in plant, nitrogen uptake, and dry-matter yield of rice grown in the greenhouse. Plant Soil 1983, 74, 223–228. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Raes, D.; Fereres, E. AquaCrop—The FAO crop model to simulate yield response to water: I. Concepts and underlying principles. Agron. J. 2009, 101, 426–437. [Google Scholar] [CrossRef]
- Raes, D.; Steduto, P.; Hsiao, T.C.; Fereres, E. AquaCrop—The FAO crop model to simulate yield response to water: II. Main algorithms and software description. Agron. J. 2009, 101, 438–447. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Heng, L.; Steduto, P.; Rojas-Lara, B.; Raes, D.; Fereres, E. AquaCrop—the FAO crop model to simulate yield response to water: III. Parameterization and testing for maize. Agron. J. 2009, 101, 448–459. [Google Scholar] [CrossRef]
- Vanuytrecht, E.; Raes, D.; Steduto, P.; Hsiao, T.C.; Fereres, E.; Heng, L.K.; Vila, M.G.; Moreno, P.M. AquaCrop: FAO’s crop water productivity and yield response model. Environ. Model. Softw. 2014, 62, 351–360. [Google Scholar] [CrossRef]
- Zeleke, K.T.; Luckett, D.; Cowley, R. Calibration and testing of the FAO AquaCrop model for canola. Agron. J. 2011, 103, 1610–1618. [Google Scholar] [CrossRef]
- Mousavizadeh, S.F.; Honar, T.; Ahmadi, S.H. Assessment of the AquaCrop Model for simulating Canola under different irrigation managements in a semiarid area. Int. J. Plant Prod. 2016, 10, 425–445. [Google Scholar]
- Ebrahimipak, N.A.; Egdernezhad, A.; Davoud, A.T.; Dehkordi, K. Evaluation of AquaCrop Model to Simulate Canola (Brassica napus) Yield under Deficit Irrigation Scenarios in Gazvin Plain. Iran. J. Soil Water Res. 2018, 49, 1003–1015. [Google Scholar]
- Egdernezhad, A.; Ebrahimipak, N.A.; Tafteh, A.; Ahmadee, M. Canola irrigation scheduling using AquaCrop model in Qazvin Plain. Water Manag. Agric. 2019, 5, 53–64. [Google Scholar]
- Dirwai, T.L.; Senzanje, A.; Mabhaudhi, T. Calibration and evaluation of the FAO AquaCrop model for Canola (Brassica napus) under varied moistube irrigation regimes. Agriculture 2021, 11, 410. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A state-of-the-art global reanalysis dataset for land applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006. World Soil Resources Report No. 103, 2nd ed.; FAO: Rome, Italy, 2006. [Google Scholar]
- Allen, R.G.; Walter, I.A.; Elliott, R.; Howell, T.A.; Itenfisu, D.; Jensen, M.E. The ASCE’s Standardized Reference Evapotranspiration Equation; American Society of Civil Engineers: Reston, VA, USA, 2005; pp. 1–214. [Google Scholar]
- Badescu, V. A new kind of cloudy sky model to compute instantaneous values of diffuse and global solar irradiance. Theor. Appl. Climatol. 2002, 72, 127–136. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and Drainage Paper No. 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; pp. 41–54. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. Available online: http://www.rstudio.com/ (accessed on 1 July 2023).
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef]
- Droux, M. Sulfur assimilation and the role of sulfur in plant metabolism: A survey. Photosynth. Res. 2004, 79, 331–348. [Google Scholar] [CrossRef]
- Clandinin, D.R. Canola Meal for Livestock and Poultry; Canola Council of Canada: Winnipeg, MB, Canada, 1989. [Google Scholar]
- Grant, C.A.; Mahli, S.S.; Karamanos, R.E. Sulfur management for rapeseed. Field Crop. Res. 2012, 128, 119–128. [Google Scholar] [CrossRef]
- Liu, Y.; Rossi, M.; Liang, X.; Zhang, H.; Zou, L.; Ong, C.N. An integrated metabolomics study of glucosinolate metabolism in different Brassicaceae genera. Metabolites 2020, 10, 313. [Google Scholar] [CrossRef]
- Süzer, S. Effects of plant nutrition on canola (Brassica napus L.) growth. Trak. Univ. J. Nat. Sci. 2015, 16, 87–90. [Google Scholar]
- Malhi, S.S.; Gill, K.S. Interactive effects of N and S fertilizers on canola yield and seed quality on S-deficient Gray Luvisol soils in northeastern Saskatchewan. Can. J. Plant Sci. 2007, 87, 211–222. [Google Scholar] [CrossRef]
- Lisowska, A.; Filipek-Mazur, B.; Kalisz, A.; Gorczyca, O.; Kowalczyk, A. Changes in Soil Sulfate Sulfur Content as an Effect of Fertilizer Granules Containing Elemental Sulfur, Halloysite and Phosphate Rock. Agronomy 2023, 13, 1410. [Google Scholar] [CrossRef]
- Janzen, H.H.; Bettany, J.R. Sulfur nutrition of rapeseed: I. Influence of fertilizer nitrogen and sulfur rates. Soil Sci. Soc. Am. J. 1984, 48, 100–107. [Google Scholar] [CrossRef]
- Eriksen, J.; Thorup-Kristensen, K.; Askegaard, M. Plant availability of catch crop sulfur following spring incorporation. J. Plant Nutr. Soil Sci. 2004, 167, 609–615. [Google Scholar] [CrossRef]
- Girma, K.; Mosali, J.; Freeman, K.W.; Raun, W.R.; Martin, K.L.; Thomason, W.E. Forage and grain yield response to applied sulfur in winter wheat as influenced by source and rate. J. Plant Nutr. 2005, 28, 1541–1553. [Google Scholar] [CrossRef]
- Schonhof, I.; Blankenburg, D.; Müller, S.; Krumbein, A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Mascagni Jr, H.J.; Harrison, S.A.; Padgett, G.B. Influence of sulfur fertility on wheat yield performance on alluvial and upland soils. Commun. Soil Sci. Plant Anal. 2008, 39, 2133–2145. [Google Scholar] [CrossRef]
- Camberato, J.; Casteel, S. Sulfur Deficiency. Purdue Univ. Dep. of Agronomy, Soil Fertility Update. 2017. Available online: https://www.agry.purdue.edu/ext/corn/news/timeless/sulfurdeficiency.pdf (accessed on 1 July 2023).
- Scherer, H.W. Sulphur in crop production. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, E.; Schnug, E. History of sulfur deficiency in crops. In Sulfur: A Missing Link between Soils, Crops, and Nutrition, 50; Jez, J., Ed.; American Society of Agronomy: Madison, WI, USA, 2008; pp. 45–58. [Google Scholar]
- IFA Task Force. The Global “4R” Nutrient Stewardship Framework. Developing Fertilizer Best Management Practices for Delivering Economic, Social and Environmental Benefits; International Fertilizer Industry Association: Paris, France, 2009. [Google Scholar]
- Nyborg, M. Sulfur deficiency in cereal grains. Can. J. Soil Sci. 1968, 48, 37–41. [Google Scholar] [CrossRef]
- Grant, C.A.; Johnston, A.M.; Clayton, G.W. Sulphur fertilizer and tillage management of canola and wheat in western Canada. Can. J. Plant Sci. 2004, 84, 453–462. [Google Scholar] [CrossRef]
- Janzen, H.H.; Bettany, J.R. Sulfur nutrition of rapeseed II. Effect of time of sulfur application. Soil Sci. Soc. Am. J. 1984, 48, 107–112. [Google Scholar] [CrossRef]
- Wen, G.; Schoenau, J.J.; Yamamoto, T.; Inoue, M. A model of oxidation of an elemental sulfur fertilizer in soils. Soil Sci. 2001, 166, 607–613. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Malhi, S.S. Sulfur forms and cycling processes in soil and their relationship to sulfur fertility. In Sulfur: A Missing Link between Soils, Crops, and Nutrition, 50; Jez, J., Ed.; American Society of Agronomy: Madison, WI, USA, 2008; pp. 1–10. [Google Scholar]
- Nyborg, M.; Bentley, C.F.; Hoyt, P.B. Effect of sulphur deficiency. Sulphur Inst. J. 1974, 10, 14–15. [Google Scholar]
- Malhi, S.S.; Gill, K.S. Effectiveness of sulphate-S fertilization at different growth stages for yield, seed quality and S uptake of canola. Can. J. Plant Sci. 2002, 82, 665–674. [Google Scholar] [CrossRef]
- Swan, M.; Soper, R.J.; Morden, G. The effect of elemental sulfur, gypsum and ammonium thiosulfate as sulfur sources on yield of rapeseed. Commun. Soil Sci. Plant Anal. 1986, 17, 1383–1390. [Google Scholar] [CrossRef]
- Giweta, M.; Dyck, M.F.; Malhi, S.S.; Puurveen, D.; Robertson, J.A. Long-term S-fertilization increases carbon sequestration in a sulfur-deficient soil. Can. J. Soil Sci. 2014, 94, 295–301. [Google Scholar] [CrossRef]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica. Crop. Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Qian, B.; De Jong, R.; Gameda, S. Multivariate analysis of water-related agroclimatic factors limiting spring wheat yields on the Canadian prairies. Eur. J. Agron. 2009, 30, 140–150. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Conservative: | |
| Crop water productivity—normalized for climate and CO (g·m) | 18.6 |
| Adjustment for yield formation (%) | 100 |
| Base temperature (°C) | 0 |
| Upper temperature (°C) | 30 |
| Canopy growth coefficient (%·day) | 8.9 |
| Canopy decline coefficient (%·day) | 5.2 |
| Soil water depletion factor for canopy expansion, upper limit | 0.2 |
| Soil water depletion factor for canopy expansion, lower limit | 0.55 |
| Shape factor for water stress coefficient for canopy expansion | 3.5 |
| Soil water depletion factor for stomatal closure, upper limit | 0.6 |
| Shape factor for water stress coefficient for stomatal closure | 5.0 |
| Soil water depletion factor for early canopy senescence, upper limit | 0.7 |
| Shape factor for water stress coefficient for early canopy senescence | 3.0 |
| Maximum crop transpiration coefficient | 0.95 |
| Non-conservative: | |
| Plant density (plants m) | 200 |
| Initial canopy cover (%) | 10 |
| Time to emergence (days) | 10 |
| Time to flowering (days) | 54 |
| Time to maximum rooting depth (days) | 59 |
| Time to maximum canopy cover (days) | 63 |
| Time to senescence (days) | 97 |
| Time to maturity (days) | 116 |
| Length building up (days) | 51 |
| Duration of flowering (days) | 19 |
| Minimum effective rooting depth (m) | 0.3 |
| Maximum effective rooting depth (m) | 1.0 |
| Reference harvest index (%) | 25 |
| SH * | Depth | TAW | PWP | FC | SAT | Ksat | Initial SMC | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | |||||||
| Ap + A/E | 0.00–0.47 | 77 | 8.5 | 16.3 | 47.0 | 1643 | 31.8 | 36.0 | 33.3 | 36.2 |
| B | 0.47–0.66 | 41 | 2.0 | 6.1 | 41.0 | 2685 | 34.0 | 39.0 | 36.0 | 38.0 |
| C1 | 0.66–0.72 | 38 | 2.3 | 6.1 | 41.0 | 2573 | 34.0 | 39.0 | 36.0 | 38.0 |
| C2 | 0.72–1.44 | 41 | 4.6 | 8.7 | 39.8 | 1695 | 37.7 | 39.6 | 37.8 | 38.6 |
| C3 | 1.44–1.70 | 31 | 0.1 | 3.2 | 42.8 | 6456 | 39.8 | 39.8 | 39.0 | 39.0 |
| Treatment | pH | SOC % | AN | P | K | S | SB cmol·kg |
|---|---|---|---|---|---|---|---|
| mg·kg | |||||||
| Control * | 4.5 ± 0.1 | 1.8 ± 0.1 | 95 ± 4 | 53 ± 10 | 37 ± 1 | 0.75 ± 0.10 | 1.90 ± 0.54 |
| NPK | 4.6 ± 0.1 | 2.0 ± 0.2 | 109 ± 16 | 63 ± 6 | 43 ± 5 | 1.04 ± 0.21 | 2.85 ± 0.72 |
| NPK | 4.7 ± 0.1 | 2.3 ± 0.1 | 118 ± 6 | 58 ± 2 | 36 ± 1 | 0.94 ± 0.11 | 3.10 ± 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrokhotov, A.; Kozyreva, L.; Fesenko, M.; Dubovitskaya, V.; Sushko, S. Soil Sulfur Deficiency Restricts Canola (Brassica napus) Productivity in Northwestern Russia Regardless of NPK Fertilization Level. Agriculture 2023, 13, 1409. https://doi.org/10.3390/agriculture13071409
Dobrokhotov A, Kozyreva L, Fesenko M, Dubovitskaya V, Sushko S. Soil Sulfur Deficiency Restricts Canola (Brassica napus) Productivity in Northwestern Russia Regardless of NPK Fertilization Level. Agriculture. 2023; 13(7):1409. https://doi.org/10.3390/agriculture13071409
Chicago/Turabian StyleDobrokhotov, Aleksei, Ludmila Kozyreva, Mariia Fesenko, Victoria Dubovitskaya, and Sofia Sushko. 2023. "Soil Sulfur Deficiency Restricts Canola (Brassica napus) Productivity in Northwestern Russia Regardless of NPK Fertilization Level" Agriculture 13, no. 7: 1409. https://doi.org/10.3390/agriculture13071409
APA StyleDobrokhotov, A., Kozyreva, L., Fesenko, M., Dubovitskaya, V., & Sushko, S. (2023). Soil Sulfur Deficiency Restricts Canola (Brassica napus) Productivity in Northwestern Russia Regardless of NPK Fertilization Level. Agriculture, 13(7), 1409. https://doi.org/10.3390/agriculture13071409





