Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Method
2.2. Fungi and Bacteria
2.3. Soil Sampling
2.4. Analyses
2.4.1. Total Organic Carbon
2.4.2. Labile Organic Matter
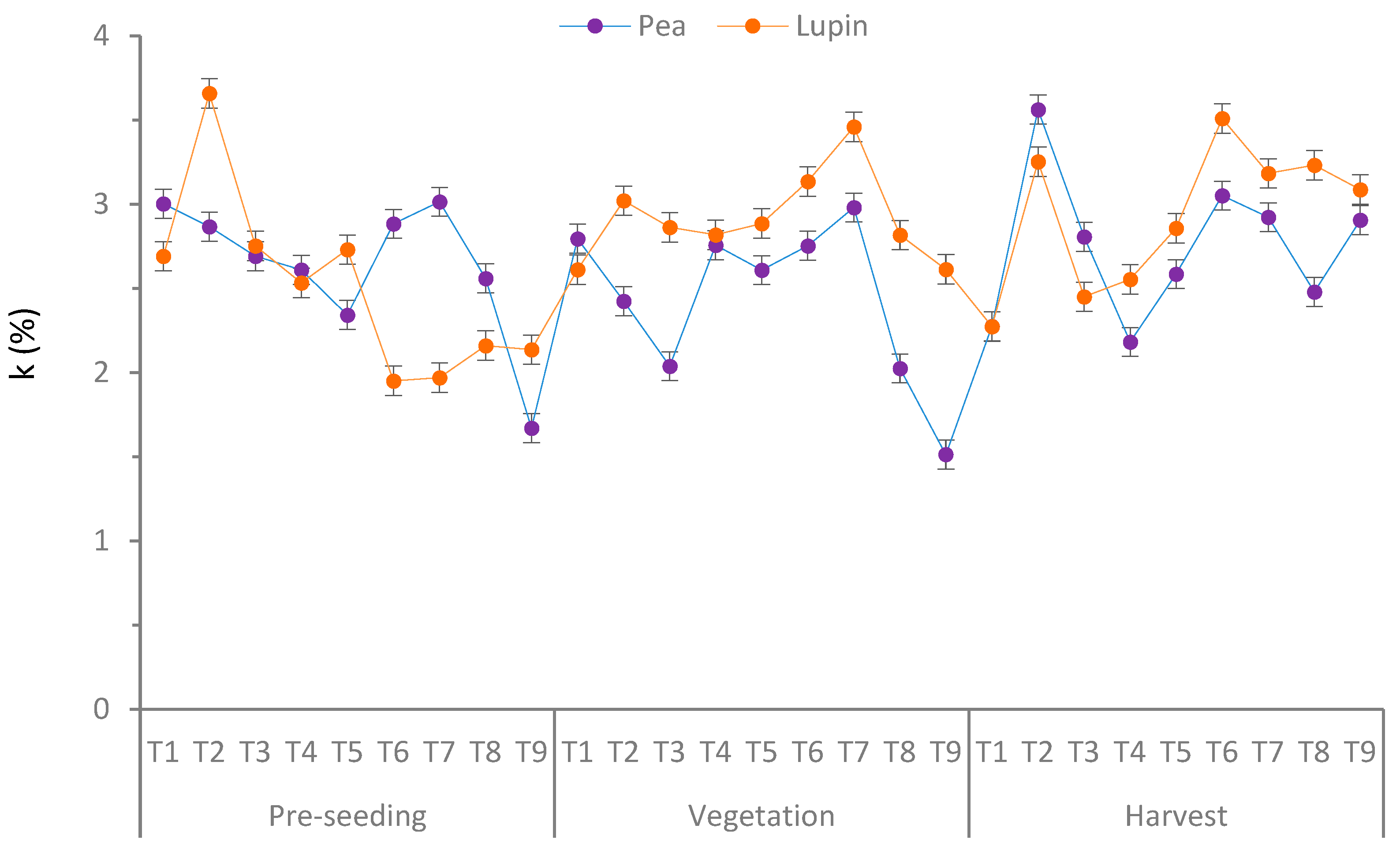
2.4.3. The Labile Fraction of the Soil Organic Matter Oxidation Reaction Rate (Speed) Constant (k)
2.4.4. Non-labile Organic Matter
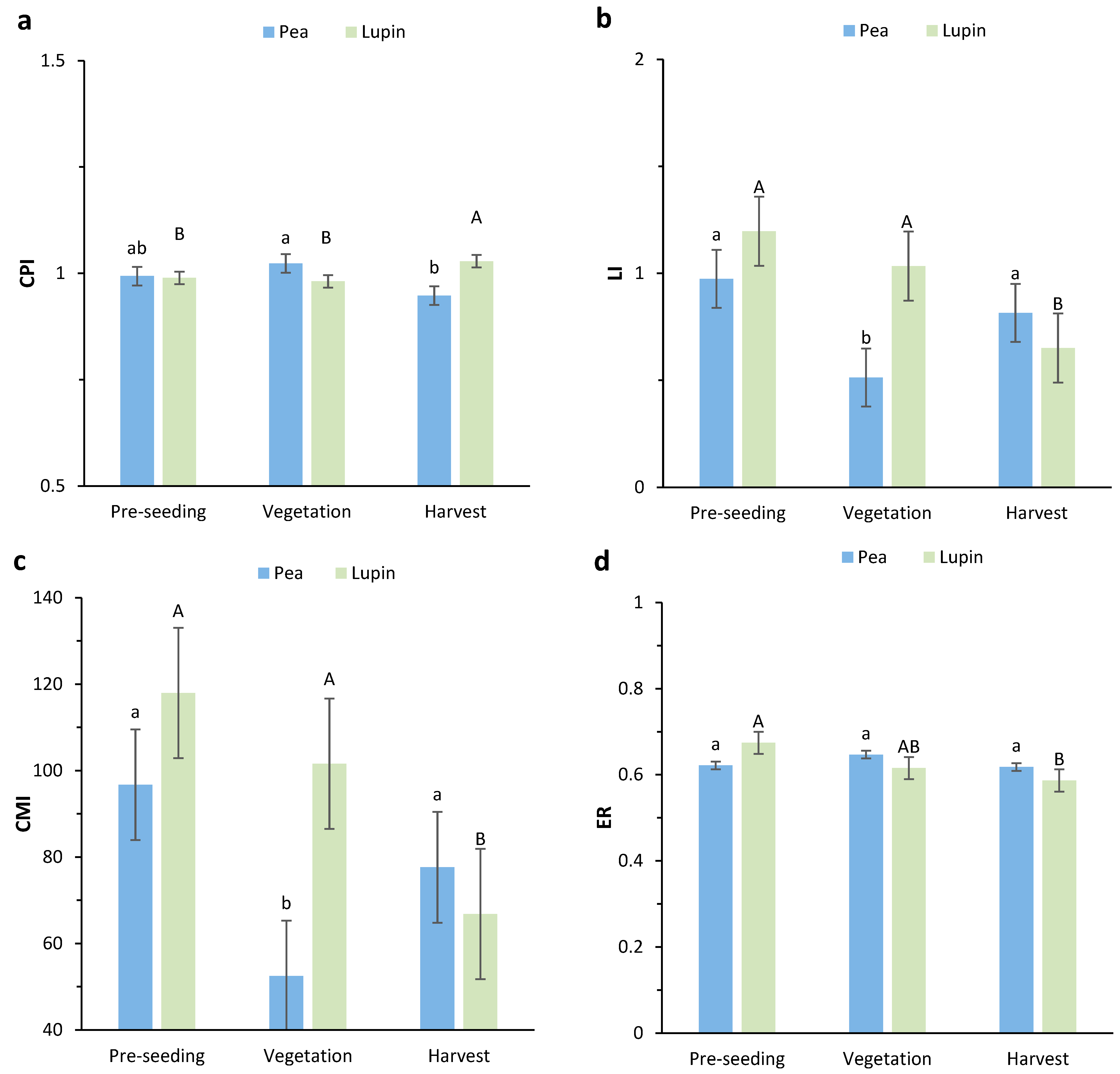
2.5. Carbon Management Index, Lability Index, and Enrichment Ratio
2.6. Statistical Analyses
3. Results
3.1. Fractions and Corresponding Carbon Content (%)
3.2. Carbon Management Index (CMI), Carbon Lability Index (LI), and Carbon Enrichment Ratio (ER)
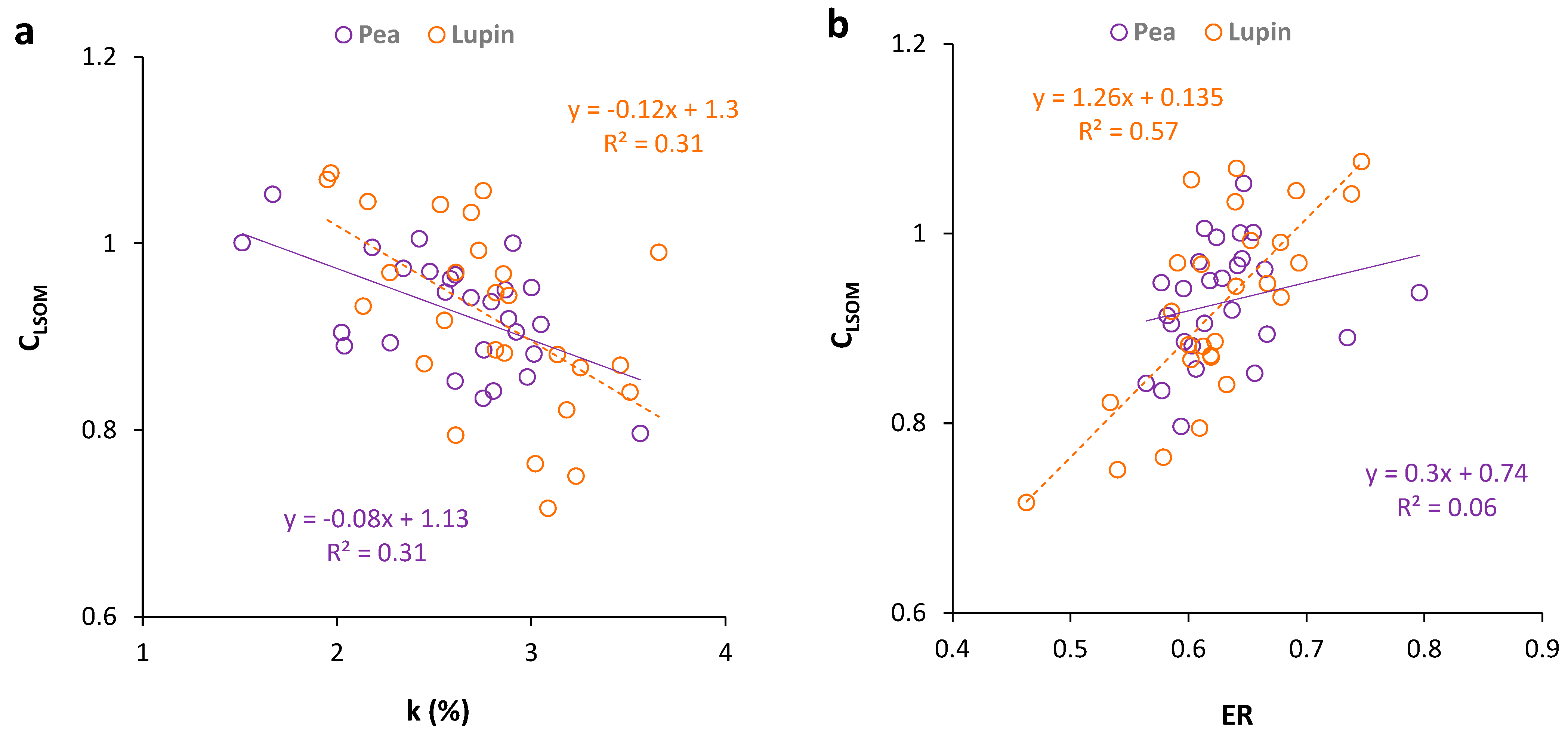
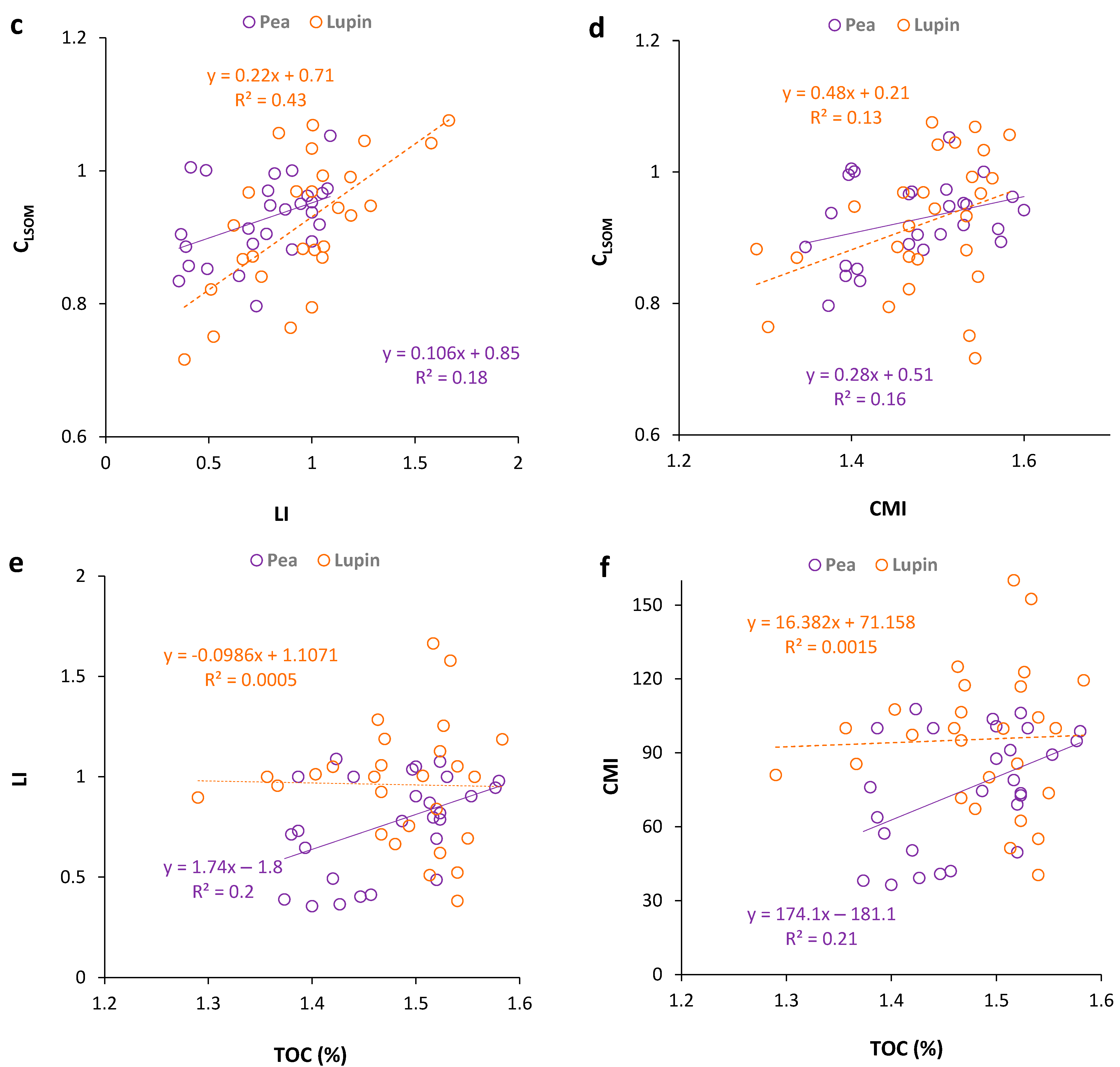
3.3. Regression Analysis between Soil Organic Matter Carbon Stocks and Selected Indicators
4. Discussion
4.1. Carbon Pool (TOC, CLSOM)
4.2. Organic Matter Pool Change and Indicators (k, ER, LI, CMI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Es, H. A New Definition of Soil. CSA News 2017, 62, 20–21. [Google Scholar] [CrossRef]
- Dror, I.; Yaron, B.; Berkowitz, B. The Human Impact on All Soil-Forming Factors during the Anthropocene. ACS Environ. Au 2022, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jenny, H.A. The Soil Resource: Origin and Behavior; Springer: New York, NY, USA, 1980; ISBN 978-1-4612-6112-4. [Google Scholar]
- Lal, R. Reducing Carbon Footprints of Agriculture and Food Systems. Carbon Footpr. 2022, 1, 3. [Google Scholar] [CrossRef]
- Kopecký, M.; Peterka, J.; Kolář, L.; Konvalina, P.; Maroušek, J.; Váchalová, R.; Herout, M.; Strunecký, O.; Batt, J.; Tran, D.K. Influence of Selected Maize Cultivation Technologies on Changes in the Labile Fraction of Soil Organic Matter Sandy-Loam Cambisol Soil Structure. Soil Tillage Res. 2021, 207, 104865. [Google Scholar] [CrossRef]
- Lal, R. Soil Conservation and Ecosystem Services. Int. Soil Water Conserv. Res. 2014, 2, 36–47. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of Soil Organic Matter on Soil Properties—A Review with Emphasis on Australian Soils. Soil Res. 2015, 53, 605. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; FAO soils bulletin; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; ISBN 978-92-5-105366-9. [Google Scholar]
- Brock, C.; Oberholzer, H.-R.; Franko, U. Soil Organic Matter Balance as a Practical Tool for Environmental Impact Assessment and Management Support in Arable Farming. Eur. J. Soil Sci. 2017, 68, 951–952. [Google Scholar] [CrossRef]
- Farid, I.M.; Abbas, M.H.H.; Beheiry, G.S.; Elcossy, S.A.E. Implications of Organic Amendments and Tillage of a Sandy Soil on Its Physical Properties and CSequestration as Well as Its Productivity of Wheat and Maize Grown Thereon. Egypt. J. Soil Sci. 2014, 54, 177–194. [Google Scholar] [CrossRef][Green Version]
- Hoang, T.N.; Konvalina, P.; Kopecký, M.; Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Ali, S.; Nguyen, T.G.; Murindangabo, Y.T.; Tran, D.K.; et al. Assessing Grain Yield and Achieving Enhanced Quality in Organic Farming: Efficiency of Winter Wheat Mixtures System. Agriculture 2023, 13, 937. [Google Scholar] [CrossRef]
- Machado, S.; Rhinhart, K.; Petrie, S. Long-Term Cropping System Effects on Carbon Sequestration in Eastern Oregon. J. Environ. Qual. 2006, 35, 1548–1553. [Google Scholar] [CrossRef]
- Widyati, E.; Nuroniah, H.S.; Tata, H.L.; Mindawati, N.; Lisnawati, Y.; Darwo; Abdulah, L.; Lelana, N.E.; Mawazin; Octavia, D.; et al. Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia. Forests 2022, 13, 1913. [Google Scholar] [CrossRef]
- Baldy, V.; Gessner, M.O.; Chauvet, E. Bacteria, Fungi and the Breakdown of Leaf Litter in a Large River. Oikos 1995, 74, 93. [Google Scholar] [CrossRef]
- Fabian, J.; Zlatanovic, S.; Mutz, M.; Premke, K. Fungal–Bacterial Dynamics and Their Contribution to Terrigenous Carbon Turnover in Relation to Organic Matter Quality. ISME J. 2017, 11, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Gulis, V.; Suberkropp, K. Interactions between Stream Fungi and Bacteria Associated with Decomposing Leaf Litter at Different Levels of Nutrient Availability. Aquat. Microb. Ecol. 2003, 30, 149–157. [Google Scholar] [CrossRef]
- Hieber, M.; Gessner, M.O. Contribution of Stream Detrivores, Fungi, and Bacteria to Leaf Breakdown Based on Biomass Estimates. Ecology 2002, 83, 1026–1038. [Google Scholar] [CrossRef]
- Pascoal, C.; Cássio, F. Contribution of Fungi and Bacteria to Leaf Litter Decomposition in a Polluted River. Appl. Environ. Microbiol. 2004, 70, 5266–5273. [Google Scholar] [CrossRef]
- Gulis, V.; Kuehn, K.A.; Schoettle, L.N.; Leach, D.; Benstead, J.P.; Rosemond, A.D. Changes in Nutrient Stoichiometry, Elemental Homeostasis and Growth Rate of Aquatic Litter-Associated Fungi in Response to Inorganic Nutrient Supply. ISME J. 2017, 11, 2729–2739. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N: P Ratios Influence Litter Decomposition and Colonization by Fungi and Bacteria in Microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Hobbie, S.E. Contrasting Effects of Substrate and Fertilizer Nitrogen on the Early Stages of Litter Decomposition. Ecosystems 2005, 8, 644–656. [Google Scholar] [CrossRef]
- Krauss, G.-J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in Freshwaters: Ecology, Physiology and Biochemical Potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Kandeler, E.; Tscherko, D.; Hobbs, P.J.; Bezemer, T.M.; Jones, T.H.; Thompson, L.J. Below-Ground Microbial Community Development in a High Temperature World. Oikos 1999, 85, 193. [Google Scholar] [CrossRef]
- Judd, K.E.; Crump, B.C.; Kling, G.W. Variation in Dissolved Organic Matter Controls Bacterial Production and Community Composition. Ecology 2006, 87, 2068–2079. [Google Scholar] [CrossRef]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Konvalina, P.; Kavková, M. Prominent Use of Lactic Acid Bacteria in Soil-Plant Systems. Appl. Soil Ecol. 2023, 189, 104955. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Yao, L. Soil Microbial Community and Physicochemical Properties Together Drive Soil Organic Carbon in Cunninghamia Lanceolata Plantations of Different Stand Ages. PeerJ 2022, 10, e13873. [Google Scholar] [CrossRef]
- Hama, J.R.; Jorgensen, D.B.G.; Diamantopoulos, E.; Bucheli, T.D.; Hansen, H. Chr.B.; Strobel, B.W. Indole and Quinolizidine Alkaloids from Blue Lupin Leach to Agricultural Drainage Water. Sci. Total Environ. 2022, 834, 155283. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Zornoza, R.; Faz, Á.; Fernández, J.A. Comparing Legumes for Use in Multiple Cropping to Enhance Soil Organic Carbon, Soil Fertility, Aggregates Stability and Vegetables Yields under Semi-Arid Conditions. Sci. Hortic. 2019, 246, 835–841. [Google Scholar] [CrossRef]
- da Silva, J.P.; Teixeira, R.d.S.; da Silva, I.R.; Soares, E.M.B.; Lima, A.M.N. Decomposition and Nutrient Release from Legume and Non-Legume Residues in a Tropical Soil. Eur. J. Soil Sci. 2022, 73, e13151. [Google Scholar] [CrossRef]
- Halvorson, J.J.; Smith, J.L.; Franz, E.H. Lupine Influence on Soil Carbon, Nitrogen and Microbial Activity in Developing Ecosystems at Mount St. Helens. Oecologia 1991, 87, 162–170. [Google Scholar] [CrossRef]
- Woźniak, A.; Rachoń, L. Yellow Lupine (Lupinus luteus L.) Response to Reduced Tillage. Arch. Agron. Soil Sci. 2022, 68, 2060–2068. [Google Scholar] [CrossRef]
- Loss, S.; Ritchie, G.; Robson, A. Effect of Lupins and Pasture on Soil Acidification and Fertility in Western Australia. Aust. J. Exp. Agric. 1993, 33, 457. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Lucas, M.M.; Pueyo, J.J. Adaptive Mechanisms Make Lupin a Choice Crop for Acidic Soils Affected by Aluminum Toxicity. Front. Plant Sci. 2022, 12, 810692. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus Plants Acquire Mercury Tolerance When Inoculated with an Hg-Resistant Bradyrhizobium Strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef]
- Xu, R.K.; Coventry, D.R. Soil PH Changes Associated with Lupin and Wheat Plant Materials Incorporated in a Red–Brown Earth Soil. Plant Soil 2003, 250, 113–119. [Google Scholar] [CrossRef]
- Kaiser, K.; Zech, W. Competitive Sorption of Dissolved Organic Matter Fractions to Soils and Related Mineral Phases. Soil Sci. Soc. Am. J. 1997, 61, 64–69. [Google Scholar] [CrossRef]
- Sun, L.; Tian, J.; Zhang, H.; Liao, H. Phytohormone Regulation of Root Growth Triggered by P Deficiency or Al Toxicity. J. Exp. Bot. 2016, 67, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Editorial: Protein Crops: Food and Feed for the Future. Front. Plant Sci. 2017, 8, 105. [Google Scholar] [CrossRef]
- Djeghri-Hocine, B.; Boukhemis, M.; Zidoune, N.; Amrane, A. Growth of Lactic Acid Bacteria on Oilseed Crop Pea- and Chickpea-Based Media. World J. Microbiol. Biotechnol. 2007, 23, 765–769. [Google Scholar] [CrossRef]
- Migiro, L.N.; Maniania, N.K.; Chabi-Olaye, A.; Vandenberg, J. Pathogenicity of Entomopathogenic Fungi Metarhizium Anisopliae and Beauveria Bassiana (Hypocreales: Clavicipitaceae) Isolates to the Adult Pea Leafminer (Diptera: Agromyzidae) and Prospects of an Autoinoculation Device for Infection in the Field. Environ. Entomol. 2010, 39, 468–475. [Google Scholar] [CrossRef]
- Senthilraja, G.; Anand, T.; Kennedy, J.S.; Raguchander, T.; Samiyappan, R. Plant Growth Promoting Rhizobacteria (PGPR) and Entomopathogenic Fungus Bioformulation Enhance the Expression of Defense Enzymes and Pathogenesis-Related Proteins in Groundnut Plants against Leafminer Insect and Collar Rot Pathogen. Physiol. Mol. Plant Pathol. 2013, 82, 10–19. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G.; Azrieli, A.; Brukental, I. Ensiling Peas, Ryegrass and Wheat with Additives of Lactic Acid Bacteria (LAB) and Cell Wall Degrading Enzymes. Grass Forage Sci. 1993, 48, 70–78. [Google Scholar] [CrossRef]
- van der Pol, L.K.; Robertson, A.; Schipanski, M.; Calderon, F.J.; Wallenstein, M.D.; Cotrufo, M.F. Addressing the Soil Carbon Dilemma: Legumes in Intensified Rotations Regenerate Soil Carbon While Maintaining Yields in Semi-Arid Dryland Wheat Farms. Agric. Ecosyst. Environ. 2022, 330, 107906. [Google Scholar] [CrossRef]
- Awale, R.; Emeson, M.A.; Machado, S. Soil Organic Carbon Pools as Early Indicators for Soil Organic Matter Stock Changes under Different Tillage Practices in Inland Pacific Northwest. Front. Ecol. Evol. 2017, 5, 96. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459. [Google Scholar] [CrossRef]
- Kopecký, M.; Kolář, L.; Perná, K.; Váchalová, R.; Mráz, P.; Konvalina, P.; Murindangabo, Y.T.; Ghorbani, M.; Menšík, L.; Dumbrovský, M. Fractionation of Soil Organic Matter into Labile and Stable Fractions. Agronomy 2021, 12, 73. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil Labile Organic Carbon Fractions and Soil Enzyme Activities after 10 Years of Continuous Fertilization and Wheat Residue Incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Horáček, J.; Čechová, V.; Borová-Batt, J.; Peterka, J. Labile Fractions of Soil Organic Matter, Their Quantity and Quality. Plant Soil Environ. 2009, 55, 245–251. [Google Scholar] [CrossRef]
- Bayer, C.; Dick, D.P.; Ribeiro, G.M.; Scheuermann, K.K. Carbon Stocks in Organic Matter Fractions as Affected by Land Use and Soil Management, with Emphasis on No-Tillage Effect. Cienc. Rural 2002, 32, 401–406. [Google Scholar] [CrossRef]
- Kolář, L.; Vaněk, V.; Kužel, S.; Peterka, J.; Borová-Batt, J.; Pezlarová, J. Relationship between Quality and Quantity of Soil Labile Fraction of the Soil Carbon in Cambisols after Limit during a 5-Years Period. Plant Soil Environ. 2011, 57, 193–200. [Google Scholar] [CrossRef]
- Shibu, M.E.; Leffelaar, P.A.; Van Keulen, H.; Aggarwal, P.K. Quantitative Description of Soil Organic Matter Dynamics—A Review of Approaches with Reference to Rice-Based Cropping Systems. Geoderma 2006, 137, 1–18. [Google Scholar] [CrossRef]
- Haynes, R.J. Interactions between Soil Organic Matter Status, Cropping History, Method of Quantification and Sample Pretreatment and Their Effects on Measured Aggregate Stability. Biol. Fertil. Soils 2000, 30, 270–275. [Google Scholar] [CrossRef]
- Lefroy, R.D.B.; Blair, G.J.; Strong, W.M. Changes in Soil Organic Matter with Cropping as Measured by Organic Carbon Fractions and 13C Natural Isotope Abundance. Plant Soil 1993, 155, 399–402. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of Soil Organic Carbon Fractions and Carbon Management Index under Different Land Use Types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef]
- Kopáček, J. (Ed.) Encyklopedie Českých Budějovic; 2., rozš. vyd.; Nebe: České Budějovice, Czech Republic, 2007; ISBN 80-239-6706-1. [Google Scholar]
- Vavruška, F. Climate. Encyclopedia of České Budějovice. 1998. Available online: http://encyklopedie.c-budejovice.cz/clanek/podnebi (accessed on 25 April 2023).
- Folorunso, E.A.; Bohatá, A.; Kavkova, M.; Gebauer, R.; Mraz, J. Potential Use of Entomopathogenic and Mycoparasitic Fungi against Powdery Mildew in Aquaponics. Front. Mar. Sci. 2022, 9, 992715. [Google Scholar] [CrossRef]
- Kavková, M.; Cihlář, J.; Dráb, V.; Bazalová, O.; Dlouhá, Z. The Interactions among Isolates of Lactiplantibacillus Plantarum and Dairy Yeast Contaminants: Towards Biocontrol Applications. Fermentation 2021, 8, 14. [Google Scholar] [CrossRef]
- Lawrence, P.G.; Roper, W.; Morris, T.F.; Guillard, K. Guiding Soil Sampling Strategies Using Classical and Spatial Statistics: A Review. Agron. J. 2020, 112, 493–510. [Google Scholar] [CrossRef]
- Maroušek, J.; Bartoš, P.; Filip, M.; Kolář, L.; Konvalina, P.; Maroušková, A.; Moudrý, J.; Peterka, J.; Šál, J.; Šoch, M.; et al. Advances in the Agrochemical Utilization of Fermentation Residues Reduce the Cost of Purpose-Grown Phytomass for Biogas Production. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From Yogurt to Yield: Potential Applications of Lactic Acid Bacteria in Plant Production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Virk, A.L.; Lin, B.-J.; Kan, Z.-R.; Qi, J.-Y.; Dang, Y.P.; Lal, R.; Zhao, X.; Zhang, H.-L. Simultaneous Effects of Legume Cultivation on Carbon and Nitrogen Accumulation in Soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2022; Volume 171, pp. 75–110. ISBN 978-0-323-98841-4. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of Straw Carbon Input on Carbon Dynamics in Agricultural Soils: A Meta-Analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, P.; Zhao, N.; Cao, W.; Zhang, S.; Li, Y.; Huang, D.; Zhai, B.; Wang, Z.; Gao, Y. Building up the Soil Carbon Pool via the Cultivation of Green Manure Crops in the Loess Plateau of China. Geoderma 2019, 337, 425–433. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in Soil Microbial Community and Organic Carbon Fractions under Short-Term Straw Return in a Rice–Wheat Cropping System. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Li, J.; Yang, X.; Guo, Z. Straw Application and Soil Organic Carbon Change: A Meta-Analysis. Soil Water Res. 2021, 16, 112–120. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Torrent, J.; Barrón, V.; Corpas, A.; Quesada-Moraga, E. Soil Properties Affect the Availability, Movement, and Virulence of Entomopathogenic Fungi Conidia against Puparia of Ceratitis Capitata (Diptera: Tephritidae). Biol. Control 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Kessler, P.; Matzke, H.; Keller, S. The Effect of Application Time and Soil Factors on the Occurrence of Beauveria Brongniartii Applied as a Biological Control Agent in Soil. J. Invertebr. Pathol. 2003, 84, 15–23. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental Controls on Soil PH in Planted Forest and Its Response to Nitrogen Deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil PH Mediates the Balance between Stochastic and Deterministic Assembly of Bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Haslam, T.C.F.; Tibbett, M. Soils of Contrasting PH Affect the Decomposition of Buried Mammalian (Ovis Aries) Skeletal Muscle Tissue. J. Forensic Sci. 2009, 54, 900–904. [Google Scholar] [CrossRef]
- Magdoff, F.; Van Es, H. Building Soils for Better Crops: Ecological Management for Healthy Soils, 4th ed.; Handbook Series; Sustainable Agriculture Research & Education: College Park, Maryland, 2021; ISBN 978-1-888626-19-3. [Google Scholar]
- Van Veen, J.A.; Kuikman, P.J. Soil Structural Aspects of Decomposition of Organic Matter by Micro-Organisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, Y.-L.; Liu, J.-D.; Lin, X.-P.; Sun, H.-Z.; Tang, X.-H.; Hu, Y.-L.; Huang, Y.-P.; Yi, Z.-G. Effects of Moisture and Temperature on Soil Organic Carbon Decomposition along a Vegetation Restoration Gradient of Subtropical China. Forests 2022, 13, 578. [Google Scholar] [CrossRef]
- Li, H.; Van Den Bulcke, J.; Mendoza, O.; Deroo, H.; Haesaert, G.; Dewitte, K.; De Neve, S.; Sleutel, S. Soil Texture Controls Added Organic Matter Mineralization by Regulating Soil Moisture—Evidence from a Field Experiment in a Maritime Climate. Geoderma 2022, 410, 115690. [Google Scholar] [CrossRef]
- Ruixue; Guo, Q.; Chen, Q.; Bernal, M.P.; Wang, Q.; Li, Y. Effect of Initial Material Bulk Density and Easily-Degraded Organic Matter Content on Temperature Changes during Composting of Cucumber Stalk. J. Environ. Sci. 2019, 80, 306–315. [Google Scholar] [CrossRef]
- Li, C.; Yue, L.; Kou, Z.; Zhang, Z.; Wang, J.; Cao, C. Short-Term Effects of Conservation Management Practices on Soil Labile Organic Carbon Fractions under a Rape–Rice Rotation in Central China. Soil Tillage Res. 2012, 119, 31–37. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Zhang, Z.; Xu, J.; Tao, B.; Meng, Y. Short-Term Responses of Soil Organic Carbon and Carbon Pool Management Index to Different Annual Straw Return Rates in a Rice–Wheat Cropping System. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Guo, L.-J.; Zhang, Z.-S.; Wang, D.-D.; Li, C.-F.; Cao, C.-G. Effects of Short-Term Conservation Management Practices on Soil Organic Carbon Fractions and Microbial Community Composition under a Rice-Wheat Rotation System. Biol. Fertil. Soils 2015, 51, 65–75. [Google Scholar] [CrossRef]
- Spedding, T.A.; Hamel, C.; Mehuys, G.R.; Madramootoo, C.A. Soil Microbial Dynamics in Maize-Growing Soil under Different Tillage and Residue Management Systems. Soil Biol. Biochem. 2004, 36, 499–512. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of Long-Term Fertilization and Residue Management on Soil Organic Carbon Changes in Paddy Soils of China: A Meta-Analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Yan, D.; Wang, D.; Yang, L. Long-Term Effect of Chemical Fertilizer, Straw, and Manure on Labile Organic Matter Fractions in a Paddy Soil. Biol. Fertil. Soils 2007, 44, 93–101. [Google Scholar] [CrossRef]
- Kay, B.D.; VandenBygaart, A.J. Conservation Tillage and Depth Stratification of Porosity and Soil Organic Matter. Soil Tillage Res. 2002, 66, 107–118. [Google Scholar] [CrossRef]
- Ahn, K.; Lee, K.-B.; Kim, Y.-J.; Koo, Y.-M. Quantitative Analysis of the Three Main Genera in Effective Microorganisms Using QPCR. Korean J. Chem. Eng. 2014, 31, 849–854. [Google Scholar] [CrossRef]
- Burford, M.A.; Costanzo, S.D.; Dennison, W.C.; Jackson, C.J.; Jones, A.B.; McKinnon, A.D.; Preston, N.P.; Trott, L.A. A Synthesis of Dominant Ecological Processes in Intensive Shrimp Ponds and Adjacent Coastal Environments in NE Australia. Mar. Pollut. Bull. 2003, 46, 1456–1469. [Google Scholar] [CrossRef]
- Danger, M.; Gessner, M.O.; Bärlocher, F. Ecological Stoichiometry of Aquatic Fungi: Current Knowledge and Perspectives. Fungal Ecol. 2016, 19, 100–111. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical Transformations of Rocks, Minerals, Metals and Radionuclides by Fungi, Bioweathering and Bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Long-Term Effective Microorganisms Application Promote Growth and Increase Yields and Nutrition of Wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Murindangabo, Y.T.; Kopecký, M.; Konvalina, P. Adoption of Conservation Agriculture in Rwanda: A Case Study of Gicumbi District Region. Agronomy 2021, 11, 1732. [Google Scholar] [CrossRef]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial Diversity at Different Stages of the Composting Process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef]
- Pineda, A.; Kaplan, I.; Bezemer, T.M. Steering Soil Microbiomes to Suppress Aboveground Insect Pests. Trends Plant Sci. 2017, 22, 770–778. [Google Scholar] [CrossRef]
- Formowitz, B.; Elango, F.; Okumoto, S.; Müller, T.; Buerkert, A. The Role of “Effective Microorganisms” in the Composting of Banana (Musa Ssp.) Residues. Z. Pflanzenernähr. Bodenk. 2007, 170, 649–656. [Google Scholar] [CrossRef]
- Giassi, V.; Kiritani, C.; Kupper, K.C. Bacteria as Growth-Promoting Agents for Citrus Rootstocks. Microbiol. Res. 2016, 190, 46–54. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effect of Compost and Chemical Fertilizer on Soil Nematode Community in a Chinese Maize Field. Eur. J. Soil Biol. 2010, 46, 230–236. [Google Scholar] [CrossRef]
- Javaid, A.; Bajwa, R. Field Evaluation of Effective Microorganisms (EM) Application for Growth, Nodulation, and Nutrition of Mung Bean. Turk. J. Agric. For. 2011, 35, 443–452. [Google Scholar] [CrossRef]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant Tissue Localization of the Endophytic Insect Pathogenic Fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic Colonisation of Tobacco, Corn, Wheat and Soybeans by the Fungal Entomopathogen Beauveria Bassiana (Ascomycota, Hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- Wang, D.; Deng, J.; Pei, Y.; Li, T.; Jin, Z.; Liang, L.; Wang, W.; Li, L.; Dong, X. Identification and Virulence Characterization of Entomopathogenic Fungus Lecanicillium Attenuatum against the Pea Aphid Acyrthosiphon Pisum (Hemiptera: Aphididae). Appl. Entomol. Zool. 2017, 52, 511–518. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Druzhinina, I.S. (Eds.) Fungal Decomposers of Plant Litter in Aquatic Ecosystems. In Environmental and Microbial Relationships; The Mycota; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 301–324. ISBN 978-3-540-71839-0. [Google Scholar]
- Romaní, A.M.; Fischer, H.; Mille-Lindblom, C.; Tranvik, L.J. Interactions of Bacteria and Fungi on Decomposing Litter: Differential Extracellular Enzyme Activities. Ecology 2006, 87, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.E.; Pringle, M.J.; Page, K.L.; Dalal, R.C. A Review of Sampling Designs for the Measurement of Soil Organic Carbon in Australian Grazing Lands. Rangel. J. 2010, 32, 227. [Google Scholar] [CrossRef]
- Smith, P. How Long before a Change in Soil Organic Carbon Can Be Detected? Time taken to measure a change in soil organic carbon. Glob. Change Biol. 2004, 10, 1878–1883. [Google Scholar] [CrossRef]
- Váchalová, R.; Borová-Batt, J.; Kolář, L.; Váchal, J. Selectivity of Ion Exchange as a Sign of Soil Quality. Commun. Soil Sci. Plant Anal. 2014, 45, 2673–2679. [Google Scholar] [CrossRef]
- Gosling, P.; Parsons, N.; Bending, G.D. What Are the Primary Factors Controlling the Light Fraction and Particulate Soil Organic Matter Content of Agricultural Soils? Biol. Fertil. Soils 2013, 49, 1001–1014. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile Organic Matter Fractions as Central Components of the Quality of Agricultural Soils: An Overview. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 85, pp. 221–268. ISBN 978-0-12-000783-7. [Google Scholar]
- Leifeld, J.; Kögel-Knabner, I. Soil Organic Matter Fractions as Early Indicators for Carbon Stock Changes under Different Land-Use? Geoderma 2005, 124, 143–155. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon Management Index Based on Physical Fractionation of Soil Organic Matter in an Acrisol under Long-Term No-till Cropping Systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Sodhi, G.P.S.; Beri, V.; Benbi, D.K. Using Carbon Management Index to Assess the Impact of Compost Application on Changes in Soil Carbon after Ten Years of Rice–Wheat Cropping. Commun. Soil Sci. Plant Anal. 2009, 40, 3491–3502. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Lanyon, L.E.; Needelman, B.A. Evaluating Soil Management Using Particulate and Chemically Labile Soil Organic Matter Fractions. Soil Sci. Soc. Am. J. 2008, 72, 180–185. [Google Scholar] [CrossRef]
- Six, J.; Guggenberger, G.; Paustian, K.; Haumaier, L.; Elliott, E.T.; Zech, W. Sources and Composition of Soil Organic Matter Fractions between and within Soil Aggregates: Sources and Composition of Soil Organic Matter Fractions. Eur. J. Soil Sci. 2001, 52, 607–618. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L.; Liu, Y.; Zhang, P.; Li, Q.; Zhou, J.; Hess, N.J.; Hazen, T.C.; Yang, W.; Chakraborty, R. Microbial Interactions with Dissolved Organic Matter Drive Carbon Dynamics and Community Succession. Front. Microbiol. 2018, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- De Bona, F.D.; Bayer, C.; Dieckow, J.; Bergamaschi, H. Soil Quality Assessed by Carbon Management Index in a Subtropical Acrisol Subjected to Tillage Systems and Irrigation. Soil Res. 2008, 46, 469. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Alam, N.M.; Dogra, P.; Bhattacharyya, R.; Sharma, N.K.; Mishra, P.K. Impact of Conservation Practices on Soil Aggregation and the Carbon Management Index after Seven Years of Maize–Wheat Cropping System in the Indian Himalayas. Agric. Ecosyst. Environ. 2016, 216, 247–257. [Google Scholar] [CrossRef]
- Kalambukattu, J.G.; Singh, R.; Patra, A.K.; Arunkumar, K. Soil Carbon Pools and Carbon Management Index under Different Land Use Systems in the Central Himalayan Region. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 200–205. [Google Scholar] [CrossRef]
- Jílková, V.; Jandová, K.; Cajthaml, T.; Devetter, M.; Kukla, J.; Starý, J.; Vacířová, A. Organic Matter Decomposition and Carbon Content in Soil Fractions as Affected by a Gradient of Labile Carbon Input to a Temperate Forest Soil. Biol. Fertil. Soils 2020, 56, 411–421. [Google Scholar] [CrossRef]



| Parameter | Description |
|---|---|
| Soil type | Orthic Luvisol |
| Soil texture | Loamy soil |
| pH (H2O) | 5.7 |
| pH (CaCl2) | 5.1 |
| Electrical conductivity | 0.0932 dS/m |
| Bulk density | 1.3 g/cm3 |
| Soil management | Conventional methods with minimum soil disturbance |
| Fertilization | Composted sheep manure, 4 T/ha (8.9 Kg N/t, 5.4 kg P2O5/t, 17.7 kg K2O/t) No synthetic inputs |
| Rotation Plan | Wheat–Oat–Barley/Clover–Peas–Lupin |
| Crops | Variables | S-W/K-S | D-W | Mean Residual | F | |
|---|---|---|---|---|---|---|
| Pea | k | CLFOM | 0.961 | 2.239 | 0.000 | F(1, 79) = 38.80 |
| ER | CLFOM | 0.139 | 1.345 | 0.000 | F(1, 25) = 54.85 | |
| LI | CLFOM | 0.200 | 1.654 | 0.000 | F(1, 25) = 18.73 | |
| CMI | CLFOM | 0.200 | 1.492 | 0.000 | F(1, 25) = 27.89 | |
| LI | TOC | 0.20 | 1.678 | 0.000 | F(1, 25) = 13.80 | |
| CMI | TOC | 0.2 | 1.03 | 0.000 | F(1, 25) = 18.74 | |
| Lupin | k | CLFOM | 0.6 | 1.431 | 0.000 | F(1, 79) = 33.40 |
| ER | CLFOM | 0.472 | 1.121 | 0.000 | F(1, 25) = 106.24 | |
| LI | CLFOM | 0.604 | 0.815 | 0.000 | F(1, 25) = 30.007 | |
| CMI | CLFOM | 0.854 | 0.69 | 0.000 | F(1, 25) = 41.314 | |
| LI | TOC | 0.60 | 0.991 | 0.000 | F(1, 25) = 0.116 | |
| CMI | TOC | 0.854 | 1.03 | 0.000 | F(1, 25) = 0.0274 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Ghorbani, M.; Konvalina, P.; Bohatá, A.; Kavková, M.; Hoang, T.N.; Kabelka, D.; et al. Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators. Agriculture 2023, 13, 1314. https://doi.org/10.3390/agriculture13071314
Murindangabo YT, Kopecký M, Perná K, Nguyen TG, Ghorbani M, Konvalina P, Bohatá A, Kavková M, Hoang TN, Kabelka D, et al. Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators. Agriculture. 2023; 13(7):1314. https://doi.org/10.3390/agriculture13071314
Chicago/Turabian StyleMurindangabo, Yves Theoneste, Marek Kopecký, Kristýna Perná, Thi Giang Nguyen, Mohammad Ghorbani, Petr Konvalina, Andrea Bohatá, Miloslava Kavková, Trong Nghia Hoang, David Kabelka, and et al. 2023. "Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators" Agriculture 13, no. 7: 1314. https://doi.org/10.3390/agriculture13071314
APA StyleMurindangabo, Y. T., Kopecký, M., Perná, K., Nguyen, T. G., Ghorbani, M., Konvalina, P., Bohatá, A., Kavková, M., Hoang, T. N., Kabelka, D., & Klenotová, E. (2023). Enhancing Soil Organic Matter Transformation through Sustainable Farming Practices: Evaluating Labile Soil Organic Matter Fraction Dynamics and Identifying Potential Early Indicators. Agriculture, 13(7), 1314. https://doi.org/10.3390/agriculture13071314








